Abstract
The National Cancer Institute’s Implementation Science Centers in Cancer Control (ISC3) Network represents a large-scale initiative to create an infrastructure to support and enable the efficient, effective, and equitable translation of approaches and evidence-based treatments to reduce cancer risk and improve outcomes. This Cancer MoonshotSM–funded ISC3 Network consists of 7 P50 Centers that support and advance the rapid development, testing, and refinement of innovative approaches to implement a range of evidence-based cancer control interventions. The Centers were designed to have research-practice partnerships at their core and to create the opportunity for a series of pilot studies that could explore new and sometimes risky ideas and embed in their infrastructure a 2-way engagement and collaboration essential to stimulating lasting change. ISC3 also seeks to enhance capacity of researchers, practitioners, and communities to apply implementation science approaches, methods, and measures. The Organizing Framework that guides the work of ISC3 highlights a collective set of 3 core areas of collaboration within and among Centers, including to 1) assess and incorporate dynamic, multilevel context; 2) develop and conduct rapid and responsive pilot and methods studies; and 3) build capacity for knowledge development and exchange. Core operating principles that undergird the Framework include open collaboration, consideration of the dynamic context, and engagement of multiple implementation partners to advance pragmatic methods and health equity and facilitate leadership and capacity building across implementation science and cancer control.
Substantial progress has been made in preventing and controlling cancer, as measured by the achievement of national cancer-related targets for Healthy People 2020, and declining cancer mortality rates for most types of cancers (1). However, gaps remain in cancer prevention, detection, diagnosis, treatment, and care of cancer patients and survivors. These gaps are in part because of the slow, insufficient, and unequal delivery of effective interventions to at-risk populations (2). Recent evaluation of the translational pathway for evidence-based cancer control programs (mammography, clinicians’ advice to quit smoking, colorectal cancer screening, human papillomavirus co-testing and vaccination) found intervention adoption by 50% of the population took an average of 15 (ranging 13-21) years from the landmark publication (3). Consideration of equitable implementation of these interventions, in which there are no differences in receipt of intervention by different population groups, would no doubt be even longer. Thus, opportunities to rapidly advance population health impacts in cancer prevention and control remain an urgent priority requiring investment in implementation science (IS). This challenge has been further exacerbated by the COVID-19 pandemic, with recent reports documenting obstacles and delays in delivery of cancer prevention, early detection, and treatment services as health and research organizations shifted, halted, and postponed services and research in response to the pandemic (4,5). The impact on low-resourced and historically underserved communities and communities experiencing health disparities is particularly pronounced (6-9), suggesting implementation efforts to address COVID-19–related delays and challenges in improving cancer control are needed.
The National Cancer Institute (NCI) Cancer MoonshotSM Blue Ribbon Panel (2) recommended conducting IS to accelerate the adoption and deployment of sustainable evidence-based cancer prevention, early detection, and control strategies at multiple levels across clinical and community settings. This agenda highlights the importance for scale-up of evidence-based cancer prevention and control interventions to expand the reach and public benefit of scientific investments in interventions across populations. The focus on IS is also seen in other research networks and priorities (eg, Clinical Translational Science Awards, Cancer Prevention and Control Research Network of the Centers for Disease Control and Prevention, the Veterans Affairs Quality Enhancement Research Initiative program) identifying IS as a fundamental step to move from discovery to impact (10-13).
The NCI Implementation Science Centers in Cancer Control (ISC3) Initiative, consisting of 7 P50 Centers, supports and advances the rapid development, testing, and refinement of innovative approaches to increase the implementation of a range of evidence-based cancer prevention and control interventions (eg, evidence-based guidelines, policies, and programs). The purpose of ISC3 is to build a network of US-based research Centers with the aim of rapidly and comprehensively advancing IS in cancer control. Over the 5 years of this initiative, ISC3 will 1) establish IS laboratories for cancer control in health care and community settings, capable of more rapidly studying innovations in implementation of evidence-based cancer control and prevention; 2) develop IS methods, measures, and study designs; 3) develop and execute innovative pilot projects to deliver evidence on optimal strategies for adopting, implementing, and sustaining evidence-based care; 4) develop data resources contributing to an IS data ecosystem; 5) incorporate community perspectives and engagement; and 6) disseminate lessons learned to National Institutes of Health (NIH) grantees, service systems, practitioners, and other key stakeholders in the field (14). To date, ISC3 is the largest targeted investment in IS at the NCI.
Central to achieving the ISC3 goals for the field of cancer prevention and control research are coordinated collaboration and leadership to translate our evidence base with community partner engagement into practice with an emphasis on health equity, so evidence is reaching populations equitably (15). In addition, many Centers are led by scientists engaged in population science and community outreach and engagement programs at their respective Cancer Centers. As such, many of the ISC3 activities are focused on addressing cancer-relevant priorities identified by their communities and supported by their Cancer Center’s leadership to help broaden the utilization of IS. In this manuscript, we describe the ISC3 Organizing Framework and highlight the overarching activities, mechanisms, and components of ISC3, as a collective effort to advance IS in cancer control and prevention within and beyond the initiative. We also describe the organizing principles for collaboration and offer examples of some of the activities within the Centers that illustrate the operationalization of the Framework. This Framework is shared in the spirit of collaboration and transparency so other networks, investigators, and community partners may learn about ISC3 and identify opportunities for collaboration, synergy, and expansion—all essential to achieve broader reach.
The ISC3 Network
The ISC3 Network includes 7 Centers, listed in Table 1. Each Center is comprised of an administrative core, research program, and implementation laboratory. ISC3 Centers represent virtually all US regions and further represent a wide range of implementation settings and populations, spanning the cancer control continuum.
Table 1.
Description of ISC3 Center themes and laboratory characteristics (2021)
| ISC3 Center | Implementation theme | Number of laboratory sites by organizational type | Number of laboratory sites by clients served |
|---|---|---|---|
| Building Research in Implementation and Dissemination to close Gaps and achieve Equity in Cancer Control Center (BRIDGE-C2) at Oregon Health & Science University and OCHIN, Inc | Advancing implementation science to improve cancer screening and prevention in underserved populations | 391 community health centers; 45 health departments; 69 safety net; 20 nonprofit; 290 other | 222 sites serve 1-500 clients; 183 sites serve 501-2000 clients; 158 sites serve 2001-5000 clients; 83 sites serve 5001-10 000 clients; 27 sites serve more than 10 000 clients; data not provided for 142 sites |
| Colorado Implementation Science Center in Cancer Control at University of Colorado (Colorado ISC3) | Using pragmatic approaches to assess and enhance the value of cancer prevention and control in rural primary care and interactive resources to enhance implementation science capacity | 4 network/health system | 3 sites serve 5001-10 000 clients; 1 site serves more than 10 000 clients |
| Implementation and Informatics—Developing Adaptable Processes and Technologies for Cancer Control (iDAPT) at Wake Forest School of Medicine and University of Massachusetts Medical School | Using technologies to support rapid cycle and real-time deployment and testing of implementation processes and adaptations within cancer control | 3 network/health system; 1 hospital | 2 sites serve 1-500 clients; 1 site serves 5001-10 000 clients; 1 site serves more than 10 000 clients |
| Implementation Science Center for Cancer Control Equity (ISCCCE) at the Harvard T.H. Chan School of Public Health | Improving community health by integrating health equity and implementation science for evidence-based cancer control | 32 community health centers | 2 sites serve 5001-10 000 clients; 10 sites serve more than 10 000 clients; data not available for 20 sites |
| Optimizing Implementation in Cancer Control (OPTICC) at University of Washington and Kaiser Permanente Washington Health Research Institute (41,42) | Developing, testing, refining, and disseminating innovative methods for optimizing the implementation of evidence-based interventions in cancer control | 6 network/health system; 1 health department | 1 site serves 1-500 clients; 6 sites serve 5001-10 000 clients |
| Penn Implementation Science Center in Cancer Control (Penn ISC3) at University of Pennsylvania | Applying insights from behavioral economics to rapidly accelerate the pace at which evidence-based practices for cancer care are deployed and to which they are delivered equitably, thereby increasing their reach and impact on the health of individuals with cancer | 6 network/health system; 6 hospitals | 7 sites serve 1-500 clients; 2 sites serve 501-2000 clients; 2 sites serve 2001-5000 clients; 1 site serves 5001-10 000 clients |
| Washington University Implementation Science Center for Cancer Control (WU-ISC3) at Washington University in St. Louis | Building a rigorous, scientific evidence base for rapid-cycle implementation research to increase the reach, external validity, and sustainability of effective cancer control interventions | 5 network/health system; 3 community health center; 11 patient advisory group/community member; 2 health department; 7 advocacy; 3 academic; 3 health association; 2 nonprofit; 2 other | 1 site serves 1-500 clients; 1 site serves 501-2000 clients; 6 sites serve more than 10 000 clients; data not available for 30 sites |
Each Center focuses on an overarching theme or opportunity to address critical scientific areas to advance IS as a field and implementation of evidence-based interventions (EBIs) in partnership with community or clinical partners. Although thematic foci are unique, a unifying theme across Centers includes reducing health disparities and advancing health equity in cancer prevention and control in urban, rural, low-resource, and racial and ethnic minority communities (16-18). Each Center’s theme can be found in Table 1.
The “implementation laboratory” is a signature research concept for ISC3 reflecting collaboration between the Center and community and/or clinical partners, united in their goal of improving implementation of EBIs across the cancer control continuum (19,20). The collaborating or “collaboratory” partner sites may reflect diverse settings (eg, oncology care, primary care, community services), but all share interest in and capacity to conduct research consistent with the Center’s IS theme. Laboratory ISC3 sites provide services across the cancer control continuum and include health-care systems, public health departments, rural primary care practices, federally qualified health centers, NCI-designated Cancer Centers, and professional networks. The composition of the implementation laboratories by organizational type and number of clients served can be found in Table 1.
The Organizing Framework prioritizes collaboration and engagement across ISC3 to advance IS, increase uptake of evidence-based cancer prevention and control interventions, and improve health equity. The Framework is based on operational principles for collaboration, and as a Cancer Moonshot–funded initiative, these principles also reflect priorities identified by the Cancer Moonshot Blue Ribbon Panel (2). These principles, described in Table 2, in part, are driven by aligning goals with the Cancer Moonshot and continue to evolve as the network expands.
Table 2.
Operational principles for research and practice for the Implementation Science Centers in Cancer Control (ISC3) Network
| Principle | Practice to operationalize principles |
|---|---|
| Open collaboration in the network through working groups, monthly and annual meetings, pilots, data sharing, and open access | Centers are required to share the underlying data from publications and make publications open access |
| Centers collaborate and share information through cross-Center pilot projects, works-in-progress presentations, in essence sharing what we learn as we learn | |
| ISC3 working group activities facilitate sharing activities across Centers on evaluation, capacity building, health equity, laboratories, and measures and methods | |
| Monthly meetings and an annual conference provide opportunities to learn about progress, new models, and opportunities for collaboration and capacity building | |
| Consideration of the dynamic context and engagement of communities, health-care settings, and practitioners to advance pragmatic methods | Engagement of partners and practitioners to stay abreast of their dynamic context and advance pragmatic methods |
| Implementation laboratories have a surveillance unit to study clinical and community setting characteristics and practices | |
| ISC3 emphasizes engagement of laboratory partners in identifying and developing pilot studies | |
| Centers contribute to advancing rapid and responsive implementation research methodologies that are tested in and/or with laboratory partners | |
| Advance health equity in the approach to conducting research with partners and inclusion in research | Development and application of metrics related to equity in research, inclusion of multisector collaboration, equity in engagement, and incorporation of social needs and risk and social determinants in implementation studies |
| Health Equity Task Force, with representation from each Center to collect case examples, share learnings, and contribute to evaluation | |
| Facilitating leadership and capacity building across ISC3 for future research and practice | Evaluation of social network of the ISC3 collaboration network on domains of planning or conducting research, capacity building for the field, and dissemination of research to nonscientific audiences and mentoring of early career investigators |
| Share learnings of the evaluation to identify cross-Center leadership opportunities |
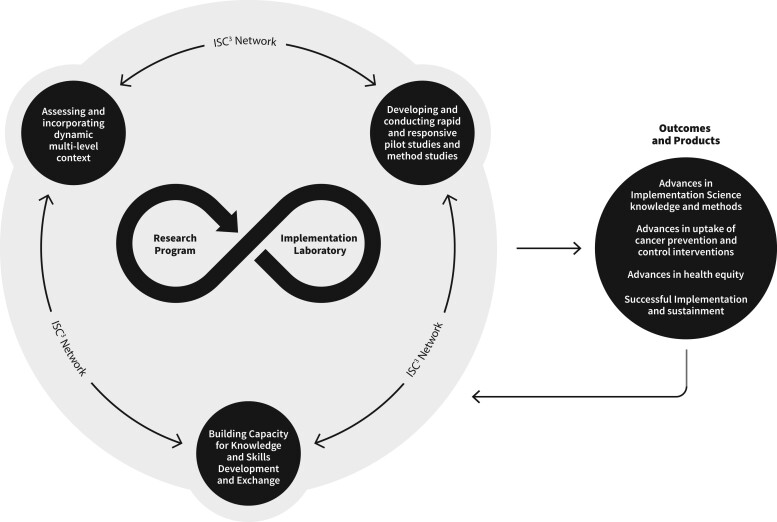
Figure 1 illustrates the Organizing Framework key components, areas of emphasis, and anticipated collaborative outcomes and impacts. ISC3 research programs and implementation laboratories partnerships are central to the model, as Centers conduct research and activities with their implementation laboratory partners. The infinity loop reflects the bidirectional, continuous flow of data, communication, and partnership for research and practice at each Center, setting the groundwork for activities to address our organizing principles (see Table 2).
Figure 1.
Implementation Science Centers in Cancer Control (ISC3) Organizing Framework.
The ISC3 Organizing Framework highlights a collective set of 3 domains of collaboration within and between Centers, as indicated in Figure 1.
Assess and incorporate dynamic, multilevel context: Centers generate research projects informed by the changing multilevel context of cancer prevention and control implementation. Work is facilitated by community advisory boards, contextual assessments, practice surveillance units, data sharing, and evaluation processes within and across Centers to inform practice and research. Many challenges and opportunities for the Centers to address inequities via policy implementation research have been highlighted in cross-Center efforts (21,22).
Build capacity for knowledge and skills development and exchange: Centers are envisioned as a marketplace of ideas and knowledge generators in cancer prevention, care, and control, with broader impact to other chronic diseases. Centers lead the field in developing and training researchers, developing and co-creating resources for research and practice partners, and including early career investigators in new and emerging areas in IS. Collaborations and capacity building are intended to support the broader ecosystem of each Center and as a network and are supported by ISC3 working groups to facilitate continuous and data-driven learning (23).
Develop and conduct rapid and responsive pilot and methods studies: The Centers are designed to be more responsive to emerging priorities and challenges than traditional NIH R01 grant mechanisms, typically 5 years long. Being responsive to partners and dynamic, multilevel context is critical for research to be useful and relevant for the laboratories and communities served. By using pragmatic research designs, emphasizing rapid generation, and sharing results, findings can be translated into faster action and decision making by ISC3 partners (24). Some Centers are advancing responsiveness by using systematic processes to engage partner ideas in pilot funding announcements and by enlisting partners to serve as reviewers of applications.
Outcomes anticipated from the Centers include 3 major areas of advancement: IS expertise and methods; uptake, successful implementation, and sustainment of cancer prevention and control interventions; and health equity. As depicted in the Framework, the overall system is learning oriented, and outcomes from Center activities undergo continued refinement, spark generation of more evidence, and capitalize on partner expertise and experiences.
Examples of Center activities across organizational domains
Dynamic multilevel context
Context is a central feature of IS, yet it is often poorly defined or unreported. Moreover, the collective field has put relatively little consideration into how outer contexts may impact implementation, or how inner and outer contexts are continuously changing. The lack of attention to dynamic and multilevel contexts may inadvertently result in missed opportunities to advance health equity. Recent calls for action posit that, to advance equity, the field needs to better account for evolving macrolevel historical, cultural, economic, and political forces that shape implementation in low-resource settings and communities (18).
ISC3 has responded to this scientific opportunity in several ways. First, through a coordinated cross-Center collaboration, the Health Equity Contextual Assessment Project illustrates efforts to create a robust set of shared measures to be used to understand and explore impacts of outer context (eg, social conditions, environmental factors, transportation, policy) on implementation outcomes in a range of settings. To our knowledge, this is the first effort to characterize the outer implementation context in a comprehensive manner. This work will inform collaborations to develop and test implementation pilot studies addressing social needs and social determinants of health identified in the outer context, as part of implementation strategies to promote equitable implementation of cancer prevention and control innovations (21).
Another example of a project responsive to dynamic multilevel context is from the Optimizing Implementation in Cancer Control (OPTICC) at University of Washington and Kaiser Permanente Washington Health Research Institute ISC3. In the project, “Operationalizing a Rideshare Intervention for Colonoscopy Completion: Barriers, Facilitators, and Process Recommendations,” a multipartner group (medical directors, nursing leaders, industry representatives, providers, patients) was part of informal community partner meetings to surface multilevel barriers to colonoscopy completion in the [Washington state] area. These meetings represent a COVID-sensitive, pragmatic procedure critical for clinical partners to ensure equitable participation in research. Questions for partners were iterative, and results from listening sessions were reviewed by partners. The nominal group technique was used to generate consensus on process recommendations. Contextual information informed the utilization of a rapid and responsive methods pilot to test a Rideshare program as an implementation strategy to promote health equity and colonoscopy completion. A 7-step concrete approach was developed to collaboratively identify and prioritize barriers and to operationalize a testable implementation strategy solution. The outcome focused on improvement in colonoscopy uptake following abnormal fecal immunochemical test screening.
Another example of responsiveness to context is demonstrated at the Harvard Implementation Science Center for Cancer Control Equity. At the start of the COVID-19 pandemic, the team and their partners in community health centers (CHCs) across Massachusetts pivoted the prepandemic pilot study to urgently address the need to screen CHC patients for social needs while simultaneously developing strategies to keep patients engaged in cancer screening. The Center’s ability to pivot to priorities facing CHCs and to navigate the reality of the pandemic and related priorities in response (eg, identifying and addressing social risks) reflects the Center’s consideration of partners’ dynamic context in which the collaborative research occurred. The Center pivoted to use its existing resources to address strategies integrating implementation of cancer screening with screening for social needs, which informed the Center how to offer efficiencies in implementation approaches to CHCs, including adaptation phase to address equity. The project has facilitated information sharing and collaboration across ISC3 and has been shared across ISC3 Centers. From this pilot, the Harvard Implementation Science Center for Cancer Control Equity developed and tested the Stakeholder and Equity Data-Driven Implementation process, which is available for use by other ISC3 Centers (25).
Building capacity for knowledge and skills development and exchange
Capacity building examples are a diverse spectrum of activities from one-to-one training in IS to the development of shared co-created tools across ISC3. Intensive trainings and leadership from ISC3 include NCI’s Training in Dissemination and Implementation Research in Cancer program and Washington University’s Institute for Implementation Science Scholars program (26,27), which include faculty and diverse trainees across the ISC3. The reach of many of the capacity building activities (eg, toolkits and webtools) include not only ISC3 Centers but other practice-based research networks and practitioners as well, with application across disease outcomes beyond cancer.
As part of ISC3, Centers coordinate capacity building activities through a Capacity Building cross-Center working group. The working group identified a unique need across the Centers to coordinate the broad range of tools and resources available to build capacity across diverse audiences in a way easily accessible across audiences. It has developed the ISC3 Capacity Building Database as an open-source repository of diverse resources to researchers and clinical and practitioner partners (28). The trainings, toolkits, and web-based tools may be of particular interest to partners including those at NCI-designated Cancer Centers and other research and practice networks seeking to enhance capacity in IS.
As the IS field has grown in the last few years in prioritizing health equity, there is a need for identification of theories, models, and frameworks emphasizing health equity. The Colorado ISC3 and the Washington University ISC3 are collaborating on an interactive web-based tool to expand the capacity of implementation scientists and practitioners to advance health equity. This webtool will serve as a resource when planning projects, selecting frameworks, carrying out work, and/or measuring outcomes. It will have a planning section highlighting dynamic context when creating a logic model to help plan a project. The tool provides guidance on planning for, selecting, adapting, combining, using, and measuring IS frameworks (https://dissemination-implementation.org). This will encourage investigators to think ahead when adapting an EBI to their setting and considering adaptations. The tool, along with a series of case studies, is intended to promote more consistent and pragmatic use of dissemination and implementation theories, models, and frameworks when conducting health equity work.
The iDAPT Center at Wake Forest School of Medicine/University of Massachusetts Medical School focuses on building capacity at the intersection of IS and informatics among members of the NCI Community Oncology Research Program. Capacity building activities include virtual workshops and in-depth consultations covering core themes related to cancer care delivery researchers, informatics, and IS. One example of this work involves NCI Community Oncology Research Program sites interested in implementing tobacco cessation during cancer screening that are developing a hybrid type II design trial to implement an evidence-based virtual exercise program for cancer survivors. The iDAPT Center has found that capacity building activities are leading to a more rigorous approach to implementation activities in practice.
Rapid and responsive pilot and methods studies
In the Centers’ research program, each site is conducting a series of pilot studies over 5 years to rapidly and iteratively test implementation strategies, new and adapted methods and frameworks, and methods and measures related to each Center’s theme. The implementation laboratory is an essential design element of the Centers, which has facilitated the capacity and ability of Centers to conduct rapid and responsive pilot studies as they leverage existing and established relationships with partners. The Centers’ research program includes an Implementation Studies Unit and a Methods Unit, with pilots to be conducted within a 1- to 2-year cycle and designed to be responsive to context of each Center’s unique laboratory. The Methods Units are designed to advance mixed methods approaches to develop, adapt, and deploy implementation strategies, as well as study implementation of EBIs in clinical and community settings (29, 30). Pilot study progress and findings are routinely shared to support cross-pollination, capitalizing on continuous learning and engagement. Four examples follow.
The Washington University ISC3 approach to the generation of rapid and responsive pilot studies is a community-driven and community-engaged process to identify the most pressing priorities related to cancer control and prevention. In a think-tank meeting twice a year, community members review and refine Center priorities, which are then included as priorities in pilot study solicitations. Think-tank principles are defined through access, participation, process and collaboration, and cancer control focus. Pilot applications must meet at least 1 of the stated priorities and are reviewed by 2 faculty members and 1 community member. Community members are invaluable in incorporating the multilevel context of the organizations and systems they work in and highlighting the feasibility and applicability of the proposals. Feedback from this process is provided to pilot applicants and used in final pilot funding decisions and serves as an opportunity for community members to gain experience in grant reviewing and to learn about IS concepts and methods.
The BRIDGE-C2 offers an example of a rapid and responsive approach to changing clinical guidelines. At the BRIDGE-C2, Center investigators were evaluating an electronic health record (EHR) tool for cervical cancer screening and abnormal follow-up. The goal of this project was to evaluate the adoption of a clinical decision support EHR tool for cervical cancer screening and abnormal results follow-up to identify the strategies needed to improve its adoption. This ongoing pilot was impacted by the release of the 2020 American Society of Colposcopy and Cervical Pathology guidelines (31), rendering the existing EHR tool obsolete. The pilot rapidly switched to support the upgrade of the tool to meet new guidelines. Through a user-centered design effort and a mixed methods analysis of the original tool, the Center identified barriers to clinical decision support use and redesigned the tool to address barriers identified. Learnings from this pilot highlighted needs for feedback data on care quality metrics currently not available (eg, rates of patients with abnormal results and missed follow-ups) and responsiveness to these data for future implementation in health information technology (HIT) projects. This work will inform the design of pilot and methods studies related to developing HIT strategies to promote adoption of EBIs.
The Penn ISC3 has integrated a rapid and responsive approach to patient and provider needs in the design of its study focused on increasing the use of serious illness conversations (SICs) within cancer care. SICs, recommended by national guidelines, elicit patients’ values, goals, and care preferences and is an evidence-based practice that improves patient well-being and quality of life. This 2-by-2 factorial, cluster-randomized pragmatic trial tests the effect of behavioral nudges to clinicians, patients, or both, compared with usual practice, to promote SICs in the context of oncology care (30). Before the trial began, the research team employed rapid cycle methods to finalize the messages embedded in the nudges to clinicians and patients and to optimize implementation strategies, including the timing and delivery modes for the messages. More specifically, this involved design meetings with behavioral economics experts, in-depth discussions with oncology clinicians across multiple sites, and focus groups with cancer patients and their caregivers. Formal and repeated usability testing ensured the study methods were responsive to the community within which it was to be conducted.
The Colorado ISC3 is exploring the use of IS frameworks (specifically Reach, Effectiveness, Adoption, Implementation, and Maintenance [RE-AIM] and the Practical, Robust Implementation and Sustainability Model [PRISM]) to inform iterative conceptual and data-driven decisions about adaptations. The iterative RE-AIM process periodically engages implementation partners from multiple perspectives to reflect on and discuss the relative importance of and progress on key implementation outcomes (32). Implementation teams then develop and implement consensus-based adaptation strategies. Pilot data suggest that the iterative RE-AIM process appears feasible and helpful. Ongoing work will assess its broad applicability across diverse prevention and control issues, interventions, contexts, and populations.
Discussion
Although progress has been made, many gaps in cancer prevention, detection, diagnosis, treatment, and the care of cancer survivors remain (33). Addressing these gaps will require real-world research on the translational roadblocks present in settings that reach large populations with inequitable access to benefit from evidence-based programs and policies. ISC3 represents a large-scale initiative with infrastructure to support efforts for rapid translation of approaches into settings to address long-standing inequities and failures. Centers were designed to have a research-practice partnership at their core and to create the opportunity for a series of smaller scale pilot studies to explore new and sometimes risky ideas.
Some cross-cutting learnings have emerged as the network has operationalized underlying principles. Pilot study structure and autonomy allows for nimble responsivity to laboratory settings and identified needs of partners, and the broad reach of laboratories allows for testing implementation strategies in diverse settings. Being responsive to contextual changes in policy, the COVID-19 pandemic and data-driven identified needs and barriers in inner and outer contexts have identified opportunities within ISC3 to compare and collaborate on the varied role of multilevel context across local and state areas.
Rapid cycle approaches allow Centers to engage in complex studies addressing multilevel dynamics in an expeditious and efficient manner, for quick learning and adaptation as needed (34). As ISC3 collaborates to build capacity in implementation research and practice, unique opportunities to create open-access tools and resources have emerged. Centers have also prioritized extending training to practitioners and community members and in doing so have developed new and leveraged existing networks such as NCI-designated Cancer Centers. Centers have been rapid and responsive to partners as needs and priorities pivot impacting implementation of cancer screening guidelines. These may be riskier but advance more pragmatic approaches to implementation contexts in real-world situations that are often not controlled as in typical randomized controlled trial effectiveness designs. ISC3 has identified core operational principles and places important responsibility on Center investigators to operationalize them. We believe the development of core operating principles, emphasis on infrastructure development, and prioritization of local partnerships that can support IS and practice will lead to important impacts and a foundation for sustainability.
The ISC3 initiative began in 2019, before the COVID-19 pandemic, and calls for racial justice from the NIH, emphasizing social and structural causes of inequalities (35,36). ISC3 has established a Health Equity Task Force with core themes consistent with and to reinforce the Organizing Framework and includes explicit emphasis on health equity in building capacity and diversity of scholars working on applying health equity in implementation research across career stages; advancing theories, models, and frameworks that include equity-related constructs; and including a focus on health equity in evaluation.
There are several other initiatives funded by the Cancer Moonshot with grounding in IS, presenting opportunities for cross-initiative learning (eg, the Accelerating Colorectal Cancer Screening, Follow-up, and Referral to Care through Implementation Science; the Cancer Center Cessation Initiative; and others) (37-39). ISC3 has opportunities to share information and resources across these networks and, over time, anticipates additional opportunities for cross-network learning and collaboration, including through the Consortium for Cancer Implementation Science (40). Ultimately, the speed of translation of cancer prevention and control evidence into practice will be determined by the learning from the various networks, and we believe the foundation built in ISC3 will bring significant value to our implementation partners and in advancing the field toward this goal.
Finally, we believe IS is significantly enriched by the 2-way learning the ISC3 structure enables and anticipate innovations identified are more likely to find their way into practice with their relevance to participating partners. It is our hope that ISC3 will not only generate science appropriate for a 7-Center initiative but also create a larger body of cross-cutting knowledge, reflecting a network working collaboratively on key domains driven by shared operational principles. In the development of the ISC3 concept and its inception, we have created an Organizing Framework for efficiently and collaboratively advancing IS. This Framework should generate findings within ISC3 and provide a template for future efforts seeking to improve the integration of research, practice, and policy toward improvements in cancer research and care across the cancer control continuum.
Contributor Information
April Y Oh, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Karen M Emmons, Department of Social and Behavioral Science, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Ross C Brownson, Prevention Research Center, Brown School, Washington University in St. Louis, St. Louis, MO, USA; Department of Surgery (Division of Public Health Sciences) and Alvin J. Siteman Cancer Center, Washington University School of Medicine, Washington University in St. Louis, St. Louis, MO, USA.
Russell E Glasgow, Dissemination and Implementation Science Program and Department of Family Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Kristie L Foley, Department of Implementation Science, Wake Forest University School of Medicine, Winston Salem, NC, USA.
Cara C Lewis, Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA.
Robert Schnoll, Department of Psychiatry and Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, USA.
Nathalie Huguet, Department of Family Medicine, Oregon Health & Science University, Portland, OR, USA.
Amy Caplon, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
David A Chambers, Division of Cancer Control and Population Sciences, National Cancer Institute, Rockville, MD, USA.
Funding
This work was supported by the National Cancer Institute (grant numbers P50CA244693, P50CA244433, P50CA244289, P50CA244688, P50CA244432, P50CA244431, and P50CA244690).
Notes
Role of the funder: The funder played a role in the conceptualization of the manuscript but did not play a role in the writing of the manuscript or the decision to submit it for publication.
Disclosures: AYO, KME, RCB, REG, KLF, CCL, RS, NH, DAC, AC have nothing to disclose.
Author contributions: AYO, KME, RCB, REG, CCL, RS, NH, DAC, AC: conceptualization. AYO, KME, RCB, KLF, REG: writing—original draft and DAC, AC, CCL, RS, NH: writing—review and editing.
Acknowledgements: The authors would like to acknowledge the ISC3 laboratory partners, ISC3 staff, investigators, trainees, and other partners to the ISC3 initiative.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the National Cancer Institute.
Data availability
No new data were generated or analyzed in support of this research.
References
- 1.Healthy People 2030. Office of Disease Prevention and Health Promotion. 2020. https://health.gov/healthypeople. Accessed September 20, 2022.
- 2. Cancer Moonshot. Blue Ribbon Panel Report 2016. National Cancer Institute. 2016. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/blue-ribbon-panel-report-2016.pdf. Accessed September 13, 2022.
- 3. Khan S, Chambers D, Neta G.. Revisiting time to translation: implementation of evidence-based practices (EBPs) in cancer control. Cancer Causes Control. 2021;32(3):221-230. [DOI] [PubMed] [Google Scholar]
- 4. Sharpless NE. COVID-19 and cancer. Science. 2020;368(6497):1290. [DOI] [PubMed] [Google Scholar]
- 5. DeVoe JE, Cheng A, Krist A.. Regional strategies for academic health centers to support primary care during the COVID-19 pandemic: a plea from the front lines. JAMA Health Forum. 2020;1(4):e200423. doi: 10.1001/jamahealthforum.2020.0423. [DOI] [PubMed] [Google Scholar]
- 6. Winkfield KM, Winn RA.. Improving equity in cancer care in the face of a public health emergency. Cancer J. 2022;28(2):138-145. [DOI] [PubMed] [Google Scholar]
- 7. Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891-1892. [DOI] [PubMed] [Google Scholar]
- 8. Diez Roux AV. Population health in the time of COVID-19: confirmations and revelations. Milbank Q. 2020;98(3):629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams DR, Cooper LA.. COVID-19 and health equity-a new kind of “herd immunity”. JAMA. 2020;323(24):2478-2480. [DOI] [PubMed] [Google Scholar]
- 10. Clinical and Translational Science Awards (CTSA) Program. National Center for Advancing Translational Sciences. 2017. https://ncats.nih.gov/ctsa. Accessed September 20, 2022.
- 11. Cancer Prevention and Control Research Network. https://cpcrn.org/. Accessed May 16, 2022.
- 12. Quality Enhancement Research Initiative (QUERI). US Department of Veterans Affairs. 2022. https://www.queri.research.va.gov/. Accessed September 20, 2022.
- 13. Dzau VJ, Balatbat BA, Ellaissi WF.. Revisiting academic health sciences systems a decade later: discovery to health to population to society. Lancet. 2021;398(10318):2300-2304. [DOI] [PubMed] [Google Scholar]
- 14. Oh A, Vinson CA, Chambers DA.. Future directions for implementation science at the National Cancer Institute: Implementation Science Centers in Cancer Control. Transl Behav Med. 2021;11(2):669-675. doi: 10.1093/tbm/ibaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adsul P, Chambers D, Brandt HM, et al. Grounding implementation science in health equity for cancer prevention and control. Implement Sci Commun. 2022;3(1):56. doi: 10.1186/s43058-022-00311-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chaiyachati KH, Beidas RS, Lane-Fall MB, Rendle KA, Shelton RC, Kaufman EJ.. Weaving equity into the fabric of medical research. J Gen Intern Med. 2022;37(8):2067-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rendle KA, Beidas RS.. Four strategic areas to advance equitable implementation of evidence-based practices in cancer care. Transl Behav Med. 2021;11(11):1980-1988. doi: 10.1093/tbm/ibab105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brownson RC, Kumanyika SK, Kreuter MW, et al. Implementation science should give higher priority to health equity. Implement Sci. 2021;16(1):28. doi: 10.1186/s13012-021-01097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oh A, Vinson C, Wolfrey C. RFA-CA-19-005: Implementation Science for Cancer Control: Developing Centers (P50 Clinical Trial Optional). US Department of Health and Human Services. 2018. https://grants.nih.gov/grants/guide/rfa-files/rfa-ca-19-005.html. Accessed May 16, 2022.
- 20. Oh A, Vinson C, Wolfrey C. RFA-CA-19-006: Implementation Science for Cancer Control: Advanced Centers (P50 Clinical Trial Optional). U.S. Department of Health and Human Services. 2018. https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-19-006.html. Accessed May 16, 2022.
- 21. Emmons KM, Chambers D, Abazeed A.. Embracing policy implementation science to ensure translation of evidence to cancer control policy. Transl Behav Med. 2021;11(11):1972-1979. doi: 10.1093/tbm/ibab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brownson RC, Shelton RC, Geng EH, Glasgow RE.. Revisiting concepts of evidence in implementation science. Implement Sci. 2022;17(1):26. doi: 10.1186/s13012-022-01201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacob RR, Korn AR, Huang GC, et al. Collaboration networks of the Implementation Science Centers for Cancer Control: a social network analysis. Implement Sci Commun. 2022;3(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Proctor E, Ramsey AT, Saldana L, Maddox TM, Chambers DA, Brownson RC.. FAST: a framework to assess speed of translation of health innovations to practice and policy. Glob Implement Res 2022;2(2):107-119. doi: 10.1007/s43477-022-00045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aschbrenner KA, Mueller NM, Banerjee S, Bartels SJ.. Applying an equity lens to characterizing the process and reasons for an adaptation to an evidenced-based practice. Implement Res Pract. 2021;2:10.1177/26334895211017252. doi: 10.1177/26334895211017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Training Institute for Dissemination and Implementation Research in cancer (TIDIRC) facilitated course. National Cancer Institute Division of Cancer Control and Population Sciences. 2021. https://cancercontrol.cancer.gov/is/training-education/TIDIRC. Accessed June 7, 2022.
- 27. Institute for Implementation Science Scholars. https://is2.wustl.edu. Accessed June 7, 2022.
- 28. Capacity Building Database. Implementation Science Centers in Cancer Control. 2022. https://iscentersincancercontrol.org/resources/. Accessed September 20, 2022.
- 29. Jenssen BP, Schnoll R, Beidas R, et al. Rationale and protocol for a cluster randomized pragmatic clinical trial testing behavioral economic implementation strategies to improve tobacco treatment rates for cancer patients who smoke. Implement Sci. 2021;16(1):72. doi: 10.1186/s13012-021-01139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takvorian SU, Bekelman J, Beidas RS, et al. Behavioral economic implementation strategies to improve serious illness communication between clinicians and high-risk patients with cancer: protocol for a cluster randomized pragmatic trial. Implement Sci. 2021;16(1):90. doi: 10.1186/s13012-021-01156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perkins RB, Guido RS, Castle PE, et al. ; for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24(2):102-131. doi:10.1097/lgt.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glasgow RE, Battaglia C, McCreight M, Ayele RA, Rabin BA.. Making implementation science more rapid: use of the RE-AIM framework for mid-course adaptations across five health services research projects in the Veterans Health Administration. Front Public Health. 2020;8:194. doi: 10.3389/fpubh.2020.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Association for Cancer Research. AACR Cancer Disparities Progress Report. Oxford University Press; 2022. http://www.CancerDisparitiesProgressReport.org/. Accessed June 14, 2022. [Google Scholar]
- 34. Chambers DA, Glasgow RE, Stange KC.. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013;8(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Collins FS, Adams AB, Aklin C, et al. ; for the NIH UNITE. Affirming NIH’s commitment to addressing structural racism in the biomedical research enterprise. Cell. 2021;184(12):3075-3079. [DOI] [PubMed] [Google Scholar]
- 36. Bernard MA, Johnson AC, Hopkins-Laboy T, Tabak LA.. The US National Institutes of Health approach to inclusive excellence. Nat Med. 2021;27(11):1861-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Accelerating Colorectal Cancer Screening and Follow-up Through Implementation Science. ACCSIS. 2022. https://accsis.rti.org/. Accessed September 20, 2022.
- 38. Cancer Center Cessation Initiative. National Cancer Institute Division of Cancer Control and Population Sciences (DCCPS). 2022. https://cancercontrol.cancer.gov/brp/tcrb/cancer-center-cessation-initiative. Accessed September 20, 2022.
- 39.Improving the Management of Symptoms During And Following Cancer Treatment. IMPACT. 2022. https://impactconsortium.org/. Accessed September 20, 2022.
- 40. Consortium for Cancer Implementation Science. 2021. https://consortiumforcanceris.org/. Accessed September 20, 2022.
- 41. Lewis CC, Hannon PA, Klasnja P, et al. ; OPTICC Consortium, represented by Bryan J. Weiner. Optimizing implementation in cancer control (OPTICC): Protocol for an implementation science center. Implement Sci Commun. 2021;2(1):44. doi: 10.1186/s43058-021-00117-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.OPTICC: Optimizing Implementation in Cancer Control. https://www.opticc.org/. Accessed May 9, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.