Dear Editors,
Rett Syndrome (RTT) is a severe neurodevelopmental disorder characterized by neural dysfunctions and a reduced lifespan, mainly in female patients, involving loss-of-function mutations in the methyl-CpG binding protein 2 (MECP2) gene [1–4]. As the MECP2 gene is X-linked, its absence leads to the neonatal or juvenile fatality of male hemizygous MECP2-/y patients [5]. Owing to the predictable limited survival, Mecp2-/y mice are ideal for the experimental assessment of candidate strategies for genetic interference with RTT [6]. Adeno-associated virus serotype 9 (AAV9) has been applied to re-introduce wild-type (WT) MECP2 by intravenous injection in vivo, to extend the lifespan of Mecp2-/y mice [7]. However, intravenous administration of AAV9-MECP2 generates lethal liver toxicity in WT mice, which raises safety concerns and casts a shadow over AAV-based genetic interference in human clinical trial [8, 9].
Here, we report that, by delivering adeno-associated virus serotype 9–methyl CpG binding protein 2 (AAV9-MECP2), which was driven by the human synapsin (hSyn) promoter or the cytomegalovirus early enhancer/chicken β-actin (CAG) promoter, into the lateral ventricle (LV) by intracerebroventricular (ICV) injection on postnatal day 2, the lifespans of Mecp2-/y mice were apparently extended. We found that the protein level of MECP2 was partially rescued in neurons throughout the brain, including the cortex, hippocampus, and hypothalamus. Besides, Mecp2-/y mice injected with AAV9-CAG-MECP2 showed higher transduction efficiency in neurons, lower local overexpression risk, and increased lifespan compared with those injected with AAV9-hSyn-MECP2. Importantly, pathological features including reduced body weight, abnormal locomotor score, and abnormal gait score were partially rescued in Mecp2-/y mice injected with AAV9-CAG-MECP2. Our results provide a novel approach for genetic interference in RTT.
To generate Mecp2-/y mice with complete Mecp2 gene knock-out (KO) without mosaicism, we injected spCas9 mRNA and 4 sgRNAs into the cytoplasm of fertilized eggs (Fig. 1A) [10]. We identified a mutant Mecp2 allele with a 4-bp deletion (a 3-bp and a 1-bp deletion) in exons, which resulted in a frameshift of the Mecp2 gene (Fig. 1A), and silencing of the MECP2 protein in brain (Fig. 1B). The Mecp2-/y mice exhibited an apparent limited lifespan with a median survival of 43.2 days, compared with that of male WT mice (P <0.0001; Gehan-Breslow-Wilcoxon test) (Fig. 1C). Interestingly, we found that the median survival of Mecp2-/y mice was shorter than that in other reports (~60 to 70 days) [8, 9, 11, 12]. We reasoned that this was potentially attributable to impaired parenting ability of the maternal Mecp2-/+ mice [13]. To minimize the influence of impaired parenting, we replaced Mecp2-/+ mothers with an ICR breeding mother on postnatal day 0 of male WT and Mecp2-/y mice.
Fig. 1.
The lifespan of Mecp2-/y mice is extended by injecting AAV9-MECP2 into the lateral ventricles (LVs). A Schematic of the generation of Mecp2-/y mice with the CRISPR/Cas9 system and Sanger sequencing of male WT and Mecp2-/y mice. B Western-blots of MECP2 and α-tubulin protein in brain lysate. C Survival of male WT and Mecp2-/y mice (n = 14 WT mice; n = 18 Mecp2-/y mice; ****P <0.0001; Gehan-Breslow-Wilcoxon test). D Schematic of AAV9-hSyn-MECP2 and AAV9-CAG-MECP2 vectors. E Schematic of AAV9 injection in the LVs. F, H Western-blots with anti-MECP2 and GAPDH antibodies using brain lysates from male WT mice injected with control AAV9 vector and Mecp2-/y mice injected with AAV9-hSyn-MECP2 (F), or AAV9-CAG-MECP2 (H). G, I Survival of male WT and Mecp2-/y mice injected with AAV9 as indicated. For G, n = 6 male WT mice, injected with control AAV9 vector; n = 6 Mecp2-/y mice, injected with control AAV9 vector; n = 6 Mecp2-/y mice, injected with AAV9-hSyn-MECP2; ***P = 0.0005; Gehan-Breslow-Wilcoxon test; For I, n = 11 male WT mice, injected with control AAV9 vector; n = 5 Mecp2-/y mice, injected with control AAV9 vector; n = 7 Mecp2-/y mice, injected with AAV9-CAG-MECP2; ****P <0.0001; Gehan-Breslow-Wilcoxon test. WT indicates male WT mice; KO indicates Mecp2-/y mice.
As previously reported, the level of MECP2 expression in WT liver was 6 times that in the brain after intravenous injection [9]. The hepatic tropism triggered by intravenous injection might be attributed to: (1) the choice of endogenous Mecp2 promoter [14]; and (2) the affinity of AAV9 capsid for liver [15]. To avoid using the endogenous Mecp2 promoter and improve the transduction efficiency in neurons where MECP2 is highly expressed, we designed a MECP2-expressing vector driven by the hSyn promoter (Fig. 1D). Considering that MECP2 might also be functional in non-neuronal cells [16], we replaced the hSyn promoter with the CAG promoter to achieve more ubiquitous expression of MECP2 across all cell types in the brain (Fig. 1D). It has been shown that re-introducing MECP2 only in the brain of Mecp2 mutant mice globally compensates the normal phenotypes [17]. Thus, we made stereotactic ICV injections bilaterally into the LVs of mice on postnatal day 2, to ensure the AAV delivery was distant from the liver (Fig. 1E).
One month after AAV injection, we collected lysates of the whole brain of male WT mice and Mecp2-/y mice for Western blotting. We found that the normalized MECP2 protein level in Mecp2-/y mice was increased 41.3% ± 7.8% for AAV9-hSyn-MECP2 (Fig. 1F), and was increased 38.9% ± 6.9% for AAV9-CAG-MECP2 (Fig. 1H) compared with male WT mice injected with control AAV9 vector. Notably, both AAV9-hSyn-MECP2 and AAV9-CAG-MECP2 delivery markedly extended the lifespan of Mecp2-/y mice (Fig. 1G, I). Specifically, AAV9-hSyn-MECP2 prolonged the median survival of Mecp2-/y mice by 28.7 days or 41.05% of the lifespan (P = 0.0005; Gehan-Breslow-Wilcoxon test), whereas the AAV9-CAG-MECP2 extraordinarily prolonged the median survival of Mecp2-/y mice by 204.83 days or 273.11% (P <0.0001; Gehan-Breslow-Wilcoxon test).
For the last decade, researchers have been devoted to improving the curative effect of AAV9-based MECP2 delivery for Mecp2-/y mice. For instance, by applying cisterna magna injection of AAV9-MECP2 with endogenous Mecp2 promoter MeP229, Sinnett et al extended the median survival of Mecp2-/y mice by 27 days or 47.37% of the lifespan (*P = 0.001; Gehan-Breslow-Wilcoxon test) [9]. Besides, Gadalla et al. prolonged the median lifespan by 51.1 days or 78.49% of the lifespan (P <0.0001; Gehan-Breslow-Wilcoxon test) by ICV injection of AAV9-MECP2 with C-terminal Myc tag under the chicken β-actin (CBA) promoter into neonatal Mecp2-/y mice [8]. Importantly, our strategy of bilateral ICV injection into neonatal mice with AAV9-CAG-MECP2 exhibited a great advantage over previous work in prolonging lifespan of Mecp2-/y mice.
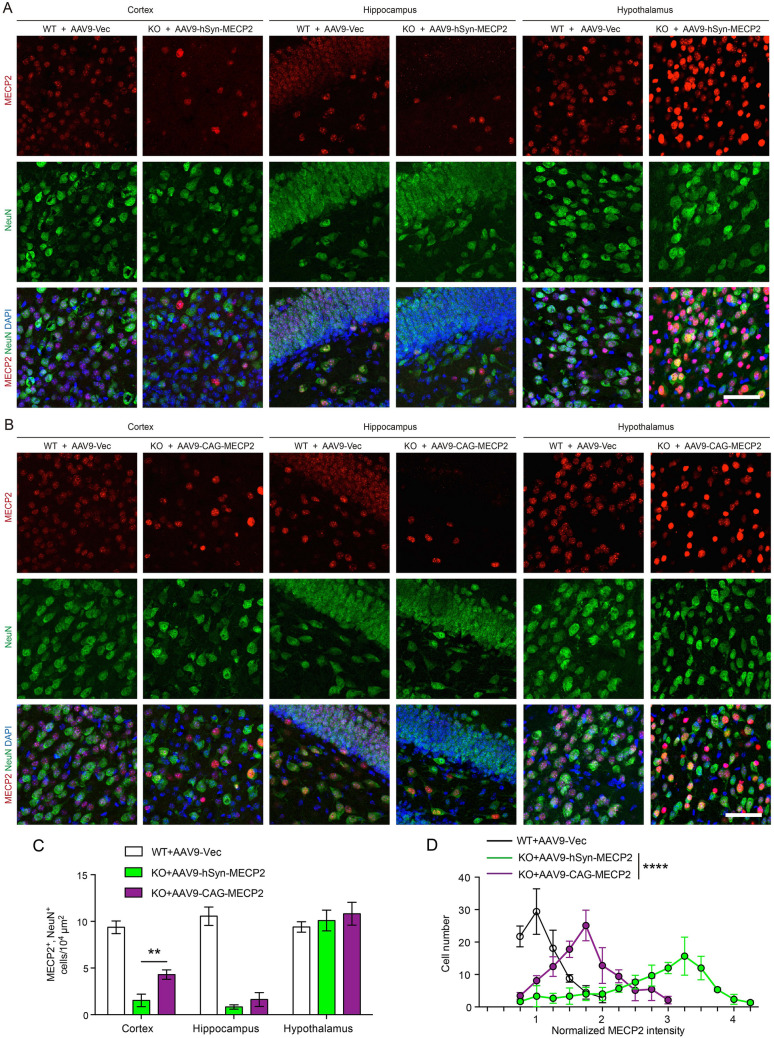
To estimate the MECP2 transduction efficiency in the neurons of Mecp2-/y mice, we applied immunofluorescence staining for both MECP2 and NeuN (Fig. 2A, B). We found that the protein level of MECP2 was partially rescued in neurons throughout the brain, including the cortex, hippocampus, and hypothalamus. However, the transduction efficiency differed among brain regions. The highest efficiency occurred in the hypothalamus while the lowest efficiency was in the hippocampus (Fig. 2A, B). Interestingly, the Mecp2-/y mice injected with AAV9-CAG-MECP2 showed a higher density of MECP2/NeuN double-positive cells than those injected with AAV9-hSyn-MECP2 (P = 0.0045; unpaired Student’s t test), showing that the CAG promoter achieved better transduction efficiency even in the neural population in our hands (Fig. 2C). Actually, we found that MECP2 was expressed in both astrocytes and oligodendrocytes in the brain of WT mice (Fig. S1A, B), indicating that MECP2 plays roles in non-neuronal cells [18]. We showed that ICV delivery of AAV9-CAG-MECP2 restored MECP2 in both astrocytes (GFAP-positive) and oligodendrocytes (IBA1-positive) in the brain of Mecp2-/y mice (Fig. S1A, B). Thus, we inferred that restoring MECP2 in non-neuronal cells might also have contributed to the better prolongation of the lifespan of Mecp2-/y mice in the AAV9-CAG-MECP2 group.
Fig. 2.
AAV9-CAG-MECP2 has higher transduction efficiency in neurons with milder local overexpression. A, B Representative images of immunofluorescence of cortex, hippocampus and hypothalamus (red, MECP2; blue, DAPI; green, NeuN; scale bars, 100 μm). For A, n = 3 slices from 3 male WT mice injected with control AAV9 vector; n = 3 slices from 3 Mecp2-/y mice injected with AAV9-hSyn-MECP2. For B, n = 3 slices from 3 male WT mice injected with control AAV9 vector; n = 3 slices from 3 Mecp2-/y mice injected with AAV9-CAG-MECP2. C Counts of MECP2/NeuN double-positive cells in the cortex, hippocampus, and hypothalamus of mice injected with AAVs as indicated (**P = 0.0045; unpaired Student’s t test). D Normalized MECP2 protein intensity in the hypothalamus of mice injected AAVs as indicated (black line, n = 254 cells from 3 slices from 3 male WT mice injected with control AAV9 vector; green line, n = 272 cells from 3 slices from 3 Mecp2-/y mice injected with AAV9-hSyn-MECP2; purple line, n = 292 cells from 3 slices from 3 Mecp2-/y mice injected with AAV9-CAG-MECP2; ****P < 0.0001; ordinary one-way ANOVA test). WT, male WT mice; KO, Mecp2-/y mice.
Then, we calculated the immunofluorescence intensity of MECP2 of each cell from each group, and found that it varied largely in hypothalamus. In the male WT mice injected with control AAV9 vector, the normalized MECP2 intensity was 1 (range, 0.5–2.0) (Fig. 2D). However, in the Mecp2-/y mice injected with AAV9-hSyn-MECP2, the normalized MECP2 intensity was 3.25 (range, 0.5–4.25), indicating strong overexpression in the hypothalamus (Fig. 2D). On the contrary, the Mecp2-/y mice injected with AAV9-CAG-MECP2 showed milder overexpression of MECP2 (1.75; range, 0.5–3) (Fig. 2D). We suggest that the expression of MECP2 driven by the CAG promoter exhibited better transduction efficiency throughout the brain with diffusion in the cerebrospinal fluid. Compared with the hSyn promoter, the CAG promoter executed a widespread and moderate overexpression of MECP2 (P <0.0001; ordinary one-way ANOVA test), which in turn enhanced the long-lasting restoration of MECP2 in Mecp2-/y mice.
In addition to reduced survival, Mecp2 -/y mice exhibit well-described neurological features including reduced body weight, abnormal locomotion, and abnormal gait [19]. Thus, we examined the rescue effect on developmental and behavioral phenotypes. We showed that bilateral ICV injection of AAV9-CAG-MECP2 into neonatal mice slightly rescued parts of the developmental and behavioral deficits. We found that Mecp2 -/y mice injected with AAV9-CAG-MECP2 showed a mild increase of body weight compared with Mecp2 -/y mice injected with AAV9-Vector, at postnatal day 45 (P = 0.0128; paired Student’s t test) (Fig. S2A). To better characterize the behavioral deficits in this mouse model, we applied an established observational aggregate phenotypic severity scoring system [20]. We found that the abnormal locomotor score was partially rescued in Mecp2 -/y mice injected with AAV9-CAG-MECP2 on postnatal day 31, compared with Mecp2 -/y mice injected with AAV9-Vector (P = 0.0377; paired Student’s t test) (Fig. S2B). Besides, we showed that the abnormal gait score was partially rescued in Mecp2 -/y mice injected with AAV9-CAG-MECP2 on postnatal day 38, compared with Mecp2 -/y mice injected with AAV9-Vector (P = 0.0377; paired Student’s t test) (Fig. S2C). These results demonstrated that bilateral ICV injection of AAV9-CAG-MECP2 into neonatal mice compensates Mecp2 -/y mice for both lifespan and neurological features.
Here, we generated a Mecp2-/y model based on CRISPR/Cas9. To avoid liver toxicity, we used bilateral ICV injection at the neonatal stage and eliminated the use of an endogenous Mecp2 promoter. We ensured the transduction efficiency in neurons by applying the hSyn promoter or the CAG promoter. We found that AAV9-CAG-MECP2 induced a widespread, long-lasting restoration of MECP2, contributing to an outstanding extension of the lifespan of Mecp2-/y mice. Then, we revealed that the protein level of MECP2 re-introduced within each neuron and the percentage of neurons transduced, as well as re-introduction into non-neuronal cells, plays a decisive role in prolonging the lifespan of Mecp2-/y mice. Finally, we showed that injection of AAV9-CAG-MECP2 compensates Mecp2 -/y mice for both lifespan and neurological features. These findings provide a potential strategy for genetic interference with RTT.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the Youth Innovation Promotion Association Chinese Academy of Sciences (2022269), the Youth Science Fund of State Key Laboratory of Neuroscience(SKLN-2022B006), the National Natural Science Foundation of China (82001211, 81941015, and 82021001), the CPSF-CAS Joint Foundation for Excellent Postdoctoral Fellows (2017LH036), the China Postdoctoral Science Foundation (2017M620173), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDBS01060200), the Program of Shanghai Academic Research Leader, the Open Large Infrastructure Research of Chinese Academy of Sciences, the Shanghai Municipal Science and Technology Major Project (2018SHZDZX05), and the Guangdong Key Scientific and Technological Project (2018B030335001).
Conflict of interest
These authors declare no competing interests.
Contributor Information
Kan Yang, Email: kanyang@ion.ac.cn.
Zilong Qiu, Email: zqiu@ion.ac.cn.
References
- 1.Qiu ZL, Sylwestrak EL, Lieberman DN, Zhang Y, Liu XY, Ghosh A. The Rett syndrome protein MeCP2 regulates synaptic scaling. J Neurosci. 2012;32:989–994. doi: 10.1523/JNEUROSCI.0175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirby RS, Lane JB, Childers J, Skinner SA, Annese F, Barrish JO, et al. Longevity in Rett syndrome: Analysis of the North American Database. J Pediatr. 2010;156:135–138.e1. doi: 10.1016/j.jpeds.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amir RE, van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZC, Han JH. The first national prevalence of autism spectrum disorder in China. Neurosci Bull. 2020;36:959–960. doi: 10.1007/s12264-020-00571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyst MJ, Bird A. Rett syndrome: A complex disorder with simple roots. Nat Rev Genet. 2015;16:261–275. doi: 10.1038/nrg3897. [DOI] [PubMed] [Google Scholar]
- 6.Zhou ZL, Hong EJ, Cohen S, Zhao WN, Ho HYH, Schmidt L, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadalla KKE, Bailey MES, Spike RC, Ross PD, Woodard KT, Kalburgi SN, et al. Improved survival and reduced phenotypic severity following AAV9/MECP2 gene transfer to neonatal and juvenile male Mecp2 knockout mice. Mol Ther. 2013;21:18–30. doi: 10.1038/mt.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinnett SE, Hector RD, Gadalla KKE, Heindel C, Chen D, Zaric V, et al. Improved MECP2 gene therapy extends the survival of MeCP2-null mice without apparent toxicity after intracisternal delivery. Mol Ther Methods Clin Dev. 2017;5:106–115. doi: 10.1016/j.omtm.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo EW, Cai YJ, Li K, Wei Y, Wang BG, Sun YD, et al. One-step generation of complete gene knockout mice and monkeys by CRISPR/Cas9-mediated gene editing with multiple sgRNAs. Cell Res. 2017;27:933–945. doi: 10.1038/cr.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg SK, Lioy DT, Cheval H, McGann JC, Bissonnette JM, Murtha MJ, et al. Systemic delivery of MeCP2 rescues behavioral and cellular deficits in female mouse models of Rett syndrome. J Neurosci. 2013;33:13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tillotson R, Selfridge J, Koerner MV, Gadalla KKE, Guy J, de Sousa D, et al. Radically truncated MeCP2 rescues Rett syndrome-like neurological defects. Nature. 2017;550:398–401. doi: 10.1038/nature24058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pohl AL, Crockford SK, Blakemore M, Allison C, Baron-Cohen S. A comparative study of autistic and non-autistic women's experience of motherhood. Mol Autism. 2020;11:3. doi: 10.1186/s13229-019-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray SJ, Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J, et al. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Gene Ther. 2011;22:1143–1153. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray SJ, Nagabhushan Kalburgi S, McCown TJ, Jude Samulski R. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20:450–459. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, et al. A role for glia in the progression of Rett's syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luikenhuis S, Giacometti E, Beard CF, Jaenisch R. Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc Natl Acad Sci USA. 2004;101:6033–6038. doi: 10.1073/pnas.0401626101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 20.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.