Abstract
Background
Growing evidence indicates the adverse effect of ultra-processed food (UPF) consumption. However, it remains unknown whether UPF consumption influences the risk of colorectal cancer (CRC) precursors, namely conventional adenomas and serrated lesions.
Methods
We drew data from the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study, comprising 142 052 participants who had undergone at least 1 lower gastrointestinal endoscopy during follow-up. To handle multiple records per participants, we used multivariable logistic regression for clustered data to calculate odds ratios (OR) and 95% confidence intervals (CIs) of colorectal polyps in relation to cumulative average consumption of UPFs. All statistical tests were 2-sided.
Results
We documented 11 644 patients with conventional adenomas and 10 478 with serrated lesions during 18-20 years of follow-up. Compared with participants in the lowest quintile of UPF consumption, those in the highest quintile had an increased risk of conventional adenomas (OR = 1.18, 95% CI = 1.11 to 1.26) and serrated lesions (OR = 1.20, 95% CI = 1.13 to 1.28). Similar results were found for high-risk polyps (ie, advanced adenomas and ≥10 mm serrated lesions; OR = 1.17, 95% CI = 1.07 to 1.28). These associations were slightly attenuated but remained statistically significant after further adjusting for body mass index, Western dietary pattern score, or individual dietary factors (fiber, folate, calcium, and vitamin D). The results remained essentially unchanged after excluding processed meat from total UPF intake.
Conclusions
Higher consumption of UPFs is associated with an increased risk of CRC precursors. UPFs might be a modifiable target for early prevention of CRC.
Colorectal cancer (CRC), the third-most common type of malignancy worldwide, is known to originate from precursor polyps (1). There are 2 main types of colorectal polyps with recognized malignant potential: conventional adenomas and serrated lesions that account for approximately 60%-80% and 20%-30% of sporadic CRC cases, respectively (2). Mounting evidence shows that diet modification can play an important role in primary prevention of CRC. During the past decades, ultra-processed food (UPF) consumption has substantially increased, which contributes to approximately 25%-60% of total daily energy intake worldwide (3). UPFs refer to products undergoing series of industrial techniques and processes and generally containing flavors, colors, sweeteners, emulsifiers, and other additives (4). It has been proposed that several characteristics of UPFs may cause health problems, including cancer. For example, UPFs commonly have a poor nutritional quality, high in total fat, saturated fat, and added sugar and salt but low in fiber and vitamins (5). Some types of sweeteners and emulsifiers may disrupt the host–microbiota interaction, leading to metabolic abnormalities and a proinflammatory environment for colonic carcinogenesis (6,7). Moreover, potential carcinogens (eg, acrylamide) may be produced during the heat processing of foods (8), and some additives (eg, sodium nitrite in processed meats and titanium dioxide used to whiten food products) have carcinogenicity as reported in in vivo and vitro studies (9,10).
Nevertheless, epidemiological evidence linking intake of UPFs to risk of cancer remains scarce and controversial. In a French cohort study, a 10% increase in the proportion of UPFs in the diet was associated with a 12% higher risk of overall cancer and an 11% higher risk of breast cancer but was not associated with the risk of CRC (11). In contrast, a subsequent multicentric case-control study in Spain reported that higher UPF consumption was associated with an increased risk of CRC (12). Our recent cohort study also found a positive association of total UPF consumption with CRC risk in men and certain UPF subgroups (eg, ready-to-eat or -heat mixed dishes) with CRC risk in women (13). Despite these data, however, to our knowledge, no prospective studies have yet examined the influence of UPFs on CRC precursors.
Therefore, to better understand the role of UPFs in CRC development, we conducted this prospective study to examine the association of UPF consumption with risk of conventional adenomas and serrated lesions in 3 large US cohorts.
Methods
Study population
Participants were derived from 3 ongoing prospective cohorts: the Nurses’ Health Study (NHS) recruiting 121 700 female nurses aged 30-55 years at baseline in 1976, the Nurses’ Health Study II (NHS II) recruiting 116 429 female nurses aged 25-42 years at baseline in 1989, and the Health Professional Follow-up Study (HPFS) recruiting 51 529 male health professionals aged 40-75 years at baseline in 1986 (14). All participants completed a questionnaire at baseline and were mailed follow-up questionnaires biennially to update their lifestyle and medical information. Diet was assessed by a validated food frequency questionnaire (FFQ) every 4 years. The average follow-up rate has been greater than 90% in all 3 cohorts (15).
In this study, we used 1992 for the NHS/HPFS and 1991 for the NHS II as baseline, because detailed histological information of polyps was collected since then. At baseline, we excluded participants with a history of cancer (except nonmelanoma skin cancer), colorectal polyp, or inflammatory bowel disease and with implausible adolescent caloric intake (ie, <800 or >4200 kcal/d for men; <600 or >3500 kcal/d for women) or extensive missing responses (>70 for food items). We also excluded those who had no colonoscopy or sigmoidoscopy over the follow-up period because colorectal polyps are generally asymptomatic and can only be detected with an endoscopy. The final sample included 55 493 women from the NHS, 58 317 women from the NHS II, and 28 242 men from the HPFS (see flowchart in Supplementary Figure 1, available online). The study protocol was approved by the institutional review board at the Brigham and Women’s Hospital, the Harvard T.H. Chan School of Public Health, and the participating registries as required.
Assessment of UPF consumption and covariates
Based on FFQs with approximately 130 food items, we applied the Nova classification to categorize foods into 4 groups: unprocessed or minimally processed foods, processed culinary ingredients, processed foods, and UPFs (Supplementary Methods, available online) (16). For 9 food items that lacked sufficient details to support their classification (ie, “popcorn,” “soy milk,” “pancakes or waffles,” “pie, home-baked or ready-made,” “beef, pork, lamb sandwich,” “tomato sauce”), we adopted a conservative approach by assigning these items to a non-UPF group as their primary categorization and to the UPF group for a sensitivity analysis. Because alcohol consumption is an established risk factor for CRC, we removed this item from the UPF group. We estimated UPF consumption as servings per day, which was energy adjusted using the residual methods (16). Covariates were selected a priori as potential confounders (Supplementary Methods, available online).
Ascertainment of colorectal polyps
Ascertainment of colorectal polyps in the 3 cohorts was previously described (Supplementary Methods, available online) (17). Conventional adenomas included tubular, tubulovillous, and villous adenomas as well as adenomas with high-grade dysplasia, and serrated lesions comprised hyperplastic polyps, traditional serrated adenomas, sessile serrated adenomas or polyps, and mixed serrated polyps. High-risk polyps included advanced conventional adenomas (at least 1 adenoma of ≥10-mm diameter or any size with tubulovillous, villous, or high-grade dysplasia) and large serrated lesions (≥10 mm) (18).
Statistical analysis
Because most polyps are asymptomatic and cannot be diagnosed without an endoscopic examination, “incident polyp” is not well defined and a time-to-event analysis (eg, Cox proportional hazards model) is not suitable for studies of polyps. Instead, we used the Andersen-Gill repeated data structure with a new record for each 2-year follow-up period during which a participant underwent an endoscopy (17,19). Participants were censored at the time of the first diagnosis of colorectal polyps, death, or the end of follow-up (June 1, 2012, for the NHS and June 1, 2011, for the NHS II), whichever occurred first. To better represent long-term habitual intake and minimize random measurement errors, we calculated the cumulative average of energy-adjusted servings of UPFs per day from preceding questionnaires up to the current cycle (20).
Multivariable logistic regression for clustered data was used to account for repeated observations (ie, multiple endoscopies) and compute odds ratios (ORs) of conventional adenomas and serrated lesions according to quintiles of UPF consumption. Model 1 was adjusted for potential confounders, including age, race, study cohort, time period of endoscopy, number of prior endoscopies, and time in years since the most recent endoscopy. Model 2 was additionally adjusted for family history of CRC, total alcohol intake, physical activity, smoking status and pack-years, regular aspirin use, menopausal status (women only), and postmenopausal hormone use (women only). Because UPFs include processed meats, an established risk factor for colorectal carcinogenesis, we also assessed the association after removing processed meats from UPFs in Model 2. In addition, to delineate whether the association was independent of adiposity and established dietary factors, we conducted a secondary analysis by further adjusting for body mass index (BMI), Western dietary pattern score, and individual dietary factors (fiber, folate, calcium, and vitamin D) that have been linked to CRC (17). We examined the dose-response relationship between UPF intake and colorectal polyps using the restricted cubic spline analysis with the default 4 knots at the 5th, 35th, 65th, and 95th percentiles of UPF intake (21).
We further performed separate analyses according to anatomic subsites and malignant potential of polyps. We also conducted stratified analyses according to age (<60, ≥60 years), sex (female, male), smoking (never, 0-20 pack-years, ≥20 pack-years), BMI (<25, ≥25 kg/m2), physical activity (<15, ≥15 metabolic equivalent-h/wk), Western dietary pattern score (less than median, median or greater), family history of CRC (yes, no), and reason for endoscopy (screening, symptom). Screening was defined by the indication for routine or asymptomatic screening and family history of CRC, and symptoms encompassed bleeding in stool, positive test for occult fecal blood, diarrhea or constipation, and abdominal pain. Potential effect modification was assessed by the likelihood ratio test comparing models with and without the interaction term between UPF consumption and the stratified variable. In addition, we categorized UPFs into 9 mutually exclusive subgroups (Supplementary Table 1, available online) (22) and assessed their independent association with colorectal polyps by mutually adjusting for the individual subgroups. All statistical tests were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC), with a 2-sided P value less than .05 indicating statistical significance.
Results
The median intake of total UPF in the study participants was 6.2 servings per day (interquartile range = 4.9-7.6), similar to that in the overall cohorts (median = 6.2, interquartile range = 5.0-7.7 servings per day). During the18-20 years of follow-up among 142 052 participants in the 3 cohorts, we documented 11 644 patients with conventional adenomas and 10 478 patients with serrated lesions. As shown in Table 1, compared with participants who did not develop polyp, those with conventional adenomas had higher proportions of males (30% vs 22%), positive family history of CRC (25% vs 21%), and current smokers (8% vs 6%); they also had higher mean BMI (26.1 vs 25.8 kg/m²), alcohol consumption (7.4 vs 6.3 g/d), Western dietary pattern score (0.02 vs −0.07), and UPF intake (6.61 vs 6.41 servings per day). Similar results were found for participants with serrated lesions compared with those without any polyps. We also presented the basic characteristics of participants by quintiles of UPF intake (Supplementary Table 2, available online).
Table 1.
Basic characteristics of participants in the 3 cohort studies (NHS, NHS II, HPFS)a
| Characteristics | Overall population | Nonpolyps | Conventional adenomas | Serrated lesions |
|---|---|---|---|---|
| Number of participants | 142 052 | 120 402 | 11 644 | 10 478 |
| Age, mean (SD), y | 60.3 (10.6) | 60.4 (10.7) | 61.1 (10.0) | 58.8 (9.9) |
| Male, % | 22 | 22 | 30 | 24 |
| White, % | 96 | 96 | 96 | 97 |
| Family history of colorectal cancer, % | 21 | 21 | 25 | 25 |
| BMI, mean (SD), kg/m² | 25.9 (4.7) | 25.8 (4.7) | 26.1 (4.7) | 26.4 (4.8) |
| Current smoker, % | 6 | 6 | 8 | 11 |
| Pack-years of smoking, mean (SD) | 9.1 (15.6) | 9.0 (15.4) | 11.0 (17.2) | 13.5 (18.9) |
| Alcohol, mean (SD), g/d | 6.3 (9.3) | 6.3 (9.3) | 7.4 (10.7) | 7.6 (10.9) |
| Physical activity, mean (SD), MET-h/wk | 22.0 (20.5) | 22.0 (20.5) | 21.7 (19.8) | 20.8 (18.5) |
| Postmenopausal, %b | 81 | 81 | 77 | 79 |
| Postmenopausal hormone use, %b | 57 | 57 | 54 | 56 |
| Regular aspirin use (2 or more tablets/wk), % | 42 | 42 | 42 | 41 |
| Dietary intake, mean (SD) | ||||
| Western dietary pattern scorec | −0.06 (0.94) | −0.07 (0.94) | 0.02 (0.94) | 0.03 (0.94) |
| Total fiber, g/d | 20.1 (5.4) | 20.2 (5.4) | 20.0 (5.3) | 19.6 (5.0) |
| Total folate intake, µg/d | 550 (216) | 551 (216) | 534 (206) | 534 (203) |
| Total calcium, mg/d | 1146 (428) | 1149 (429) | 1094 (405) | 1114 (407) |
| Total vitamin D, IU/d | 436 (227) | 437 (227) | 412 (213) | 412 (209) |
| Ultra-processed foodd | 6.43 (2.2) | 6.41 (2.19) | 6.61 (2.22) | 6.60 (2.19) |
| Ultra-processed breads and breakfast foodd | 1.57 (0.80) | 1.56 (0.79) | 1.62 (0.81) | 1.60 (0.78) |
| Fat, condiment, and saucesd | 1.45 (0.99) | 1.45 (0.99) | 1.50 (1.01) | 1.55 (1.03) |
| Packaged sweet snacks and dessertsd | 0.99 (0.67) | 0.99 (0.67) | 1.03 (0.69) | 1.00 (0.65) |
| Beveragesd | 0.95 (1.02) | 0.95 (1.02) | 0.97 (1.04) | 0.96 (1.00) |
| Ready-to-eat/-heat mixed dishesd | 0.21 (0.15) | 0.21 (0.15) | 0.22 (0.15) | 0.22 (0.14) |
| Meat-, poultry-, seafood-based ready-to-eat productsd | 0.23 (0.20) | 0.23 (0.20) | 0.24 (0.21) | 0.24 (0.21) |
| Packaged savory snacksd | 0.25 (0.36) | 0.25 (0.36) | 0.26 (0.36) | 0.26 (0.36) |
| Yogurt and dairy-based dessertsd | 0.26 (0.23) | 0.26 (0.23) | 0.26 (0.23) | 0.25 (0.22) |
All variables were adjusted for age except for age itself. Because there are synchronous conventional adenomas and serrated lesions, the sum of the number of nonpolyps and polyps is not equal to the overall number. BMI = body mass index; HPFS = Health Professionals Follow-up Study; MET = metabolic equivalent tasks; NHS = Nurses’ Health Study; NHS II = the Nurses’ Health Study II.
Calculated among women.
Western dietary patten score was derived from principal component analysis.
Energy adjusted servings per day.
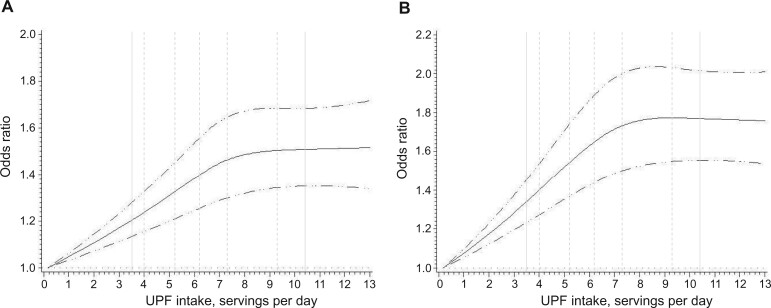
The dose-response analysis showed a nonlinear relationship for both conventional adenomas and serrated polyps (Pnonlinearity less than .0001), with the risk elevation peaked at roughly 8 servings per day and no further increase observed at higher intake (Figure 1). Compared with those who consumed UPFs in the lowest quintile (Q1), those in the highest quintile (Q5) had an 18% increased risk of developing conventional adenomas (Model 2: OR = 1.18, 95% CI = 1.11 to 1.26) and a 20% increased risk of developing serrated lesions (OR = 1.20, 95% CI = 1.13 to 1.28) (Table 2). These associations were slightly attenuated but remained statistically significant after further adjusting for BMI (OR = 1.16 for conventional adenomas and 1.15 serrated lesions), Western dietary pattern score (OR = 1.12 for conventional adenomas and 1.14 for serrated lesions), or individual dietary factors (fiber, folate, calcium, and vitamin D) (OR = 1.16 for conventional adenomas and 1.18 for serrated lesions). The results remained essentially unchanged after excluding processed meats from total UPF intake. Also, the associations were similar after categorizing the undetermined food items as UPFs (Supplementary Table 3, available online).
Figure 1.
Dose-response relationship between ultra-processed food (UPF) consumption and conventional adenomas (A) and serrated lesions (B). The dot–dash curves represent the 95% confidence intervals of the odds ratio. The vertical solid lines represent 5th and 95th percentile levels of UPF intake, and the vertical dash lines represent the median UPF intakes in each of the quintiles. Multivariable model was adjusted for the same set of covariates as in Model 2. For conventional adenomas in A, P for nonlinear relation less than .0001 and P for overall statistical significance of the curve less than .0001. For serrated lesions in B, P for nonlinear relation less than .0001 and P for overall statistical significance of the curve less than .0001. UPF intake over 99% was not plotted due to wide confidence intervals at the extremes.
Table 2.
Association between UPF consumption and risk of conventional adenomas and serrated lesions in the 3 cohort studies (NHS, NHS II, HPFS)
| Energy-adjusted servings per day of UPF intake, OR (95% CI)a |
P nonlinearity b | P overall significance b | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |||
| Median intake | 4.0 | 5.2 | 6.2 | 7.3 | 9.3 | ||
| Conventional adenomas | |||||||
| Cases | 2064 | 2347 | 2364 | 2514 | 2355 | ||
| Model 1 | 1 (referent) | 1.05 (0.99 to 1.12) | 1.07 (1.01 to 1.14) | 1.17 (1.10 to 1.24) | 1.17 (1.10 to 1.25) | <.001 | <.001 |
| Model 2 | 1 (referent) | 1.06 (1.00 to 1.13) | 1.08 (1.01 to 1.15) | 1.18 (1.11 to 1.25) | 1.18 (1.11 to 1.26) | <.001 | <.001 |
| Model 2 (UPF excluding processed meat)c | 1 (referent) | 1.09 (1.03 to 1.16) | 1.07 (1.01 to 1.14) | 1.20 (1.13 to 1.28) | 1.18 (1.11 to 1.26) | <.001 | <.001 |
| Model 2 + BMI | 1 (referent) | 1.06 (0.99 to 1.12) | 1.07 (1.00 to 1.13) | 1.16 (1.09 to 1.23) | 1.16 (1.09 to 1.23) | <.001 | <.001 |
| Model 2 + Western dietary pattern score | 1 (referent) | 1.04 (0.98 to 1.10) | 1.04 (0.98 to 1.11) | 1.12 (1.06 to 1.20) | 1.12 (1.05 to 1.20) | .002 | <.001 |
| Model 2 + dietary fiber, folate, calcium, and vitamin D | 1 (referent) | 1.05 (0.99 to 1.11) | 1.06 (1.00 to 1.13) | 1.15 (1.08 to 1.23) | 1.16 (1.09 to 1.24) | <.001 | <.001 |
| Serrated lesions | |||||||
| Cases | 1827 | 2113 | 2111 | 2279 | 2148 | ||
| Model 1 | 1 (referent) | 1.06 (1.00 to 1.13) | 1.07 (1.00 to 1.14) | 1.18 (1.11 to 1.26) | 1.20 (1.13 to 1.28) | <.001 | <.001 |
| Model 2 | 1 (referent) | 1.08 (1.02 to 1.16) | 1.10 (1.03 to 1.17) | 1.21 (1.13 to 1.29) | 1.20 (1.13 to 1.28) | <.001 | <.001 |
| Model 2 (UPF excluding processed meat) | 1 (referent) | 1.13 (1.06 to 1.20) | 1.09 (1.02 to 1.17) | 1.23 (1.15 to 1.31) | 1.22 (1.14 to 1.30) | <.001 | <.001 |
| Model 2 + BMI | 1 (referent) | 1.07 (1.01 to 1.14) | 1.07 (1.01 to 1.14) | 1.17 (1.10 to 1.25) | 1.15 (1.08 to 1.23) | <.001 | <.001 |
| Model 2 + Western dietary pattern score | 1 (referent) | 1.06 (0.99 to 1.13) | 1.06 (0.99 to 1.13) | 1.15 (1.08 to 1.23) | 1.14 (1.07 to 1.22) | <.001 | <.001 |
| Model 2 + dietary fiber, folate, calcium, and vitamin D | 1 (referent) | 1.07 (1.00 to 1.14) | 1.07 (1.01 to 1.15) | 1.18 (1.11 to 1.26) | 1.18 (1.11 to 1.26) | <.001 | <.001 |
Model 1 was adjusted for age (years), race (Caucasian or non-Caucasian), cohort (NHS, NHS II, or HPFS), time period of endoscopy (in 2-year intervals), number of prior endoscopies (continuous), and time in years since the most recent endoscopy (continuous). CI = confidence interval; BMI = body mass index; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; NHS II = Nurses’ Health Study II; OR = odds ratio; UPF = ultra-processed food.
Model 2 was further adjusted for family history of colorectal cancer (yes or no), total alcohol intake (in g/d, <5, 5-10, 10-15, 15-30, or ≥30), physical activity (in metabolic equivalent-h/wk; <3, 3-9, 9-18, 18-27, or ≥27), smoking status and pack-years of smoking (never, past smoker with pack-years <5, past smoker with pack-years ≥5, current smoker with pack-years <20, current smoker with pack-years ≥20), regular aspirin use (yes or no), and additionally for menopausal status (yes or no) and postmenopausal hormone use (never or ever) in women.
The other models were further adjusted for BMI (continuous), Western dietary pattern score (continuous), or individual dietary factors (quintiles), respectively.
Derived from the restricted cubic spline analysis.
UPF items excluding bacon, beef, pork hotdogs; chicken or turkey hotdogs; salami, bologna, processed meat sandwiches; processed meats, sausages.
In the subsite analysis, we found that the odds ratio comparing Q5 with Q1 of UPF intake was statistically higher for distal colon polyps (Model 2: 1.26, 95% CI = 1.18 to 1.35) than proximal colon polyps (1.13, 95% CI = 1.05 to 1.21) (Pheterogeneity = .01) (Table 3). When analyzed by malignant potential, a higher intake of UPFs was associated with both high-risk (OR for Q5 vs Q1 = 1.17, 95% CI = 1.07 to 1.28) and low-risk polyps (OR for Q5 vs Q1 = 1.19, 95% CI = 1.13 to 1.26; Pheterogeneity = .47).
Table 3.
Association between UPF consumption and risk of colorectal polyps according to polyp features in the 3 cohort studies (NHS, NHS II, HPFS)a
| Energy-adjusted servings per day of UPF intake, OR (95% CI)a |
P nonlinearity b | P overall significance b | P heterogeneity c | |||||
|---|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||||
| Median intake | 4.0 | 5.2 | 6.2 | 7.3 | 9.3 | |||
| Anatomic subsite | ||||||||
| Proximal colon | ||||||||
| Cases | 1613 | 1720 | 1791 | 1867 | 1753 | |||
| Model 1 | 1 (referent) | 0.98 (0.92 to 1.05) | 1.03 (0.96 to 1.11) | 1.10 (1.03 to 1.18) | 1.12 (1.04 to 1.20) | .001 | <.001 | |
| Model 2 | 1 (referent) | 0.99 (0.92 to 1.06) | 1.04 (0.97 to 1.11) | 1.11 (1.04 to 1.19) | 1.13 (1.05 to 1.21) | .001 | <.001 | |
| Distal colon | ||||||||
| Cases | 1664 | 1971 | 1964 | 2134 | 2045 | |||
| Model 1 | 1 (referent) | 1.10 (1.03 to 1.17) | 1.10 (1.03 to 1.18) | 1.23 (1.15 to 1.31) | 1.26 (1.18 to 1.35) | <.001 | <.001 | .01 |
| Model 2 | 1 (referent) | 1.11 (1.04 to 1.19) | 1.12 (1.05 to 1.20) | 1.24 (1.16 to 1.33) | 1.26 (1.18 to 1.35) | <.001 | <.001 | .01 |
| Rectum | ||||||||
| Cases | 971 | 1155 | 1081 | 1201 | 1094 | |||
| Model 1 | 1 (referent) | 1.09 (1.00 to 1.19) | 1.03 (0.94 to 1.12) | 1.17 (1.07 to 1.28) | 1.15 (1.05 to 1.25) | .03 | .001 | .79 |
| Model 2 | 1 (referent) | 1.12 (1.03 to 1.22) | 1.05 (0.97 to 1.15) | 1.20 (1.10 to 1.31) | 1.15 (1.05 to 1.25) | .003 | .001 | .85 |
| Feature | ||||||||
| High-risk polypsd | ||||||||
| Cases | 907 | 1002 | 1018 | 1115 | 1030 | |||
| Model 1 | 1 (referent) | 1.03 (0.94 to 1.12) | 1.05 (0.96 to 1.15) | 1.18 (1.08 to 1.29) | 1.16 (1.06 to 1.27) | .01 | <.001 | |
| Model 2 | 1 (referent) | 1.04 (0.95 to 1.14) | 1.07 (0.97 to 1.17) | 1.20 (1.09 to 1.31) | 1.17 (1.07 to 1.28) | .001 | <.001 | |
| Low-risk polyps | ||||||||
| Cases | 2637 | 3030 | 3032 | 3220 | 3058 | |||
| Model 1 | 1 (referent) | 1.06 (1.00 to 1.12) | 1.07 (1.01 to 1.13) | 1.16 (1.10 to 1.23) | 1.19 (1.13 to 1.26) | <.001 | <.001 | .36 |
| Model 2 | 1 (referent) | 1.07 (1.01 to 1.13) | 1.08 (1.02 to 1.14) | 1.18 (1.11 to 1.24) | 1.19 (1.13 to 1.26) | <.001 | <.001 | .47 |
Model 1 was adjusted for age (years), race (Caucasian or non-Caucasian), cohort (NHS, NHS II, or HPFS), time period of endoscopy (in 2-year intervals), number of prior endoscopies (continuous), and time in years since the most recent endoscopy (continuous). CI = confidence interval; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; NHS II = Nurses’ Health Study II; OR = odds ratio; UPF = ultra-processed food.
Model 2 was further adjusted for family history of colorectal cancer (yes or no), total alcohol intake (in g/d, <5, 5-10, 10-15, 15-30, or ≥30), physical activity (in metabolic equivalent-h/wk; <3, 3-9, 9-18, 18-27, or ≥27), smoking status and pack-years of smoking (never, past smoker with pack-years <5, past smoker with pack-years ≥5, current smoker with pack-years <20, current smoker with pack-years ≥20), regular aspirin use (yes or no), and additionally for menopausal status (yes or no) and postmenopausal hormone use (never or ever) in women.
Derived from the restricted cubic spline analysis.
P heterogeneity between odds ratio for Q5 vs Q1 was calculated through case-only analysis (distal colon or rectum vs proximal colon; high-risk vs low-risk).
High-risk polyps included advanced conventional adenomas (defined as at least 1 adenoma ≥10-mm diameter or any size with tubulovillous, villous, or high-grade dysplasia) and large serrated lesions (≥10 mm).
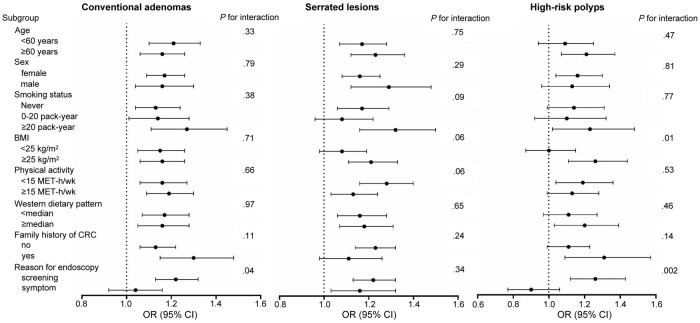
In the stratified analysis, the association between UPF consumption and high-risk polyps appeared stronger in those with a BMI 25 kg/m2 or greater vs less than 25 kg/m2 (Pinteraction = .01) (Figure 2). Also, the associations for conventional adenomas and high-risk polyps were stronger in those who had the endoscopy for screening only vs symptoms (Pinteraction = .04 and .002, respectively). No other interactions were statistically significant.
Figure 2.
Stratified analysis for the association between ultra-processed food (UPF) consumption and colorectal polyp risk. Odds ratios (OR) for the highest vs lowest quintiles of UPF consumption were calculated in the multivariable logistic regression model (Model 2). Pinteraction was calculated by comparing the models with and without the product term between UPF consumption (dichotomous) and the stratified variable (categorical). BMI = body mass index; CI = confidence interval; CRC = colorectal cancer; MET = metabolic equivalent tasks; OR = odds ratio; UPF = ultra-processed food.
Among different UPF subgroups (Table 4), a positive association with high-risk polyps was observed for meat-, poultry-, and seafood-based ready-to-eat products (OR for Q5 vs Q1 = 1.20, 95% CI = 1.09 to 1.32); fat, condiment, and sauces (1.18, 95% CI = 1.08 to 1.30); packaged sweet snacks and desserts (1.13, 95% CI = 1.03 to 1.24); and ultra-processed breads and breakfast food (1.13, 95% CI = 1.03 to 1.24).
Table 4.
Multivariable-adjusted associations of UPF subgroups with polyp risk in the 3 cohort studies (NHS, NHS II, HPFS)
| Energy-adjusted servings of UPF subgroup intake, OR (95% CI)a |
P nonlinearity b | P overall significance b | |||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |||
| Ultra-processed breads and breakfast food | |||||||
| Median intake | 0.69 | 1.09 | 1.43 | 1.82 | 2.61 | ||
| Conventional adenomas | 1 (referent) | 1.07 (1.00 to 1.13) | 1.12 (1.05 to 1.19) | 1.15 (1.08 to 1.22) | 1.16 (1.09 to 1.23) | <.001 | <.001 |
| Serrated lesions | 1 (referent) | 1.13 (1.06 to 1.21) | 1.23 (1.16 to 1.31) | 1.20 (1.12 to 1.28) | 1.22 (1.14 to 1.31) | .001 | <.001 |
| High-risk polypsc | 1 (referent) | 1.07 (0.97 to 1.17) | 1.14 (1.04 to 1.25) | 1.10 (1.01 to 1.21) | 1.13 (1.03 to 1.24) | .001 | <.001 |
| Fat, condiment, and sauces | |||||||
| Median intake | 0.48 | 0.87 | 1.22 | 1.69 | 2.68 | ||
| Conventional adenomas | 1 (referent) | 1.07 (1.01 to 1.14) | 1.06 (0.99 to 1.12) | 1.13 (1.06 to 1.20) | 1.15 (1.08 to 1.22) | <.001 | <.001 |
| Serrated lesions | 1 (referent) | 1.08 (1.01 to 1.16) | 1.11 (1.04 to 1.19) | 1.21 (1.13 to 1.29) | 1.24 (1.16 to 1.32) | .004 | <.001 |
| High-risk polyps | 1 (referent) | 1.00 (0.91 to 1.10) | 1.11 (1.01 to 1.22) | 1.10 (1.01 to 1.21) | 1.18 (1.08 to 1.30) | <.001 | <.001 |
| Packaged sweet snacks and desserts | |||||||
| Median intake | 0.32 | 0.59 | 0.83 | 1.14 | 1.82 | ||
| Conventional adenomas | 1 (referent) | 1.01 (0.95 to 1.07) | 1.02 (0.96 to 1.09) | 1.07 (1.01 to 1.13) | 1.11 (1.04 to 1.18) | <.001 | <.001 |
| Serrated lesions | 1 (referent) | 1.10 (1.03 to 1.17) | 1.09 (1.02 to 1.16) | 1.10 (1.03 to 1.17) | 1.12 (1.05 to 1.19) | <.001 | <.001 |
| High-risk polyps | 1 (referent) | 1.02 (0.93 to 1.11) | 1.03 (0.94 to 1.13) | 1.06 (0.97 to 1.16) | 1.13 (1.03 to 1.24) | .002 | <.001 |
| Beverages | |||||||
| Median intake | 0.08 | 0.32 | 0.62 | 1.06 | 2.19 | ||
| Conventional adenomas | 1 (referent) | 0.99 (0.93 to 1.05) | 1.01 (0.95 to 1.07) | 1.00 (0.94 to 1.07) | 1.00 (0.94 to 1.07) | — | .51 |
| Serrated lesions | 1 (referent) | 1.06 (1.00 to 1.13) | 1.02 (0.96 to 1.09) | 1.05 (0.98 to 1.11) | 1.04 (0.97 to 1.11) | .01 | .002 |
| High-risk polyps | 1 (referent) | 1.00 (0.91 to 1.09) | 1.00 (0.91 to 1.09) | 1.04 (0.95 to 1.14) | 1.03 (0.94 to 1.13) | — | .11 |
| Ready-to-eat/-heat mixed dishes | |||||||
| Median intake | 0.07 | 0.14 | 0.19 | 0.24 | 0.37 | ||
| Conventional adenomas | 1 (referent) | 1.07 (1.00 to 1.13) | 1.05 (0.98 to 1.11) | 1.06 (1.00 to 1.13) | 1.03 (0.97 to 1.10) | <.001 | <.001 |
| Serrated lesions | 1 (referent) | 1.09 (1.02 to 1.16) | 1.08 (1.02 to 1.16) | 1.06 (0.99 to 1.13) | 1.04 (0.98 to 1.12) | .01 | <.001 |
| High-risk polyps | 1 (referent) | 1.05 (0.96 to 1.15) | 1.01 (0.92 to 1.11) | 1.05 (0.96 to 1.15) | 1.03 (0.94 to 1.14) | .02 | .10 |
| Meat-, poultry-, seafood-based ready-to-eat products | |||||||
| Median intake | 0.04 | 0.11 | 0.19 | 0.28 | 0.49 | ||
| Conventional adenomas | 1 (referent) | 1.07 (1.01 to 1.13) | 1.06 (1.00 to 1.13) | 1.05 (0.99 to 1.12) | 1.08 (1.01 to 1.15) | .02 | .001 |
| Serrated lesions | 1 (referent) | 1.12 (1.05 to 1.19) | 1.14 (1.07 to 1.22) | 1.11 (1.04 to 1.18) | 1.13 (1.06 to 1.21) | <.001 | <.001 |
| High-risk polyps | 1 (referent) | 1.11 (1.02 to 1.22) | 1.11 (1.02 to 1.22) | 1.17 (1.07 to 1.28) | 1.20 (1.09 to 1.32) | <.001 | <.001 |
| Packaged savory snacks | |||||||
| Median intake | 0.03 | 0.08 | 0.15 | 0.26 | 0.52 | ||
| Conventional adenomas | 1 (referent) | 1.04 (0.97 to 1.10) | 1.09 (1.02 to 1.16) | 1.11 (1.04 to 1.18) | 1.09 (1.03 to 1.16) | <.001 | <.001 |
| Serrated lesions | 1 (referent) | 1.12 (1.04 to 1.19) | 1.10 (1.03 to 1.17) | 1.15 (1.08 to 1.23) | 1.11 (1.04 to 1.19) | <.001 | <.001 |
| High-risk polyps | 1 (referent) | 0.99 (0.91 to 1.09) | 1.04 (0.95 to 1.14) | 1.06 (0.97 to 1.16) | 1.00 (0.91 to 1.10) | — | .76 |
| Yogurt and dairy-based desserts | |||||||
| Median intake | 0.05 | 0.11 | 0.19 | 0.31 | 0.54 | ||
| Conventional adenomas | 1 (referent) | 1.07 (1.01 to 1.14) | 1.06 (1.00 to 1.13) | 1.11 (1.04 to 1.18) | 1.07 (1.01 to 1.14) | .02 | .002 |
| Serrated lesions | 1 (referent) | 1.08 (1.02 to 1.15) | 1.08 (1.01 to 1.15) | 1.09 (1.03 to 1.17) | 0.99 (0.92 to 1.05) | .01 | .001 |
| High-risk polyps | 1 (referent) | 1.08 (0.99 to 1.19) | 1.03 (0.94 to 1.13) | 1.12 (1.02 to 1.22) | 1.04 (0.95 to 1.14) | .003 | .01 |
| Other UPFs | |||||||
| Median intake | 0.01 | 0.01 | 0.01 | 0.13 | 0.76 | ||
| Conventional adenomas | 1 (referent) | 0.87 (0.82 to 0.93) | 0.90 (0.85 to 0.95) | 1.01 (0.96 to 1.07) | 0.95 (0.90 to 1.01) | — | .77 |
| Serrated lesions | 1 (referent) | 0.88 (0.82 to 0.94) | 0.85 (0.79 to 0.90) | 0.99 (0.93 to 1.05) | 0.97 (0.91 to 1.03) | — | .39 |
| High-risk polyps | 1 (referent) | 0.82 (0.74 to 0.90) | 0.86 (0.79 to 0.94) | 1.00 (0.91 to 1.08) | 0.87 (0.80 to 0.95) | — | .05 |
Adjusted for age (years), race (Caucasian or non-Caucasian), cohort (NHS, NHS II, or HPFS), time period of endoscopy (in 2-year intervals), number of prior endoscopies (continuous), and time in years since the most recent endoscopy (continuous), family history of colorectal cancer (yes or no), total alcohol intake (in g/day, <5, 5-10, 10-15, 15-30, or ≥30), physical activity (in metabolic equivalent-hours/week; <3, 3-9, 9-18, 18-27, or ≥27), smoking status and pack-years of smoking (never, past smoker with pack-years <5, past smoker with pack-years ≥5, current smoker with pack-years <20, current smoker with pack-years ≥20), regular aspirin use (yes or no), and additionally for menopausal status (yes or no) and postmenopausal hormone use (never or ever) in women. The analysis was also mutually adjusted for the individual subgroups. CI = confidence interval; HPFS = Health Professionals Follow-up Study; NHS = Nurses’ Health Study; NHS II = Nurses’ Health Study II; OR = odds ratio; UPF = ultra-processed food.
Derived from the restricted cubic spline analysis. The em-dash denotes not available because no spline variables were selected.
High-risk polyps included advanced conventional adenomas (defined as at least 1 adenoma ≥10-mm diameter or any size with tubulovillous, villous, or high-grade dysplasia) and large serrated lesions (≥10 mm).
Discussion
To the best of our knowledge, this study represents the first effort to prospectively investigate UPF consumption in relation to risk of colorectal premalignant lesions. We found that higher UPF consumption was nonlinearly associated with an increased risk of conventional adenomas and serrated lesions. The results were robust to multivariable adjustment and similar for polyps of different anatomic subsites and malignant potential. Moreover, we identified certain subgroups of UPFs associated with high-risk polyps. These findings lend strong epidemiologic support to the detrimental role of UPFs in the early stages of colorectal carcinogenesis.
In support of our observations, a case-control study conducted in an Israel medical center reported that patients diagnosed with colorectal adenomas had a higher intake of UPFs than controls (23). In addition, a multicentric case-control Spanish study (12) and our recent study within the 3 US cohorts (NHS, NHS II, and HPFS) (13) have linked higher UPF consumption to an increased risk of CRC. The evidence together indicates that UPFs may play a role in the initiation of CRC. Furthermore, we found a stronger association for UPF intake with risk of distal colon polyps than polyps at other subsites. This is consistent with our previous results showing that the positive association between UPF intake and CRC risk was restricted to distal colon cancer (13). The differential associations by subsite might be explained by the anatomic differences in susceptibility to neoplastic transformation (24), in microbial communities and immune niches (25), and in exposures to metabolites that may influence CRC development, such as short-chain fatty acids and bile acids (26). Additionally, we observed a similar association across sex for polyps in contrast with our previous study reporting the association between UPF intake and CRC risk only in men (13). The exact reasons for the inconsistency are unclear. It is possible that UPFs increase the risk of tumor initiation in both men and women, and, once the tumorigenic process is started, other factors such as sex hormones can affect individuals’ susceptibility to tumor progression and thereby influence the outcome of CRC development. This hypothesis needs to be confirmed in future studies.
There is an ongoing debate whether the detrimental effect of UPFs observed in epidemiological studies are primarily driven by poor nutritional quality (27). In this study, the observed association between UPF consumption and colorectal polyps was only slightly attenuated after adjustment for Western dietary pattern or major CRC-related nutrients, including dietary fiber, folate, calcium, and vitamin D. These results indicate that poor diet quality cannot fully explain the association of UPF with higher risk of colorectal neoplasia. Furthermore, UPF consumption has been linked to an increased risk of weight gain and obesity (28,29), which is an established risk factor for colorectal polyps (17). Adjustment for BMI showed a minimal impact on the association between UPFs and polyps, suggesting that the adverse effect of UPFs on colorectal carcinogenesis is largely independent of adiposity.
Beyond nutritional composition, UPFs commonly contain food additives such as emulsifiers, preservatives, colors, and flavors that may account for the potential carcinogenicity of UPFs. For example, animal studies have demonstrated that 2 commonly used emulsifiers—carboxymethylcellulose and polysorbate-80—can increase the proinflammation potential of the microbiome (7,30) and alter intestinal expression of genes involved in proliferation and apoptosis, thereby initiating colonic carcinogenesis (7). Increasing evidence also suggests that sodium nitrite, known as a preservative and coloring substance in processed meats, may increase the risk of colorectal neoplasia (31,32). In addition, allura red, a sulfonated mono azo dye widely used in foods, can induce inflammation and DNA damage in the colon of mice (33,34). In an animal study, administration of monosodium glutamate, a popular food flavoring agent, was able to produce obese and diabetic mice highly susceptible to azoxymethane-induced colorectal carcinogenesis (35). Notably, we found positive associations between certain UPF subgroups and high-risk polyps, namely, meat-, poultry-, and seafood-based ready-to-eat products; fat, condiment, and sauces, packaged sweet snacks and desserts; and ultra-processed breads and breakfast food. Many food items included in these subgroups contain considerable amounts of the above-mentioned additives. For example, margarine, mayonnaise, soy sauce, and cake are rich in emulsifiers, and snacks, desserts, breakfast cereals, and bakery goods are rich in coloring and flavoring agents (36). Although food additives are approved for human consumption after toxicological studies and safety assessment, the health impact of long-term exposure to such chemicals and their combined effect remain largely unknown and warrant attention.
Major strengths of this study include the prospective design with a high follow-up rate, detailed and repeated measurement of diet and other covariates to minimize measurement errors and reduce residual confounding, and confirmed diagnosis of different subtypes of colorectal polyps with detailed histopathological data through central medical record review. Moreover, the large number of colorectal polyp cases enabled us to examine the association between UPFs and CRC precursors by anatomic subsites and malignant potential with sufficient statistical power. Several limitations need to be acknowledged as well. First, as in any observational study, unmeasured confounders cannot be ruled out despite robust adjustment for established risk factors. Second, because colorectal polyps are usually asymptomatic and cannot be diagnosed until an endoscopic exam, this study was restricted to participants who had undergone endoscopy. Although this raises concerns about selection bias, the similar consumption of UPF intake in this study and in the overall cohorts indicates little influence of selection bias on our findings. Third, the FFQs are unable to cover the full spectrum of UPF consumption. Also, the FFQs used in the cohorts were not specifically designed to classify foods according to the extent of processing; however, given the prospective design, nondifferential misclassification of the exposure likely could have biased our results toward the null. Finally, the study participants were all health professionals and predominantly White, which may limit the generalizability of our findings. However, the homogeneity of our study population reduces the likelihood of uncontrolled confounding. There are no prior data indicating that UPFs have different carcinogenic effects according to race and ethnicity. Nevertheless, we acknowledge the need for further studies in more diverse study populations.
In conclusion, our study indicates that high intake of UPFs and certain subgroups is independently associated with an increased risk of conventional adenomas and serrated lesions. Future studies are necessary to confirm our findings, elucidate the underlying mechanisms, and determine the potential of limiting UPF consumption for CRC prevention.
Supplementary Material
Contributor Information
Dong Hang, Department of Epidemiology, Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, School of Public Health, Gusu School, Nanjing Medical University, Nanjing, China; Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Lu Wang, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Zhe Fang, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Mengxi Du, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Kai Wang, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Xiaosheng He, Department of Colorectal Surgery, the Six Affiliated Hospital, Sun Yat-sen University, Guangzhou, China.
Neha Khandpur, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Nutrition, School of Public Health, University of São Paulo, São Paulo, Brazil; Center for Epidemiological Studies in Health and Nutrition (NUPENS), Faculty of Public Health, University of São Paulo, Brazil.
Sinara L Rossato, Department of Nutrition, School of Public Health, University of São Paulo, São Paulo, Brazil; Institute of Geography, Universidade Federal de Uberlândia, Minas Gerais, Brazil.
Kana Wu, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Zhibin Hu, Department of Epidemiology, Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, School of Public Health, Gusu School, Nanjing Medical University, Nanjing, China.
Hongbing Shen, Department of Epidemiology, Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, School of Public Health, Gusu School, Nanjing Medical University, Nanjing, China.
Shuji Ogino, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, MA, USA; Cancer Immunology Program, Dana-Farber Harvard Cancer Center, Boston, MA, USA; Program in MPE Molecular Pathological Epidemiology, Department of Pathology, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Andrew T Chan, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; Clinical and Translational Epidemiology Unit and Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Edward L Giovannucci, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Fang Fang Zhang, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
Mingyang Song, Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Clinical and Translational Epidemiology Unit and Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Funding
This work was supported by grant MRSG-17-220-01-NEC from the American Cancer Society (MS), grants UM1 CA186107, P01 CA87969, U01 CA176726, U01 CA167552, R00 CA215314 (MS), R35 CA253185 (ATC), R03CA197879 (KW), R21CA230873 (KW), and R21CA222940 (KW) from the National Institutes of Health, grant 81973127 from the National Natural Science Foundation of China (DH), and grant BK20190083 from the Natural Science Foundation of Jiangsu Province (DH). ATC is an American Cancer Society Clinical Research Professor and Stuart and Suzanne Steele MGH Research Scholar. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Notes
Role of the funder: The founders had no role in the design, conduct, analysis, or reporting of this study. The funding sources did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: None disclosed. MS, a JNCI Associate Editor and coauthor on this article, was not involved in the editorial review or decision to publish the manuscript.
Author contributions: Conceptualization: DH, MS. Data curation: DH, NK, SR, KW, SO, ATC, ELG, MS. Formal analysis: DH. Funding acquisition: MS, KW, ATC, DH. Writing—original draft: DH. Writing—review & editing: LW, ZF, MD, KW, XH, NK, SR, KW, ZH, HS, SO, ATC, ELG, FZ, MS. Resources: SO, ATC, ELG, MS. Supervision: MS.
Acknowledgements: The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming.
Data availability
Because of participant confidentiality and privacy concerns, data are available upon reasonable written request. According to standard controlled access procedure, applications to use NHS/NHS II/HPFS resources will be reviewed by our External Collaborators Committee for scientific aims, evaluation of the fit of the data for the proposed methodology, and verification that the proposed use meets the guidelines of the Ethics and Governance Framework and the consent that was provided by the participants. Investigators wishing to use NHS/NHS II/HPFS data are asked to submit a brief description of the proposed project (go to https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/ for details).
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2. De Palma F, D’Argenio V, Pol J, et al. The molecular hallmarks of the serrated pathway in colorectal cancer. Cancers (Basel). 2019;11(7):1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Srour B, Fezeu LK, Kesse-Guyot E, et al. Ultraprocessed food consumption and risk of type 2 diabetes among participants of the NutriNet-Sante prospective cohort. JAMA Intern Med. 2020;180(2):283-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monteiro CA, Cannon G, Moubarac JC, et al. The UN decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;21(1):5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luiten CM, Steenhuis IH, Eyles H, et al. Ultra-processed foods have the worst nutrient profile, yet they are the most available packaged products in a sample of New Zealand supermarkets--CORRIGENDUM. Public Health Nutr. 2016;19(3):539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181-186. [DOI] [PubMed] [Google Scholar]
- 7. Viennois E, Merlin D, Gewirtz AT, et al. Dietary emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 2017;77(1):27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jagerstad M, Skog K.. Genotoxicity of heat-processed foods. Mutat Res. 2005;574(1-2):156-172. [DOI] [PubMed] [Google Scholar]
- 9. Bouvard V, Loomis D, Guyton KZ, et al. ; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599-1600. [DOI] [PubMed] [Google Scholar]
- 10. Barreau F, Tisseyre C, Menard S, et al. Titanium dioxide particles from the diet: involvement in the genesis of inflammatory bowel diseases and colorectal cancer. Part Fibre Toxicol. 2021;18(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiolet T, Srour B, Sellem L, et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Sante prospective cohort. BMJ. 2018;360:k322- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Romaguera D, Fernandez-Barres S, Gracia-Lavedan E, et al. Consumption of ultra-processed foods and drinks and colorectal, breast, and prostate cancer. Clin Nutr. 2021;40(4):1537-1545. [DOI] [PubMed] [Google Scholar]
- 13. Wang L, Du M, Wang K, et al. Association of ultra-processed food consumption with colorectal cancer risk among men and women: results from three prospective US cohort studies. BMJ. 2022;378:e068921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327-334. [DOI] [PubMed] [Google Scholar]
- 15. Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three nurses' health studies. Am J Public Health. 2016;106(9):1573-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khandpur N, Rossato S, Drouin-Chartier JP, et al. Categorising ultra-processed foods in large-scale cohort studies: evidence from the Nurses' Health Studies, the Health Professionals Follow-up Study, and the Growing Up Today Study. J Nutr Sci. 2021;10:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He X, Wu K, Ogino S, et al. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology. 2018;155(2):355-373.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844-857. [DOI] [PubMed] [Google Scholar]
- 19. Andersen PK, Gill RD.. Cox's regression model for counting processes: a large sample study. Ann Statist. 1982;10(4):1100-1120. [Google Scholar]
- 20. Lo CH, Khandpur N, Rossato SL, et al. Ultra-processed foods and risk of Crohn's disease and ulcerative colitis: a prospective cohort study. Clin Gastroenterol Hepatol. 2022;20(6):e1323-e1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Durrleman S, Simon R.. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551-561. [DOI] [PubMed] [Google Scholar]
- 22. Martinez Steele E, Baraldi LG, Louzada ML, et al. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016;6(3):e009892- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fliss-Isakov N, Zelber-Sagi S, Ivancovsky-Wajcman D, et al. Ultra-processed food intake and smoking interact in relation with colorectal adenomas. Nutrients. 2020;12(11):3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113(10):779-788. [DOI] [PubMed] [Google Scholar]
- 25. James KR, Gomes T, Elmentaite R, et al. Distinct microbial and immune niches of the human colon. Nat Immunol. 2020;21(3):343-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macfarlane GT, Gibson GR, Cummings JH.. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol. 1992;72(1):57-64. [DOI] [PubMed] [Google Scholar]
- 27. Dicken SJ, Batterham RL.. The role of diet quality in mediating the association between ultra-processed food intake, obesity and health-related outcomes: a review of prospective cohort studies. Nutrients. 2021;14(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67-77.e3. e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moradi S, Entezari MH, Mohammadi H, et al. Ultra-processed food consumption and adult obesity risk: a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr. 2022;63(2):249-260. [DOI] [PubMed] [Google Scholar]
- 30. Naimi S, Viennois E, Gewirtz AT, et al. Direct impact of commonly used dietary emulsifiers on human gut microbiota. Microbiome. 2021;9(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santarelli RL, Vendeuvre JL, Naud N, et al. Meat processing and colon carcinogenesis: cooked, nitrite-treated, and oxidized high-heme cured meat promotes mucin-depleted foci in rats. Cancer Prev Res (Phila). 2010;3(7):852-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crowe W, Elliott CT, Green BD.. A review of the in vivo evidence investigating the role of nitrite exposure from processed meat consumption in the development of colorectal cancer. Nutrients. 2019;11(11):2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsuda S, Murakami M, Matsusaka N, et al. DNA damage induced by red food dyes orally administered to pregnant and male mice. Toxicol Sci. 2001;61(1):92-99. [DOI] [PubMed] [Google Scholar]
- 34. Hofseth LJ, Hebert JR, Chanda A, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17(6):352-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hata K, Kubota M, Shimizu M, et al. Monosodium glutamate-induced diabetic mice are susceptible to azoxymethane-induced colon tumorigenesis. Carcinogenesis. 2012;33(3):702-707. [DOI] [PubMed] [Google Scholar]
- 36. Soni VK, Amit A, Chandra V, et al. Role of food additives and intestinal microflora in colorectal cancer. In: Shukla D, Vishvakarma NK, Nagaraju GP, editors. Colon Cancer Diagnosis and Therapy. Vol. 3. Cham: Springer International Publishing; 2022:307-324. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Because of participant confidentiality and privacy concerns, data are available upon reasonable written request. According to standard controlled access procedure, applications to use NHS/NHS II/HPFS resources will be reviewed by our External Collaborators Committee for scientific aims, evaluation of the fit of the data for the proposed methodology, and verification that the proposed use meets the guidelines of the Ethics and Governance Framework and the consent that was provided by the participants. Investigators wishing to use NHS/NHS II/HPFS data are asked to submit a brief description of the proposed project (go to https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/ for details).