Abstract
It is uncontested that perivascular spaces play critical roles in maintaining homeostasis and priming neuroinflammation. However, despite more than a century of intense research on perivascular spaces, many open questions remain about the anatomical compartment surrounding blood vessels within the central nervous system. The goal of this comprehensive review is to summarize the literature on perivascular spaces in human neuroinflammation and associated animal disease models. We describe the cell types taking part in the morphological and functional aspects of perivascular spaces and how those spaces can be visualized. Based on this, we propose a model of the cascade of events occurring during neuroinflammatory pathology. We also discuss current knowledge gaps and limitations of the available evidence. An improved understanding of perivascular spaces could advance our comprehension of the pathophysiology of neuroinflammation and open a new therapeutic window for neuroinflammatory diseases such as multiple sclerosis.
Keywords: multiple sclerosis, neuroinflammation, perivascular spaces, paravascular spaces, Virchow-Robin spaces, review
In Brief
In this review, Ineichen and colleagues summarize the role of the perivascular space in neuroinflammation including involved cell types and how those spaces can be visualized. Based on this, they propose a model of the cascade of events during neuroinflammation.
1. A brief history of perivascular spaces
Physician-pathologist Rudolf Virchow was the first to publish a detailed description of a space surrounding certain brain vessels1. He termed it “disseziierende Ektasie” (dissecting ectasia) and drew a comparison with the pathology of an aortic aneurysm. Charles-Philippe Robin soon extended Virchow’s findings, postulating that these spaces were part of normal brain vessel anatomy2. Later, perivascular spaces became eponymous as Virchow-Robin spaces3. However, it must be noted that the term “Virchow-Robin space” now only relates to the prominent perivascular spaces surrounding larger arteries and veins, which can be seen macroscopically or on magnetic resonance imaging (MRI).
The potential functional significance of perivascular spaces was first suggested by Virchow’s pupil, Wilhelm His, who considered them the lymphatic analogue of the central nervous system (CNS)4. From then on, much research was devoted to perivascular spaces’ role in health and disease. This then somewhat abated until recent technological advances and the emergence of the glymphatic hypothesis once again fostered an interest in perivascular spaces.
This review provides an overview of the role of perivascular spaces in neuroinflammation. We first set the stage by discussing current concepts involving perivascular spaces and their associated controversies. Next, we summarize the literature on the role of perivascular spaces in neuroinflammation. Finally, we integrate the sum of the evidence on perivascular spaces, present a model of their role in neuroinflammation, and address unresolved gaps in knowledge.
2. Anatomy and visualization of perivascular spaces
2.1. Definition of perivascular spaces
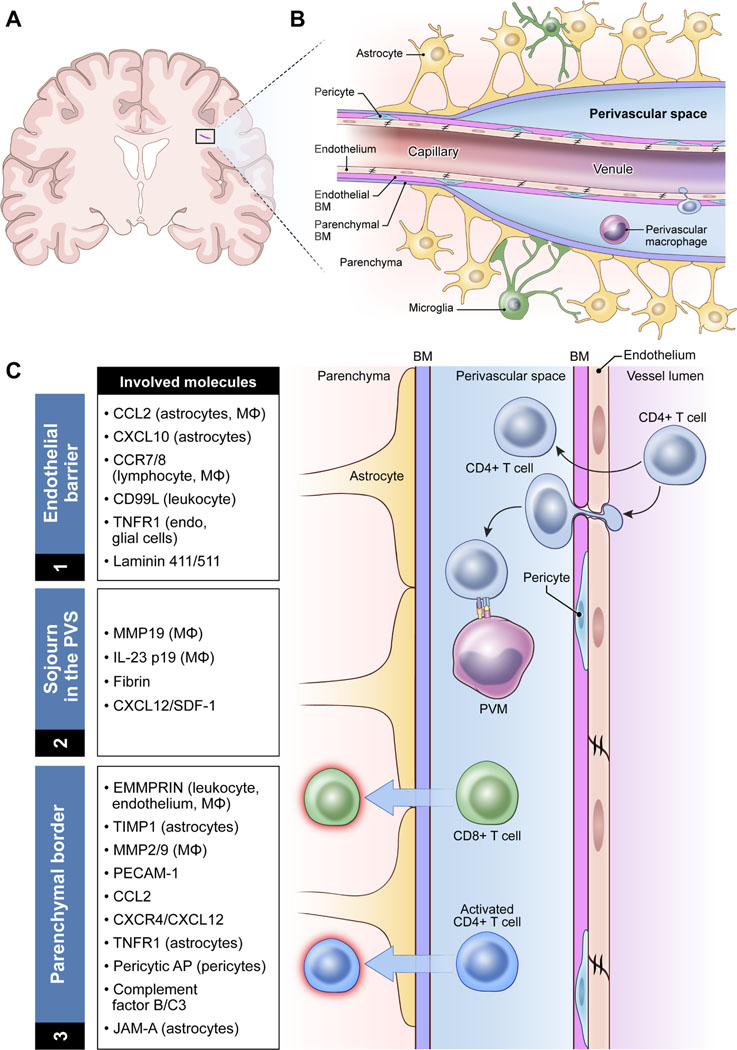
The perivascular space is defined as the compartment surrounding the brain’s blood vessels — its arteries, arterioles, venules, and veins5 (see Table 1 for definitions). There is a large body of historical and contemporary literature assessing the exact anatomical configuration of perivascular spaces, with considerable inconsistencies across studies, including in nomenclature. Indeed, there is an ongoing debate about whether these are true “spaces” or potential spaces that are normally filled with connective tissue. The most acknowledged current anatomical concept suggests that perivascular spaces are the compartments between the parenchymal basement membrane of the glia limitans (the outer boundary, formed by compacted astrocyte foot processes and an overlying parenchymal basement membrane) and the endothelial basement membrane of the blood vessel (inner boundary)6–8 (Figures 1A and 1B). Perivascular spaces are widely considered to be confined by the boundaries of the pia mater1 (i.e., intracerebrally) and to follow the vascular tree down to the capillary level. At this point, the glial and endothelial basement membranes become closely juxtaposed and thus appearing as one structure7 and obliterating the space9–11.
Table 1:
Definition of key terms used in this review.
| Term | Definition |
|---|---|
| Perivascular space | The compartment surrounding (from the Ancient Greek: peri – around) a brain or spinal cord blood vessel, that is, arteries, arterioles, venules, and veins, and located within the parenchyma This is not necessarily a fluid space but could also be filled with extracellular matrix. |
| Periarterial and perivenous space | The perivascular space surrounding an artery or vein, respectively. |
| ‘Paravascular’ space | From the Ancient Greek para (next to), the non-concentric compartment alongside blood vessels in the subarachnoid or subpial space on the surface of brain and spinal cord, as recently visualized during intravital microscopy. |
| Virchow-Robin space | Large perivascular spaces that are visible macroscopically or on magnetic resonance imaging (MRI). |
| Glia limitans | Formed by compacted astrocyte foot processes and an overlying parenchymal basement membrane |
Figure 1:
Anatomy and functions of the perivascular space at the level of a postcapillary venule.
A: Location of perivascular spaces within the brain. B: Anatomical model of a perivascular space at the level of the postcapillary venule. C: Functional model of immune cell trafficking within the perivascular space of a postcapillary venule, including the relevant molecules identified to date. Note that for a more comprehensive list of adhesion molecules on the endothelium, we refer to other reviews.112–114.
Abbreviations: AP, amino peptidase; BM, basement membrane; EMMPRIN, Extracellular matrix metalloproteinase inducer; Endo, endothelial cells; IL, interleukin; JAM-A, junctional adhesion molecule-A; Leuko, leukocytes; MΦ, macrophage; MMP, matrix metalloproteinase; PECAM, platelet endothelial fundingcell adhesion molecule; PVM, perivascular macrophage; TIMP, tissue inhibitor metalloproteinase; TNFR, tumor necrosis factor receptor.
2.2. Histopathology and electron microscopy
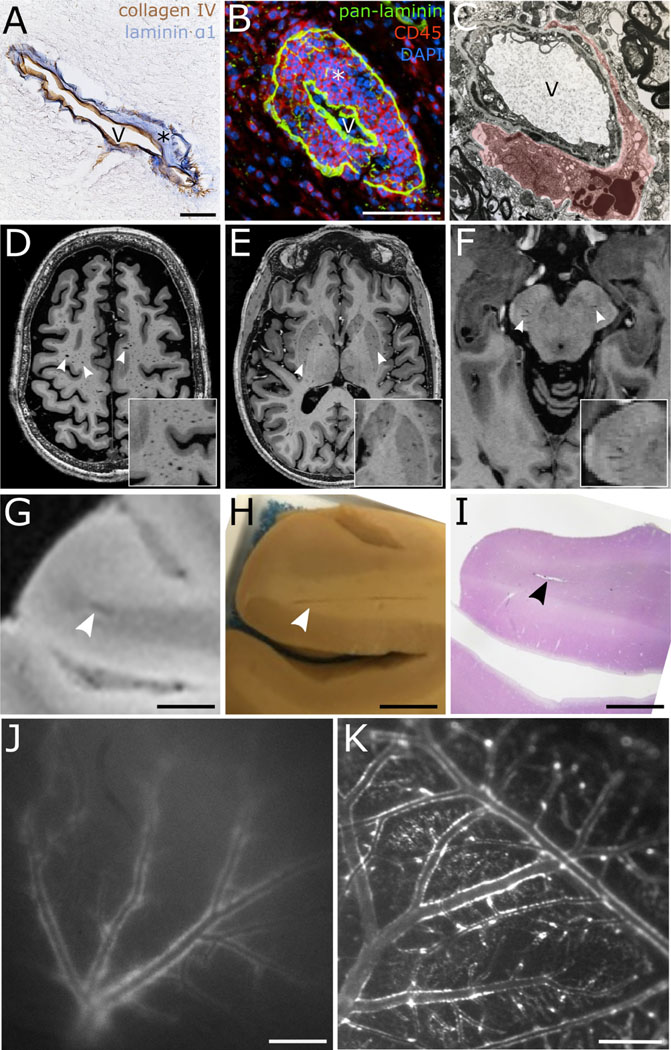
Because of their small dimensions, perivascular spaces have predominantly been studied using histological methods. Earlier studies employed less specific histological methods to visualize them12; more recent studies have used immunostaining. This has enabled a more specific identification of perivascular spaces, mostly by targeting antigens on the surface of astrocytic foot processes, e.g., aquaporins13,14 and β-dystroglycan15, but also certain laminin isoforms (of the parenchymal and endothelial basement membranes or of smooth muscle/mural cells) (Figure 2A)7 and type IV collagen (a component of both basement membranes)16. Another solution has been the pan-laminin antibody that recognizes laminin α1, β1, and γ1 chains equally well, thereby labelling both acellular borders of the perivascular space8 (Figure 2B). Electron microscopy has also been used to acquire ultrastructural insights into perivascular space anatomy and function12,17,18 (Figure 2C).
Figure 2:
Visualization of (enlarged) perivascular and ‘paravascular’ spaces.
A: Visualization of a perivascular space using double immunohistochemistry for collagen type IV (brown, vascular basement membrane) and laminin α1 (blue, parenchymal basement membrane) (*labels the perivascular space, V = vessel lumen; magnification bar: 50 μm). B: Histological visualization of a perivascular space at the level of a postcapillary venule using triple immunofluorescence staining for pan-laminin (green, labeling both the parenchymal and vascular basement membranes), leukocytes (CD45, red), and cell nuclei (DAPI, blue). On the induction of experimental autoimmune encephalomyelitis (EAE) in mice, leukocytes accumulate in perivascular spaces before parenchymal invasion, as in this example (magnification bar: 50 μm). C: Electron microscopy image of a perivascular space and containing two macrophages in the lower portion (red shading labels the perivascular space; magnification: 15,750x). D–F: 7-tesla magnetic resonance imaging (MRI) of enlarged perivascular spaces (EPVS, T1-weighted MRI scans, white arrowheads). EPVS have signal intensities similar to cerebrospinal fluid and are commonly observed in the centrum semiovale/supratentorial white matter (D), the basal ganglia (E), and/or at the pontomesencephalic junction (F). Inset images show higher magnifications of EPVS. G–I: MRI-histology correlation of an MRI-visible Virchow-Robin space (magnification bars: 5 mm). Postmortem 7-tesla MRI depicting a juxtacortical Virchow-Robin space (G, arrowhead) with a corresponding Virchow-Robin space on gross pathology (H) and on histology (I, H&E staining). J: Tracer-filled ‘paravascular’ spaces of superficial brain blood vessels as visualized by in vivo near-infrared imaging through an intact mouse skull (tracer size: 40 kDa; magnification bar: 500 μm). K: Ex vivo distribution of tracer around arteries and veins on the surface of the cortex in the ‘paravascular’ spaces (tracer size: 40 kDa, magnification bar: 500 μm). Note that the tracer distribution becomes more prominent at the time of death55.
2.3. Magnetic resonance imaging
In recent decades, literature on perivascular space histology and ultrastructure has been complemented by MRI studies19. Although MRI provides inferior resolution than tissue-based approaches for detecting perivascular spaces and thus only depicts the larger Virchow-Robin spaces, it does enable repeated noninvasive imaging of the entire brain. This is especially important considering potential changes in the configuration of perivascular spaces.
MRI identifies perivascular spaces as mostly linear structures with a signal intensity similar to cerebrospinal fluid (CSF)20. This has led to the identification and classification of enlarged perivascular spaces (EPVS) in specific brain regions into three types (Figures 2D–2F). Type 1 EPVS are situated in the basal ganglia along the lenticulostriate blood vessels (lentiform nucleus, external/internal capsule). In extensive cases, these are termed “état criblé” or “status cribrosus” and can also be seen on autopsy21. Type 2 EPVS are found in the centrum semiovale, and Type 3 in the midbrain (pontomesencephalic junction). Additionally, anterior temporal lobe perivascular spaces have been reported as a possibly distinct type22. Importantly, cortical perivascular spaces are difficult to detect using MRI, likely due to compaction of the glia limitans, pia mater, and vessel wall, as it has been shown in human and rodent cortices23,24.
Recently, advanced MRI techniques using high static magnetic field strengths have improved the detection of even smaller perivascular spaces25, enabling the classification of perivascular space location within the vascular tree, i.e., surrounding arteries or veins. By combining high-resolution MRI with susceptibility-based imaging to detect veins, one small study found that 80–90% of perivascular spaces adjoined arterial vessels26, in line with findings from another small study employing high-resolution MRI27.
Associations between enlarged perivascular spaces and neuroinflammatory diseases
Insights from MRI have confirmed previous knowledge from neuropathology by showing associations between EPVS and certain demographic parameters and/or diseases. Age28 and hypertension29 have been linked to higher numbers of arteries with enlarged perivascular spaces, and a recent large meta-analysis confirmed this19. Similar associations have been assessed in neuroinflammatory diseases: several studies have found greater numbers of EPVS in human inflammatory CNS diseases in general30, in systemic lupus erythematosus (SLE)31,32, and particularly in multiple sclerosis (MS)33. These findings in MS were recently corroborated by a systematic review and meta-analysis34.
In MS, several studies have assessed associations between EPVS and clinical or imaging outcomes. Greater numbers of EPVS have been associated with worse cognitive performance35, fatigue36, the presence of gadolinium-enhancing lesions indicating opening of the blood–brain barrier (BBB)37, lower brain volumes25, and a lower percentage of central vein sign–positive (i.e., perivenular) lesions38. However, none of these findings has been replicated. Similarly, although one study found that higher numbers of basal ganglia EPVS were associated with lower expanded disability status scale (EDSS) scores, a composite measure of MS disability39, these findings were not reproduced by other studies25,35,37,40.
In SLE, greater numbers of centrum semiovale EPVS were associated with more disease activity41. Smaller studies and/or case reports have observed contrast enhancement around perivascular spaces in progressive multifocal leukoencephalopathy (PML)42,43, (neuro)sarcoidosis44, neuromyelitis optica spectrum disorder (NMOSD)45, and primary CNS angiitis46.
Perivascular spaces and cuffs in animal models
MRI has also been harnessed to study perivascular cuffs in experimental autoimmune encephalomyelitis (EAE), a commonly used neuroinflammatory animal model. Perivascular cuffs are leukocyte conglomerates within the perivascular spaces around postcapillary venules, formed before immune cells infiltrate the parenchyma. These perivascular cuffs are associated with opening of the BBB, as shown by the observation of iron-based contrast agent within perivascular cuffs at the level of postcapillary venules in murine EAE47. In marmoset EAE, such perivenular contrast enhancement can also precede the impending emergence of inflammatory plaques48. These early changes in vascular pathology corroborate the importance of the perivascular compartment also in experimental neuroinflammation.
3. Fluid circulation in perivascular spaces
3.1. Perivascular fluid dynamics
Following Goldmann’s initial studies using vital dyes49, the use of horseradish peroxidase and electron microscopy indicated that there was no barrier preventing the diffusion of macromolecules < 70 kDa between the subarachnoid space, perivascular spaces, and the brain’s extracellular spaces50,51. The notion of perivascular fluid dynamics was confirmed by data from animal52–55 and human studies56–58. This perivascular fluid drainage seems to decrease under certain conditions, e.g., with aging59 or reduced vascular pulsations60.
The presence of an intramural drainage pathway, i.e., within the vesse’s muscular walls, further increases the complexity of what is referred to as perivascular fluid drainage61,62 (reviewed in10).
Despite solid evidence of fluid dynamics within perivascular spaces, the potential route of entry for fluid into perivascular spaces is a controversial subject. The pia mater is the innermost layer of the meninges, coating veins and arteries in the subarachnoid space, and there is some evidence of a separation between the subarachnoid space and the subpial space63. The perivascular space containing interstitial fluid also seems to be separated from the subarachnoid space containing CSF24. This separation mainly involves cells and particulate material: in subarachnoid hemorrhage, erythrocytes do not enter the perivascular space. In contrast, there is no such barrier for inflammatory cells in meningitis64. There may be specialized pores (termed stomata) in the adventitial lining of leptomeningeal vessels, which could facilitate the exchange of cells or fluid between the subarachnoid and subpial/perivascular spaces65,66. Similar pores have also been observed in canine and human spinal cord pia mater, thus providing an additional fluid exchange route67,68.
3.2. Molecular transport in the perivascular space
Tracer experiments have shown that molecules of up to 150 kDa can be transported within the intramural drainage pathway, whereas larger molecules, particularly cells or particulate material, track outside of arteries, adjacent to the glia limitans62,69,70. It has also been shown that tracers can be taken up by smooth muscle cells and perivascular macrophages along their passage62. It is possible that antigenic CNS material is delivered to perivascular macrophages in a similar fashion for presentation to trafficking lymphocytes71.
Interestingly, this perivascular pathway might also be relevant for the distribution of gadolinium-based MRI contrast agents upon systemic application. Concerns about the use of such contrast agents were raised after gadolinium deposition was observed within deep gray matter structures72–74, and the gadolinium may reach them via the perivascular fluid drainage system (reviewed in75).
Finally, it has been shown that β-amyloid is drained along this fluid drainage system but that its drainage becomes impaired with aging76. This impairment leaves insoluble β-amyloid deposits within basement membranes of the vessel walls, potentially further impeding efficient perivascular fluid exchange77 and potentially giving rise to cerebral amyloid angiopathy and amyloid-associated imaging abnormalities78.
3.3. The glymphatic hypothesis
CSF influx through perivascular spaces penetrating the CNS parenchyma was reported to be dependent on aquaporin-4 expressed on astrocyte end-feet52,79. Similarly, it has also been proposed that low-molecular-weight tracers (but also proteins such as HRP, albumin, and immunoglobulins) traverse the glia limitans to enter the parenchyma23,52,53. This process was originally termed convective tracer influx53, but the concept was then extended and renamed the glymphatic system52. The glymphatic hypothesis suggests a periarterial influx of fluid followed by aquaporin-4-facilitated, convective, trans-parenchymal fluid drainage and, finally, perivenous efflux, thus representing a CNS-specific drainage circuit with a similar function to lymphatic vessels in other tissues79,80.
Although this is an interesting concept, several lines of evidence do not support the glymphatic hypothesis, including those relating to the proposed role of aquaporin-4 (reviewed in81 and82). For example, the high hydraulic resistance of the brain extracellular space might restrict convective flow in favor of diffusion83,84. Furthermore, pressure-dependent, aquaporin-4-mediated water entry into astrocytes might be prevented by the resulting oppositely-directed osmotic gradient85. In addition, flow from periarterial to perivenous spaces should depend on a hydrostatic pressure gradient, for which there is no evidence86, and it has been suggested that net parenchymal flow could be explained by diffusion alone87. Finally, the existence of perivascular fluid influx and efflux remains controversial as animal studies utilizing injected tracers have reported conflicting findings on flow direction23,52,88 and changes in tracer distribution at the time of death, limiting the value of post-mortem analysis55. Indeed, key aspects of the original studies52 have proved difficult to reproduce, such as the reduced perivascular fluid flow in aquaporin-4-null mice87. Taken together, although there is strong evidence of fluid dynamics along and through perivascular spaces, more research is needed to elucidate the nature and direction of the parenchymal fluid exchange pathways proposed by the glymphatic hypothesis.
4. Immune cells and other cells in perivascular spaces
In addition to their role in fluid dynamics, immunocytochemical studies have confirmed the presence of scattered CD45-expressing cells in perivascular spaces (reviewed in89 and90) (Figure 1C). The presence of myeloid cells with macrophage-like properties or dendritic cells in the perivascular space has two potential implications: first, it provides an opportunity for foreign antigens to be taken up and processed by these resident antigen presenting cells (APCs)91–93; second, it allows for interactions between antigen-loaded macrophages/dendritic cells and lymphocytes from adjacent blood vessels or CSF94.
The second notion requires that lymphocytes enter perivascular spaces. During physiological conditions, such lymphocytes may be recruited from the CSF and seem to actively surveil the perivascular spaces (reviewed in95). Lymphocytes can also enter the perivascular spaces from the bloodstream across the endothelial barrier96. Many researchers have contributed to analyzing such CNS barriers, including Stern97,98, Lewandowsky99, Goldmann49, and Reese and Karnovsky100 (reviewed in101). However, Stern and Gautier were the first to use the term blood–brain barrier (BBB), in 1918, in their study of how a wide range of molecules from the blood penetrated into CSF101,102.
Lymphocytes such as activated or effector/memory T cells can enter the meninges and perivascular spaces independently of their antigen-specificity and are further activated when they recognize their cognate antigen present on local border-associated macrophages or dendritic cells in the subarachnoid space. This was shown in murine spinal cords using two-photon intravital microscopy71,103–105. Upon antigen-specific activation in subarachnoid or perivascular spaces, T cells gain the ability to enter the CNS parenchyma via migration across the glia limitans, potentially causing inflammatory tissue damage that results in clinical symptoms8,106 (reviewed in107). This has been shown in EAE models in which T cells recognized an antigen present within the CNS parenchyma (reviewed in108).
Importantly, during BBB disturbance, immune cell recruitment occurs at postcapillary venules61,109. This is in contrast to the diffusion of soluble molecules across the cerebral endothelium which is governed by different mechanisms and which is mostly controlled at the capillary level110.
5. Functional aspects of perivascular spaces in neuroinflammation
The opening of the BBB is a key process of neuroinflammation. Although traditionally considered a single entity, the BBB comprises two anatomical layers, the endothelial cell/endothelial basement membrane layer and the glia limitans (formed by compacted astrocyte foot processes and an overlying parenchymal basement membrane), which are separated by a distinct perivascular space except at the capillary level (Table 1 and Figure 1). Interestingly, data suggest that penetration of immune cells through the endothelial layer and their process of parenchymal invasion across the glia limitans are distinct processes, independent of one another111. Thus, for leukocytes, perivascular spaces are not merely another compartment to cross to access the CNS parenchyma; rather, they provide the critical components needed to initiate neuroinflammation and CNS immune surveillance.
(1). Endothelial barrier
Immune cell migration through the endothelium is a tightly controlled process involving various cell types and key molecules, reviewed in112–114.
Chemokines and their receptors
Several chemokine receptors have been shown to govern immune cell recruitment to perivascular spaces through the endothelium during neuroinflammation, among them chemokine receptors 7 and 8 (CCR7 and CCR8), which are upregulated during early EAE relapses and expressed on inflammatory cells in perivascular spaces115.
Chemokine ligands such as CCL2, secreted by microglia116 and astrocytes117, also play a role. In murine EAE, the lack of astroglial CCL2 reduced the capacity of CD4+ T cells to transit from perivascular spaces into the spinal cord parenchyma117. Another series of studies in a transgenic mouse model overexpressing CCL2 found that inflammatory cells were confined to the perivascular space118. However, additionally challenging these mice using pertussis toxin induced cellular infiltration and fluid leakage to the parenchyma119,120. Together, these findings emphasize that CCL2 overexpression is insufficient to induce active CNS inflammation; additional cues are required such as other chemokine ligands, e.g., CXCL10. In murine EAE, the deletion of astroglial CXCL10 reduced the influx of CD4+ T cells into spinal cord perivascular spaces, but macrophage accumulation was unaffected121.
Endothelial cells and molecules
It is not surprising that aside from leukocytes and glial cells, endothelial cells also regulate the transmigration of immune cells into perivascular spaces. When membrane proteins of inflammatory cells, such as CD99L2122, that are typically expressed on inflammatory cells, are also expressed on endothelial cells, they seem to be critical molecules for enabling transmigration through the endothelial barrier into perivascular spaces.
Additional endothelial molecules important for immune cells to cross the endothelial barrier are claudin-5 and TNF receptor 1 (TNFR1). In EAE, endothelial cells are able to transmit molecules like claudin-5 to circulating leukocytes, thereby enabling their passage through the endothelial cell layer123. Finally, TNFR1, which is expressed on endothelial cells and a variety of glial cells, is important for other peripheral inflammatory cells, such as macrophages, to reach the perivascular space, also resulting in lower production of proinflammatory chemokines116.
(2). Sojourn in the perivascular space
Data from rodent8,124 and marmoset EAE48 models suggest that inflammatory cells can be present within perivascular cuffs for up to several weeks before inflammatory parenchymal demyelination. Yet only a few studies have addressed the processes occurring during this sojourn.
Observations from in vivo microscopy of rodent EAE lesions showed that CD4+ T cells seem to be compartmentalized within perivascular spaces unless they recognize their cognate antigen on perivascular APCs71. A follow-up study in a similar model and employing in vivo microscopy of spinal meningeal vessels found that CD4+ T cells were activated by macrophages and potentially other APCs125. This would be in line with observations from murine viral encephalomyelitis models where CD8+ T cells were directly recruited to the parenchyma, whereas CD4+ T cells accumulated in perivascular spaces before transiting to the parenchyma126. A similar mechanism has also been proposed for myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), in which MOG-loaded macrophages were observed in perivascular spaces127.
But macrophages may also serve additional purposes besides antigen presenting properties: they can secrete proinflammatory cytokines such as interleukin-23, which has been found in active and chronic MS lesions and which could further stimulate autoreactive T cells locally128. Furthermore, fibrin deposition associated with astrocytic swelling has been reported in perivascular spaces in MS, which might also promote the parenchymal infiltration of immune cells129,130.
(3). Parenchymal border
Similar to the endothelial barrier, migration through the parenchymal border is a highly controlled process governed by various key molecules.
Matrix metalloproteinases
MMPs also seem critical to immune cell trafficking from the perivascular space to the CNS parenchyma. Gelatinase activity in early neuroinflammatory lesions has initially been shown in murine EAE15. This notion is further supported by the observation that there is significant MMP expression in early, active MS lesions131. Also, molecular imaging of gelatinase activity has been used as early marker of leukocyte infiltration in MS132. Indeed, in EAE, both MMP2 and MMP9 (also known as gelatinases A and B) aid leukocyte transmigration by modulating chemokine activity and thus generating a chemotactic gradient that attracts T cells from the perivascular space to the parenchyma133. Consistent with these observations, MMP-antagonizing molecules are also involved in parenchymal infiltration: upregulation of tissue inhibitor of metalloproteinase 1 (TIMP-1) by astrocytes inhibits parenchymal leukocyte infiltration from perivascular spaces134. Similarly, depletion of peripheral macrophages, which are potential secretors of MMPs, resulted in higher numbers of T cells being confined to perivascular spaces in EAE111,135.
MMP expression is governed by the MMP-inducer EMMPRIN (CD147), which is expressed on endothelial cells, leukocytes, and microglia. Its upregulation was associated with more severe EAE136,137, and its pharmacological inhibition dampened EAE severity138. Intriguingly, EMMPRIN also seems to regulate monocarboxylate transporter 4 (MCT-4), a potent secretor of lactate139. Indeed, it has been shown that macrophages in the perivascular cuff are highly glycolytic, expressing both lactate dehydrogenase A (converting pyruvate to lactate) and MCT-4. Knockdown of these molecules resulted in decreased lactate secretion and reduced transmigration from perivascular spaces to the parenchyma.
Among other substrates, dystroglycan, which anchors astrocyte end-feet to the parenchymal basement membrane, is a major substrate for MMPs15. It has also been shown that the platelet endothelial cell adhesion molecule (PECAM-1) might complement MMPs during leukocyte transmigration140.
Chemokines and their receptors
Chemokine receptors and their ligands are also involved in parenchymal infiltration of immune cells. Just as it facilitates transendothelial transmigration, CCL2 regulates trafficking of inflammatory cells to the parenchyma from perivascular spaces. In EAE, dendritic cells and T cells migrate across the glia limitans into the parenchyma in a CCL2-dependent manner141. In a murine viral neuroinflammatory model, CCL2 ablation resulted in CD4+ and CD8+ T cells being retained in perivascular spaces, causing a delayed control of viral neuroinflammation142.
The chemokine ligand CXCL12 and its receptor CXCR4 also play an important role in migration of inflammatory cells to the CNS parenchyma143,144. It has been shown in EAE that T cells which express CXCR4 are confined to the CXCL12-enriched perivascular space143. Upon inflammation, CXCR7 is upregulated on the endothelium resulting in uptake and degradation of CXCL12145. These reduced CXCL12 concentrations in the perivascular space promote a pro-inflammatory environment by allowing T cells to migrate towards the CNS parenchyma in a chemokine-dependent manner133. Of note, MMP2 and MMP9 act to inactivate CXCL12 in the perivascular cuff thereby enabling immune cells to respond to other chemokines expressed outside of the perivascular cuff132,146. These gelatinases also selectively cleave several CCL chemokines, including CCL2, antagonizing CCR receptors147. Hence, MMP activity at the parenchymal border acts to fine tune chemotactic signals across the parenchymal border148.
Additionally involved molecules
Another relevant process is TNFR1-dependent VCAM expression on astrocytes, which is involved in T cell trafficking from perivascular spaces to the parenchyma106. In line with this observation, TNF-deficient mice with EAE show inflammatory cells entrapped within their perivascular spaces149.
Several additional molecules have been suggested as promoters of immune cell trafficking from perivascular spaces to the parenchyma in neuroinflammatory animal models, including pericytic aminopeptidase N150, complement factors B and C3151, astrocytic junctional adhesion molecule-A152, and certain chondroitin sulfate proteoglycans153. These proteoglycans153, urokinase plasminogen activator, urokinase receptor, and plasminogen activator inhibitor-1 were all upregulated in acute MS lesions154. The urokinase plasminogen activator-urokinase receptor complex concentrates in the inflammatory cells in perivascular spaces, potentially facilitating cellular infiltration into the CNS. Interestingly, in the same study, tissue plasminogen activator (the most abundant plasminogen activator in MS lesions) was reduced. Finally, laminin isoforms are involved in perivascular cuffing: inflammatory cuffs only occur around endothelial basement membranes containing laminin 8 (now known as laminin 411) and laminin 10 (now known as laminin 511)8.Of note, the differential laminin expression of laminin 511 in different parts of the vasculature defines sites of low/no laminin 511 expression only at postcapillary venules as permissive for leukocyte extravasation.
Based on this and prior reviews108,155,156 , we have synthesized an anatomical and functional model of perivascular spaces in neuroinflammation (Figure 1C). While this review focuses on the perivascular space, for a detailed overview of BBB pathophysiology, including the adhesion molecules involved in leukocyte transmigration, please refer to other comprehensive reviews by some of the present paper’s authors112,114 and others157,158.
6. Perivascular spaces and the central vein
The perivascular spaces surrounding postcapillary venules, which are the usual site of parenchymal lymphocyte infiltration (reviewed in159), play a particularly important role in neuroinflammation. Initially described by Eduard Rindfleisch160, many authors have since reported central venules in the white matter of MS lesions in autopsy samples (reviewed in161). However, it has only recently become possible to image the perivenular topography of an MS lesion in vivo using high-resolution MRI162 (reviewed in163). Far from being merely a morphological feature of MS lesions, the central vein sign is currently being implemented in clinical research studies in order to increase the specificity of MS diagnostic criteria164. Such lesion-centered veins are not commonly observed in MS-mimicking neuroinflammatory or non-inflammatory conditions165.
The underlying cause of the central vein’s prominence within MS lesions is still a matter of debate. Higher oxygen requirements within the lesion parenchyma (with a concomitant increase in deoxyhemoglobin) and vessel-size alterations have been suggested166,167. Recent data from studies of marmoset neuroinflammatory models and MS have indicated both luminal enlargement and eccentric thickening of perivascular spaces via type I fibrillar collagen deposition168. However, none of these points explains why the central vein is so prominent in MS lesions in particular, despite being very rare in other neuroinflammatory diseases in which lesions also arise through perivenous inflammation. For example, the central vein sign is rare or absent in MOGAD, despite a pathology involving brain inflammation and widespread demyelination, i.e., similar pathological hallmarks as in MS127,169. The major difference is that one key feature of MS — the formation of large, perivascular, lymphocyte-rich, inflammatory aggregates170 — is very rare in MOGAD, with small perivascular infiltrates around venules dominating169. It is possible that large inflammatory aggregates can only develop in connective tissue spaces of sufficient size, which, in the human brain, are only available in the meninges and large perivascular spaces171.
7. Translational aspects of perivascular spaces
It is noteworthy that many of the findings described above were gleaned from rodents, especially EAE studies. This results in key caveats regarding the translation of perivascular space function and anatomy from rodents to humans. Regarding perivascular space function, although EAE is the most frequently used animal model for mimicking MS172, its ability to model every aspect of MS has been disputed173. Most notably, the nature of the immune response leading to brain inflammation is different between rodent neuroinflammation and MS173. For example, in contrast to MS in which CD8+ T cells are suggested to play a key role during pathogenesis, EAE is mostly driven by CD4+ T cells. Antigen recognition, the mode of immune cell activation, the mechanisms of immune-mediated tissue injury, and, associated with this, the type of inflammatory reaction is supposed to be fundamentally different in inflammatory brain diseases propagated by CD4+ T-cell-mediated, CD8+ T-cell-mediated, and T-cell plus antibody-mediated inflammatory CNS diseases. In addition, no EAE model has ever convincingly described the full clinical course and pathological characteristics of progressive MS173,174. Thus, it has been suggested that EAE is a more accurate model for acute or relapsing disseminating encephalomyelitis, including MOGAD174. This is further supported by the finding that autoimmunization of humans using brain tissue (which leads to widespread MS-like demyelination) is associated with an intrathecal antibody response against MOG175.
Regarding perivascular space anatomy, there are several similarities between rodents and humans. These include similar composition of the two cellular layers and basement membranes as well as the presence of perivascular cuffs in both MS and EAE61. However, there are also key differences in perivascular space anatomy between these species. Most obviously, the considerably larger size of human brains can be associated with much larger perivascular spaces, especially in periventricular white matter171. This should lead to substantially longer trafficking distances for the immune cells within those perivascular spaces61, and it suggests that the pathogenetic mechanisms behind neuroinflammation (including T cell surveillance and their activation within perivascular spaces) could involve different pathways in humans. In summary, more research is warranted to link findings from rodent studies to the human population.
8. Knowledge gaps
There are critical gaps in knowledge about perivascular space anatomy and function (Table 2). First, despite over a century of study, the exact anatomy of perivascular spaces is still debated, and the literature is hampered by inconsistent terminology. Open questions include whether there are anatomical differences between perivascular spaces at different levels along the CNS vascular tree, i.e., around veins, postcapillary venules, and arteries5, between perivascular spaces in the centrum semiovale and the basal ganglia176, or between cerebral and spinal cord perivascular spaces177. High-resolution MRI has enabled noninvasive insights into macroscopically visible EPVS, thus partially addressing these questions26,27. However, histological validation of these findings is largely lacking, and postmortem fixation artifacts (which can affect extracellular spaces) compound the problem. In addition, to date, MRI has been unable to identify spinal cord perivascular spaces, likely in part due to the technically limited resolution of spinal cord MRI178. Another cause could be anatomical differences between cerebral and spinal cord perivascular spaces177. Increasing spatial resolution and modern MRI techniques, including dynamic image acquisition methods, might provide pioneering insights into the anatomy of microscopic perivascular spaces and into their function in general.
Table 2:
Key gaps in knowledge gaps on perivascular space pathophysiology and limitations of the available literature.
| Key knowledge gaps |
|---|
|
|
|
1) Perivascular space anatomy
-What is the exact anatomy of perivascular spaces? -Are there anatomical differences between periarterial, periarteriolar, perivenular (including peripostcapillary venule), and perivenous spaces? |
|
2) Magnetic resonance imaging (MRI)-visible enlarged perivascular space (EPVS) etiopathogenesis
-How do EPVS relate to arteries and/or veins? -What is the temporal evolution of EPVS and why do they become enlarged, i.e., MRI-visible? -How do EPVS relate to perivascular spaces around postcapillary venules where immune cell trafficking occurs? |
|
3) Fluid dynamics of perivascular spaces
-Is there directional fluid influx along periarterial and outflow along perivenous spaces? -Are fluid dynamics within perivascular spaces disrupted in neuroinflammatory diseases such as multiple sclerosis (MS)? |
|
4) Neuroinflammatory priming within the perivascular space
-Which cells serve as antigen-presenting cells in the perivascular space, e.g., macrophages, dendritic cells, B cells? -Which mechanisms govern differences between homeostatic immune-cell trafficking within the perivascular space and the trafficking that occurs during a neuroinflammatory outbreak? |
|
|
| Limitations of the available literature |
|
|
|
1) Inconsistent terminology
-“Paravascular” versus “perivascular” -Leukocytes “cross the blood-brain barrier” |
| 2) Insufficient anatomical delineation of the perivascular space/compartment, including insufficient definition of locations within the vascular tree (artery, arteriole, venule, vein) |
|
3) Unclear translatability of animal study findings
-Caused by anatomical differences, e.g., between human and rodents |
MRI studies have identified several neuroinflammatory disorders with greater numbers of EPVS, mostly MS34 and, to a lesser extent, systemic lupus erythematosus31. Yet, a second gap in knowledge surrounds the continuing debate over the exact role of these imaging biomarkers. Based on longitudinal MRI data, one study hypothesized that the dilation of EPVS might represent a local accumulation of immune cells prior to the emergence of a neuroinflammatory lesion37. However, these findings have yet to be reproduced34. High-resolution MRI data suggest that EPVS might, at least in part, be signs of focal ex vacuo brain atrophy25. Determining the role of EPVS in the pathology of MS and other neuroinflammatory disorders will require more neuroimaging studies, particularly longitudinal ones. Furthermore, despite its inherent difficulty, correlating EPVS with their corresponding histopathology could give key insights into their pathophysiology (Figures 2G–2I).
Although the existence of the glymphatic system remains controversial, especially in humans and particularly with respect to the fluid drainage pathways and proposed role of aquaporin-4, there is a large body of evidence supporting the existence of a convective fluid flow within perivascular spaces (though not necessarily within the parenchyma). MRI data suggest that certain CNS diseases, such as normal pressure hydrocephalus, might be associated with reduced convective flow in perivascular spaces58. Indeed, it has been speculated that there are similar mechanisms in Alzheimer’s diseases, based on rodent studies179 and postmortem human studies180 (reviewed in181). Also, dynamic PET-tracer studies have shown altered ventricular CSF flow in MS182. However, a third gap in knowledge is the current uncertainty about whether fluid circulation within perivascular spaces is also altered in neuroinflammation, and, if this were the case, whether this is a primary cause of neuroinflammatory pathology or a bystander effect. In addition, particularly in MS (with its predominant perivenular pathology), peri2 arterial and perivenous convective flow might be altered in distinct ways.
Fourth, even though several molecules have been identified as governing the priming of immune cells in perivascular spaces, this process, including the exact type of APCs, is still a matter of debate. Both dendritic cells and perivascular macrophages have been implicated as those APCs, but resident microglia may also play this role183 by extending their processes to take part in the neurovascular unit184. The small dimensions of this anatomical compartment certainly impede any detailed assessment of the cells involved. However, it is clear that the involvement of perivascular spaces and their cells depends upon the type of inflammation, the pathogenic roles of different immune cells, and the nature and location of the target antigen that triggers the inflammatory reaction. Thus, more emphasis needs to be placed on disease-specific aspects of the immune reaction within the CNS compartment.
9. Limitations of the available evidence
There are several limitations in the available studies on perivascular spaces (Table 2). A first limitation is that the terms ‘perivascular’ and ‘paravascular’ are inconsistently used throughout the literature. We recommend using ‘perivascular’ to denote the compartments between the parenchymal basement membrane of the glia limitans and the vessel’s outer border81,107 (Table 1). In addition, phrases such as “leukocytes cross the blood–brain barrier” were used in many of the studies discussed here, neglecting the presence of perivascular spaces and thus diminishing the clinical relevance of distinguishing between whether these cells had passed through the vessel walls of postcapillary venules into the perivascular space or had in addition progressed through the glia limitans into the neuropil109. Furthermore, inflammatory cells may remain in perivascular spaces but induce clinical disease and tissue damage by producing soluble factors. Anti-MOG antibodies in EAE and MOGAD are examples of this mechanism169,185.
A second limitation is that many of the discussed studies assessing perivascular spaces did not properly define the exact delineations of their perivascular spaces by means of specific immunostaining and/or electron microscopy. We recommend that future studies include such immunostainings, e.g., for pan-laminin8 (Figure 2B) or other markers labelling specific components of perivascular spaces, such as collagen type IV (for labelling the basement membranes of the perivascular space) and laminin-alpha 1, β-dystroglycan, or aquaporin-4 (for its outer boundary). In this context, it is also important that MRI detection of perivascular spaces is restricted to macroscopically visible Virchow-Robin spaces, which form only a fraction of perivascular spaces in the CNS. Thus, findings from MRI studies require validation by histopathology before results can be generalized.
Many similarities between rodent and human perivascular space anatomy exist, yet critical distinguishing features, such as size differences, warrant a more careful interpretation of the findings from preclinical studies. This limitation may reduce the relevance of rodent data for human physiology and disease186. A focus should therefore be put on performing more human studies, particularly using modern, noninvasive neuroimaging approaches and, if possible, combining them with neuropathology in direct comparative investigations of postmortem material. The advent of ultra-high-resolution MRI enabling near-microscopic insights into perivascular space anatomy, and potentially even a visualization of the fluid dynamics within them (e.g., via phase-contrast imaging), might lead to the next generation of perivascular space studies. Additionally, studies in nonhuman primates, such as marmosets, might further bridge the gap between rodents and humans.
10. Conclusions
Our review corroborates the key role of perivascular spaces in neuroinflammatory pathologies such as MS but also during immune surveillance. However, despite many years of intense research on this topic, many critical gaps in knowledge remain, such as the exact anatomy of perivascular spaces, the etiopathogenesis of EPVS in neuroinflammatory diseases, and the role of fluid dynamics within perivascular spaces. Furthermore, technical limitations and uncertainties as to how findings translate from animal disease models to humans suggest the need for careful interpretation of the available evidence. An improved understanding of these anatomical compartments could lead into promising yet uncharted therapeutic territory.
Acknowledgments
We thank Erin Beck, Govind Bhagavatheeshwaran, and the NINDS Quantitative MRI Core Facility for the acquisition of 7-tesla MRI scans, Hartwig and Karen Wolburg for providing electron microscopy images, and Emma-Lotta Säätelä and Carl Gornitzki for their expert help with the medical library database search. We thank Darren Hart for copy-editing our manuscript.
Funding
This work was supported by grants from the University of Zurich (Forschungskredit Postdoc [FK-20-050] and UZH Alumni, to BVI) and the Swiss National Science Foundation (P400PM_183884, to BVI). This study was partially supported by the NIH’s NINDS Intramural Research Program.
Footnotes
Compliance with Ethical Standards
Although this review focuses on perivascular spaces, for the sake of completeness, we also mention the term ‘paravascular’ spaces (from Ancient Greek: para – alongside). We suggest it be reserved for the spaces that are visualized alongside vessels in the subarachnoid or subpial space that have not been shown experimentally to completely surround the vessel. We use quotation marks around this term as it remains to be shown if this is a true anatomical compartment (see Table 1 and Figures 2J and 2K)
Conflicts of interest
The authors declare no conflicts of interest related to the conduct of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Virchow R. (1851). Ueber die Erweiterung kleinerer Gefässe. Virchows Archiv 3, 427–462. [Google Scholar]
- 2.Robin C. (1859). Recherches sur quelques particularites de la structure des capillaires de l’encephale. J. Physiol. Homme. Anim 2, 537–548. [Google Scholar]
- 3.Bruce A, and Dawson JW. (1911). On the relations of the lymphatics of the spinal cord. The Journal of Pathology and Bacteriology 15, 169–178. [Google Scholar]
- 4.His W. (1865). Über ein perivasculäres Canalsystem in den nervösen Centralorganen und über dessen Beziehungen zum Lymphsystem (W. Engelmann). [Google Scholar]
- 5.Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, Doubal FN, Brown R, Ramirez J, MacIntosh BJ, et al. (2020). Perivascular spaces in the brain: anatomy, physiology and pathology. Nature reviews. Neurology 10.1038/s41582-020-0312-z. [DOI] [PubMed] [Google Scholar]
- 6.Bacyinski A, Xu M, Wang W, and Hu J. (2017). The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy. Frontiers in neuroanatomy 11, 101. 10.3389/fnana.2017.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannocks MJ., Pizzo ME., Huppert J., Deshpande T., Abbott NJ., Thorne RG., and Sorokin L. (2018). Molecular characterization of perivascular drainage pathways in the murine brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 38, 669–686. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, and Sorokin LM (2001). Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood–brain barrier in experimental autoimmune encephalomyelitis. The Journal of cell biology 153, 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones EG (1970). On the mode of entry of blood vessels into the cerebral cortex. Journal of anatomy 106, 507–520. [PMC free article] [PubMed] [Google Scholar]
- 10.Proulx ST, and Engelhardt B. (2022). Central nervous system zoning: How brain barriers establish subdivisions for CNS immune privilege and immune surveillance. Journal of internal medicine. 10.1111/joim.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimoda A. (1961). [Electronoptic studies on the perivascular structure of the brain with special reference to changes in cerebral edema and swelling]. Deutsche Zeitschrift fur Nervenheilkunde 183, 78–98. [PubMed] [Google Scholar]
- 12.Luse SA, and McDougal DB Jr. (1960). Electron microscopic observations on allergic encephalomyelitis in the rabbit. The Journal of experimental medicine 112, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palazzo C, Buccoliero C, Mola MG, Abbrescia P, Nicchia GP, Trojano M, and Frigeri A. (2019). AQP4ex is crucial for the anchoring of AQP4 at the astrocyte end-feet and for neuromyelitis optica antibody binding. Acta Neuropathologica Communications 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolburg H, and Paulus W. (2010). Choroid plexus: biology and pathology. Acta neuropathologica 119, 75–88. 10.1007/s00401-009-0627-8. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, and Sorokin LM (2006). Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. Journal of Experimental Medicine 203, 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gay FW (2006). Early cellular events in multiple sclerosis. Intimations of an extrinsic myelinolytic antigen. Clinical neurology and neurosurgery 108, 234–240. [DOI] [PubMed] [Google Scholar]
- 17.Butter C, O’Neill JK, Baker D, Gschmeissner SE, and Turk JL (1991). An immunoelectron microscopical study of the expression of class II major histocompatibility complex during chronic relapsing experimental allergic encephalomyelitis in Biozzi AB/H mice. Journal of neuroimmunology 33, 37–42. [DOI] [PubMed] [Google Scholar]
- 18.Esiri MM, Taylor CR, and Mason DY (1976). Application of an immunoperoxidase method to a study of the central nervous system: preliminary findings in a study of human formalin fixed material. NEUROPATHOL.APPL.NEUROBIOL 2, 233–246. [Google Scholar]
- 19.Francis F, Ballerini L, and Wardlaw JM (2019). Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: A systematic review and meta-analysis. International journal of stroke : official journal of the International Stroke Society, 1747493019830321. 10.1177/1747493019830321. [DOI] [PubMed] [Google Scholar]
- 20.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet. Neurology 12, 822–838. 10.1016/s1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Román GC (2002). On the history of lacunes, etat crible, and the white matter lesions of vascular dementia. Cerebrovascular Diseases 13, 1–6. [DOI] [PubMed] [Google Scholar]
- 22.Lim AT, Chandra RV, Trost NM, McKelvie PA, and Stuckey SL (2015). Large anterior temporal Virchow-Robin spaces: unique MR imaging features. Neuroradiology 57, 491–499. 10.1007/s00234-015-1491-y. [DOI] [PubMed] [Google Scholar]
- 23.Morris AW, Sharp MM, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, Weller RO, and Carare RO (2016). Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta neuropathologica 131, 725–736. 10.1007/s00401-0161555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang E, Inman C, and Weller R. (1990). Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. Journal of anatomy 170, 111. [PMC free article] [PubMed] [Google Scholar]
- 25.Kilsdonk ID., Steenwijk MD., Pouwels PJW., Zwanenburg JJM., Visser F., Luijten PR., Geurts JJG., Barkhof F., and Wattjes MP. (2015). Perivascular spaces in MS patients at 7 Tesla MRI: a marker of neurodegeneration? Multiple sclerosis (Houndmills, Basingstoke, England) 21, 155–162. [DOI] [PubMed] [Google Scholar]
- 26.George I, Arrighi-Allisan A, Delman B, Balchandani P, Horng S, and Feldman R. (2021). A Novel Method to Measure Venular Perivascular Spaces in Patients with MS on 7T MRI. American Journal of Neuroradiology 42, 1069–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, and Zwanenburg JJ (2014). Visualization of perivascular spaces and perforating arteries with 7 T magnetic resonance imaging. Investigative radiology 49, 307–313. [DOI] [PubMed] [Google Scholar]
- 28.Huang P, Zhu Z, Zhang R, Wu X, Jiaerken Y, Wang S, Yu W, Hong H, Lian C, and Li K. (2021). Factors associated with the dilation of perivascular space in healthy elderly subjects. Frontiers in Aging Neuroscience 13, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, Ayres A, Schwab KM, Martinez-Ramirez S, and Goldstein JN (2017). MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 88, 1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spielmeyer W. (2013). Histopathologie des Nervensystems: Erster Band Allgemeiner Teil (Springer-Verlag; ). [Google Scholar]
- 31.Barraclough M, Elliott R, Parker B, McKie S, Jackson A, Pemberton P, and Bruce IN (2018). Altered cognitive function in systemic lupus erythematosus and associations with inflammation and functional brain changes. Arthritis and Rheumatology 70, 2170–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiseman SJ, Bastin ME, Jardine CL, Barclay G, Hamilton IF, Sandeman E, Hunt D, Amft EN, Thomson S, Belch JFF, et al. (2016). Cerebral Small Vessel Disease Burden Is Increased in Systemic Lupus Erythematosus. Stroke 47, 2722–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achiron A, and Faibel M. (2002). Sandlike appearance of Virchow-Robin spaces in early multiple sclerosis: a novel neuroradiologic marker. AJNR. American journal of neuroradiology 23, 376–380. [PMC free article] [PubMed] [Google Scholar]
- 34.Granberg T, Moridi T, Brand JS, Neumann S, Hlavica M, Piehl F, and Ineichen BV (2020). Enlarged perivascular spaces in multiple sclerosis on magnetic resonance imaging: a systematic review and meta-analysis. Journal of neurology 267, 3199–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Favaretto A, Lazzarotto A, Riccardi A, Pravato S, Margoni M, Causin F, Anglani MG, Seppi D, Poggiali D, and Gallo P. (2017). Enlarged Virchow Robin spaces associate with cognitive decline in multiple sclerosis. PloS one 12, e0185626. 10.1371/journal.pone.0185626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conforti R, Cirillo M, Sardaro A, Caiazzo G, Negro A, Paccone A, Sacco R, Sparaco M, Gallo A, Lavorgna L, et al. (2016). Dilated perivascular spaces and fatigue: is there a link? Magnetic resonance retrospective 3Tesla study. Neuroradiology 58, 859–866. 10.1007/s00234-016-1711-0. [DOI] [PubMed] [Google Scholar]
- 37.Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD, Zipp F, and Paul F. (2008). Perivascular spaces--MRI marker of inflammatory activity in the brain? Brain : a journal of neurology 131, 2332–2340. [DOI] [PubMed] [Google Scholar]
- 38.Guisset F, Lolli V, Bugli C, Perrotta G, Absil J, Dachy B, Pot C, Théaudin M, Pasi M, van Pesch V, and Maggi P. (2020). The central vein sign in multiple sclerosis patients with vascular comorbidities. Multiple Sclerosis Journal. [DOI] [PubMed] [Google Scholar]
- 39.Wooliscroft L, Boespflug E, Hildebrand A, Shangraw K, Silbermann E, Bourdette D, and Spain R. (2020). Enlarged perivascular spaces are not associated with vascular comorbidities, clinical outcomes, and brain volumes in people with secondary progressive multiple sclerosis. Multiple Sclerosis Journal - Experimental, Translational and Clinical 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conforti R, Cirillo M, Saturnino PP, Gallo A, Sacco R, Negro A, Paccone A, Caiazzo G, Bisecco A, Bonavita S, and Cirillo S. (2014). Dilated Virchow-Robin spaces and multiple sclerosis: 3 T magnetic resonance study. La Radiologia medica 119, 408–414. [DOI] [PubMed] [Google Scholar]
- 41.Miyata M, Kakeda S, Iwata S, Nakayamada S, Ide S, Watanabe K, Moriya J, Tanaka Y, and Korogi Y. (2017). Enlarged perivascular spaces are associated with the disease activity in systemic lupus erythematosus. Scientific reports 7, 12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahani L, Shah M, and Tavakoli-Tabasi S. (2015). Immune reconstitution inflammatory syndrome in a patient with Progressive multifocal leukoencephalopathy. BMJ Case Reports 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wattjes MP., Verhoeff L., Zentjens W., Killestein J., van Munster ET., Barkhof F., and van Eijk JJJ. (2013). Punctate lesion pattern suggestive of perivascular inflammation in acute natalizumab-associated progressive multifocal leukoencephalopathy: productive JC virus infection or preclinical PML-IRIS manifestation? Journal of neurology, neurosurgery, and psychiatry 84, 1176–1177. [DOI] [PubMed] [Google Scholar]
- 44.Tabuchi S, and Uno T. (2013). Hydrocephalus with panventricular enlargement as the primary manifestation of neurosarcoidosis: a case report. Journal of medical case reports 7, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pekcevik Y, and Izbudak I. (2015). Perivascular Enhancement in a Patient with Neuromyelitis Optica Spectrum Disease during an Optic Neuritis Attack. Journal of neuroimaging : official journal of the American Society of Neuroimaging 25, 686–687. [DOI] [PubMed] [Google Scholar]
- 46.Campi A, Benndorf G, Filippi M, Reganati P, Martinelli V, and Terreni MR (2001). Primary angiitis of the central nervous system: serial MRI of brain and spinal cord. Neuroradiology 43, 599–607. [DOI] [PubMed] [Google Scholar]
- 47.Xu S, Jordan EK, Brocke S, Bulte JW, Quigley L, Tresser N, Ostuni JL, Yang Y, McFarland HF, and Frank JA (1998). Study of relapsing remitting experimental allergic encephalomyelitis SJL mouse model using MION-46L enhanced in vivo MRI: early histopathological correlation. Journal of neuroscience research 52, 549–558. [DOI] [PubMed] [Google Scholar]
- 48.Maggi P, Macri SMC, Gaitán MI, Leibovitch E, Wholer JE, Knight HL, Ellis M, Wu T, Silva AC, and Massacesi L. (2014). The formation of inflammatory demyelinated lesions in cerebral white matter. Annals of neurology 76, 594–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldmann E. (1913). Vitalfärbungen am Zentralnervensystem. Beitrag zur Physiologie des Plexus choroideus und der Hirnhäute. Hirschwald, Berlin [Google Scholar]
- 50.Delorme P, Gayet J, and Grignon G. (1975). Diffusion of horseradish peroxidase perfused through the lateral ventricle of the chick telencephalon. Cell and Tissue Research 157, 535540. [DOI] [PubMed] [Google Scholar]
- 51.Brightman MW (1968). The intracerebral movement of proteins injected into blood and cerebrospinal fluid of mice. Progress in brain research 29, 19–40. [DOI] [PubMed] [Google Scholar]
- 52.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine 4, 147ra111. 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, and Grady PA (1985). Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain research 326, 47–63. [DOI] [PubMed] [Google Scholar]
- 54.Bedussi B, Almasian M, de Vos J, VanBavel E, and Bakker EN (2018). Paravascular spaces at the brain surface: Low resistance pathways for cerebrospinal fluid flow. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 38, 719–726. 10.1177/0271678x17737984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma Q, Ries M, Decker Y, Muller A, Riner C, Bucker A, Fassbender K, Detmar M, and Proulx ST (2019). Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta neuropathologica 137, 151–165. 10.1007/s00401-018-1916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE, Mardal KA, and Eide PK (2018). Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI insight 3. 10.1172/jci.insight.121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eide PK, Vatnehol SAS, Emblem KE, and Ringstad G. (2018). Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Scientific reports 8, 7194. 10.1038/s41598-018-25666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ringstad G, Vatnehol SAS, and Eide PK (2017). Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain : a journal of neurology 140, 2691–2705. 10.1093/brain/awx191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawkes CA, Härtig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, and Carare RO (2011). Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta neuropathologica 121, 431–443. [DOI] [PubMed] [Google Scholar]
- 60.Arbel-Ornath M., Hudry E., Eikermann-Haerter K., Hou S., Gregory JL., Zhao L., Betensky RA., Frosch MP., Greenberg SM., and Bacskai BJ. (2013). Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer’s disease mouse models. Acta neuropathologica 126, 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engelhardt B, Vajkoczy P, and Weller RO (2017). The movers and shapers in immune privilege of the CNS. Nature immunology 18, 123–131. 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 62.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JAR, Perry VH, and Weller RO (2008). Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathology and applied neurobiology 34, 131–144. [DOI] [PubMed] [Google Scholar]
- 63.Engelhardt B. (2018). Cluster: barriers of the central nervous system. Acta neuropathologica 135, 307–310. 10.1007/s00401-018-1816-0. [DOI] [PubMed] [Google Scholar]
- 64.Hutchings M, and Weller RO (1986). Anatomical relationships of the pia mater to cerebral blood vessels in man. Journal of neurosurgery 65, 316–325. [DOI] [PubMed] [Google Scholar]
- 65.Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, Abbott NJ, Meyerand ME, Sorokin L, and Stanimirovic DB (2018). Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. The Journal of physiology 596, 445–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zervas NT, Liszczak TM, Mayberg MR, and Black PM (1982). Cerebrospinal fluid may nourish cerebral vessels through pathways in the adventitia that may be analogous to systemic vasa vasorum. Journal of neurosurgery 56, 475–481. [DOI] [PubMed] [Google Scholar]
- 67.Cloyd MW, and Low FN (1974). Scanning electron microscopy of the subarachnoid space in the dog. I. Spinal cord levels. Journal of Comparative Neurology 153, 325–367. [DOI] [PubMed] [Google Scholar]
- 68.Reina MA, Casasola ODL, Villanueva M, López A, Machés F, and De Andrés JA (2004). Ultrastructural findings in human spinal pia mater in relation to subarachnoid anesthesia. Anesthesia & Analgesia 98, 1479–1485. [DOI] [PubMed] [Google Scholar]
- 69.Barua NU, Bienemann AS, Hesketh S, Wyatt MJ, Castrique E, Love S, and Gill SS (2012). Intrastriatal convection-enhanced delivery results in widespread perivascular distribution in a pre-clinical model. Fluids and barriers of the CNS 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang E, Richards H, Kida S, and Weller R. (1992). Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta neuropathologica 83, 233–239. [DOI] [PubMed] [Google Scholar]
- 71.Kawakami N, gerl UV, Odoardi F, Bonhoeffer T, Wekerle H, and Flügel A. (2005). Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. The Journal of experimental medicine 201, 1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kanda T., Ishii K., Kawaguchi H., Kitajima K., and Takenaka D. (2014). High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270, 834–841. [DOI] [PubMed] [Google Scholar]
- 73.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, and Eckel LJ (2015). Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275, 772–782. [DOI] [PubMed] [Google Scholar]
- 74.Malayeri AA, Brooks KM, Bryant LH, Evers R, Kumar P, Reich DS, and Bluemke DA (2016). National Institutes of Health Perspective on Reports of Gadolinium Deposition in the Brain. Journal of the American College of Radiology : JACR 13, 237–241. 10.1016/j.jacr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taoka T, and Naganawa S. (2018). Gadolinium-based contrast media, cerebrospinal fluid and the glymphatic system: possible mechanisms for the deposition of gadolinium in the brain. Magnetic Resonance in Medical Sciences 17, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weller RO, Subash M, Preston SD, Mazanti I, and Carare RO (2008). Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain pathology (Zurich, Switzerland) 18, 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, and Liew JA (2014). Impairment of paravascular clearance pathways in the aging brain. Annals of neurology 76, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, Raskind M, Sabbagh M, Honig LS, and Porsteinsson AP (2012). Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. The Lancet Neurology 11, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, Monai H, Murlidharan G, Castellanos Rivera RM, Simon MJ, et al. (2018). Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 7. 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jessen NA, Munk AS, Lundgaard I, and Nedergaard M. (2015). The Glymphatic System: A Beginner’s Guide. Neurochemical research 40, 2583–2599. 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abbott NJ, Pizzo ME, Preston JE, Janigro D, and Thorne RG (2018). The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’system? Acta neuropathologica 135, 387–407. [DOI] [PubMed] [Google Scholar]
- 82.Hladky SB, and Barrand MA (2022). The glymphatic hypothesis: the theory and the evidence. Fluids and barriers of the CNS 19, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holter KE, Kehlet B, Devor A, Sejnowski TJ, Dale AM, Omholt SW, Ottersen OP, Nagelhus EA, Mardal K-A, and Pettersen KH (2017). Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proceedings of the National Academy of Sciences 114, 9894–9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolak DJ, and Thorne RG (2013). Diffusion of macromolecules in the brain: implications for drug delivery. Molecular pharmaceutics 10, 1492–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacAulay N. (2021). Reply to ‘Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis’. Nature reviews. Neuroscience 10.1038/s41583-021-00515-y. [DOI] [PubMed] [Google Scholar]
- 86.Smith AJ, Jin B-J, and Verkman AS (2015). Muddying the water in brain edema? Trends in neurosciences 38, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith AJ, Yao X, Dix JA, Jin BJ, and Verkman AS (2017). Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6. 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, and Weller RO (2008). Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathology and applied neurobiology 34, 131–144. 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 89.Esiri MM, and Gay D. (1990). Immunological and neuropathological significance of the Virchow-Robin space. Journal of the neurological sciences 100, 3–8. [DOI] [PubMed] [Google Scholar]
- 90.Goldmann T, Wieghofer P, Jordão MJC, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, and Krueger M. (2016). Origin, fate and dynamics of macrophages at central nervous system interfaces. Nature immunology 17, 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kida S, Steart PV, Zhang E-T, and Weller RO (1993). Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta neuropathologica 85, 646–652. [DOI] [PubMed] [Google Scholar]
- 92.de Vos AF., van Meurs M., Brok HP., Boven LA., Hintzen RQ., van der Valk P., Ravid R., Rensing S., Boon L., and Bert A. (2002). Transfer of central nervous system autoantigens and presentation in secondary lymphoid organs. The Journal of Immunology 169, 5415–5423. [DOI] [PubMed] [Google Scholar]
- 93.Fabriek BO, Zwemmer JN, Teunissen CE, Dijkstra CD, Polman CH, Laman JD, and Castelijns JA (2005). In vivo detection of myelin proteins in cervical lymph nodes of MS patients using ultrasound-guided fine-needle aspiration cytology. Journal of neuroimmunology 161, 190–194. [DOI] [PubMed] [Google Scholar]
- 94.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, and Becher B. (2005). Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nature medicine 11, 328–334. [DOI] [PubMed] [Google Scholar]
- 95.Mundt S, Greter M, Flügel A, and Becher B. (2019). The CNS immune landscape from the viewpoint of a T cell. Trends in neurosciences 42, 667–679. [DOI] [PubMed] [Google Scholar]
- 96.Vajkoczy P, Laschinger M, and Engelhardt B. (2001). α4-integrin-VCAM-1 binding mediates G protein–independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. The Journal of clinical investigation 108, 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stern L, and Gautier R. (1918). Le passage dans le liquide céphalo-rachidien de substances introduites dans la circulation et leur action sur le système nerveux central chez les différentes espèces animales. RCR d. Ia Soc. de Phys. et d’hist. natur. de Genève 35, 91–94. [Google Scholar]
- 98.Stern L, and Gautier R. (1921). Recherches sur Le liquide céphalo-rachidien: I.–Les rapports entre Le liquide céphalo-rachidien et la circulation sanguine. Archives Internationales de Physiologie 17, 138–192. [Google Scholar]
- 99.Lewandowsky M. (1909). Zur lehre der cerebrospinalflussigkeit. Z. klin. Med 40, 480–494. [Google Scholar]
- 100.Reese T, and Karnovsky MJ (1967). Fine structural localization of a blood-brain barrier to exogenous peroxidase. Journal of Cell Biology 34, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saunders NR, Dreifuss J-J, Dziegielewska KM, Johansson PA, Habgood MD, Møllgård K, and Bauer H-C (2014). The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Frontiers in neuroscience 8, 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stern L, and Gautier R. (1918). Passage simultané des substances dans le liquide céphalo-rachidien et dans les centres nerveux. RCR d. Ia Soc. de Phys. et d’hist. natur. de Genève 35, 58–60. [Google Scholar]
- 103.Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WE, Flügel-Koch C, Issekutz TB, and Wekerle H. (2009). Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98. [DOI] [PubMed] [Google Scholar]
- 104.Mues M, Bartholomäus I, Thestrup T, Griesbeck O, Wekerle H, Kawakami N, and Krishnamoorthy G. (2013). Real-time in vivo analysis of T cell activation in the central nervous system using a genetically encoded calcium indicator. Nature medicine 19, 778–783. [DOI] [PubMed] [Google Scholar]
- 105.Kyratsous NI, Bauer IJ, Zhang G, Pesic M, Bartholomäus I, Mues M, Fang P, Wörner M, Everts S, and Ellwart JW (2017). Visualizing context-dependent calcium signaling in encephalitogenic T cells in vivo by two-photon microscopy. Proceedings of the National Academy of Sciences 114, E6381–E6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Archambault AS, Sim J, Gimenez MAT, and Russell JH (2005). Defining antigendependent stages of T cell migration from the blood to the central nervous system parenchyma. European journal of immunology 35, 1076–1085. [DOI] [PubMed] [Google Scholar]
- 107.Engelhardt B, Carare RO, Bechmann I, Flügel A, Laman JD, and Weller RO (2016). Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta neuropathologica 132, 317–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Owens T, Bechmann I, and Engelhardt B. (2008). Perivascular spaces and the two steps to neuroinflammation. Journal of Neuropathology & Experimental Neurology 67, 1113–1121. [DOI] [PubMed] [Google Scholar]
- 109.Bechmann I, Galea I, and Perry VH (2007). What is the blood–brain barrier (not)? Trends in immunology 28, 5–11. [DOI] [PubMed] [Google Scholar]
- 110.Dyrna F, Hanske S, Krueger M, and Bechmann I. (2013). The blood-brain barrier. Journal of Neuroimmune Pharmacology 8, 763–773. [DOI] [PubMed] [Google Scholar]
- 111.Tran EH., Hoekstra K., van Rooijen N., Dijkstra CD., and Owens T. (1998). Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. The Journal of Immunology 161, 3767–3775. [PubMed] [Google Scholar]
- 112.Castro Dias M, Mapunda JA, Vladymyrov M, and Engelhardt B. (2019). Structure and junctional complexes of endothelial, epithelial and glial brain barriers. International journal of molecular sciences 20, 5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sweeney MD, Zhao Z, Montagne A, Nelson AR, and Zlokovic BV (2019). Blood-Brain Barrier: From Physiology to Disease and Back. Physiological reviews 99, 21–78. 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marchetti L, and Engelhardt B. (2020). Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vascular Biology 2, H1–H18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bielecki B, Mazurek A, Wolinski P, and Glabinski A. (2007). Expression of chemokine receptors CCR7 and CCR8 in the CNS during ChREAE. Scandinavian journal of immunology 66, 383–392. [DOI] [PubMed] [Google Scholar]
- 116.Gimenez MA, Sim J, Archambault AS, Klein RS, and Russell JH (2006). A tumor necrosis factor receptor 1-dependent conversation between central nervous system-specific T cells and the central nervous system is required for inflammatory infiltration of the spinal cord. The American journal of pathology 168, 1200–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, and Pleasure D. (2014). Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 8175–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toft-Hansen H, Buist R, Sun XJ, Schellenberg A, Peeling J, and Owens T. (2006). Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. Journal of immunology (Baltimore, Md. : 1950) 177, 7242–7249. [DOI] [PubMed] [Google Scholar]
- 119.Fuchtbauer L, Toft-Hansen H, Khorooshi R, and Owens T. (2010). Expression of Astrocytic Type 2 Angiotensin Receptor in Central Nervous System Inflammation Correlates With Blood-Brain Barrier Breakdown. Journal of Molecular Neuroscience 42, 89–98. [DOI] [PubMed] [Google Scholar]
- 120.Schellenberg AE, Buist R, Del Bigio MR, Toft-Hansen H, Khorooshi R, Owens T, and Peeling J. (2012). Blood-brain barrier disruption in CCL2 transgenic mice during pertussis toxin-induced brain inflammation. Fluids and Barriers of the CNS 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mills Ko E, Ma JH, Guo F, Miers L, Lee E, Bannerman P, Burns T, Ko D, Sohn J, Soulika AM, and Pleasure D. (2014). Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. Journal of neuroinflammation 11, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Samus M, Seelige R, Schäfer K, Sorokin L, and Vestweber D. (2018). CD99L2 deficiency inhibits leukocyte entry into the central nervous system and ameliorates neuroinflammation. Journal of leukocyte biology 104, 787–797. [DOI] [PubMed] [Google Scholar]
- 123.Paul D, Baena V, Ge S, Jiang X, Jellison ER, Kiprono T, Agalliu D, and Pachter JS (2016). Appearance of claudin-5+ leukocytes in the central nervous system during neuroinflammation: a novel role for endothelial-derived extracellular vesicles. Journal of neuroinflammation 13, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alvarez JI, Saint-Laurent O, Godschalk A, Terouz S, Briels C, Larouche S, Bourbonnière L, Larochelle C, and Prat A. (2015). Focal disturbances in the blood–brain barrier are associated with formation of neuroinflammatory lesions. Neurobiology of disease 74, 14–24. [DOI] [PubMed] [Google Scholar]
- 125.Pesic M, Bartholomaus I, Kyratsous NI, Heissmeyer V, Wekerle H, and Kawakami N. (2013). 2-photon imaging of phagocyte-mediated T cell activation in the CNS. The Journal of clinical investigation 123, 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stohlman SA, Bergmann CC, Lin MT, Cua DJ, and Hinton DR (1998). CTL effector function within the central nervous system requires CD4+ T cells. The Journal of Immunology 160, 2896–2904. [PubMed] [Google Scholar]
- 127.Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S, Nishida H, Komori T, Seki M, Komatsu T, et al. (2020). Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain : a journal of neurology 143, 1431–1446. [DOI] [PubMed] [Google Scholar]
- 128.Li Y, Chu N, Hu A, Gran B, Rostami A, and Zhang G-X (2007). Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain 130, 490–501. [DOI] [PubMed] [Google Scholar]
- 129.Claudio L, Raine CS, and Brosnan CF (1995). Evidence of persistent blood-brain barrier abnormalities in chronic-progressive multiple sclerosis. Acta neuropathologica 90, 228–238. [DOI] [PubMed] [Google Scholar]
- 130.Davalos D., Kyu Ryu J., Merlini M., Baeten KM., Le Moan N., Petersen MA., Deerinck TJ., Smirnoff DS., Bedard C., Hakozaki H., and Akassoglou K. (2012). Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nature communications 3, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van Horssen J, Vos CMP, Admiraal L, van Haastert ES, Montagne L, van der Valk P, and de Vries HE (2006). Matrix metalloproteinase-19 is highly expressed in active multiple sclerosis lesions. Neuropathology and applied neurobiology 32, 585–593. [DOI] [PubMed] [Google Scholar]
- 132.Gerwien H, Hermann S, Zhang X, Korpos E, Song J, Kopka K, Faust A, Wenning C, Gross CC, and Honold L. (2016). Imaging matrix metalloproteinase activity in multiple sclerosis as a specific marker of leukocyte penetration of the blood-brain barrier. Science translational medicine 8, 364ra152–364ra152. [DOI] [PubMed] [Google Scholar]
- 133.Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, Faber C, Schäfers M, Körner H, Opdenakker G, et al. (2015). Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep 10, 1040–1054. 10.1016/j.celrep.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 134.Althoff GE, Wolfer DP, Timmesfeld N, Kanzler B, Schrewe H, and Pagenstecher A. (2010). Long-term expression of tissue-inhibitor of matrix metalloproteinase-1 in the murine central nervous system does not alter the morphological and behavioral phenotype but alleviates the course of experimental allergic encephalomyelitis. The American journal of pathology 177, 840–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, and Dijkstra CD (1995). The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia 15, 437–446. [DOI] [PubMed] [Google Scholar]
- 136.Agrawal SM, Williamson J, Sharma R, Kebir H, Patel K, Prat A, and Yong VW (2013). Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain : a journal of neurology 136, 1760–1777. [DOI] [PubMed] [Google Scholar]
- 137.Agrawal SM, Silva C, Tourtellotte WW, and Yong VW (2011). EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. Journal of Neuroscience 31, 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hahn JN, Kaushik DK, Mishra MK, Wang J, Silva C, and Yong VW (2016). Impact of minocycline on extracellular matrix metalloproteinase inducer, a factor implicated in multiple sclerosis immunopathogenesis. The Journal of Immunology 197, 3850–3860. [DOI] [PubMed] [Google Scholar]
- 139.Kaushik DK, Bhattacharya A, Mirzaei R, Rawji KS, Ahn Y, Rho JM, and Yong VW (2019). Enhanced glycolytic metabolism supports transmigration of brain-infiltrating macrophages in multiple sclerosis. The Journal of clinical investigation 129, 3277–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nelissen I, Gveric D, Van Noort J, Cuzner M, and Opdenakker G. (2006). PECAM-1 and gelatinase B coexist in vascular cuffs of multiple sclerosis lesions. Neuropathology and applied neurobiology 32, 15–22. [DOI] [PubMed] [Google Scholar]
- 141.Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, and Jain P. (2012). Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. Journal of neuroinflammation 9, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Savarin C, Stohlman SA, Atkinson R, Ransohoff RM, and Bergmann CC (2010). Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis. Journal of virology 84, 4878–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCandless EE., Wang Q., Woerner BM., Harper JM., and Klein RS. (2006). CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. Journal of immunology (Baltimore, Md. : 1950) 177, 8053–8064. [DOI] [PubMed] [Google Scholar]
- 144.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, and Klein RS (2008). Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. The American journal of pathology 172, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, and Littman DR (2011). CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. Journal of Experimental Medicine 208, 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vergote D, Butler GS, Ooms M, Cox JH, Silva C, Hollenberg MD, Jhamandas JH, Overall CM, and Power C. (2006). Proteolytic processing of SDF-1α reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proceedings of the National Academy of Sciences 103, 19182–19187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, and Overall CM (2008). Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2,−7,−8, and-13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood, The Journal of the American Society of Hematology 112, 3455–3464. [DOI] [PubMed] [Google Scholar]
- 148.Song J, Zhang X, Buscher K, Wang Y, Wang H, Di Russo J, Li L, Lütke-Enking S, Zarbock A, and Stadtmann A. (2017). Endothelial basement membrane laminin 511 contributes to endothelial junctional tightness and thereby inhibits leukocyte transmigration. Cell reports 18, 1256–1269. [DOI] [PubMed] [Google Scholar]
- 149.Körner H, Riminton DS, Strickland DH, Lemckert FA, Pollard JD, and Sedgwick JD (1997). Critical points of tumor necrosis factor action in central nervous system autoimmune inflammation defined by gene targeting. The Journal of experimental medicine 186, 15851590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kunz J, Krause D, Gehrmann J, and Dermietzel R. (1995). CHANGES IN THE EXPRESSION PATTERN OF BLOOD-BRAIN BARRIER-ASSOCIATED PERICYTIC AMINOPEPTIDASE-N (PAP-N) IN THE COURSE OF ACUTE EXPERIMENTAL AUTOIMMUNE ENCEPHALOMYELITIS. Journal of Neuroimmunology 59, 41–55. [DOI] [PubMed] [Google Scholar]
- 151.Nataf S, Carroll SL, Wetsel RA, Szalai AJ, and Barnum SR (2000). Attenuation of experimental autoimmune demyelination in complement-deficient mice. Journal of immunology (Baltimore, Md. : 1950) 165, 5867–5873. [DOI] [PubMed] [Google Scholar]
- 152.Amatruda M, Chapouly C, Woo V, Safavi F, Zhang J, Dai D, Therattil A, Moon C, Villavicencio J, Gordon A, et al. (2022). Astrocytic junctional adhesion molecule-A regulates T-cell entry past the glia limitans to promote central nervous system autoimmune attack. Brain Commun 4, fcac044. 10.1093/braincomms/fcac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stephenson EL, Mishra MK, Moussienko D, Laflamme N, Rivest S, Ling C-C, and Yong VW (2018). Chondroitin sulfate proteoglycans as novel drivers of leucocyte infiltration in multiple sclerosis. Brain 141, 1094–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gveric D, Hanemaaijer R, Newcombe J, van Lent NA, Sier CF, and Cuzner ML (2001). Plasminogen activators in multiple sclerosis lesions: implications for the inflammatory response and axonal damage. Brain 124, 1978–1988. [DOI] [PubMed] [Google Scholar]
- 155.Engelhardt B, and Sorokin L. (2009). The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. In 4. (Springer; ), pp. 497–511. [DOI] [PubMed] [Google Scholar]
- 156.Sorokin L. (2010). The impact of the extracellular matrix on inflammation. Nature Reviews Immunology 10, 712–723. [DOI] [PubMed] [Google Scholar]
- 157.Profaci CP, Munji RN, Pulido RS, and Daneman R. (2020). The blood–brain barrier in health and disease: Important unanswered questions Journal of Experimental Medicine 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sweeney MD, Zhao Z, Montagne A, Nelson AR, and Zlokovic BV (2019). Blood-brain barrier: from physiology to disease and back. Physiological reviews 99, 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Steinman L. (2014). Immunology of relapse and remission in multiple sclerosis. Annual review of immunology 32, 257–281. [DOI] [PubMed] [Google Scholar]
- 160.Rindfleisch E. (1863). Histologisches Detail zu der grauen Degeneration von Gehirn und Rückenmark.(Zugleich ein Beitrag zu der Lehre von der Entstehung und Verwandlung der Zelle.). Virchows Archiv 26, 474–483. [Google Scholar]
- 161.Rae-Grant AD, Wong C, Bernatowicz R, and Fox RJ (2014). Observations on the brain vasculature in multiple sclerosis: A historical perspective. Multiple sclerosis and related disorders 3, 156–162. 10.1016/j.msard.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 162.Tan IL., van Schijndel RA., Pouwels PJ., van Walderveen MA., Reichenbach JR., Manoliu RA., and Barkhof F. (2000). MR venography of multiple sclerosis. AJNR. American journal of neuroradiology 21, 1039–1042. [PMC free article] [PubMed] [Google Scholar]
- 163.Absinta M, Sati P, and Reich DS (2016). Advanced MRI and staging of multiple sclerosis lesions. Nature reviews. Neurology 12, 358–368. 10.1038/nrneurol.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sati P, Oh J, Constable RT, Evangelou N, Guttmann CR, Henry RG, Klawiter EC, Mainero C, Massacesi L, McFarland H, et al. (2016). The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nature reviews. Neurology 12, 714–722. 10.1038/nrneurol.2016.166. [DOI] [PubMed] [Google Scholar]
- 165.Maggi P, Absinta M, Grammatico M, Vuolo L, Emmi G, Carlucci G, Spagni G, Barilaro A, Repice AM, and Emmi L. (2018). Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Annals of neurology 83, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dal-Bianco A, Hametner S, Grabner G, Schernthaner M, Kronnerwetter C, Reitner A, Vass C, Kircher K, Auff E, Leutmezer F, et al. (2015). Veins in plaques of multiple sclerosis patients - a longitudinal magnetic resonance imaging study at 7 Tesla. European radiology 25, 2913–2920. 10.1007/s00330-015-3719-y. [DOI] [PubMed] [Google Scholar]
- 167.Gaitan MI, de Alwis MP, Sati P, Nair G, and Reich DS (2013). Multiple sclerosis shrinks intralesional, and enlarges extralesional, brain parenchymal veins. Neurology 80, 145–151. 10.1212/WNL.0b013e31827b916f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Absinta M, Nair G, Monaco MCG, Maric D, Lee NJ, Ha SK, Luciano NJ, Sati P, Jacobson S, and Reich DS (2019). The “Central Vein Sign” in inflammatory demyelination: The role of fibrillar collagen type I. Annals of neurology. 10.1002/ana.25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Höftberger R, Guo Y, Flanagan EP, Lopez-Chiriboga AS, Endmayr V, Hochmeister S, Joldic D, Pittock SJ, Tillema JM, Gorman M, et al. (2020). The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta neuropathologica 139, 875–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, and Aloisi F. (2004). Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain pathology 14, 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lassmann H. (2019). Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Frontiers in immunology, 3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ineichen BV, Sati P, Granberg T, Absinta M, Lee NJ, Lefeuvre JA, and Reich DS (2020). Magnetic resonance imaging in multiple sclerosis animal models: A systematic review, meta-analysis, and white paper. NeuroImage: Clinical, 102371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lassmann H, and Bradl M. (2016). Multiple sclerosis: experimental models and reality. Acta neuropathologica. 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Young NP., Weinshenker BG., Parisi JE., Scheithauer B., Giannini C., Roemer SF., Thomsen KM., Mandrekar JN., Erickson BJ., and Lucchinetti CF. (2010). Perivenous demyelination: association with clinically defined acute disseminated encephalomyelitis and comparison with pathologically confirmed multiple sclerosis. Brain : a journal of neurology 133, 333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Beltrán E, Paunovic M, Gebert D, Cesur E, Jeitler M, Höftberger R, Malotka J, Mader S, Kawakami N, and Meinl E. (2021). Archeological neuroimmunology: resurrection of a pathogenic immune response from a historical case sheds light on human autoimmune encephalomyelitis and multiple sclerosis. Acta neuropathologica 141, 67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Groeschel S, Chong WK, Surtees R, and Hanefeld F. (2006). Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology 48, 745–754. 10.1007/s00234-006-0112-1. [DOI] [PubMed] [Google Scholar]
- 177.Lam MA, Hemley SJ, Najafi E, Vella NG, Bilston LE, and Stoodley MA (2017). The ultrastructure of spinal cord perivascular spaces: implications for the circulation of cerebrospinal fluid. Scientific reports 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ineichen BV, Beck ES, Piccirelli M, and Reich DS (2021). New Prospects for Ultra-HighField Magnetic Resonance Imaging in Multiple Sclerosis. Invest Radiol. 10.1097/rli.0000000000000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, and Xiao M. (2015). Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Molecular neurodegeneration 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, Grafe MR, Woltjer RL, Kaye J, and Iliff JJ (2017). Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA neurology 74, 91–99. [DOI] [PubMed] [Google Scholar]
- 181.Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, Alper SL, Lundgaard I, Nedergaard M, and Kahle KT (2020). Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends in molecular medicine 26, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schubert JJ, Veronese M, Marchitelli L, Bodini B, Tonietto M, Stankoff B, Brooks DJ, Bertoldo A, Edison P, and Turkheimer F. (2019). Dynamic (11)C-PiB PET shows cerebrospinal fluid flow alterations in Alzheimer’s disease and multiple sclerosis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 10.2967/jnumed.118.223834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wlodarczyk A, Løbner M, Cédile O, and Owens T. (2014). Comparison of microglia and infiltrating CD11c+ cells as antigen presenting cells for T cell proliferation and cytokine response. Journal of neuroinflammation 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao X, Eyo UB, Murugan M, and Wu LJ (2018). Microglial interactions with the neurovascular system in physiology and pathology. Developmental neurobiology 78, 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Linington C, Bradl M, Lassmann H, Brunner C, and Vass K. (1988). Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. The American journal of pathology 130, 443. [PMC free article] [PubMed] [Google Scholar]
- 186.Ioannidis JP (2012). Extrapolating from animals to humans. Science translational medicine 4, 151ps115. 10.1126/scitranslmed.3004631. [DOI] [PubMed] [Google Scholar]