The continuous development of various gene therapies has brought new light to the treatment of genetic diseases. Among them, therapies targeting monogenic diseases are relatively progressive and promising due to the explicit and clear pathogenesis. The discovery and development of clustered regularly interspaced short palindromic repeats/associated nuclease (CRISPR/Cas) and related technologies was undoubtedly an extraordinary leap forward for gene therapy, especially the rise of base editors which provide a safer method with site-directed mutation for the treatment of monogenic diseases. However, these methods requiring the CRISPR/Cas system or other exogenous protein interventions such as viral capsid, the exogenous overexpressed enzyme leads to potential problems including off-target effects, the immune response, or inefficient delivery.
Recently, Chandra Vargeese et al. have designed an efficient tool for adenine (A)-to-inosine (I) RNA base editing by recruiting the endogenous adenosine deaminases acting on RNA (ADAR), short chemically modified oligonucleotides named AIMers which do not need an exogenous enzyme and vector for delivery [1]. The team applied a series of chemical modifications to synthesize various stereopure and stereorandom AIMers, screening for effective endogenous ADAR-recruiting and base editing based on the oligonucleotide chemistry platform they had previously developed [2]. Here, we briefly introduce its characteristics and discuss the perspective on neurological disorders.
This biotechnology demonstrated two main breakthroughs. On the one hand, stereopure AIMers presented efficient editing in the absence of double-stranded ADAR-recruiting domains and the introduction of exogenous proteins. In previous studies, the researchers adopted diverse strategies to resolve the restriction of the double-stranded RNA binding domain of ADAR to recognize dsRNA, such as designing a specific guide RNA or optimizing the structure of an antisense oligonucleotide (ASO) [3, 4]. However, these guide RNAs and ASOs were relatively too long to be conveniently synthesized, modified, or delivered. Here, the design AIMer was inspired by the representative endogenous substrate GluR; its full length was truncated to only 30 mers after chemical modification and purification. On the other hand, to achieve organ targeting, they applied the conjugation of N-acetylgalactosamine (GalNAc) with stereopure AIMers and achieved liver-specific editing in non-human primates (NHPs) without relying on extra exogenous delivery vehicles [5].
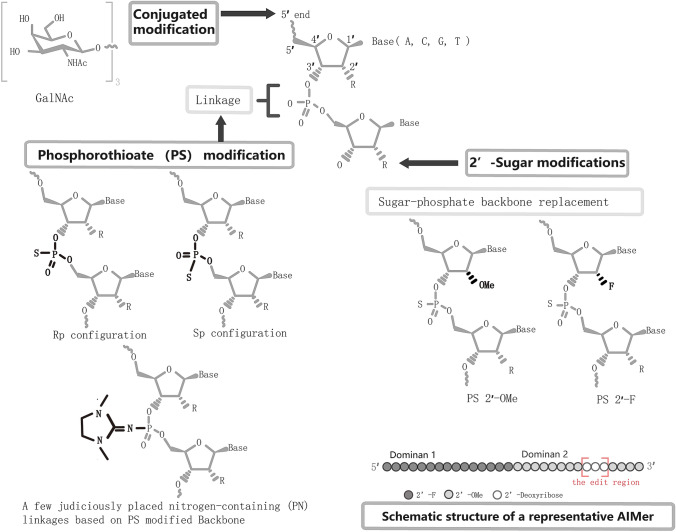
To optimize AIMers, three chemical modifications (Fig. 1) were tested to improve editing efficiency. First, stereorandom phosphorothioate (PS) modification of the backbone, a clinically promising modification widely applied in ASOs [5], was introduced into AIMers, and it was found that this kind of AIMer edited the target luciferase RNA effectively. In the subsequent test of chirality, the PS-modified backbone of Sp configuration had a boosted ability to increase the editing of both ADAR1 isoforms. To confirm the effect of stereopurity, the ADAR1-optimized stereopure AIMers were then tested in editing experiments on 7 endogenous transcripts in primary human retinal pigmented epithelial cells and primary human hepatocytes and showed higher efficiency than their control stereorandom AIMers. Next, the addition of a few judiciously-placed nitrogen-containing (PN) linkages based on the PS-modified backbone was verified to further enhance the editing of stereopure PS-modified AIMers. The second chemical modification in AIMer was 2′-ribose modification: 2′-deoxyfluoro (2′-F) modifications and 2′-O-methyl (2’-OMe) modifications. The appropriate combination of 2’-F and 2’-OMe based on the PS-modified backbone further acted as a positive reaction. Third, 5′-end GalNAc-conjugated modification, which has been reported to increase the uptake of ASOs in hepatocytes, was added and tested both in vitro and in vivo. Significantly, its subcutaneous injection into Cynomolgus monkeys was well tolerant and provides valuable pharmacological data for future reference. Moreover, deep RNA-seq demonstrated its low off-target effects without bystander editing.
Fig. 1.
Schematic chemical modifications tested on AIMers. The structure of an AIMer includes domain 1 and domain 2, 30 mers in total, and the edit region marked by the red box in domain 2. A total of 3 chemical modification were tested on AIMers: phosphorothioate (PS) modified backbone with exploration of the effect of chirality and placing a few nitrogen-containing (PN) linkages, 2′-ribose modification, and the conjugation of N-acetylgalactosamine (GalNAc).
Therapeutic RNA editing was first termed by Woolf et al. in 1995. Editing at the mRNA level is considered to be promising because it can reversibly and temporarily perturb specific gene expression without genome modification. The transformation from C:G to T:A was the most common mutation in human genes, covering a large proportion of pathogenic mutations. Therefore, therapeutic ADAR-mediated A-to-I editing has broad clinical application prospects. To improve its targetability, biological activity, delivery efficiency and biological security, various editing strategies have emerged, such as RESTORE [4] and LEAPER [3]. However, we still know little about their effects in vivo. Meanwhile, some studies have reported that chemical modifications (e.g., PS modifications, 2′-ribose modification, GalNAc-conjugated modification) can improve the targetability, biological activity, and pharmacokinetic kinetics of ASOs during in vivo administration and function [6]. The stereopure chemical modifications of AIMer were similar or even optimized compared to ASOs. In addition, the pharmacological data of the stereopure AIMer manifested the basic feasibility of A-to-I RNA editing in NHPs.
The verification of the editing effects in NHPs is a crucial step before entering clinical trials. Before the stereopure AIMer, Kathiresan et al. once performed a near-complete knockdown of PCSK9 in the liver to lower cholesterol in cynomolgus monkeys using lipid nanoparticles that encapsulated CRISPR base editors for the treatment of atherosclerotic cardiovascular disease [7]. Although the editing of CRISPR base editors was efficient with a low-level off-target effects and did not make double-strand breaks, an exogenous delivery vehicle for CRISPR tools was requisite. Meanwhile, whether targeting the genome by CRISPR base editors escapes potential biosafety issues remains uncertain.
Genetic neurological disorders remain intractable problems. Many of them are caused by the G > A monogenic mutation. Typically, SHANK family gene-related autism (c.2621G>A, p.Arg874His) [8], MYO6-mutated nonsyndromic hearing loss in an autosomal dominant pattern (NSAD) (DFNA22) (c.1325G>A, p.Cys442Tyr) [9], and SLC20A2-related Fahr disease (c.1492G>A, p.Gly498Arg) [10] were separately identified by sequencing. A-to-I RNA editing may bring some new therapeutic insights into these diseases. Although the pharmacokinetic data of stereopure AIMer has not been explored in the nervous system, the previous work on ASO drugs can provide some hints. Generally, chemically-modified ASOs are mainly distributed and accumulate in the liver when administered intravenously, resulting in insufficient pharmacological activity in cerebrospinal fluid [6]. Notably, intrathecal and intracerebroventricular administration can produce a significant pharmacokinetic manifestation with broad distribution, long-term effects, and a characteristic half-life in the central nervous system tissue of mice and NHPs [6, 11]. Clinically, intrathecal injection of the ASO drug, Spinraza®, was approved for spinal muscular atrophy by the U.S. Food and Drug Administration in 2018. Moreover, some specific encapsulations of ASOs could dramatically improve transportability across the blood-brain barrier by minor invasive administration, such as an ASO-loaded glucose‐modified polymeric nanocarrier which can be transported by glucose transporter‐1 expressed on brain capillary endothelial cells through transcytosis and exhibited a satisfactory effect on targeting long non‐coding RNA in various brain regions [12]. Therefore, we may extend the application of stereopure AIMers in neurological disorders by complementary encapsulation or specific administration methods (e.g., stereotactic administration and intrathecal or intracerebroventricular injection). To sum up, stereopure AIMer, as a novel efficient A-to-I RNA base-editing tool, takes another crucial leap forward in optimizing the strategy of therapeutic ADAR-mediated RNA editing.
Acknowledgements
We thank Yong Chen from Rib Lab for cooperation, discussion, and schematic drawing during this investigation. This work was supported by grants from the Ministry of Science and Technology of China (2018YFA0107900, 92168103, 32171417, 82001140 and 2019CXJQ01), the National Nature Science Foundation, and Shanghai Municipal Government, Peak Disciplines (Type IV) of Institutions of Higher Leaning in Shanghai.
Footnotes
Jingyu Yu and Tianwen Li have contributed equally to this work.
References
- 1.Monian P, Shivalila C, Lu G, Shimizu M, Boulay D, Bussow K, et al. Endogenous ADAR-mediated RNA editing in non-human Primates using stereopure chemically modified oligonucleotides. Nat Biotechnol 2022: 1–10. [DOI] [PubMed]
- 2.Iwamoto N, Butler DCD, Svrzikapa N, Mohapatra S, Zlatev I, Sah DWY, et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat Biotechnol. 2017;35:845–851. doi: 10.1038/nbt.3948. [DOI] [PubMed] [Google Scholar]
- 3.Qu L, Yi Z, Zhu S, Wang C, Cao Z, Zhou Z, et al. Programmable RNA editing by recruiting endogenous ADAR using engineered RNAs. Nat Biotechnol. 2019;37:1059–1069. doi: 10.1038/s41587-019-0178-z. [DOI] [PubMed] [Google Scholar]
- 4.Merkle T, Merz S, Reautschnig P, Blaha A, Li Q, Vogel P, et al. Precise RNA editing by recruiting endogenous ADARs with antisense oligonucleotides. Nat Biotechnol. 2019;37:133–138. doi: 10.1038/s41587-019-0013-6. [DOI] [PubMed] [Google Scholar]
- 5.Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: An overview and prospectus. Nat Rev Drug Discov. 2021;20:427–453. doi: 10.1038/s41573-021-00162-z. [DOI] [PubMed] [Google Scholar]
- 6.Bennett CF, Krainer AR, Cleveland DW. Antisense oligonucleotide therapies for neurodegenerative diseases. Annu Rev Neurosci. 2019;42:385–406. doi: 10.1146/annurev-neuro-070918-050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musunuru K, Chadwick AC, Mizoguchi T, Garcia SP, DeNizio JE, Reiss CW, et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in Primates. Nature. 2021;593:429–434. doi: 10.1038/s41586-021-03534-y. [DOI] [PubMed] [Google Scholar]
- 8.Qin Y, Du Y, Chen L, Liu Y, Xu W, Liu Y, et al. A recurrent SHANK1 mutation implicated in autism spectrum disorder causes autistic-like core behaviors in mice via downregulation of mGluR1-IP3R1-calcium signaling. Mol Psychiatry. 2022;27:2985–2998. doi: 10.1038/s41380-022-01539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melchionda S, Ahituv N, Bisceglia L, Sobe T, Glaser F, Rabionet R, et al. MYO6, the human homologue of the gene responsible for deafness in Snell's waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am J Hum Genet. 2001;69:635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Li Y, Shi L, Ren J, Patti M, Wang T, et al. Mutations in SLC20A2 link familial idiopathic basal Ganglia calcification with phosphate homeostasis. Nat Genet. 2012;44:254–256. doi: 10.1038/ng.1077. [DOI] [PubMed] [Google Scholar]
- 11.Kuijper EC, Bergsma AJ, Pijnappel WWMP, Aartsma-Rus A. Opportunities and challenges for antisense oligonucleotide therapies. J Inherit Metab Dis. 2021;44:72–87. doi: 10.1002/jimd.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min HS, Kim HJ, Naito M, Ogura S, Toh K, Hayashi K, et al. Systemic brain delivery of antisense oligonucleotides across the blood-brain barrier with a glucose-coated polymeric nanocarrier. Angew Chem Int Ed Engl. 2020;59:8173–8180. doi: 10.1002/anie.201914751. [DOI] [PMC free article] [PubMed] [Google Scholar]