Abstract
Objective
This study aimed to explore possible associations between molecular subtypes and site of distant metastasis in advanced breast cancer (ABC).
Methods
3577 ABC patients were selected from 21 hospitals of seven geographic regions in China from 2012-2014. A questionnaire was designed to collect medical information regarding demographic characteristics, risk factors, molecular subtype, recurrence/metastasis information, and disease-free survival (DFS). The cancers were classified into Luminal A, Luminal B, HER2-enriched and Triple Negative subtypes. Chi-square test and multivariate Cox proportional hazard models were performed to explore the associations between molecular subtypes and distant metastasis sites.
Results
A total of 2393 cases with molecular subtypes information were finally examined. Patients with Luminal A (51.1%) and Luminal B (44.7%) were most prone to bone metastasis, whereas liver metastasis was more frequently observed in HER2-enriched ABC patients (29.1%).The cumulative recurrence and metastasis rates of ABC patients at 36 months of DFS were the most significant within molecular types, of which Triple Negative was the highest (82.7%), while that of Luminal A was the lowest (58.4%). In the adjusted Cox regression analysis, Luminal B, HER2-enriched and Triple Negative subtypes increased the risk of visceral metastasis by 23%, 46% and 87% respectively. In addition, Triple Negative patients had a higher probability of brain metastasis (HR 3.07, 95% CI: 1.04-9.07).
Conclusion
Molecular subtypes can predict the preferential sites of distant metastasis, emphasizing that these associations were of great help in choices for surveillance, developing appropriate screening and cancer management strategies for follow-up and personalized therapy in ABC patients.
Keywords: advanced breast cancer, molecular subtype, site of distant metastasis, retrospective study, clinical epidemiological characteristic
Introduction
Breast cancer is the most common malignancy in women worldwide. Approximately 2,261,419 new cases and 416,371 deaths occurred in 2020 (1). Although the prognosis of breast cancer patients is generally favorable and mortality has declined due to early detection, optimal surgery and improved adjuvant therapies, 20%–30% of patients will still develop distant metastases and cases with progressive stage only have a median two-year survival time (2, 3). The distant organs to which breast cancer preferentially metastasizes, of which bone, liver, lung, and brain are among the most common sites, are associated with the patients’ survival outcomes (4, 5). Patients with brain metastases or metastases at multiple sites generally have relatively poor prognostic outcome compared with lung and other visceral metastases (6). Breast cancer is no longer seen as a single disease but rather a multifaceted disease comprised of distinct biological subtypes with diverse natural history, presenting a varied spectrum of clinical, pathologic and molecular features with different prognostic and therapeutic implications.
Breast cancer is widely recognized as a heterogeneous disease with a variable clinical and pathological behavior and also different prognosis and response to cancer therapies, which makes it difficult not only to define prognosis of this disease, but also predict the risk of metastasis (7, 8). It has long been accepted that common risk factors such as tumor size, histologic grade, clinical stage and hormone receptor status have with increasing importance influenced the progression of malignancy and the pattern of distant metastasis (9, 10). Also, molecular subtype is being increasingly considered as an indicator to further reveal distinct characteristics and mechanisms for different organ-specific metastatic breast cancer variants (11). Four major molecular subtypes of breast cancer have been identified based on their gene expression profiling and immunohistochemical results: Luminal A, Luminal B, HER2-enriched and Triple Negative. There is growing evidence indicating that patterns of metastasis and clinical outcome are different between different subtypes: Luminal tumors are associated with a better prognosis compared with HER2-enriched or Triple negative tumors which have a more aggressive clinical outcome (12, 13). Our research team has already conducted a 10-year retrospective multi-center study of breast cancer in China (14). However, data are limited concerning advanced breast cancer (ABC) at a national level. Therefore, in the present study, we sought to investigate the possible relationship between molecular subtypes and the preferential distant metastasis sites among ABC patients to guide treatment decision making and develop appropriate surveillance strategies.
Materials and methods
Institutional review board
This study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (CHCAMS). Patient consent was not required as there were no risks anticipated to the participants of the study. All data were stripped of any patient identifiers.
Study design and hospital selection
This study was a 3-year retrospective multi-center analysis in China from January 1st, 2012 to December 31st, 2014. Female ABC patients were selected randomly from 21 tertiary hospitals of seven geographic regions. Traditionally, China was divided into seven geographical regions: north, northeast, central, south, east, northwest, and southwest, three tertiary hospitals were selected from each region randomly. The aim of this method was to establish a large group of participants in ABC with complete pathological diagnosis and treatment information, which could fully represent the differences between urban and rural areas, including women of different living habits and socio-economic status in different geographical regions.
Patients and inclusion criteria
The subjects of this study were pathologically confirmed ABC patients with recurrence and distant metastasis hospitalized within the specified period. The number of collected cases were determined proportionally according to the inherent population capacity of each geographical region. In each hospital, one month was randomly selected for each year according to the random number and all pathologically diagnosed ABC cases for that month were reviewed. January and February were excluded from the random selection to decrease any confounding effects of the Chinese New Year holiday (the longest holiday period of the year). Firstly, all eligible cases were recruited in 2012, if inpatients admissions were less than predetermined numbers, more cases from the neighboring months were reviewed in that year, otherwise, the remaining cases continued to be collected in 2013 and 2014, until the total number in that year reaches the target quantity.
All patients enrolled in this study must meet 3 key inclusion criteria: (1) pathology confirmed female ABC, when diagnosed as stage IV or local recurrence/distant metastasis after diagnosis; (2) inpatient admission date was within the selected month in the study hospital and (3) full information of surgical pathology results, time and location of metastasis, detailed therapeutic methods and protocols after metastasis.
Data collection and quality control
Medical records were reviewed by local clerks within each hospital according to the designated protocol and a questionnaire was designed by epidemiologists and breast physicians in CHCAMS to collect information from each enrolled patient’s chart regarding (1) General information; (2) Demographic characteristics and breast cancer risk factors; (3) Clinical and imaging diagnostic information at first diagnosis; (4) Molecular subtype of patients; (5) Surgical and adjuvant treatment information; (6) Recurrence and metastasis treatment information including distant metastasis sites, disease-free survival (DFS), endocrine therapy, targeted therapy and chemotherapy after metastasis.
Two data-input clerks from each hospital were recruited to independently double-enter all above information from the paper to computer-based database. Then all finished double-entry databases were sent to CHCAMS for validation by running EpiData. Inconsistencies between the two databases revealed by CHCAMS were reported to the local clerks for adjudication until the databases were consistent. As a final inspection, one of the databases was chosen to undergo a final consistency check. Logical errors (e.g. the first recurrence and metastasis date were earlier than the date of the first diagnosis of breast cancer) were reported back to the local hospital, and the local collaborators reviewed the original medical chart again. After checking with the original medical record, the local staff sent the revised database back to CHCAMS for a final analysis. During the consistency check, 5% of the medical charts were randomly selected based on the study ID and sent to CHCAMS for quality control review.
Molecular subtypes
The cancers were classified into four molecular subtypes according to the St-gallen Guidelines, 2013 (15): (1) Luminal A: ER+, PR+, HER2- and Ki-67<14%; (2) Luminal B: ① ER+, HER2- and at least one of: Ki-67 > 14%, PR- or low expression, low recurrence risk based on polygene expression analysis (if applicable); ② ER+, HER2+, any Ki-67 and any PR; (3) HER2-enriched: ER-, PR- and HER2+; (4) triple negative: ER-, PR- and HER2-.
Recurrence and distant metastasis
Recurrence and distant metastasis were diagnosed by clinical evaluations including imaging studies or biopsy. Distant metastasis was defined as metastasis of breast cancer developing beyond the ipsilateral or contralateral breast, chest wall, or regional lymph node including ipsilateral axillary, supraclavicular, or internal mammary lymph node. Five patterns of the distant metastasis were mainly classified: bone metastasis only, metastases excluding bone and brain, bone metastasis + others (excluding brain metastasis), brain metastasis only and brain metastasis + others. Cumulative frequency of bone, liver, lung, brain and visceral metastases (including liver, lung, brain, adrenal gland, ovary, etc.) in this study was analyzed regarding to the molecular subtypes. Disease free survival (DFS) was defined as the time from the first diagnosis of breast cancer by surgery or puncture to recurrence and/or metastasis, whichever was the earliest.
Statistical analysis
Patients’ demographic and clinicopathological characteristics, pattern of the distant metastasis and cumulative frequency of the metastasis sites were compared within subtypes using chi-square (χ2) test or fisher for categorical variables as appropriate and analysis of variance (ANOVA) for continuous variables. The Kaplan-Meier analysis was used for cumulative recurrence and metastasis rates with a log-rank test to assess the significance among the four molecular subtypes. Cox proportional hazards regression models were used to determine hazard ratios (HRs) and 95% confidence intervals (Cis) for the associations between molecular subtypes and the risk of distant metastasis at specific sites. Potential confounders included age at diagnosis, tumor size, number of lymph node metastasis, menstrual status, education and family history. In the pairwise comparisons, Luminal A was set as the referral group for the better prognosis, all other subtypes compared with it. Statistical significance was assessed using two-tailed tests with a significant level of 0.05. Analyses were conducted using SPSS statistical software version 22.0 (IBM Corp, Armonk, NY, http://www.ibm.com).
Results
Clinicopathological characteristics
A total of 3577 female patients with pathologically confirmed ABC from January 1st, 2012 to December 31st, 2014 were identified. 1184 patients were excluded from analysis with missing information of molecular subtypes. Therefore, 2393 patients included in the final analysis. The most common subtype in ABC patients was Luminal types (1361/2393, 56.9%), followed by HER2-enriched (523/2393, 21.9%), Triple Negative (509/2393, 21.3%). Clinicopathological characteristics of ABC patients according to molecular subtypes are shown in Table 1 . Age at diagnosis, household register, education, surgery, adjuvant chemotherapy, adjuvant endocrine therapy and DFS were found to be significantly different among the four molecular subtypes (P<0.05). The subtype of Triple negative was older at first diagnosis and rural household registration, was more likely to have lower level of education and a relatively shorter DFS, compared with other three subtypes.
Table 1.
Clinicopathological characteristics of ABC patients according to molecular subtypes.
| Total | Luminal A | Luminal B | HER2-enriched | Triple Negative | P value | |
|---|---|---|---|---|---|---|
| Age at diagnosis (years) | ||||||
| Mean ± SD | 46.51 ± 10.40 | 45.70 ± 10.53 | 45.86 ± 10.47 | 47.19 ± 10.71 | 47.65 ± 9.69 | 0.002 |
| ≤45 | 1125 (47.0%) | 182 (52.3%) | 504 (49.8%) | 240 (45.9%) | 199 (39.1%) | <0.001 |
| >45 | 1268 (53.0%) | 166 (47.7%) | 509 (50.2%) | 283 (54.1%) | 310 (60.9%) | |
| BMI (Kg/m2) | ||||||
| Mean ± SD | 23.89 ± 3.37 | 23.96 ± 3.42 | 23.68 ± 3.33 | 24.09 ± 3.19 | 24.07 ± 3.58 | 0.083 |
| Underweight (≤18.49) | 87 (4.1%) | 9 (2.9%) | 42 (4.6%) | 15 (3.2%) | 21 (4.6%) | 0.479 |
| Normal Weight (18.50~24.99) | 1313 (61.5%) | 194 (62.2%) | 573 (63.4%) | 276 (59.7%) | 270 (59.2%) | |
| Overweight (25.00~29.99) | 626 (29.3%) | 93 (29.8%) | 246 (27.2%) | 150 (32.5%) | 137 (30.0%) | |
| Obesity (>29.99) | 108 (5.1%) | 16 (5.1%) | 43 (4.8%) | 21(4.5%) | 28 (6.1%) | |
| Household register | ||||||
| Urban | 1146 (48.1%) | 152 (44.1%) | 490 (48.4%) | 269 (51.6%) | 235 (46.4%) | 0.010 |
| Rural | 872 (36.6%) | 123 (35.7%) | 375 (37.1%) | 167 (32.1%) | 207 (40.8%) | |
| Unknown | 367 (15.4%) | 70(20.3%) | 147(14.5%) | 85 (16.3%) | 65 (12.8%) | |
| Education | ||||||
| ≤Primary school education | 244 (10.2%) | 33 (9.6%) | 110 (10.9%) | 42 (8.0%) | 59 (11.7%) | 0.005 |
| Middle school education | 191 (8.0%) | 17 (5.0%) | 88 (8.7%) | 37 (7.1%) | 49 (9.7%) | |
| ≥ High school education | 276 (11.6%) | 33 (9.6%) | 135 (13.4%) | 48 (9.2%) | 60 (11.9%) | |
| Unknown | 1670 (70.1%) | 260(75.8%) | 677 (67.0%) | 395 (75.7%) | 338 (66.8%) | |
| Marital status (%) | ||||||
| Unmarried | 73 (3.1%) | 9 (2.6%) | 32 (3.2%) | 18 (3.5%) | 14 (2.8%) | 0.983 |
| Married | 2269 (95.7%) | 328 (96.2%) | 959 (95.6%) | 496 (95.6%) | 486 (95.9%) | |
| Widowed/divorced | 28 (1.2%) | 4 (1.2%) | 12 (1.2%) | 5 (1.0%) | 7 (1.4%) | |
| Menstrual status (%) | ||||||
| Premenopausal | 1097 (49.1%) | 163 (50.3%) | 477 (50.3%) | 246 (50.4%) | 211 (44.5%) | 0.170 |
| Postmenopausal | 1138 (50.9%) | 161 (49.7%) | 47 2(49.7%) | 242 (49.6%) | 263(55.5%) | |
| Family history | ||||||
| Yes | 111 (4.9%) | 14 (4.2%) | 48 (5.0%) | 30 (6.0%) | 19 (3.9%) | 0.442 |
| No | 2162 (95.1%) | 317 (95.8%) | 914 (95.0%) | 467 (94.0%) | 464 (96.1%) | |
| Smoking status | ||||||
| Yes | 26 (1.2%) | 4 (1.3%) | 7 (0.8%) | 7 (1.5%) | 8 (1.7%) | 0.415 |
| No | 2143 (98.8%) | 308 (98.7%) | 908 (99.2%) | 473 (98.5%) | 454 (98.3%) | |
| Drinking status | ||||||
| Yes | 36 (1.7%) | 5 (1.6%) | 15 (1.6%) | 9 (1.9%) | 7 (1.5%) | 0.976 |
| No | 2133 (98.3%) | 307 (98.4%) | 900 (98.4%) | 470 (98.1%) | 456 (98.5%) | |
| Tumor size (cm) | ||||||
| ≤ 2 | 440 (26.0%) | 80 (31.7%) | 179 (25.4%) | 95 (25.9%%) | 86 (23.1%) | 0.220 |
| 2-5 | 986 (58.2%) | 138 (54.8%) | 415 (58.9%) | 217 (59.1%) | 216 (58.1%) | |
| >5 | 269 (15.9%) | 34 (13.5%) | 110 (15.6%) | 55 (15.0%) | 70 (18.8%) | |
| Number of lymph node metastasis | ||||||
| 0 | 849 (35.5%) | 120 (34.5%) | 345 (34.1%) | 202 (38.6%) | 182 (35.8%) | 0.188 |
| 1-3 | 611 (25.5%) | 103 (29.6%) | 255 (25.2%) | 138 (26.4%) | 115 (22.6%) | |
| 3-9 | 485 (20.3%) | 70 (20.1%) | 207 (20.4%) | 97 (18.5%) | 111 (21.8%) | |
| >9 | 448 (18.7%) | 55 (15.8%) | 206 (20.3%) | 86 (16.4%) | 101 (19.8%) | |
| Pathological type (%) | ||||||
| Carcinoma in situ | 57 (2.4%) | 6 (1.8%) | 18 (1.8%) | 16 (3.1%) | 17 (3.4%) | 0.102 |
| Invasive ductal carcinoma | 1877 (80.4%) | 265 (79.3%) | 795 (80.3%) | 415 (81.2%) | 402 (80.2%) | |
| Other invasive carcinoma | 254 (10.9%) | 47 (14.1%) | 116 (11.7%) | 43 (8.4%) | 48 (9.6%) | |
| Others | 148 (6.3%) | 16 (4.8%) | 61 (6.2%) | 37 (7.2%) | 34 (6.8%) | |
| Surgery | ||||||
| Yes | 2155 (90.5%) | 316 (92.1%) | 890 (88.1%) | 495 (94.8%) | 454 (89.7%) | <0.001 |
| No | 223 (9.4%) | 27 (7.9%) | 119 (11.8%) | 25 (4.8%) | 52 (10.3%) | |
| Surgical method | ||||||
| Mastectomy | 1902 (90.6%) | 278 (92.4%) | 774 (88.9%) | 440 (90.3%) | 410 (93.2%) | 0.053 |
| Conservative surgery | 197 (9.4%) | 23 (7.6%) | 97 (11.1%) | 47 (9.7%) | 30 (6.8%) | |
| Adjuvant chemotherapy | ||||||
| Yes | 1930 (82.8%) | 271 (80.4%) | 804 (81.5%) | 454 (88.5%) | 401 (81.0%) | 0.002 |
| No | 402 (17.2%) | 66 (19.6%) | 183 (18.5%) | 59 (11.5%) | 94 (19.0%) | |
| Adjuvant radiotherapy | ||||||
| Yes | 960 (41.6%) | 128 (38.6%) | 412 (42.3%) | 226 (44.7%) | 194 (39.2%) | 0.205 |
| No | 1348 (58.4%) | 204 (61.4%) | 563 (57.7%) | 280 (55.3%) | 301 (60.8%) | |
| Adjuvant endocrine therapy | ||||||
| Yes | 923 (39.6%) | 212 (63.7%) | 610 (61.7%) | 42 (8.3%) | 59 (11.8%) | <0.001 |
| No | 1406 (60.4) | 121 (36.3%) | 379 (38.3%) | 466 (91.7%) | 440 (88.2%) | |
| DFS (months) | ||||||
| Median | 29.0 | 36.0 | 27.0 | 20.0 | 18.5 | <0.001 |
| ≤24 | 998 (50.4%) | 86 (30.7%) | 369 (45.4%) | 286 (60.2%) | 257 (62.1%) | <0.001 |
| >24 | 984 (49.6%) | 194 (69.3%) | 444 (54.6%) | 189 (39.8%) | 157 (37.9%) | |
Pattern of distant metastasis and molecular subtypes
The difference of the pattern of distant metastasis was significantly different among subtypes (P<0.001, Table 2 ). The pattern of bone metastasis only was more frequently observed in Luminal A and Luminal B subtypes (24.4% and 18.9%, respectively), the same was true of the pattern of Bone metastasis + others (excluding brain metastasis). While the pattern of metastases excluding bone and brain was the most common type in HER2-enriched and Triple Negative subtypes (68.5% and 65.6%, respectively). In addition, brain-related metastasis patterns accounted for a smaller proportion and were also more common in HER2-enriched and Triple Negative subtypes.
Table 2.
Pattern of distant metastasis according to molecular subtypes.
| Total (%) | Luminal A (%) | Luminal B (%) | HER2-enriched (%) | Triple negative (%) | P value | |
|---|---|---|---|---|---|---|
| Bone metastasis only | 377 (16.0%) | 83 (24.4%) | 188 (18.9%) | 48 (9.2%) | 58 (11.5%) | <0.001 |
| Metastases excluding bone and brain | 1369 (58.1%) | 158 (46.5%) | 525 (52.8%) | 356 (68.5%) | 330 (65.6%) | |
| Bone metastasis + others (excluding brain metastasis) | 530 (22.5%) | 93 (27.4%) | 249 (25.1%) | 96 (18.5%) | 92 (18.3%) | |
| Brain metastasis only | 25 (1.1%) | 1 (0.3%) | 5 (0.5%) | 6 (1.2%) | 13 (2.6%) | |
| Brain metastasis + others | 56 (2.4%) | 5 (1.5%) | 27 (2.7%) | 14 (2.7%) | 10 (2.0%) |
Cumulative data of distant metastasis and molecular subtypes
Cumulative data of distant metastasis were shown in Table 3 . Nearly half of ABC patients presented multiple metastases simultaneously. Bone was the most common site of distant metastasis (39.1%), and patients who were found to be positive in Luminal A and Luminal B accounted for 51.1% and 44.7% of patients who developed bone metastasis, respectively. Liver metastasis was more frequently observed in HER2-enriched ABC patients, accounting for 29.1%, and patients with HER2-enriched and Triple negative primarily presented lung metastasis more. Further, Triple Negative subtype was also more likely to have brain metastasis, but no statistically significant differences were found with other molecular subtypes.
Table 3.
Cumulative frequency of distant metastasis sites according to molecular subtypes.
| Total (%) | Luminal A (%) | Luminal B (%) | HER2-enriched (%) | Triple negative (%) | P value | |
|---|---|---|---|---|---|---|
| Number of distant metastasis (%) | ||||||
| 1 | 1249 (53.0%) | 187 (55.0%) | 528 (53.1%) | 261 (50.2%) | 273 (54.3%) | 0.472 |
| ≥2 | 1109 (47.0%) | 153 (45.0%) | 467 (46.9%) | 259 (49.8%) | 230 (45.7%) | |
| Bone | 935 (39.1%) | 178 (51.1%) | 452 (44.7%) | 150 (28.7%) | 155 (30.5%) | <0.001 |
| Liver | 569 (23.8%) | 69 (19.8%) | 265 (26.2%) | 148 (29.1%) | 87 (16.6%) | <0.001 |
| Lung | 729 (30.5%) | 85 (24.4%) | 299 (29.5%) | 189 (36.1%) | 156 (30.6%) | 0.002 |
| Brain | 81 (3.4%) | 6 (1.7%) | 32 (3.2%) | 20 (3.8%) | 23 (4.5%) | 0.145 |
| visceral metastases* | 1845 (77.1%) | 275 (79.0%) | 811 (80.1%) | 369 (70.6%) | 390 (76.6%) | <0.001 |
* including liver, lung, brain, adrenal gland and ovary, etc.
Cumulative recurrence and metastasis rate
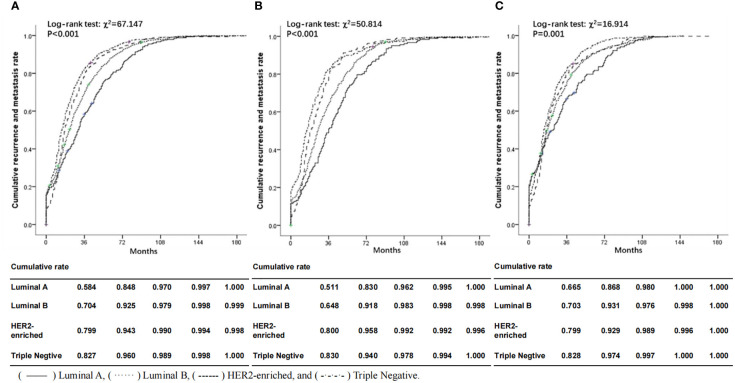
Figure 1 illustrates the significant difference in cumulative recurrence and metastasis rate according to molecular subtypes. The median time of DFS was 29.0 months (range 0.1-38.7). Luminal B subtype had a poorer prognosis than Luminal A compared with Triple Negative, HER2-enriched subtype tended to spread more aggressively and was associated with higher cumulative recurrence and metastasis rates, whether from the whole or divided into two age subgroups (< 45 years and ≥45 years subgroups). It can be clearly seen from the curves that the cumulative recurrence and metastasis rates of ABC patients at 36 months of DFS were the most significant in ABC patients of all molecular types. The recurrence and metastasis rate of Triple Negative was the highest (82.7%), while that of Luminal A was the lowest, 58.4% of the tumors were recorded as recurrence and distant metastasis. Luminal B and HER2-enriched were between them. These differences still existed in the age subgroup analysis, especially in <45 years age group (Triple Negative: 83.0% versus. Luminal A: 51.1% at the 36-month time point). Both HER2-enriched and Triple Negative patients in younger age group showed higher cumulative recurrence and metastasis rates than those in older age group, but the Luminal subtype was the opposite.
Figure 1.
Comparisons of cumulative recurrence and metastasis rate according to molecular subtypes, overall (A) and in subgroups aged <45 (B) and ≥45 (C) at first diagnosis. Solid lines represent ABC patients with Luminal A subtype; dotted lines represent ABC patients with Luminal B subtype; dashed lines represent ABC patients with HER2-enriched subtype; dash-dot lines represent ABC patients with Triple Negative subtype. () Luminal A, () Luminal B, () HER2-enriched and () Triple Negative.
Association between molecular subtypes and distant metastasis sites
In Cox regression analyses, crude and fully adjusted HRs and 95% CIs for the associations between molecular subtypes and distant metastasis sites are shown in Table 4 . ABC patients with HER2-enriched and Luminal B significantly increased the risk of liver metastasis (HR HER2-enriched 2.27, 95% CI 1.65-3.14, HR Luminal B 1.66, 95% CI 1.24–2.23), and the HRs remained statistically significant after extended adjustment to the model (HR HER2-enriched 2.14, 95% CI 1.47-3.12, HR Luminal B 1.40, 95% CI 1.01–1.96). Luminal B, HER2-enriched and Triple Negative were positively correlated to lung metastasis in the univariate as well as in the multivariate analyses. Further, these types of molecular subtypes also increased the risk of visceral metastasis by 23%, 46% and 87% respectively. In terms of brain metastasis, the results showed that triple Negative was associated with a significantly increased risk of brain metastasis (HR 3.99, 95% CI 1.58–10.12) and this association remained significant after full adjustment (HR 3.07, 95% CI 1.04–9.07). However, it made no statistical difference in the risk of bone metastasis of the three subtypes compared with Luminal A, whether by univariate or multivariate analyses.
Table 4.
HRs and 95% CIs for the associations between molecular subtypes and the sites of distant metastasis in advanced breast cancer.
| Sites of distant metastasis | Univariate analysis HR (95%CI) |
P-value | Multivariate analysis* HR (95%CI) |
P-value |
|---|---|---|---|---|
| Bone | ||||
| Luminal A | 1.00 | 1.00 | ||
| Luminal B | 1.17 (0.97-1.43) | 0.109 | 1.06 (0.84-1.35) | 0.628 |
| HER2-enriched | 0.99 (0.78-1.26) | 0.968 | 0.94 (0.70-1.27) | 0.686 |
| Triple Negative | 1.14 (0.89-1.46) | 0.295 | 1.29 (0.96-1.73) | 0.094 |
| Liver | ||||
| Luminal A | 1.00 | 1.00 | ||
| Luminal B | 1.66 (1.24-2.23) | 0.001 | 1.40 (1.01-1.96) | 0.047 |
| HER2-enriched | 2.27 (1.65-3.14) | 0.256 | 2.14 (1.47-3.12) | <0.001 |
| Triple Negative | 1.30 (0.63-2.67) | 0.478 | 1.18 (0.90-1.53) | 0.225 |
| Lung | ||||
| Luminal A | 1.00 | 1.00 | ||
| Luminal B | 1.74 (1.33-2.29) | <0.001 | 1.63 (1.18-2.24) | 0.003 |
| HER2-enriched | 2.76 (2.07-3.68) | <0.001 | 2.46 (1.74-3.47) | <0.001 |
| Triple Negative | 2.65 (1.96-3.57) | <0.001 | 2.65 (1.86-3.78) | <0.001 |
| Viscus** | ||||
| Luminal A | 1.00 | 1.00 | ||
| Luminal B | 1.35 (1.16-1.57) | <0.001 | 1.23 (1.02-1.48) | 0.032 |
| HER2-enriched | 1.54 (1.29-1.83) | <0.001 | 1.46 (1.18-1.80) | 0.001 |
| Triple Negative | 1.82 (1.53-2.16) | <0.001 | 1.87 (1.51-2.30) | <0.001 |
| Brain | ||||
| Luminal A | 1.00 | 1.00 | ||
| Luminal B | 1.87 (0.76-4.58) | 0.171 | 1.72 (0.64-4.62) | 0.285 |
| HER2-enriched | 3.00 (1.18-7.63) | 0.021 | 1.94 (0.64-5.87) | 0.242 |
| Triple Negative | 3.99 (1.58-10.12) | 0.004 | 3.07 (1.04-9.07) | 0.042 |
* Adjusted for age at diagnosis, tumor size, number of lymph node metastasis, menstrual status, education and family history.
** including liver, lung, brain, adrenal gland and ovary, etc.Bold text in this manuscript indicates statistical significance.
Discussion
The complex biological behavior of breast cancer is determined by the heterogeneity of its intrinsic molecular phenotypes. There is marked variability in the time interval between treatment of the primary tumor and the occurrence of distant metastases, in the organs involved with distant metastases and in the response to systemic treatment in patients with metastatic breast cancer (16). Prior studies have reported that the general mechanism of tumor metastasis was composed of reciprocal interactions between tumor cells and host tissues, which included alterations in adhesion, proteolysis, invasion and angiogenesis (17, 18). However, only a few studies have described unique predilections and intrinsic association between molecular subtypes and distant metastasis sites among ABC patients, especially in Chinese native women. In this study, ABC patients in multiple centers in China were included, and the internal relationship between molecular subtypes and sites of distant metastasis was deeply discussed. In general, patients with Luminal A tended to develop bone metastasis and they had better survival outcomes than Luminal B and HER2-enriched with tendency of having visceral metastasis, Triple Negative with brain metastasis. Therefore, this study may fully represent the overall situation of distant metastasis pattern and prognosis of ABC patients at the national level. Further, these results lend support to the hypothesis that molecular subtypes predict the preferential sites of distant metastasis in ABC and may give valuable guidance to the formulation of personal targeted therapy strategy and the prediction of clinical prognosis.
Molecular subtypes of breast cancer, as an important complement to TNM staging, have the propensity to give rise to distant metastasis at specific sites. Recent publications had shown that newer molecular subtypes which used microarrays for gene expression analysis, was the best way to perform such molecular classification (19–21). However, such assays were mostly limited to the research laboratories and were not available for the routine clinical practice. Moreover, most of the archived clinical specimens were not suitable for this analysis. Therefore, the IHC-based classification systems are now still useful in clinical practice especially when performed by inexperienced centers, and have proven to be highly correlated with intrinsic classification using gene expression microarrays (22, 23). Also, there is significant agreement in the site of distant metastasis and outcome predictions for individual patients by these tests (24). For classification of molecular subtypes to be more helpful, ongoing efforts should be directed at standardization of current testing and development of more reliable and reproducible testing for ER/PR and Her2 gene expression (25).
Previous studies had demonstrated significant difference in the time of recurrence or distant metastasis within different molecular subtypes. In this study as expected, ABC patients with Luminal subtypes had relatively longer DFS and were prone to bone metastasis with good prognosis. While Triple Negative had a higher cumulative rate than other subtypes and more of them had a propensity of brain metastasis with the shortest DFS, highlighting a substantial clinical burden and unmet need for more effective treatment for these high-risk patients. These results indicated that distant metastasis of breast cancer was a non-random process, and ABC patients with different molecular subtypes have different distant metastasis mechanisms. In our study, we further observed the cumulative recurrence and metastasis rate of different subtypes on age subgroups, showing that the difference of cumulative rates were more significant in younger age group, manifested that the rates of HER2 and TNBC patients were higher than those of older age group, while rates were just opposite in Luminal types with good prognosis at the 3-year time point. Another retrospective study showed that DFS after adjuvant chemotherapy in younger patients with ER-positive tumors was significantly longer than patients with ER-negative disease (26). Colak et al. also described different gene expression profiles in breast tumors from younger women (27). They found lower expression of ER alpha and beta mRNA, with higher expression of HER2 and epidermal growth factor receptor (EGFR) compared to older patients. Moreover, elevated HER2 and EGFR were reported to be associated with distant metastasis especially brain metastasis (28). This finding could help to explain the disease course that we observed in young patients.
Characterization of breast cancer metastasis to bone has been most extensively studied since bone is the most common site of distant metastasis (29). With the arriving era of molecular profiling, it has become evident that bone metastasis was most common in the Luminal subtypes in one or more organ systems (30), as recapitulated in the current study. Bone metastasis is generally determined by spinal vein system which has the characteristics of no venous flap and low venous pressure, so cancer cells can be transferred to spinal vein prior to vena cava system, resulting in bone metastasis. Therefore, we speculate that this may be the primary reason for the highest rate of bone metastasis. In addition, there is increasing evidence that breast cancer cells have the ability to activate osteoclasts similar to that of normal breast gland epithelial cells during lactation, so breast cancer cells have the inherent characteristics of mutual benefit with bone tissue (31). Kroepil et al. also reported that SNAI1 was a zinc finger transcriptional repressor of CDH1, which encoded E-cadherin. Downregulation of E-cadherin was crucial to the dissemination and invasion of cancer cells, which might augment breast cancer metastasis into the bone (32). Moreover, the results of collective studies so far indicated that breast cancer with bone metastatic potential could be divided into two groups: those with bone metastasis only (ie, Luminal subtypes) and those with bone + other sites, the latter showed biological behavior similar to that of extra skeletal metastasis (33). This idea was supported in part by previous genetic analysis studies, but the underlying mechanism remained unclear. Further molecular studies are needed to explain the differences in biological behavior.
HER2 over-expression plays an important role in the proliferation, apoptosis, and angiogenesis of many solid tumors, so it is thought that the prognosis for patients with HER2 gene amplification should be poor. Some studies have shown that HER2-enriched was more likely to have multiple site and visceral metastases, among which liver metastasis was the most common (34). These findings were partly in keeping with the observations in our study. The observed liver-seeking characteristic of the HER2-enriched subtype was further strengthened by its significant association with liver metastasis in ABC patients. The mechanisms of liver metastasis in HER2-enriched ABC are complex and still largely unclear. Li et al. (35) reported that HER2 overexpression mediated a chemokine receptor, CXCR4 associated metastases. Therefore, we speculated that HER2 overexpression involved or promoted liver metastasis, but not peritoneal and lymph node metastases. Further study to clarify the mechanism of preferential liver metastases using HER2-enriched breast cancer cell lines will still be carried out.
To date, the association between breast cancer subtypes and lung metastasis in patients with breast cancer have been preliminarily identified (36). A tissue microarray analysis had showed HER2-enriched subtype, TNBC and the Luminal-HER2 all exhibited higher lung metastasis rates compared with Luminal A cancers (37). On multivariate analysis in this study, the probability of lung metastasis in HER2-enriched and Triple Negative subtypes was higher than Luminal type tumors. This was consistent with observations from previous studies describing that several signature genes was associated with increased lung metastasis risk (38, 39). These signature included EGFR, COX2, and the matrix metalloproteinases 1 (MMP1), CXCL1 and IDI1 which were highly expressed in the Triple Negative subtype and HER2-positive cancers. These genes collectively allow angiogenesis, tumor intravasation into the circulation, and breaching of lung capillaries by circulating tumor cells to seed the pulmonary parenchyma (40). This is the main reason why we infer the patients with the above molecular subtypes have the propensity of lung metastasis. In addition, miRNA profiling revealed that miR-629-3p was most commonly upregulated in both metastatic lesions and primary carcinomas in TNBC patients with lung metastasis compared with normal breast tissue (41). It should be hypothesized that miR-629-3p can create a specific microenvironment surrounding the metastasizing cells, necessary for invading and proliferating in lung issue. Therefore, accurate clinical testing of HER2, EGFR and other genomic makers may become necessary to provide complementary information for predicting the possibility of lung metastasis.
It is obvious from studies discussed that Triple Negative is a subtype of breast cancer that is associated with high risk of developing brain metastases, with an associated subsequent poor survival outcome (42–45). It has been reported that breast cancer, especially metastatic breast cancer, had a higher rate of brain metastasis, which was considered secondary because many new therapeutic drugs may not be able to penetrate the blood-brain barrier (46). In agreement with prior studies, brain metastasis was infrequent as the first site of distant metastasis in the present series. However, an increasing rate of brain metastasis was reported in other studies (47–49), and we also found a strong association between Triple Negative and elevated brain metastasis risk in the Cox regression model. This expected result has meaningful implications for communication and care decisions and is worth further molecular investigation. Furthermore, the median DFS of all patients with Triple Negative ABC in the present study was less than 2 years (18.5 months) and the 3-year cumulative recurrence and metastasis rate was 82.7%. The results were comparable to those by Lin et al. (50) in which median DFS of Triple Negative breast cancer was 19.9 months and 75% of metastases occurred within 3 years of the diagnosis of breast cancer. The reason may be related to the special biological characteristics and gene expression in different molecular subtypes. Previous studies have suggested that cell chemokines (CXCR4/CXCL12), PTHrP and NF-κB were important molecular markers affecting the metastasis rate of patients (51). The results of genome studies (52–54) showed that these markers were overexpressed in Triple Negative subtypes, suggesting that it was reasonable for Triple Negative breast cancer patients to have a higher distant metastasis rate. Therefore, exploring new therapeutic targets will become a new hotspot in Triple Negative breast cancer research.
Adding to the previous literature, the current study could provide important evidence for decision-makers. From the perspective of physicians, molecular subtypes can be used as an important tool for prognosis assessment of ABC patients, and it is an important supplement in assessing the time and site distribution of postoperative distant metastasis, developing targeted preventive therapeutic schedules for patients, and contributing to personalized screening during postoperative follow-up. For ABC patients, the difference in the distant metastasis pattern and overall prognosis of different molecular subtypes can provide guidance to choosing a reasonable and cost-effective approach for treatment, so as to improve the quality of life and promote rehabilitation. While for health policy maker, understanding the burden of different molecular subtypes of ABC could help in optimizing resource allocation for those who in greater need of advanced treatment.
The present study exhibits several strengths. Firstly, our study was the first multi-center hospital-based clinical epidemiological investigations to explore the characteristics of ABC for women in China. All the data was collected from 21 tertiary hospitals in seven geographical regions, representing different cancer burden levels, diagnoses, and treatments. Covering different geographic regions in China would be more informative and less subject to practices specific to individual hospitals which can provided important insights about real-world clinical outcomes for ABC patients from a large nationwide sample. Secondly, convenience sampling was adopted, and the sample size was allocated according to the month to reduce the bias caused by the time of initial diagnosis. In addition, a full review of the medical records permitted a check of the detailed documented metastasis sites, thereby providing a more apparent pattern as well as cumulative frequency of distant metastasis sites.
However, several potential limitations of this study should be considered inherent to hospital-based retrospective study. Firstly, the results might be subject to selection bias if confounding factors were not identified or adjusted for in the analyses. In the current study, we adjusted for confounders such as age at diagnosis, tumor size, menstrual status and family history which were thought to have the potential to affect the prognostic outcomes. In addition, molecular typing characteristics still need to be evaluated in conjunction with conventional or established gold criteria such as imaging and pathology.Secondly, the consistency of molecular typing between relapsed and metastatic tumor and primary tumor was not examined in this study, as there have been reported the rate of subtype conversion was 0% in basal-like tumors, 23.1% in HER2-enriched tumors, 30.0% in Luminal B tumors, and 55.3% in Luminal A tumors (55). The main reason was that the subtypes of some distant sites in medical records were unknown. However, given the clinical consequences of discordance, it urgently requires to deeply understand the differences between primary and metastatic tumors and develops the proper management of cancer patients in future studies. Thirdly, modern chemotherapy and adjuvant trastuzumab have reduced metastasis risk in HER2-positive disease (56, 57). In this study, adjuvant chemotherapy was only prescribed for 11.5% and 18.5% of ABC patients with Luminal B and HER2-enriched tumors. Therefore, it may be plausible if we consider that the results may be underestimated when discussing the distant metastasis risk with HER2-positive subtypes. Further studies to clarify this point are warranted. Due to these limitations and confounders, the study results need to be viewed with some caution, although several of the findings are well in line with prior clinical studies or with experimental data.
Conclusions
In conclusion, this study provided data of ABC patients to clearly show that molecular subtypes were significant different in metastatic behavior with regard to the sites of distant metastasis as well as recurrence and metastasis rate, suggesting that molecular subtypes could predict the preferential site of distant metastasis in female ABC patients. Despite improving breast cancer outcomes, distant metastasis remains common and incurable, especially for the ABC patients. These observations could potentially be used in determining the appropriate schemes for follow-up of ABC patients and hopefully shed light on the development of effective surveillance strategies and targeted therapies. Therefore, future studies are warranted and can ultimately lead to the tailoring of individualized comprehensive treatment fields based on molecular subtypes combined with the conventional clinicopathologic characteristics.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to You-lin Qiao, qiaoy@cicams.ac.cn.
Ethics statement
The studies involving human participants were reviewed and approved by Cancer Hospital, Chinese Academy of Medical Sciences. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Y-LQ, J-YW, and B-HX had access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Y-LQ and B-HX participated in the study concept and design. SZ acquired and analyzed data. J-HF, SZ, and HY were responsible for interpreting the data. J-HF, SZ, and HY took the lead in drafting the report. The other co-authors collected the data from the corresponding study hospital. Every author participated in editing and finalization. The work reported in the paper has been performed by the authors, unless clearly specified in the text. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was supported by the Investigator initiated program of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (CH-BC-045).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QZ declared a shared parent affiliation with the author TH to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Redig AJ, Mcallister SS. Breast cancer as a systemic disease: a view of metastasis. J Internal Med (2013) 274(2):113–26. doi: 10.1111/joim.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster RS, Costanza MC. Breast self-examination practices and breast cancer survival. Cancer. (2015) 53(4):999–1005. doi: [DOI] [PubMed] [Google Scholar]
- 4. Chen MT, Sun HF, Zhao Y, Fu WY, Yang LP, Gao SP, et al. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep (2017) 7(1):9254. doi: 10.1038/s41598-017-10166-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bishop AJ, Ensor J, Moulder SL, Shaitelman SF, Edson MA, Whitman GJ, et al. Prognosis for patients with metastatic breast cancer who achieve a no-evidence-of-disease status after systemic or local therapy. Cancer. (2016) 121(24):4324–32. doi: 10.1002/cncr.29681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Ieso PB, Schick U, Rosenfelder N, Mohammed K, Ross GM. Breast cancer brain metastases – a 12 year review of treatment outcomes. Breast. (2015) 24(4):426–33. doi: 10.1016/j.breast.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 7. Caliari D, Zappulli V, Rasotto R, Cardazzo B, Frassineti F, Goldschmidt MH, et al. Triple-negative vimentin-positive heterogeneous feline mammary carcinomas as a potential comparative model for breast cancer. BMC Veterinary Res (2014) 10(1):1–12. doi: 10.1186/s12917-014-0185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X, Mingyue LI, Yin Y, Liang LI, Tao Y, Chen D, et al. Profiling of alternative polyadenylation sites in luminal b breast cancer using the SAPAS method. Int J Mol Med (2015) 35(1):39–50. doi: 10.3892/ijmm.2014.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sofi GN, Sofi JN, Nadeem R, Shiekh RY, Khan FA, Sofi AA, et al. Estrogen receptor and progesterone receptor status in breast cancer in relation to age, histological grade, size of lesion and lymph node involvement. Asian Pacific J Cancer Prev Apjcp. (2012) 13(10):5047. doi: 10.7314/APJCP.2012.13.10.5047 [DOI] [PubMed] [Google Scholar]
- 10. Rakha EA, Pigera M, Shin SJ, D'Alfonso T, Ellis IO, Lee AH. HER2 testing in invasive breast cancer: should histological grade, type and oestrogen receptor status influence the decision to repeat testing? Histopathology (2015) 69(1):20. doi: 10.1111/his.12900 [DOI] [PubMed] [Google Scholar]
- 11. Tobin NP, Harrell JC, Lövrot J, Brage SE, Frostvik Stolt M, Carlsson L, et al. Molecular subtype and tumor characteristics of breast cancer metastases as assessed by gene expression significantly influence patient post-relapse survival. Ann Oncol (2015) 26(1):81–8. doi: 10.1093/annonc/mdu498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu X, Baig A, Kasymjanova G, Kafi K, Holcroft C, Mekouar H, et al. Pattern of local recurrence and distant metastasis in breast cancer by molecular subtype. Cureus. (2016) 8(12):e924. doi: 10.7759/cureus.924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Errahhali ME, Errahhali ME, Ouarzane M, Harroudi TE, Afqir S, Bellaoui M. First report on molecular breast cancer subtypes and their clinico-pathological characteristics in Eastern Morocco: series of 2260 cases. BMC Womens Health (2017) 17(1):3. doi: 10.1186/s12905-016-0361-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song Q, Huang R, Li J, Fan J, Zheng S, Zhang B, et al. The diverse distribution of risk factors between breast cancer subtypes of ER, PR and HER2: a 10-year retrospective multi-center study in China. PLoS One (2013) 8(8):e72175. doi: 10.1371/journal.pone.0072175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harbeck N, Thomssen C, Gnant M. St. gallen 2013: Brief preliminary summary of the consensus discussion. Breast Care (2013) 8(2):102–9. doi: 10.1159/000351193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dilara SH C, Hans H, Hooijer GKJ, Horlings HM, Jelle W, van de Vijver MJ. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res Treat (2015) 150(3):547–57. doi: 10.1007/s10549-015-3352-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li DM, Feng YM. Signaling mechanism of cell adhesion molecules in breast cancer metastasis: potential therapeutic targets. Breast Cancer Res Treat (2011) 128(1):7–21. doi: 10.1007/s10549-011-1499-x [DOI] [PubMed] [Google Scholar]
- 18. Mittempergher L, Saghatchian M, Wolf DM, Michiels S, Canisius S, Dessen P, et al. A gene signature for late distant metastasis in breast cancer identifies a potential mechanism of late recurrences. Mol Oncol (2013) 7(5):987–99. doi: 10.1016/j.molonc.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng J, Zhang L, Yuan C, Zhou L, Xu S, Lin Y, et al. Expression profile analysis of long noncoding RNA in ER-positive subtype breast cancer using microarray technique and bioinformatics. Cancer Manage Res (2017) 9:891–901. doi: 10.2147/CMAR.S151120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: Comparison of clinicopathologic features and survival. Clin Med Res (2009) 7(2):4–13. doi: 10.3121/cmr.2008.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sandhu R, Parker JS, Jones WD, Livasy CA, Coleman WB. Microarray-based gene expression profiling for molecular classification of breast cancer and identification of new targets for therapy. Lab Med (2010) 41(6):364–73. doi: 10.1309/LMLIK0VIE3CJK0WD [DOI] [Google Scholar]
- 22. Meißner T, Seckinger A, Rème T, Hielscher T, Möhler T, Kai N, et al. Gene expression profiling in multiple myeloma–reporting of entities, risk, and targets in clinical routine. Clin Cancer Res Off J Am Assoc Cancer Res (2011) 17(23):7240–7. doi: 10.1158/1078-0432.CCR-11-1628 [DOI] [PubMed] [Google Scholar]
- 23. Lee HJ, Kim JY, Song IH, Park IA, Yu JH, Gong G. Expression of NY-ESO-1 in triple-negative breast cancer is associated with tumor-infiltrating lymphocytes and a good prognosis. Oncology. (2015) 89(6):337–44. doi: 10.1159/000439535 [DOI] [PubMed] [Google Scholar]
- 24. Carlino MS, Haydu LE, Kakavand H, Menzies AM, Hamilton AL, Yu B, et al. Correlation of BRAF and NRAS mutation status with outcome, site of distant metastasis and response to chemotherapy in metastatic melanoma. Br J Cancer. (2014) 111(2):292–9. doi: 10.1038/bjc.2014.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seshie B, Adu-Aryee NA, Dedey F, Calys-Tagoe B, Clegg-Lamptey JN. A retrospective analysis of breast cancer subtype based on ER/PR and HER2 status in ghanaian patients at the korle bu teaching hospital, Ghana. BMC Clin Pathology. (2015) 15(1):14. doi: 10.1186/s12907-015-0014-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wapnir IL, Price KN, Anderson SJ, Robidoux A, Martín M, Nortier JWR, et al. Efficacy of chemotherapy for ER-negative and ER-positive isolated locoregional recurrence of breast cancer: Final analysis of the CALOR trial. J Clin Oncol (2018) 36(11):JCO2017765719. doi: 10.1200/JCO.2017.76.5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colak D, Nofal A, Albakheet A, Nirmal M, Jeprel H, Eldali A, et al. Age-specific gene expression signatures for breast tumors and cross-species conserved potential cancer progression markers in young women. PLoS One (2013) 8(5):e63204. doi: 10.1371/journal.pone.0063204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sirkisoon SR, Carpenter RL, Rimkus T, Miller L, Methenybarlow L, Lo HW. EGFR and HER2 signaling in breast cancer brain metastasis. Front Bioscience. (2016) 8(2):245. doi: 10.2741/E765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brockton NT, Gill SJ, Laborge SL, Paterson AHG, Cook LS, Vogel HJ, et al. The breast cancer to bone (B2B) metastases research program: a multi-disciplinary investigation of bone metastases from breast cancer. BMC Cancer. (2015) 15(1):1–15. doi: 10.1186/s12885-015-1528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pulido C, Vendrell I, Ferreira AR, Casimiro S, Mansinho A, Alho I, et al. Bone metastasis risk factors in breast cancer. Ecancermedicalscience. (2017) 11:715. doi: 10.3332/ecancer.2017.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Closhisy DR, Palkert D, Ramnaraine MLR, Pekurovsky I, Oursler MJ. Human breast cancer induces osteoclast activation and increases the number of osteoclasts at sites of tumor osteolysis. J Orthopaedic Res (2010) 14(3):396–402. doi: 10.1002/jor.1100140309 [DOI] [PubMed] [Google Scholar]
- 32. Kroepil F, Fluegen G, Totikov Z, Baldus SE, Vay C, Schauer M, et al. Down-regulation of CDH1 is associated with expression of SNAI1 in colorectal adenomas. PLoS One (2012) 7(9):e46665. doi: 10.1371/journal.pone.0046665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Soni A, Ren Z, Hameed O, Chanda D, Morgan CJ, Siegal GP, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathology. (2015) 143(4):471–8. doi: 10.1309/AJCPYO5FSV3UPEXS [DOI] [PubMed] [Google Scholar]
- 34. Seho P, Ja Seung K, Suk KM, Hyung Seok P, Jun Sang L, Jong Seok L, et al. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. (2012) 21(1):50–7. doi: 10.1016/j.breast.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 35. Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell (2004) 6(5):459–69. doi: 10.1016/j.ccr.2004.09.027 [DOI] [PubMed] [Google Scholar]
- 36. Minn AJ, Gupta GP, Siegel PM, Bos PD, Weiping S, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature (2005) 436(7050):518–24. doi: 10.1038/nature03799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28(20):3271–7. doi: 10.1200/JCO.2009.25.9820 [DOI] [PubMed] [Google Scholar]
- 38. Bachelot T, Romieu G, Campone M, Diéras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol (2013) 14(1):64–71. doi: 10.1016/S1470-2045(12)70432-1 [DOI] [PubMed] [Google Scholar]
- 39. Molnár IA, Molnár BÁ, Vízkeleti L, Fekete K, Tamás J, Deák P, et al. Breast carcinoma subtypes show different patterns of metastatic behavior. Virchows Archiv. (2017) 470(3):275–83. doi: 10.1007/s00428-017-2065-7 [DOI] [PubMed] [Google Scholar]
- 40. Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. (2007) 446(7137):765. doi: 10.1038/nature05760 [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Song C, Tang H, Zhang C, Tang J, Li X, et al. miR-629-3p may serve as a novel biomarker and potential therapeutic target for lung metastases of triple-negative breast cancer. Breast Cancer Res Bcr. (2017) 19(1):72. doi: 10.1186/s13058-017-0865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Niwińska A, Olszewski W, Murawska M, Pogoda K. Triple-negative breast cancer with brain metastases: a comparison between basal-like and non-basal-like biological subtypes. J Neuro-Oncology. (2011) 105(3):547–53. doi: 10.1007/s11060-011-0616-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morris PG, Murphy CG, Mallam D, Accordino M, Patil S, Howard J, et al. Limited overall survival in patients with brain metastases from triple negative breast cancer. Breast J (2012) 18(4):345–50. doi: 10.1111/j.1524-4741.2012.01246.x [DOI] [PubMed] [Google Scholar]
- 44. Lin NU, Ann V, Hughes ME, Theriault RL, Edge SB, Yu-Ning W, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the national comprehensive cancer network. Cancer. (2012) 118(22):5463–72. doi: 10.1002/cncr.27581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaheenah D, Xiudong L, Litton JK, Buchholz TA, Hortobagyi GN, Gonzalez-Angulo AM. Incidence of brain metastases as a first site of recurrence among women with triple receptor-negative breast cancer. Cancer. (2012) 118(19):4652–9. doi: 10.1002/cncr.27434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matsuo S, Watanabe J, Mitsuya K, Hayashi N, Nakasu Y, Hayashi M. Brain metastasis in patients with metastatic breast cancer in the real world: a single-institution, retrospective review of 12-year follow-up. Breast Cancer Res Treat (2017) 162(1):169–79. doi: 10.1007/s10549-017-4107-x [DOI] [PubMed] [Google Scholar]
- 47. Distefano A, Yap HY, Hortobagyi GN, Blumenschein GR. The natural history of breast cancer patients with brain metastases. Cancer. (2015) 44(5):1913–8. doi: 10.1002/1097-0142(197911)44:5 [DOI] [PubMed] [Google Scholar]
- 48. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Breast Dis A Year Book Quarterly. (2012) 82(5):2111–7. doi: 10.1016/j.ijrobp.2011.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gil-Gil MJ, Martinez-Garcia M, Sierra A, Conesa G, Barco SD, González-Jimenez S, et al. Breast cancer brain metastases: a review of the literature and a current multidisciplinary management guideline. Clin Trans Oncol (2014) 16(5):436–46. doi: 10.1007/s12094-013-1110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin N, Claus E J, Razzak A, Arnaout A, Winer E. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. (2010) 113(10):2638–45. doi: 10.1002/cncr.23930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carey LA, Claire D E, Lynda S, Lisa G, Moore DT, Frances C, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res (2007) 13(8):2329–34. doi: 10.1158/1078-0432.CCR-06-1109 [DOI] [PubMed] [Google Scholar]
- 52. Wu W, Qian L, Chen X, Ding B. Prognostic significance of CXCL12, CXCR4, and CXCR7 in patients with breast cancer. Int J Clin Exp Pathol (2015) 8(10):13217–24. [PMC free article] [PubMed] [Google Scholar]
- 53. Camirand A, Fadhil I, Luco AL, Ochietti B, Kremer RB. Enhancement of taxol, doxorubicin and zoledronate anti-proliferation action on triple-negative breast cancer cells by a PTHrP blocking monoclonal antibody. Am J Cancer Res (2013) 3(3):500–8. [PMC free article] [PubMed] [Google Scholar]
- 54. Zhu H, Bhaijee F, Ishaq N, Pepper DJ, Backus K, Brown AS, et al. Correlation of Notch1, pAKT and nuclear NF-κB expression in triple negative breast cancer. Am J Cancer Res (2013) 3(2):230–9. [PMC free article] [PubMed] [Google Scholar]
- 55. Cejalvo JM, dDE Martínez, Galván P, García-Recio S, Burgués GO, Paré L, et al. Intrinsic subtypes and gene expression profiles in primary and metastatic breast cancer. Cancer Res (2017) 77(9):2213. doi: 10.1158/0008-5472.CAN-16-2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martins M, Holmes FA, Ejlertsen B, Delaloge S, Moy B, Iwata H, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2017) 18(12):1688-1700. doi: 10.1016/S1470-2045(17)30717-9 [DOI] [PubMed] [Google Scholar]
- 57. Cameron D, Piccartgebhart MJ, Gelber RD, Procter M, Goldhirsch A, De AE, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin adjuvant (HERA) trial. Lancet. (2017) 389(10075):1195–205. doi: 10.1016/S0140-6736(16)32616-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to You-lin Qiao, qiaoy@cicams.ac.cn.