Abstract
Study Objectives
To naturalistically measure sleep disturbances following stress exposure (i.e. sleep reactivity) and stress responses following sleep disturbances (i.e. stress reactivity) at the daily level and prospectively examine these reactivity measures as individual risk factors for insomnia.
Methods
The study assessed 392 nurses’ sleep and stress for 14 days using daily diaries and actigraphy. Self-reported insomnia symptoms were assessed at the end of the 14 days, as well as 6 and 11 months later.
Results
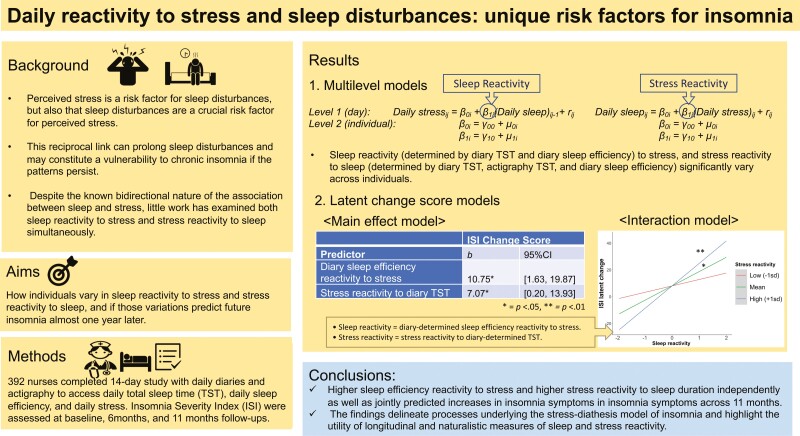
In multilevel modeling, while negative fixed effects indicated that shorter total sleep time (TST) and lower sleep efficiency led to greater stress and vice versa, significant random effects indicated individual variability in sleep reactivity and stress reactivity. In latent score change modeling, greater sleep reactivity (lower diary-determined sleep efficiency following greater stress) and greater stress reactivity (greater stress following shorter diary-determined TST) at baseline were associated with greater insomnia symptoms at 11 months (b = 10.34, p = .026; b = 7.83, p = .03). Sleep reactivity and stress reactivity also interacted to predict insomnia symptoms, such that sleep reactivity was significantly associated with insomnia symptoms for those with high (+1 SD) stress reactivity (b = 17.23, p = .001), but not for those with low (−1 SD) stress reactivity (b = 5.16, p = .315).
Conclusions
Baseline stress reactivity and sleep reactivity independently as well as jointly predict greater insomnia symptoms 11 months later. The findings delineate processes underlying the stress-diathesis model of insomnia and highlight the utility of longitudinal and naturalistic measures of sleep and stress reactivity.
Keywords: sleep, stress, sleep reactivity, stress reactivity, insomnia, predisposition
Graphical Abstract
Graphical Abstract.
Statement of Significance.
Using naturalistic and longitudinal measures of daily stress and sleep, we identified that nurses vary in the degree to which their stress and sleep co-fluctuate—defined as stress reactivity (to sleep) and sleep reactivity (to stress). Importantly, nurses with higher stress reactivity to sleep loss and higher sleep efficiency reactivity to stress had greater increases in insomnia symptoms across a year. Furthermore, these two measures jointly predicted insomnia, such that greater sleep reactivity to stress predicted the greatest increase in insomnia when combined with high levels of stress reactivity to short sleep. These findings highlight that daily stress reactivity and sleep reactivity may be important risk factors for predicting insomnia among nurses, and potential targets for cognitive-behavioral interventions.
Introduction
Daily stress and sleep are closely related and likely have a bidirectional relationship [1–6]. Anxious or depressed emotional states can heighten arousal and generate negative thoughts and worries, thereby worsening sleep duration, efficiency, depth, and quality [7–11]. Worse sleep duration or efficiency can conversely trigger or exacerbate negative emotions such as anxiety, depression, fatigue, and stress [12, 13]. Sleep problems (e.g. sleep loss and transient insomnia) impair inhibitory control and coping abilities, thereby amplifying stress responses [14, 15]. This reciprocal link can prolong sleep disturbances and may constitute a vulnerability to chronic insomnia and other stress pathologies if the patterns persist.
However, sleep reactivity, defined as sleep disturbance following stress exposure, has been shown to differ across individuals in a trait-like pattern [16, 17]. Some individuals experience significantly worse quality of sleep (e.g. low sleep efficiency or short duration) following stress exposure, whereas others’ sleep remains relatively unchanged [18]. These patterns of sleep reactivity responses tend to be consistent across different stress exposure stimuli [19].
A person with greater sleep reactivity may be more vulnerable to developing chronic insomnia, due to repeated heightened psychological, autonomic, and neuroendocrine responses to stress. For instance, empirical studies show that greater baseline sleep reactivity increases the risk of insomnia one year later [20, 21]. However, most of the previous studies have measured sleep reactivity in a cross-sectional and retrospective way. The Ford Insomnia Response to Stress (FIRST), the most widely used measure of self-reported sleep reactivity [16, 22–25], asks how much a person thinks their sleep would likely be affected by stress as a proxy for “reactivity.” One limitation of this measure is that it is largely a measure of a history of sleep disturbances in response to stress (i.e. How could a participant know a particular event might cause sleep disturbances unless they have experienced said effect previously?). That said, studies using the FIRST show it correlates with polysomnography measures of sleep reactivity [2, 16, 24]. However, retrospective and subjective measures such as the FIRST are susceptible to attribution and recall biases [26–28]. A person may think that their sleep is highly sensitive to stressful events because of their mood and/or transient sleep problems at the time of survey completion. One measurement approach that attenuates many of these limitations and provides more inferential strength is an assessment of sleep reactivity on a daily basis (i.e. relationship between stress exposure and next day sleep).
Similarly, stress reactivity, defined as increased emotional or general perceived stress responses following sleep disturbances (e.g. short sleep duration or reduced sleep efficiency), also varies across individuals in a trait-like pattern [29]. Several studies show that shorter sleep duration and reduced sleep efficiency are associated with next-day increases in perceived stress [12, 13]. Individual differences in stress regulation difficulties have been highlighted in the etiological theories of insomnia [30, 31], with some individuals being predisposed to experience more emotional arousal after transient insomnia than others. Studies have also documented individual differences in the extent to which sleep deprivation (i.e. short duration) and insomnia predicts impairment of cognitive and behavioral functioning, which are key factors for stress and emotion regulation [17, 25, 32]. It is plausible that repeated emotion dysregulation, paired with next day psychophysiological and cognitive hyperarousal in response to sleep disturbance leads to the development of chronic insomnia [30]. In addition, stress reactivity to reduced sleep or sleep disturbance/fragmentation is likely accompanied by dysfunctional beliefs and attitudes about sleep, such as unrealistic expectations about sleep and excessive worries about the consequences of disturbed sleep, which have been suggested as perpetuating factors of insomnia [25, 33, 34].
To date, no work has examined acute stress reactivity to sleep loss or worse sleep efficiency as a predictor of long-term insomnia risk. However, one longitudinal study with multiple waves showed that non-restorative sleep predicted work stress prospectively, which in turn predicted insomnia symptoms at the following wave [3]. Thus, it is plausible that exaggerated and repeated stress reactivity to transient sleep disturbances may be a catalyst or precipitant for chronic sleep disorders such as insomnia.
Despite the known bidirectional nature of the association between sleep and stress, little work has examined both sleep reactivity to stress and stress reactivity to sleep within the same study or sample. Prior studies suggest stress reactivity and sleep reactivity are interrelated yet separable and may each be associated with distinct processes underlying insomnia [4, 5]. Heightened sleep reactivity can be accounted for by a greater tendency toward pre-sleep rumination and worry [35], which has been shown to predict long-term sleep problems [36]. Stress reactivity can activate stress pathophysiological processes (e.g. hyperarousal, neuroendocrine, and cardiovascular activities) that further interfere with sleep. In addition, the reciprocal nature of sleep disturbances and emotional stress suggests they may have an interactive effect on insomnia by creating a downward, mutually reinforcing spiral.
To address these gaps (i.e. lack of rigorous, multi-method daily measurement, and a dearth of research on bidirectional reactivity between sleep and stress), the present study aims to examine how individuals vary in co-fluctuation of daily stress and nightly sleep, and if those variations predict future insomnia almost one year later. Daily dairies and actigraphy were used to obtain repeated-measures of sleep and stress in naturalistic settings. We examined a sample of nurses who are vulnerable to sleep disturbances due to their demanding work environment and high frequency of shift (i.e. night and early morning) work. Nurses also experience high levels of work-related stress exposure, making them an excellent population in which to examine sleep reactivity and stress reactivity in concert.
We predicted that there would be significant individual differences (i.e. trait-like) in both daily sleep reactivity and daily stress reactivity. We further hypothesized that individuals with higher daily sleep reactivity to stress and higher daily stress reactivity to sleep at baseline would show a greater increase in insomnia symptoms across a year. In addition to the main effects, we predicted that stress reactivity to sleep and sleep reactivity to stress would have an interactive effect. That is, given the reinforcing nature of stress and sleep disturbances, individuals who have higher scores of both sleep reactivity to stress and stress reactivity to sleep would have greater increases in insomnia across a year, compared to those with higher sleep reactivity to stress but lower stress reactivity to sleep (and vice versa).
Methods
Participants
Participants were recruited from two Dallas, Texas regional hospitals. Interested nurses were first screened for inclusion/exclusion criteria, and eligible participants were invited to participate in the study. Inclusion criteria were: (1) not yet received the current season’s influenza vaccine, (2) between the ages of 18 and 65, and (3) registered nurses actively working at least part-time at one of the two regional hospitals. Exclusion criteria were: (1) pregnant/nursing or planning to become pregnant or (2) having an egg allergy. Twenty-six percent of the sample reported working at least one night shift (work between 9 pm and 6 am) during the 14-day daily diary period. Table 1 reports the demographic characteristics for the sample. Most participants were female (92%), White (78%), non-Hispanic/Latinx (89%), married (63%), and had children (65%). The detailed sample characteristics broken down by the shiftwork status were published elsewhere [37].
Table 1.
Sample characteristics.
| M (SD) | |
|---|---|
| Age | 39.54 (11.15) |
| Sleep | |
| Actigraphy TST | 402.07 (50.36) |
| Actigraphy SE | 86.97 (4.87) |
| Diary TST | 432.36 (48.54) |
| Diary SE | 91.04 (5.14) |
| Daily stress | 0.81 (0.60) |
| Total number of night shifts | 1.34 (2.62) |
| N (%) | |
| Gender (female) | 360 (91.8) |
| Ethnicity (Hispanic/Latinx) | 42 (10.8) |
| Race | |
| White | 305 (77.8) |
| African American/Black | 26 (6.6) |
| American Indian/Alaska Native | 6 (1.5) |
| Asian | 41 (10.5) |
| Multiracial | 7 (1.8) |
| Other | 7 (1.8) |
| Marital status | |
| Married | 248 (63.3) |
| Single | 101 (25.8) |
| Divorced | 33 (8.4) |
| Separated | 7 (1.8) |
| Widowed | 3 (0.8) |
Procedures
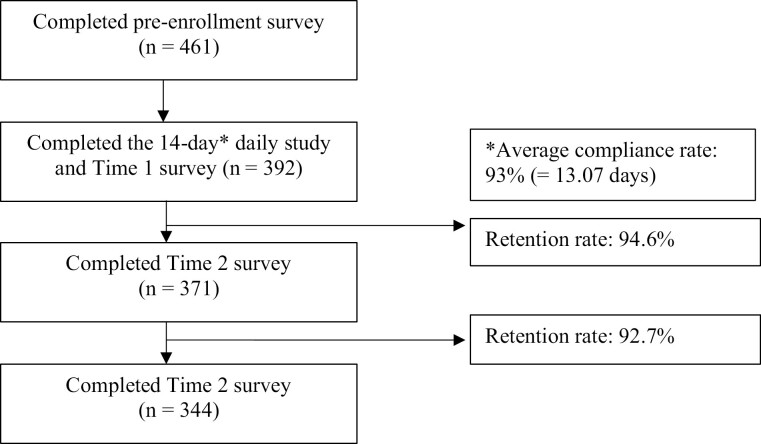
This study was part of a larger investigation on the effects of sleep on antibody response to the influenza vaccine that occurred between September 2018 and November 2018. Participants were recruited through nursing staff presentations, notification through employee email systems, and flyers that directed them to an initial online consent form. Nurses (N = 461) provided online consent and were asked to complete initial online Qualtrics surveys to collect demographic information as well as retrospective self-report estimates of recent health. Participants were then invited to enroll in the main portion of the study in the early fall (i.e. the start of the influenza season), which included completion of in-person informed consent approximately 1 month later. Three hundred ninety-two enrolled in the main portion of the study and completed the daily measures. For the main portion of the study, participants were given instructions on completing the stress surveys, sleep diaries, and wearing the actigraphy, which they completed for the subsequent 14 days.1 Participants then reported on their insomnia symptoms at approximately 6 months and 11 months following the end of the 14 days (see Figure 1). All study procedures were approved by the Medical City Plano and University of North Texas Institutional Review Boards.
Figure 1.
Flow of participants through the study.
Measures
Diary stress (baseline)
Upon awakening, participants reported on their stress severity levels during the previous day using the item “I felt stressed,” rated on a scale of 0 = not at all to 4 = extremely. Previous studies have used similar single-item approaches to capture daily stress (Petersen, Kecklund, D’Onofrio, Nilsson, & Åkerstedt, 2013).
Diary sleep (baseline)
An electronic version of the Consensus Sleep Diary—Core (Carney et al., 2012) was completed by participants each morning upon awakening using REDCap (Harris et al., 2009). Diaries were used to determine total sleep time (TST; i.e. time in bed [with the intention of sleeping] minus the sum of sleep onset latency, wake after sleep onset, and terminal wakefulness) and sleep efficiency (i.e. TST divided by time in bed, multiplied by 100). Sleep diaries provide reliable and valid assessments of TST and sleep efficiency and correlate significantly with actigraphy (r = 0.36 to 0.60), electroencephalogram ([EEG] r = 0.18 to 0.63), and polysomnography ([PSG] r = 0.36 to 0.59) measures (Dietch & Taylor, 2021; Lichstein et al., 2006; Williams et al., 2020).
Actigraphy sleep (baseline)
For 14 days, participants were instructed to continuously wear an Actiwatch Spectrum Pro (Philips Respironics, Bend, OR) on their non-dominant wrist. The Actiwatch is a watch-like device used to infer objective sleep/wake patterns. Participants were asked to push an “event marker” button when they intended to go to sleep and when they got out of bed. Rest intervals were manually set in Actiware software (Version 6.0.8) by two trained individuals using a protocol that systematically relies on a combination of event markers, sleep diary data, activity data, and light levels to determine sleep interval and offset times [38]. Discrepancies between the two scorers in setting the rest intervals were resolved by a third person. Data were exported using default settings (10 immobile minutes for sleep onset and offset, medium wake threshold [40 activity counts]). Exported actigraphy data were used to determine total sleep time (TST; i.e. total number of minutes in a rest interval that are scored as sleep by the sleep interval detection algorithm) and sleep efficiency (TST divided by time elapsed between the start and end time of a given rest interval, multiplied by 100), which were used in the current analyses.
Insomnia severity (baseline, 6-month, and 11-month follow-up)
Insomnia symptoms were measured using the Insomnia Severity Index (ISI) [39], which is a seven-item self-report measure that assesses the perceived severity of insomnia. Each item uses a four-point Likert type scale from 0 (e.g. not at all satisfied) to 4 (e.g. very much satisfied). The ISI has been shown to have good reliability (α = 0.74) [40], validity to detect clinical insomnia [41], and convergence with other measures such as the Pittsburgh Sleep Quality Index (r = 0.67) and the Dysfunctional Beliefs and Attitudes about Sleep (r = 0.55) [40]. In the current study, the ISI had good internal consistency across all time points (α = 0.86–0.89).
Night shift work (baseline, 6-month, and 11-month follow-up)
In the daily sleep diaries, participants reported on whether they worked a night shift (“Did you have to be at work past 9pm OR before 6am?”), a day shift, or were off work during the previous 24-hour period. The total number of night shifts worked across the 14-day period was calculated for each person. For the follow-ups, participants are asked to report the number of nights they had to work past 9 pm or before 6 am in the past month.
Depression (enrollment)
Pre-existing depression was measured with the Patient Health Questionnaire-9 and included as a covariate as it has been considered as a risk factor for insomnia. It consists of nine items that assess both affective and somatic symptoms related to depression and depressive disorders over the last two weeks; these nine items correspond to the diagnostic criteria for DSM Major Depressive Disorder.
Analytic approach
The data and the code for this article have been made publicly available via the Open Science Framework and can be accessed at https://osf.io/wt7bd/. We first identified individual differences in nightly sleep reactivity and daily stress reactivity. Multilevel modeling was employed to examine reactivity based on the daily covariation between sleep and stress during the 14 days. Stress reactivity (to sleep) was defined as an individual’s changes in daily stress on the next day, predicted by changes in sleep the previous night. Sleep reactivity (to stress) was defined as an individual’s changes in sleep, predicted by changes in stress experienced on the same day before bedtime. The reactivity scores were computed based on the methods employed by prior daily studies with multilevel models [42, 43]. For example, the following equation was used to compute sleep reactivity to stress.
The Level 1 equation represents the daily, within-person variation in stress. β0i is the intercept representing individual i’s average level of stress (across the diary period). β1i is the slope representing individual i’s change in stress associated with changes in sleep. rij is the residual representing the deviation from the model prediction for each individual observation. The Level 2 equation allows the estimation of individual variances from the sample average intercept and slope. γ00 is the sample average intercept (i.e. the grand mean of stress), and γ10 is the average association between sleep and stress across individuals. μ0i and μ1i are the deviations of individual i from the overall intercept and the overall slope, respectively. The within-person slopes (i.e. β1i) were computed to indicate individuals’ unique reactivity scores.
Eight models were fitted to obtain the reactivity of four measures of sleep (i.e. diary-determined TST, actigraphy-determined TST, diary-determined sleep efficiency, and actigraphy-determined sleep efficiency) to stress, and vice versa. All coefficients were standardized to aid in computation and interpretation across different measures. For example, a stress reactivity to diary-determined TST score of −0.11 means that a person’s next-day stress levels increased by 0.11 SD for every 1 SD decrease in sleep duration. For each reactivity index, the model fit was compared to the alternative model without the random slope to whether there was meaningful variation between individuals in their daily sleep and stress reactivity. The models were compared using the likelihood ratio test of model reduction. The non-significance of the difference in the model fits indicates that allowing the reactivity to vary across individuals did not account for additional variance in the model. Thus, only the reactivity indices of which individual differences significantly increased model fit were examined for further analysis.
Next, using latent change score analysis, we examined whether these reactivity coefficients predicted changes in insomnia symptoms approximately 11 months later. Latent change score models estimated the associations between the reactivity indices and change in insomnia symptoms over 11 months in a structural equation modeling framework. This modeling approach decomposes latent change scores into a proportional change that is explained by the baseline level, and a constant change that is not dependent on the baseline level. Thus, the model allows examination of how an exogeneous variable (i.e. predictor) is associated with change in an endogenous (i.e. outcome) variable that is not attributed to the endogenous variable itself. This feature is particularly valuable for disentangling the role of sleep and stress reactivity and baseline insomnia in predicting how insomnia symptoms change over time. A latent change score model has multiple advantages over other analytic approaches (e.g. difference score approach, residual change approach, and auto-regressive multilevel model), such as explicit modeling of error-free changes and accounting for individual differences in change patterns [44]. For the convergence and parsimony of the model, covariates were selectively entered only if their associations with insomnia symptoms were statistically significant.
First, main effect models were fitted to determine the independent roles of stress reactivity and sleep reactivity in accounting for the change in insomnia severity (i.e. ISI scores). All reactivity indices were be entered as between-participant, time-invariant predictors of both constant latent change in ISI scores and the initial latent ISI scores. Next, to test the interaction hypothesis, sleep reactivity and stress reactivity indices that showed significant main effects were examined, with their interaction term included as time invariant predictors of the constant latent change and initial latent ISI scores.
Results
Daily stress reactivity and daily sleep reactivity
Multilevel models estimated daily sleep and stress reactivity. The negative fixed effects indicated that on average, a decrease in sleep efficiency and TST predicted a subsequent increase in stress, and a decrease in stress predicted a subsequent increase in sleep efficiency and TST. However, as predicted, individual differences existed in the associations. Model comparisons with the log likelihood ratio test showed that the within-person variability in reactivity was significant for five indices out of eight indices: sleep reactivity (determined by diary TST and diary sleep efficiency) to stress, and stress reactivity to sleep (determined by diary TST, actigraphy TST, and diary sleep efficiency; Table 2).
Table 2.
Multilevel models of daily associations between sleep and stress.
| Predictor | Outcome | Fixed effect | Model comparison test | |
|---|---|---|---|---|
| b (SE) | LRT (df = 2) | |||
| Sleep reactivity (to stress) | Stress(1–5 scale) | Diary TST(min) | −9.44 (2.07)*** | 18.32, p < .001 |
| Stress | Acti TST (min) | −3.97 (1.87)* | 2.52, p = .2 | |
| Stress | Diary sleep efficiency(%) | −0.57 (0.18)*** | 19.44, p < .001 | |
| Stress | Acti sleep efficiency (%) | −0.07 (0.13) | 0.001, p = 1.0 | |
| Stress reactivity (to sleep) | Diary TST(min) | Stress(1–5 scale) | −0.001 (0.0001)*** | 25.63, p < .001 |
| Acti TST(min) | Stress | −0.001 (0.0001)*** | 13.43, p = .001 | |
| Diary sleep efficiency(%) | Stress | −0.004 (0.0016)* | 7.31, p = .03 | |
| Acti sleep efficiency (%) | Stress | −0.002 (0.0018) | 0.07, p = .96 |
Bolded reactivity indices indicate that the random slope significantly improved the model fit (i.e. the variability of the reactivity across individuals), based on the model comparison test. The significance of the fixed slopes: *p < . 05, ***p<.001.
acti, actigraphy; diary, sleep diary; LRT, Log-likelihood ratio test.
We obtained reactivity scores for each individual (i.e. within-partictipant standardized coefficients between sleep and stress) to use as between-person predictors of insomnia symptoms for subsequent analyses. The means and SD of the reactivity scores are presented in Table 3. For example, among people with high sleep reactivity (i.e. people whose diary TST reactivity to stress is 1 SD above the mean level), approximately 23 minutes of diary-determined TST were lost for every 1 SD increase in daily stress (1 SD = 0.98 on a scale from “0 = not at all” to “4 = extremely”). In contrast, those with moderate sleep reactivity lost only about 5 minutes for every 1 SD increase in daily. In the most extreme cases, the model predicted that people with high sleep reactivity risk losing well over an hour of sleep on extremely stressful days relative to no stress days, whereas those with mid-level sleep reactivity might lose 20 minutes of sleep at most with such a change in daily stress. Stress reactivity effects were less pronounced. The model predicted that people with high stress reactivity would have had to lose about 2 hours of sleep to register a 0.2 SD increase in stress the next day.
Table 3.
Descriptive statistics of daily stress and sleep reactivity.
| Predictor | Outcome | M (SD) | |
|---|---|---|---|
| Sleep reactivity (to stress) | Stress | Diary TST | −0.08 (0.072) |
| Stress | Acti TST | −0.036 (0.028) | |
| Stress | Diary sleep efficiency | −0.058 (0.068) | |
| Stress | Acti sleep efficiency | −0.008 (0.001) | |
| Stress reactivity (to sleep) | Diary TST | Stress | −0.108 (0.078) |
| Acti TST | Stress | −0.089 (0.068) | |
| Diary sleep efficiency | Stress | −0.036 (0.039) | |
| Acti sleep efficiency | Stress | −0.016 (0.001) |
All reactivity scores are standardized. Bolded reactivity indices are the ones that significantly differ across individuals.
Latent change analyses for insomnia symptoms
Main effect models
Latent change scores models were fitted to test whether reactivity measures predicted changes in insomnia over 11 months (see Figure 2). Reactivity indices were entered as between-participant, time-invariant predictors of both constant latent change in ISI scores and the initial latent ISI scores. All five indices were entered at the same time to determine their independent role in accounting for a change in ISI scores. For interpretability, reactivity scores were multiplied by −1 so that higher scores indicated higher reactivity (i.e. decreased TST and sleep efficiency on the night following a more stressful day, increased stress after a night of lower TST and sleep efficiency). Age, night shift work status, and depression were included as covariates due to their potentially confounding role in affecting stress and sleep.
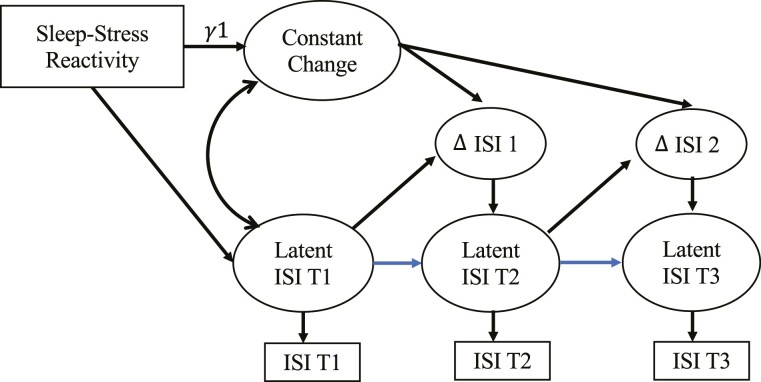
Figure 2.
Latent change score model for insomnia severity index (ISI) predicted by sleep–stress reactivity. Notes. Δ ISI 1 represents the latent change from baseline (T1) to 6 months later (T2). Δ ISI 2 represents the latent change from T2 to 11 months later (T3). This is a simplified representation of a univariate dual latent change score model. ISI denotes insomnia severity index. The error variances and regression coefficient for control variables (for age and night-shift status) were estimated but not included in the figure.
The model was an excellent fit to the data, χ2 (27, n = 333) = 521.4, p < .001, comparative fit index(CFI) = 0.99, root mean square error of approximation (RMSEA) = 0.032, standardized root mean square residual (SRMR) = 0.026. Of central interest to the hypothesis was the test of the coefficients (γ1) for reactivity predicting latent change score of insomnia symptoms, measured with the ISI. The results showed that three reactivity indices obtained from diary sleep measures predicted changes in latent ISI scores. Table 4(a) presents the model coefficients. As hypothesized, for sleep reactivity, shorter TST and lower sleep efficiency after experiencing more daily stress also predicted greater increases in ISI scores over 11 months (diary-determined TST reactivity to stress: b = 11.48, 95% confidence interval [CI] = [1.05, 21.91], p = .031; diary-determined sleep efficiency reactivity to stress: b = 10.34, 95% CI = [1.25, 19.42], p = .026). For stress reactivity, people who experienced more daily stress after shorter TST showed greater increases in ISI scores over 11 months (stress reactivity to diary-determined TST: b = 7.83, 95% CI[0.78, 14.89], p = .03). The hypothesized association was not found for stress reactivity to actigraphy-determined TST or stress reactivity to diary-determined sleep efficiency (b = −17.67, 95% CI = [−41.59, 0.15], p = .15; b = −5.99, 95% CI = [−19.56, 7.60], p = .39; respectively).
Table 4.
Model coefficients predicting constant ISI change in latent change scores models.
| (a) | |||
|---|---|---|---|
| ISI change score | |||
| Predictor | b | 95% CI | p |
| Diary TST reactivity to stress | 11.48 | (1.05, 21.91) | .031 |
| Diary sleep efficiency reactivity to stress | 10.34 | (1.25, 19.42) | .026 |
| Stress reactivity to diary TST | 7.83 | (0.78, 14.89) | .030 |
| Stress reactivity to acti TST | −17.67 | (−41.59, 0.15) | .15 |
| Stress reactivity to diary sleep efficiency | −5.99 | (−19.56, 7.60) | .39 |
| (b) | |||
|---|---|---|---|
| ISI change score | |||
| Predictor | b | 95% CI | p |
| Diary TST reactivity to stress | 6.22 | (−0.89, 13.32) | .086 |
| Diary sleep efficiency reactivity to stress | 10.75 | (1.63, 19.87) | .021 |
| Stress reactivity to diary TST | 7.07 | (0.20, 13.93) | .044 |
| Stress reactivity to diary sleep efficiency | −4.41 | (−17.68, 8.87) | .515 |
| (c) | |||
|---|---|---|---|
| ISI change score | |||
| Predictor | b | 95% CI | p |
| Diary TST reactivity to stress | 5.30 | (−2.27, 12.86) | .170 |
| Diary sleep efficiency reactivity to stress | 13.35 | (4.01, 22.69) | .005 |
| Stress reactivity to acti TST | −0.96 | (−13.55, 11.62) | .881 |
| Stress reactivity to diary sleep efficiency | 2.48 | (−13.93, 18.87) | .767 |
Regression coefficients that predict constant change in ISI score in the latent change score models. (a) presents the model in which all sleep and stress reactivity indices are entered as predictors. For multicollinearity concerns (b) and (c) each presents the model has one measure of the stress reactivity to TST indices at a time (diary-measured and actigraphy-measured, respectively). Bolded are p < .05.
Although not part of the hypotheses, reactivity indices were also associated with baseline ISI scores. Specifically, diary-determined sleep efficiency reactivity to stress and stress reactivity to diary-determined TST were associated with higher baseline ISI scores (b = 13.89, 95% CI = [5.69, 22.10], p < .001; b = 10.64, 95% CI = [4.37 16.90], p < .001), whereas diary-determined TST reactivity to stress and stress reactivity to diary-determined sleep efficiency were not associated with baseline ISI scores (b = −13.04, 95% CI = [0.41, 20.19], p = .054; b = −23.09, 95% CI = [−46.31, 0.13], p = .10). However, the coefficient for the proportional change was negative (b = −1.11, 95% CI = [−2.30, −0.74], p < .001), indicating higher baseline ISI scores were associated with decreases in ISI scores across time. Thus, bidirectional sleep and stress reactivity (i.e. diary-determined sleep efficiency and TST reactivity to stress and stress reactivity to diary-determined TST) predicted greater increases in ISI scores that were not attributed to its positive associations with the initial ISI scores.
Given the high correlation between stress reactivity to diary-determined and actigraphy-determined TST (see Supplementary Table S2 for the correlations of the reactivity indices), we also tested the model with only one stress reactivity index at a time as a sensitivity analysis to rule out the compounding effect of multicollinearity. Table 4(b) and 4(c) present the model coefficients. While the diary-determined sleep efficiency reactivity to stress and stress reactivity to diary-determined TST continued to predict a greater increase in ISI scores (b = 10.75, 95% CI = [1.627, 19.87], p = .021; b = 7.07, 95% CI = [0.20, 13.94], p = .044), diary-determined TST reactivity to stress no longer predicted changes in ISI scores when stress reactivity to actigraphy-determined TST was removed from the model (b = 3.63, p = .086). Taken together, diary-determined sleep efficiency reactivity to stress and stress reactivity to diary-determined TST robustly predicted increases in ISI scores.
Interaction models
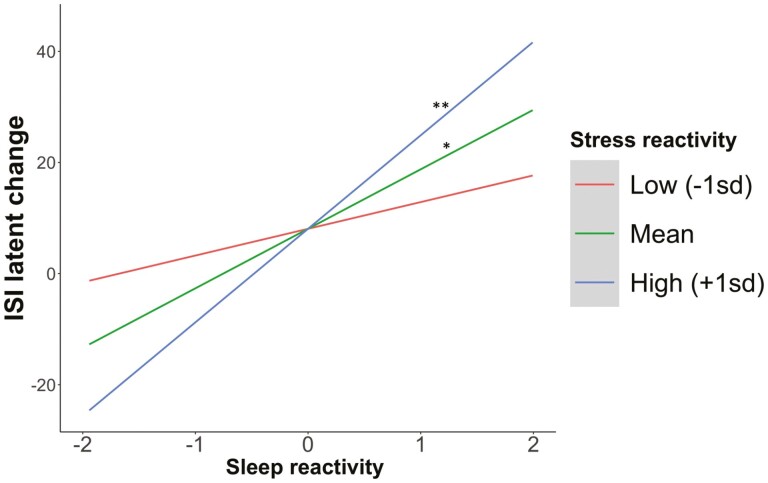
To test the interaction hypothesis, diary-determined sleep efficiency reactivity to stress, stress reactivity to diary-determined TST, and their interaction term were entered into the model.2 The model fit was excellent, χ2 (21, n = 333) = 739.76, p < .001, CFI = 0.99, RMSEA = 0.046, SRMR = 0.031. Supporting the hypothesis, the interaction between sleep reactivity and stress reactivity term predicted latent change of ISI scores, b = 78.01, 95% CI = [−0.21, 154.23], p = .051. To probe the pattern of the interaction, simple slope analyses were conducted among people with high versus low (1 SD above and below the mean) stress reactivity to diary-determined TST (see Figure 3). As predicted, among people with high stress reactivity to diary-determined TST, higher diary-determined sleep efficiency reactivity to stress predicted increases in ISI scores 11 months later (b = 17.23, 95% CI = [6.80, 27.67], p = .001). In contrast, among people with low stress reactivity to diary-determined TST, diary-determined sleep efficiency reactivity to stress was not associated with changes in ISI scores (b = 5.16, 95% CI = [−4.90, 15.22], p = .315). The interaction term did not predict baseline ISI latent scores.
Figure 3.
The association between sleep reactivity and ISI latent change scores moderated by stress reactivity. Notes. Sleep reactivity refers to diary-determined sleep efficiency reactivity to stress. Stress reactivity refers to stress reactivity to diary-determined TST. The slope with statistical significance lower than 0.01 and 0.05 was indicated by ** and *, respectively.
Discussion
To the best of our knowledge, this is the first study to examine how individual differences in daily reactivity to stress and transient sleep disturbances (i.e. shorter duration or worse efficiency) determined via diaries and actigraphy predict the development of insomnia symptoms across time. Both daily stress reactivity to sleep duration and daily sleep efficiency and duration reactivity to stress significantly differed across nurses in this study. The major finding of the study is that these individual differences in daily sleep and stress reactivity predicted increases in insomnia symptoms across 11 months. Higher sleep efficiency reactivity to stress and higher stress reactivity to sleep duration each predicted increases in insomnia symptoms over time, independently from one another. Furthermore, sleep reactivity and stress reactivity interacted to predict insomnia symptoms, such that nurses who had both higher sleep reactivity (i.e. worse sleep efficiency) to stress and higher stress reactivity to sleep loss had the greatest increases in chronic insomnia symptoms across the year. These findings highlight that higher stress reactivity and sleep reactivity may each be important risk factors for impaired sleep health, and potential preventative targets.
Our study updates and extends prior research on sleep and stress reactivity in three key ways. First, it employed rigorous measurement of sleep and stress reactivity. With a multi-method approach (i.e. using diaries and actigraphy), the study assessed sleep and stress at the daily level across 14 days, which allows for a better understanding of the dynamics between sleep and stress than cross-sectional examination. Daily measures of reactivity may capture individual traits more accurately and validly than retrospective measures, but this is an empirical question that requires additional psychometric examination. Second, we examined both sleep reactivity and stress reactivity simultaneously. Despite these constructs’ bidirectionality and their distinctive roles in insomnia pathology, to the best of our knowledge, no study has examined them together, which allowed us to begin to disentangle their independent contributions to the development or exacerbation of insomnia symptomatology. Third, the study used latent change score modeling to analyze the association between reactivity and change in insomnia symptoms that was not affected by baseline levels in insomnia symptoms [45]. Latent change score modeling overcomes limitations of the residualized change approach, where “controlling for” baseline may yield biased estimation in non-randomized longitudinal studies [44]. Modeling for two separate sources of latent changes allows us to separate the influence of baseline characteristics from the constant changes in insomnia symptoms. This is important when we determine the role of sleep and stress reactivity as a risk factors for insomnia, as the reverse pathway from insomnia symptoms to sleep and stress reactivity is also possible.
Consistent with prior findings on sleep reactivity assessed via one-time retrospective questionnaires, our measure of reactivity derived from 14 daily assessments of stress and sleep revealed that some individuals were more vulnerable to stress and sleep disturbances than others. The present study went further to identify the specific aspects of sleep that co-fluctuated with stress and predicted future insomnia symptoms. Among multiple reactivity indices based on daily diary and actigraphy measures of sleep duration and efficiency, nurses who had greater decreases in diary-determined sleep efficiency after greater daily stress, and greater increases in daily stress after shorter diary-determined sleep duration had greater increases in insomnia symptoms 11 months later. In contrast, we found no associations between insomnia symptoms and actigraphy-determined reactivity indices. Although diary and actigraphy measures are overlapping constructs, different sleep parameters may represent distinct psychological processes [13, 46, 47]. Insomnia is a disorder of subjective perception, and actigraphy has shown less accuracy in people with insomnia symptoms [13, 48]. In addition, our findings were not consistent across the two different facets of sleep (i.e. sleep duration and sleep efficiency). For stress, its increase in response to shorter sleep duration was a predictor of insomnia symptoms 11 months later. For sleep, both worse sleep efficiency and shorter sleep duration in response to stress were predictors of insomnia symptoms 11 months later. Our findings suggest that perception of daily sleep experiences is critical for predicting insomnia, and that it is important to examine different sleep parameters (i.e. diary measures vs. actigraphy measures, sleep duration vs. efficiency) as separate constructs.
Despite the importance of subjective perception in measurement of insomnia, our diary-determined reactivity indices share limitations of biases of self-report measures (e.g. FIRST) in that they are still subjective and retrospective. Moreover, because of the shared methodology (i.e. questionnaire) between the outcome variable and the predictor variables, the associations we observed might have been attributed to their common-method variance. That said, although the diary provided a retrospective and subjective measure of the previous night’s sleep, daily dairy methods yield greater validity and attenuate recall biases compared to other questionnaires asking about overall tendencies or history [49]. In addition, whereas previous measures of reactivity (e.g. the FIRST) may be inflated by lay beliefs of how stress and sleep are related, the current approach objectively computed the co-fluctuation of sleep and stress, overcoming this limitation.
The present study also expanded prior work by examining the role of stress reactivity to sleep in the risk of insomnia. Although the bidirectional influence of stress and sleep has been proposed as a key component of the stress–sleep diathesis models, unlike sleep reactivity, stress reactivity to disturbed sleep has not been studied in the context of insomnia [1, 2, 12]. Addressing this gap is important, as the extent to which individuals are distressed by daily sleep disturbances may play a key role in how transient sleep problems develop into chronic insomnia. Consistent with this idea, in our study, stress reactivity to sleep predicted increases in insomnia symptoms independently from sleep reactivity to stress, suggesting that sleep disturbances due to stress may only be the half of the stress–sleep cycle underlying insomnia. The other half of the cycle may be how perceptions of stress changes in response to daily sleep disturbances and feed into subsequent sleep.
In addition to the main effect, stress reactivity interacted with sleep reactivity to predict change in insomnia symptoms over 11 months. Prior work suggests that the extent to which sleep reactivity is a risk factor for insomnia varies by environmental stressor exposure and individual characteristics that affect sleep–stress dysregulation [20, 50]. The present study provides empirical support for this idea by identifying stress reactivity as a between-person moderator of the association between sleep reactivity and insomnia symptoms. Sleep reactivity predicted increases in insomnia symptoms among nurses with high stress reactivity at baseline but not among nurses with low stress reactivity. The results suggest that stress reactivity may amplify the influence of environmental triggers and high sleep reactivity on subsequent sleep problems, highlighting the importance of reciprocal reactivity of sleep and stress as a target of potential intervention.
While the current study focused on determining the role of sleep and stress reactivity in insomnia, it will be important for future research to examine pathways through which the reactivity leads to the progression of chronic sleep problems. The literature suggests that high sleep and stress reactivity involves cognitive, physiological, and behavioral patterns such as rumination, arousal, and harmful health behaviors (e.g. substance use), which increase insomnia risk over time [51]. The neurobiological and hyperarousal models of insomnia posit that high sleep and stress reactivity may be linked to an imbalance between sleep-promoting and arousing-promoting brain activity. The “3P” model of insomnia also illuminates factors that may be involved in the associations between sleep and stress reactivity and insomnia symptoms. This model states that chronic insomnia develops when predisposing factors (e.g. high arousal levels) interact with precipitating factors (e.g. major life stressors and repeated daily stressors) and perpetuating factors (e.g. poor sleep hygiene, like drinking caffeine and napping to compensate for lost sleep, or ruminating about stress and sleep loss) [52]. High sleep and stress reactivity may constitute a combination of all of these factors, including high arousal, high levels of stress, and maladaptive thoughts and behaviors that perpetuate daily stress and sleep dysregulation [51]. Finally, sleep and stress reactivity may contribute to the development of insomnia via heritable physiological tendency. People with high sleep reactivity show greater sympathetic activation and parasympathetic deactivation, and twin and sibling studies have shown that 29%–37% of sleep reactivity to stress is heritable [53–55]. Together, this work suggests that genetic predisposition to autonomic nervous system dysregulation may underlie the link between sleep–stress reactivity and insomnia.
The present study has several strengths, including a large sample of nurses and 14 days of daily stress and sleep measures, as determined by diaries and actigraphy. However, the study is not without limitations. The relative homogeneity of the sample in demographic variables such as gender and socioeconomic status calls for replication in more diverse samples. A strength, however, is that nurses face frequent stressor exposures and demanding work environments that may impair sleep [56]. Thus, although broad generalizations cannot be made, our findings may have public health implications for nurses and other populations at high risk of insomnia. Our sample did not exclude nurses based on pre-existing sleep disorders or other health conditions. Although this strengthens statistical power and external validity, pre-existing health conditions may affect the results. Nonetheless, the latent score modeling delineated the effect of our key predictors from the effect of baseline insomnia symptoms, diminishing concerns for possible confounds.
Our sample was made up with individuals whose circadian rhythms were likely disrupted due to their shift work schedules and could affect the findings. The current study did not have a measure of circadian rhythms other than bed/wake times, which are confounded with the shift work measure that we included as a covariate. When we examined the bivariate correlation between the number of shift work nights and reactivity indices, the results were mixed. For stress reactivity to diary TST and actigraphy TST, the correlations were negative (r = −0.23, −0.21, respectively), whereas for diary TST reactivity to stress, the correlation was positive (r = 0.23). No associations were found with the reactivity indices measured with sleep efficiency. Potentially, On the one hand, there could be a selection bias such that people with frequent shift work schedules show more stress resilience [37]. On the other hand, circadian rhythm disruptions (i.e. shift work) could be a unique stressor that exacerbates sleep reactivity to stress and its effect on insomnia. Future research may benefit from using a more precise measure of circadian rhythms to unpack the effect of circadian rhythm on the reactivity.
Our measure of stress is limited in that it was assessed only once per day retrospectively. The real-time and multiple assessments of stress across a day using experiential sampling methods would allow more nuanced understanding of stress experiences. For example, future work may examine how dynamics of stress regulation (i.e. reactivity and recovery) would be linked to sleep and prospective insomnia symptoms. In addition, the measure did not ask sources of daily stress (i.e. types of stressors). Although sleep reactivity tends to be consistent across different situational stressors [19], it is possible that the different stressors may vary in specific aspects of sleep reactivity (e.g. duration and efficiency) that they trigger. We also did not measure whether participants were exposed to on-going life stressors (e.g. caregiving responsibility and work-life conflict). Stress chronicity predicts poorer sleep quality prospectively, suggesting it may have cumulative “wear-and-tear” effects on sleep systems [57, 58]. The assessment of chronic stress, in addition to repeated-measures of daily stress would be ideal to clarify the role of stress within different time-frames in the progression of insomnia.
Given the overlap between the possible mechanisms linking the sleep and stress reactivity to insomnia and the general stress-diathesis model, future research may benefit from examining sleep and stress reactivity in relation to other health outcomes. Affective and physiological reactivity to stressors have been linked to chronic medical conditions (e.g. cardiovascular problems) and mortality [59–62]. Poor sleep mediates the effects of stress on impaired immune function (e.g. fewer NK cells) and adverse health outcomes (e.g. diabetes) [63–65]. Furthermore, a prospective study found that stress reactivity to short sleep predicts increases in chronic health conditions 8 years later [66]. Together, these studies suggest that sleep and stress reactivity may be linked to dysregulation of autonomic and neuroendocrine systems, predicting a wide range of health outcomes. Incorporating measures of physiological reactivity (e.g. cortisol, heart rate, and blood pressure) may reveal common biological mechanisms that account for the potential associations between stress and sleep reactivity and a range of health outcomes, including chronic insomnia.
Finally, the methodological strengths of the study (i.e. multiple daily assessments, objective measures of sleep, and computation of reactivity scores) may reduce its practical application in future studies. However, our rigorous approach provides strong evidence that daily stress and sleep reactivity can be used to predict insomnia. To reduce impacts and enhance practical application in future studies, researchers may be able to use fewer daily measures (e.g. <7 days) or only self-report measures of daily sleep to capture similar measures of reactivity. By making our daily reactivity calculation code publicly available, we hope other researchers may be able to apply similar methods to their datasets.
Conclusion
In summary, this study identified patterns of daily co-fluctuation between sleep and stress to prospectively predict chronic insomnia symptoms among nurses. We found that stress reactivity to sleep is an independent predictor of insomnia and that it also moderates the effect of sleep reactivity, providing new understandings of how reciprocal relationships between short-term stress and sleep disturbances may exacerbate the risk for chronic insomnia. The study provides reliable support for the hyperarousal and stress diathesis models of insomnia with methodological advances: using a longitudinal design with repeated-measures of stress and sleep in a naturalistic setting, obtaining behavioral indicators of sleep and stress reactivity, and modeling latent changes in insomnia symptoms across a year. Our results highlight that helping individuals cope with daily stress and manage perceptions of daily sleep disturbances may reduce chronic insomnia risk. It is possible that these naturalistic measures of reactivity may identify those at risk for insomnia better than existing retrospective measures, although this remains to be tested empirically and is a promising area for future research.
Supplementary Material
Footnotes
This study was conducted as a part of a large study that examined the effect of sleep on antibody response to the influenza vaccine, and the vaccination was administered on the seventh day of the daily study. Because there was no control group, the effect of the vaccination on sleep was explored by comparing sleep data from before versus after the seventh day and found inconsistent findings across sleep outcomes (see Supplementary Table S1). Importantly, because it is unclear whether these findings are artifacts of any temporal trends over days (1–14), the vaccination was not discussed further in this manuscript.
Out of five reactivity indices, stress reactivity to diary-determined TST and diary-determined sleep efficiency reactivity to stress showed statistically significant associations with ISI scores across different sets of covariates and thus further tested for their interaction effect.
Contributor Information
Jiah Yoo, Department of Psychology, University of Arizona, 1503 E University Blvd. Tucson, AZ 85721, USA.
Danica Slavish, Department of Psychology, University of North Texas, 1155 Union Circle #311280, Denton, TX 76203, USA.
Jessica R Dietch, School of Psychological Science, Oregon State University, 2950 SW Jefferson Way, Corvallis, OR 97331, USA.
Kimberly Kelly, Department of Psychology, University of North Texas, 1155 Union Circle #311280, Denton, TX 76203, USA.
Camilo Ruggero, Department of Psychology, University of North Texas, 1155 Union Circle #311280, Denton, TX 76203, USA.
Daniel J Taylor, Department of Psychology, University of Arizona, 1503 E University Blvd. Tucson, AZ 85721, USA.
Funding
This study was part of a larger investigation “Sleep and Vaccine Response in Nurses (SAV-RN)” supported by National Institutes of Health (R01AI128359-01).
Disclosure Statement
None declared.
References
- 1. Basta M, et al. Chronic insomnia and the stress system. Sleep Med Clin. 2007;2(2):279–291. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drake CL, et al. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37(8):1295–1304. doi: 10.5665/sleep.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garefelt J, et al. Reciprocal relations between work stress and insomnia symptoms: a prospective study. J Sleep Res. 2020;29(2):e12949. doi: 10.1111/jsr.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kahn M, et al. Sleep and emotions: bidirectional links and underlying mechanisms. Int J Psychophysiol. 2013;89(2):218–228. doi: 10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 5. Kalmbach DA, et al. The impact of stress on sleep: pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res. 2018;27(6):e12710. doi: 10.1111/jsr.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alvaro PK, et al. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–1068. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernández-Mendoza J, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med. 2010;72(4):397–403. doi: 10.1097/PSY.0b013e3181d75319. [DOI] [PubMed] [Google Scholar]
- 8. Han KS, et al. Stress and sleep disorder. Exp Neurobiol. 2012;21(4):141–150. doi: 10.5607/en.2012.21.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sladek MR, et al. Daily rumination about stress, sleep, and diurnal cortisol activity. Cognition & Emotion. 2020;34(2):188–200. doi: 10.1080/02699931.2019.1601617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallace DD, et al. Multilevel analysis exploring the links between stress, depression, and sleep problems among two-year college students. J Am Coll Health. 2017;65(3):187–196. doi: 10.1080/07448481.2016.1269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vgontzas AN, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes. 2008;32(5):801–809. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 12. Yap Y, et al. Bi-directional relations between stress and self-reported and actigraphy-assessed sleep: a daily intensive longitudinal study. Sleep. 2020;43(3):1–10. doi: 10.1093/sleep/zsz250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Slavish DC, et al. The cycle of daily stress and sleep: sleep measurement matters. Ann Behav Med. 2021;55(5):413–423. doi: 10.1093/abm/kaaa053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mrug S, et al. Sleep problems predict cortisol reactivity to stress in urban adolescents. Physiol Behav. 2016;155:95–101. doi: 10.1016/j.physbeh.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 15. Killgore WDS, et al. Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med. 2008;9(5):517–526. doi: 10.1016/j.sleep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 16. Drake CL, et al. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–291. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 17. Petersen H, et al. Stress vulnerability and the effects of moderate daily stress on sleep polysomnography and subjective sleepiness. J Sleep Res. 2013;22(1):50–57. doi: 10.1111/j.1365-2869.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 18. Sadeh A, et al. Effects of stress on sleep: the moderating role of coping style. Health Psychol. 2004;23(5):542–545. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- 19. Bonnet MH, et al. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26(8):1029–1036. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 20. Vargas I, et al. Vulnerability to stress-related sleep disturbance and insomnia: investigating the link with comorbid depressive symptoms. Trans Issues Psychol Sci. 2015;1(1):57–66. doi: 10.1037/tps0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Griffiths MF, et al. Risk factors for chronic insomnia following hospitalization. J Adv Nurs. 2005;49(3):245–253. doi: 10.1111/j.1365-2648.2004.03283.x. [DOI] [PubMed] [Google Scholar]
- 22. Jarrin DC, et al. Temporal stability of the Ford Insomnia Response to Stress Test (FIRST). J Clin Sleep Med. 2016;12(10):1373–1378. doi: 10.5664/jcsm.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalmbach DA, et al. Identifying at-risk individuals for insomnia using the Ford Insomnia Response to Stress Test. Sleep. 2016;39(2):449–456. doi: 10.5665/sleep.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakajima S, et al. Validation of the Japanese version of the Ford Insomnia Response to Stress Test and the association of sleep reactivity with trait anxiety and insomnia. Sleep Med. 2014;15(2):196–202. doi: 10.1016/j.sleep.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 25. Palagini L, et al. Association between stress-related sleep reactivity and cognitive processes in insomnia disorder and insomnia subgroups: preliminary results. Sleep Med. 2016;19:101–107. doi: 10.1016/j.sleep.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 26. Cohen LH, et al. Effects of induced mood on self-reported life events and perceived and received social support. J Pers Soc Psychol. 1988;55(4):669–674. doi: 10.1037//0022-3514.55.4.669. [DOI] [PubMed] [Google Scholar]
- 27. Forgas JP. On being happy and mistaken: mood effects on the fundamental attribution error. J Pers Soc Psychol. 1998;75(2):318–331. doi: 10.1037//0022-3514.75.2.318. [DOI] [PubMed] [Google Scholar]
- 28. Forgas JP. Affective influences on self-disclosure: mood effects on the intimacy and reciprocity of disclosing personal information. J Pers Soc Psychol. 2011;100(3):449–461. doi: 10.1037/a0021129. [DOI] [PubMed] [Google Scholar]
- 29. Sin NL, et al. Sleep duration and affective reactivity to stressors and positive events in daily life. Health Psychol. 2020;39(12):1078–1088. doi: 10.1037/hea0001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riemann D, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 31. Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53(1):215–243. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 32. Morin CM, et al. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65(2):259–267. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 33. Perlis ML, et al. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. Vol. 1. New York:Springer Science; & Business Media; 2006. [Google Scholar]
- 34. Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40(8):869–893. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 35. Zoccola PM, et al. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosom Med. 2009;71(7):771–775. doi: 10.1097/PSY.0b013e3181ae58e8. [DOI] [PubMed] [Google Scholar]
- 36. Harvey AG, et al. Cognitive approaches to insomnia. Clin Psychol Rev. 2005;25(5):593–611. doi: 10.1016/j.cpr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 37. Slavish DC, et al. Daily stress and sleep associations vary by work schedule: a between- and within-person analysis in nurses. J Sleep Res. 2022;31(3):1–13. doi: 10.1111/jsr.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rijsketic JM, et al. Actigraphy (Actiware) Scoring Hierarchy Manual. insomnia.arizona.edu/actigraphy. [Google Scholar]
- 39. Morin CM. Insomnia: Psychological Assessment and Management. New York:Guilford Press; 1993. [Google Scholar]
- 40. Bastien CH, et al. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 41. Morin CM, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sin NL, et al. Linking daily stress processes and laboratory-based heart rate variability in a national sample of midlife and older adults. Psychosom Med. 2016;78(5):573–582. doi: 10.1097/PSY.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sin NL, et al. Affective reactivity to daily stressors is associated with elevated inflammation. Health Psychol. 2015;34(12):1154–1165. doi: 10.1037/hea0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Castro-Schilo L, et al. Using residualized change versus difference scores for longitudinal research. J Soc Pers Relat. 2018;35(1):32–58. doi: 10.1177/0265407517718387. [DOI] [Google Scholar]
- 45. McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol. 2009;60(1):577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- 46. Dietch JR, et al. The enigma of objective and subjective measurement of response to cognitive behavioral therapy for insomnia: call to action. Sleep Med Rev. 2019;47:119–121. doi: 10.1016/j.smrv.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 47. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams JM, et al. Validity of actigraphy in young adults with insomnia. Behav Sleep Med. 2020;18(1):91–106. doi: 10.1080/15402002.2018.1545653. [DOI] [PubMed] [Google Scholar]
- 49. Dietch JR, et al. Validity of two retrospective questionnaire versions of the Consensus Sleep Diary: the whole week and split week Self-Assessment of Sleep Surveys. Sleep Med. 2019;63:127–136. doi: 10.1016/j.sleep.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 50. Kobasa SC, et al. Personality and constitution as mediators in the stress-illness relationship. J Health Soc Behav. 1981;22(4):368–378. doi: 10.2307/2136678. [DOI] [PubMed] [Google Scholar]
- 51. Kalmbach DA, et al. Hyperarousal and sleep reactivity in insomnia: current insights. Nat Sci Sleep. 2018;10:193–201. doi: 10.2147/NSS.S138823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spielman AJ, et al. A behavioral perspective on insomnia treatment. Psychiatr Clin N Am. 1987;10(4):541–553. doi: 10.1016/s0193-953x(18)30532-x. [DOI] [PubMed] [Google Scholar]
- 53. Drake CL, et al. Vulnerability to insomnia: the role of familial aggregation. Sleep Med. 2008;9(3):297–302. doi: 10.1016/j.sleep.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fernandez-Mendoza J, et al. Cognitive-emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family study. J Sleep Res. 2014;23(5):489–498. doi: 10.1111/jsr.12168. [DOI] [PubMed] [Google Scholar]
- 55. Drake CL, et al. Sleep reactivity and insomnia: genetic and environmental influences. Sleep. 2011;34(9):1179–1188. doi: 10.5665/SLEEP.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Øyane NMF, et al. Associations between night work and anxiety, depression, insomnia, sleepiness and fatigue in a sample of norwegian nurses. PLoS One. 2013;8(8):e70228. doi: 10.1371/journal.pone.0070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shaver JLF, et al. Stress exposure, psychological distress, and physiological stress activation in midlife women with insomnia. Psychosom Med. 2002;64(5):793–802. doi: 10.1097/01.psy.0000024235.11538.9a. [DOI] [PubMed] [Google Scholar]
- 58. Hall MH, et al. Chronic stress is prospectively associated with sleep in midlife women: the SWAN sleep study. Sleep. 2015;38(10):1645–1654. doi: 10.5665/sleep.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mroczek DK, et al. Emotional reactivity and mortality: longitudinal findings from the VA normative aging study. J Gerontol B Psychol Sci Soc Sci. 2015;70(3):398–406. doi: 10.1093/geronb/gbt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Piazza JR, et al. Affective reactivity to daily stressors and long-term risk of reporting a chronic physical health condition. Ann Behav Med. 2013;45(1):110–120. doi: 10.1007/s12160-012-9423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lovallo WR. Cardiovascular reactivity: mechanisms and pathways to cardiovascular disease. Int J Psychophysiol. 2005;58(2–3):119–132. doi: 10.1016/j.ijpsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 62. Chida Y, et al. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 63. Ambrasat J, et al. Consensus and stratification in the affective meaning of human sociality. PNAS. 2014;111(22):8001–8006. doi: 10.1073/pnas.1313321111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hall M, et al. Sleep as a mediator of the stress-immune relationship. Psychosom Med. 1998;60(1):48–51. doi: 10.1097/00006842-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 65. Mohr D, et al. The mediating effects of sleep in the relationship between traumatic stress and health symptoms in urban police officers. Psychosom Med. 2003;65(3):485–489. doi: 10.1097/01.psy.0000041404.96597.38. [DOI] [PubMed] [Google Scholar]
- 66. Sin NL, et al. Emotional vulnerability to short sleep predicts increases in chronic health conditions across 8 years. Ann Behav Med. 2021;55(12):kaab018. doi: 10.1093/abm/kaab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.