Abstract
Background
Breast cancers (BCs) that arise in individuals heterozygous for a germline pathogenic variant in a susceptibility gene, such as BRCA1 and BRCA2, PALB2, and RAD51C, have been shown to exhibit biallelic loss in the respective genes and be associated with triple-negative breast cancer (TNBC) and distinctive somatic mutational signatures. Tumor sequencing thus presents an orthogonal approach to assess the role of candidate genes in BC development.
Methods
Exome sequencing was performed on paired normal-breast tumor DNA from 124 carriers of germline loss-of-function (LoF) or missense variant carriers in 15 known and candidate BC predisposition genes identified in the BEACCON case-control study. Biallelic inactivation and association with tumor genome features including mutational signatures and homologous recombination deficiency (HRD) score were investigated.
Results
BARD1-carrying TNBC (4 of 5) displayed biallelic loss and associated high HRD scores and mutational signature 3, as did a RAD51D-carrying TNBC and ovarian cancer. Biallelic loss was less frequent in BRIP1 BCs (4 of 13) and had low HRD scores. In contrast to other established BC genes, BCs from carriers of CHEK2 LoF (6 of 17) or missense (2 of 20) variant had low rates of biallelic loss. Exploratory analysis of BC from carriers of LoF variants in candidate genes such as BLM, FANCM, PARP2, and RAD50 found little evidence of biallelic inactivation.
Conclusions
BARD1 and RAD51D behave as classic BRCA-like predisposition genes with biallelic inactivation, but this was not observed for any of the candidate genes. However, as demonstrated for CHEK2, the absence of biallelic inactivation does not provide definitive evidence against the gene’s involvement in BC predisposition.
Hereditary breast cancer (HBC) often clusters within families and can be attributed to germline variants in susceptibility genes directly or indirectly involved in DNA repair. The major contributors—BRCA1, BRCA2, and PALB2 (1,2)—collectively explain less than half of the familial aggregation of BC (3). Exploratory case-control studies in the past have found that potentially pathogenic variants in individual candidate genes are rare (3-5), precluding any confident conclusion about their role in HBC based solely on this approach.
An orthogonal approach to assess if a candidate gene is driving tumorigenesis is through genomic analysis of the cancers from carriers of germline mutations. For example, approximately 90% of BRCA1 and 50%-60% of BRCA2 breast tumors from germline mutation carriers have a somatic “second-hit” (6-9), resulting in biallelic inactivation. Most commonly, this occurs through loss of heterozygosity (LOH) or, less frequently, through protein truncating somatic point mutations or promoter hypermethylation. Biallelic inactivation of genes such as BRCA1 and BRCA2 is almost invariably associated with specific somatic mutational signatures (10). The presence or absence of these tumor genomic features can provide strong evidence for or against a gene’s cancer predisposition role, even if based on relatively few cancers as previously demonstrated for PALB2, RAD51C, and ATM (11-13). Recent large case-control studies involving more than 65 000 participants each confirmed the association of moderate risk genes RAD51C, RAD51D, and BARD1 but not BRIP1 with breast cancer (4,5).
In this study, we extend the tumor sequencing approach by performing exome sequencing on 124 BCs from individuals harboring germline variants in proposed and candidate HBC genes identified in the BEACCON case-control study (hereditary BrEAst Case CONtrol study) (3) to look for evidence of biallelic inactivation as a means of validating the role of these genes in BC predisposition.
Methods
Case-control study and tumor sequencing
A total of 124 unique breast tumors were selected from cancers arising in individuals with a germline loss-of-function (LoF) or rare, likely pathogenic missense (MS) variant of interest in a known (BARD1, BRIP1, CHEK2, and RAD51D) or candidate (BLM, CDK9, CTH, ERCC5, FANCM, MUTYH, PARP2, RAD50, RAD51B, WRN, and XRCC2) BC predisposition gene detected in the BEACCON case-control study (3). LoF variants included stop-gained, frame-shift, or essential splice-site variants, and MS variants of interest were identified based on a combination of population frequency, in silico prediction, and location in key functional domains as detailed in Supplementary Tables 1-4 (available online). Two ovarian cancers from carriers of BRIP1 and 1 of RAD51D LoF variants, respectively, were also included as these genes are known to be ovarian cancer predisposing genes. Since last reporting (5), this study has been expanded to include 6689 BRCA-negative female index familial BC patients and 14 381 cancer-free female participants (Supplementary Table 5, available online). Candidate genes were selected for this analysis based on an excess of rare coding variants in the case group. Microdissection, DNA extraction, and exome sequencing are described in the Supplementary Methods (available online). Tumor characteristics and personal and family history of the individuals selected for the current study are summarized in Supplementary Table 6 (available online).
Determination of variant allelic status and potential biallelic inactivation
For each tumor, the somatic sequencing data were assessed for the presence of somatic LoF or MS point mutations in the gene of interest as well as the allelic status of the germline variant as described previously (11,12). In summary, locus-specific LOH was determined by tumor variant allele frequency comparisons as adjusted according to estimated tumor purity. All cases had matched germline sequencing data for the gene of interest. Promoter hypermethylation, using targeted Twist Custom Panel methylation sequencing or Sanger sequencing on bisulfite converted DNA, was also assessed for cases where there was no somatic mutation or LOH across the gene of interest. Homologous recombination deficiency (HRD) scores were calculated for each tumor sample using copy number plots as a sum of the occurrence of telomeric allelic imbalances, large-scale state transitions, and homologous recombination deficiency–loss of heterozygosity from copy number plots as described previously (12), where a threshold of an HRD score of 42 or higher is defined as high-HRD (14,15). Mutational signatures were generated against COSMIC v2 catalogue (https://cancer.sanger.ac.uk/signatures/signatures_v2/) using the DeconstructSig package in R (16) on whole-exome sequenced samples.
Statistical analyses
Odds ratios and Fisher exact test (2-sided) were calculated in case-control analyses, with a 2-tailed P value of .05 or less defined as statistically significant. Confidence intervals (CIs) were calculated using a conditional maximum likelihood estimate. All calculations were carried out using R-in built function in R 3.3.2 (17).
Results
Tumor sequencing in individuals harboring a germline variant in known BC genes
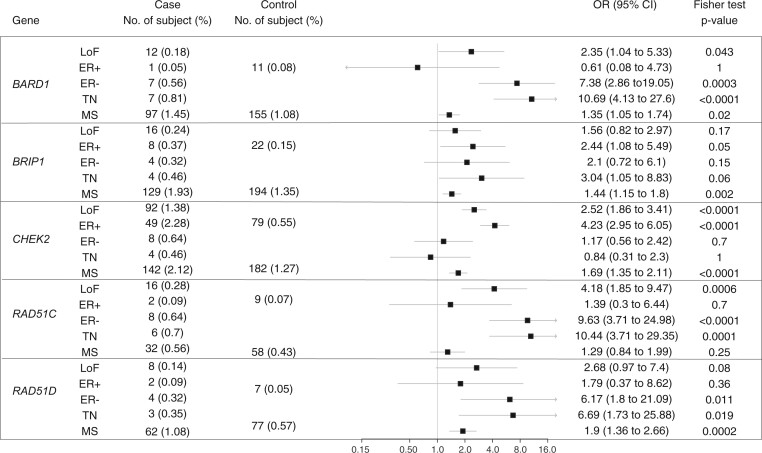
Whole or targeted exome sequencing was performed on 41 tumors from individuals harboring germline LoF variants in genes commonly present in HBC panels: BARD1 (n = 7), BRIP1 (n = 13), RAD51D (n = 4), and CHEK2 (n = 17) (Table 1). BARD1, which in the BEACCON case-control data (Figure 1) and other published data (4) is associated specifically with triple-negative breast cancer (TNBC), showed loss of the wild-type (WT) allele via LOH in 4 of 5 assessable triple negative (TN) tumors. A sixth TN tumor also had LOH, but it was not possible to determine which allele had been lost, and the only BARD1 tumor to show loss of the mutant allele was estrogen receptor (ER) positive. The 6 BARD1 TN tumors including 1 in heterozygous status showed high HRD scores and 4 with strong HRD-related mutational signature 3. Carriers of RAD51D LoF mutations, which are also associated with TNBC, were rare in the BEACCON study. One of the 2 TNBC showed LOH of the WT allele, whereas 2 ER-positive tumors remained heterozygous. An additional high-grade serous ovarian cancer (HGSOC) that was available for analysis (Supplementary Table 5, available online) showed biallelic inactivation through LOH. Nine carriers of rare RAD51D MS variants shortlisted based on likely pathogenicity assessment (Supplementary Table 1, available online) were also analyzed, but only 2 cases showed loss of the WT allele with only 1 of these being a TNBC. This case was a compound heterozygote that showed loss of the p.Ala313Val and retention of the p.Ala52Val allele; it had a high HRD score and a strong mutational signature 3.
Table 1.
Tumor sequencing data for 69 tumors from individuals heterozygous for a germline LoF (n = 41) or MS (n = 29) variant in a known breast cancer predisposition gene (BARD1, BRIP1, CHEK2, and RAD51D)
| IDa | Gene | Germline variant | Variant type | Variant allelic statusb | BC subtype | HRD | Somatic TP53 | Somatic PIK3CA | Mutation signature 3c | Dominant mutation signature | Promoter hypermethylation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3530 | BARD1 | c.1135A>T, p.Lys379Ter | LoF | Mutant loss | ER+/HER2- | na | na | na | na | na | Failed |
| 3977 | BARD1 | c.1212C>G, p.Tyr404Ter | LoF | WT loss | TN | 50 | na | na | Strong | 3, 11 | DNT |
| 1531 | BARD1 | c.1652C>G, p.Ser551Ter | LoF | WT loss | TN | 83 | LoF | — | Weak | 19, 30 | DNT |
| 3828 | BARD1 | c.1652C>G, p.Ser551Ter | LoF | WT loss | TN | 92 | MS | — | Strong | 1, 3 | DNT |
| 425 | BARD1 | c.1652C>G, p.Ser551Ter | LoF | LOH | TN | 80 | na | na | Strong | na | |
| 3496 | BARD1 | c.1905G>A, p.Trp635Ter | LoF | Het | TN | 82 | MS | — | Strong | 3 | Failed |
| 1272 | BARD1 | c.2078_2079insTAATA, p.Lys693AsnfsTer23 | LoF | WT loss | TN | 76 | LoF | — | No | 19 | DNT |
| 2439 | BRIP1 | c.93 + 1G>T | LoF | WT loss | TN | 59 | LoF | — | Strong | 3, 19 | DNT |
| 4160 | BRIP1 | c.103G>T, p.Gly35Ter | LoF | Mutant loss | ER-/HER2+ | 30 | MS | — | Strong | 1, 3 | na |
| 3259 | BRIP1 | c.1426del, p.Thr476LeufsTer50 | LoF | WT loss | ER+/HER2- | 41 | — | MS | No | 12, 20 | DNT |
| 3597 | BRIP1 | c.1888dup, p.Thr630AsnfsTer9 | LoF | Het | ER+/HER2- | 12 | — | MS | No | 30 | Negative |
| 3093 | BRIP1 | c.2298_2301delTGAG, p.Ser766ArgfsTer14 | LoF | Het | ER+/HER2- | 5 | — | MS | na | na | na |
| 227 | BRIP1 | c.2392C>T, p.Arg798Ter | LoF | WT loss | TN | 58 | MS | — | No | 20, 21 | DNT |
| 1325 | BRIP1 | c.2392C>T, p.Arg798Ter | LoF | Mutant loss | TN | 57 | LoF | — | na | na | na |
| 786 | BRIP1 | c.2392C>T, p.Arg798Ter | LoF | Het | ER+/HER2- | 1 | LoF | — | na | na | Failed |
| 1928 | BRIP1 | c.2392C>T, p.Arg798Ter | LoF | Het | ER+/HER2- | 11 | MS | — | na | na | Negative |
| 3829 | BRIP1 | c.2400C>G, p.Tyr800Ter | LoF | Mutant loss | TN | 50 | — | — | Weak | 6 | na |
| 3635 | BRIP1 | c.2400C>G, p.Tyr800Ter | LoF | Het | ER+/HER2- | 32 | LoF | — | Weak | 1 | na |
| 3354 | BRIP1 | c.2492_2492 + 5delGGTAAG | LoF | WT loss | ER+/HER2- | 31 | MS | MS | Weak | 1 | DNT |
| 3468 | BRIP1 | c.3715del, p.Ser1239ProfsTer15 | LoF | Mutant loss | ER+/HER2- | 37 | LoF | — | Weak | 1, 13 | na |
| 4152 | CHEK2 | c.629_732delCAGT, p.Ser210PhefsTer6 | LoF | Mutant loss | ER+/HER2- | 47 | MS | — | No | 3 | Negative |
| 2320 | CHEK2 | c.630delA, p.Val211PhefsTer6 | LoF | Het | na | 7 | — | — | Weak | 5, 30 | na |
| 290 | CHEK2 | c.902delT, p.Leu301TrpfsTer3 | LoF | Mutant loss | ER+/HER2- | 27 | — | — | Weak | 1,6 | na |
| 3587 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | WT loss | ER+/HER2- | 34 | na | na | No | 11 | DNT |
| 1825 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | Het | ER+/HER2- | 32 | LoF | — | Strong | 3 | na |
| 3174 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | Het | ER+/HER2- | 29 | — | — | No | 6, 30 | na |
| 2182 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | Het | ER+/HER2- | 45 | — | — | No | 11, 19 | Failed |
| 2410 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | Het | ER+/HER2- | 27 | — | — | No | 1, 30 | Failed |
| 2475 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | WT loss | ER+/HER2- | 80 | — | — | No | 6, 19 | DNT |
| 1300 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | WT loss | ER+/HER2- | 36 | — | MS | No | 10 | Failed |
| 2326 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | WT loss | ER+/HER2- | 21 | — | — | No | 19, 30 | Failed |
| 2711 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | WT loss | ER+/HER2+ | 10 | — | — | No | 1, 11 | Failed |
| 3500 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | Het | ER+/HER2unknown | 37 | — | — | Strong | 3 | na |
| 2351 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | Het | ER-/HER2+ | 4 | na | na | Weak | 19, 30 | DNT |
| 3076 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | Het | ER-/HER2+ | 1 | — | — | No | 1 | na |
| 1732 | CHEK2 | c.1100delC, p.Thr367MetfsTer15 | LoF | WT Loss | TN | 17 | MS | MS | No | 6 | DNT |
| 1853 | CHEK2 | c.1696delC, p.Thr533GlnfsTer33 | LoF | Het | ER+/HER2- | 28 | MS | — | na | na | na |
| 2625 | CHEK2 | c.14C>T, p.Ser5Leu | MS | Het | ER+/HER2- | 85 | — | — | Weak | 19 | Failed |
| 1993 | CHEK2 | c.190G>A, p.Glu64Lys | MS | Het | ER+/HER2- | 91 | LoF | — | Weak | 1, 6 | na |
| 811 | CHEK2 | c.349A>G, p.Arg117Gly | MS | Het | ER+/HER2+ | 0 | — | MS | Weak | 5 | Negative |
| 1103 | CHEK2 | c.349A>G, p.Arg117Gly | MS | Mutant loss | ER+/HER2- | 16 | — | MS | Weak | 3 | Negative |
| 616 | CHEK2 | c.349A>G, p.Arg117Gly | MS | Het | ER-/HER2+ | 17 | — | MS | Strong | 1, 3 | na |
| 787 | CHEK2 | c.442A>G, p.Arg148Gly | MS | Het | ER+/HER2- | na | na | na | na | na | Failed |
| 2531 | CHEK2 | c.470T>C, p.Ile157Thr | MS | Het | ER+/HER2- | 34 | — | — | Weak | 19 | Negative |
| 1420 | CHEK2 | c.470T>C, p.Ile157Thr | MS | Het | ER+/HER2- | 28 | MS | MS | No | 6 | Negative |
| 3240 | CHEK2 | c.470T>C, p.Ile157Thr | MS | Het | ER+/HER2- | 16 | — | — | Strong | 3, 6 | na |
| 807 | CHEK2 | c.1036C>T, p.Arg346Cys; c.499G>A, p. Gly167Arg | MS MS |
WT loss; mutant loss | ER+/HER2unknown | 52 | — | — | Weak | 1 | na |
| 2091 | CHEK2 | c.1067C>T, p.Ser356Leu | MS | Het | ER+/HER2- | 47 | — | — | Weak | 1, 19 | Failed |
| 2689 | CHEK2 | c.1211A>G, p.Tyr404Cys | MS | WT loss | ER+/HER2+ | 28 | — | MS | Strong | 3, 30 | DNT |
| 2221 | CHEK2 | c.1270T>C, p.Tyr424His | MS | WT loss | ER+/HER2- | 13 | — | MS | No | 20 | DNT |
| 1830 | CHEK2 | c.1312G>T, p.Asp438Tyr | MS | Het | ER+/HER2- | 17 | — | — | na | na | na |
| 2257 | CHEK2 | c.1312G>T, p.Asp438Tyr | MS | Het | ER+/HER2- | 0 | — | MS | Weak | 6 | Negative |
| 200 | CHEK2 | c.1312G>T, p.Asp438Tyr | MS | Het | na | 5 | — | — | Strong | 3 | na |
| 4164 | CHEK2 | c.1427C>T, p.Thr476Met | MS | Het | ER+/HER2- | 13 | — | — | No | 11, 19 | na |
| 1410 | CHEK2 | c.1447C>T, p.His483Tyr | MS | Mutant loss | ER+/HER2- | 30 | MS | — | na | na | DNT |
| 2345 | CHEK2 | c.1525C>T, p.Pro509Ser | MS | Mutant loss | ER+/HER2+ | 7 | LoF | — | na | na | Negative |
| 1198 | CHEK2 | c.1604G>A, p.Arg535His | MS | Het | TN | 22 | MS | — | No | 6, 30 | Failed |
| 1897 | RAD51D | c.616C>T, p.Arg206Ter | LoF | Het | ER+/HER2- | 4 | — | — | na | na | Negative |
| 2734 | RAD51D | c.754C>T, p.Arg252Ter | LoF | WT loss | TN | 42 | LoF | — | na | na | DNT |
| 2866 | RAD51D | c.808delC, p.His270ThrfsTer2 | LoF | Het | TN | 7 | — | — | na | na | na |
| 3500 | RAD51D | c.863G>A, p.Trp288Ter | LoF | Het | ER+/HER2- | 8 | — | — | Weak | 30 | na |
| 506 | RAD51D | c.26G>C, p.Cys9Ser | MS | Het | ER+/HER2- | 0 | — | — | na | na | na |
| 30 | RAD51D | c.26G>C, p.Cys9Ser | MS | Het | ER+/HER2- | 20 | MS | — | na | na | na |
| 2936 | RAD51D | c.26G>C, p.Cys9Ser | MS | Het | ER+/HER2- | 8 | — | — | No | 5 | DNT |
| 1980 | RAD51D | c.26G>C, p.Cys9Ser | MS | Het | TN | 3 | LoF | — | No | 1 | Negative |
| 2224 | RAD51D | c.137C>G, p.Ser46Cys | MS | Het | ER+/HER2- | 4 | — | — | na | na | Negative |
| 2219 | RAD51D | c.155C>T, p.Ala52Val; c.938C>T, p. Ala313Val | MS compound homozygous | WT loss; mutant loss | TN | 42 | LoF | — | Strong | 3 | Negative |
| 1686 | RAD51D | c.308C>T, p.Ala103Val | MS | Het | TN | 17 | MS | — | na | na | Negative |
| 3095 | RAD51D | c.472A>C, p.Asn158His | MS | Mutant Loss | ER+/HER2+ | 20 | — | — | na | na | na |
| 2606 | RAD51D | c.551T>C, p.Leu184Pro | MS | WT Loss | ER+/HER2- | 11 | MS | — | na | na | DNT |
Subject 3093 carried 2 variants of interest in BRIP1; subject 2219 carried 2 variants in RAD51C; subject 3500 carried a variant of interest in both CHEK2 and RAD51D. “—” signifies feature not present. BC = breast cancer; DNT = did not test; na = not available; ER+ = estrogen receptor—positive breast cancer; HER2- = HER2 negative; HER2+ = HER2 positive; HRD = homologous recombination deficiency score; LoF = loss of function; MS = missense; TN = triple-negative; WT = wild type.
WT loss, somatic loss of the WT allele; mutant loss, somatic loss of the allele carrying the known germline variant; Het, heterozygous. Case 425 showed loss of heterozygosity across the gene regions but unable to determine which allele had been lost.
Proportion of mutational signature 3 (COSMIC v2, assessed on whole-exome sequenced tumors only) above 25% is classified as “strong”, under 25% as “weak”.
Figure 1.
Case-control analysis of rare LoF variants (minor allele frequency [MAF] ≤ 0.005) and MS variants (MAF ≤ 0.001) in known or strongly proposed breast cancer genes, including subcategories of estrogen receptor–positive (ER+), ER-negative (ER-), and triple-negative (TN) breast tumor where diagnosis was available. ER+ and ER- groups were mutually exclusive, and the ER- groups include the TN samples. Participants without sufficient pathological information were only included in the overall LoF group and excluded from the subcategory analysis. CHEK2, BARD1, and BRIP1 were screened in 6689 cases and 14 381 controls; RAD51C and RAD51D were screened in 5726 cases and 13 428 controls. The sample sizes of ER+, ER-, and TN were 2146, 1246, and 862, respectively. CI = confidence interval; LoF = loss of function; MS = missense; OR = odds ratio (3,4,11,12,18,19).
The role of BRIP1 in breast cancer predisposition is debated, and our analysis of BRIP1 LoF variants identified an equal number of tumors showing loss of the WT or mutant alleles (4 cases each) with the remaining 5 remaining heterozygous. By comparison, analysis of 2 HGSOCs showed both had loss of the WT (Supplementary Table 5, available online), consistent with the established role of BRIP1 in ovarian cancer predisposition. Both HGSOCs also showed high HRD scores compared with only 2 of 4 BRIP1-null BCs. Mutational signature 3 was observed only in 1 BRIP1-null BC and not in the HGSOCs.
LoF mutations in CHEK2, predominantly the c.1100delC variant, are well established to confer a two- to threefold increase in BC risk (20), with the association being strongest for ER-positive BC. The current tumor data do not provide evidence that CHEK2 requires biallelic inactivation with the majority (9 of 17) of tumors remaining heterozygous, whereas only 6 showed loss of the WT allele, and 2 ER-positive tumors showed loss of the LoF allele. It appears that the 2 tumors with loss of pathogenic variants were not driven by CHEK2 LoF, and given that CHEK2 is only a moderate risk gene, several of the tumors without LOH could also not be driven by the CHEK2 pathogenic variants. In addition, 20 tumors from individuals with rare germline CHEK2 MS variants of interest (Table 1; Supplementary Table 2, available online) were analyzed. Most of these variants except for p.Ile157Thr and p.Arg117Gly are currently classified as variants of unknown significance, and tumor sequencing showed that most retained heterozygosity with only 2 showing loss of the WT allele and 2 showing loss of the variant allele. In particular, all 3 carriers of the known pathogenic, but reduced penetrance, variant CHEK2 p.Ile157Thr retained heterozygosity, and in the 2 tumors that were tested, neither were found to have promoter methylation.
Tumor sequencing in individuals with germline variants in candidate HBC genes
Tumor sequencing was performed on 57 BCs (Table 2) carrying LoF (n = 45) or rare MS (n = 16) variants in 11 genes that have been the subject of debate in the literature (FANCM, RAD50, RAD51B, and XRCC2) or were shortlisted from the BEACCON study (BLM, CDK9, CTH, ERCC5, MUTYH, PARP2, and WRN) (Supplementary Figure 1, available online). The 4 BCs from RAD50 LoF mutation carriers remained heterozygous, consistent with the accumulating literature that it does not predispose to BC (21). Literature support for a role of XRCC2 in BC predisposition is weak but with a potential association with ER-negative cancer (4,22,23). Of the 2 BCs from XRCC2 LoF variant carriers, only 1 (a TNBC) showed biallelic inactivation with both a high HRD score and mutational signature 3. For RAD51B, only tumors from carriers of rare MS variants were available with 2 remaining heterozygous and 2 showing loss of the variant allele.
Table 2.
Sequencing results of 57 tumors from individuals heterozygous for a germline LoF (n = 43) or MS (n = 16) variant in a candidate breast cancer predisposition gene (BLM, CDK9, CTH, ERCC5, FANCM, MUTYH, PARP2, RAD50, RAD51B, WRN, and PARP2)
| IDa | Germline gene | Variant | Variant type | Variant statusb | Subtype | HRD | Somatic TP53 | Somatic PIK3CA | Mutation signature 3c | Dominant signature | Hypermutated |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2660 | BLM | c.318_319insT, p.Leu107PhefsTer36 | LoF | Het | ER+/HER2- | 1 | — | — | na | na | Negative |
| 1471 | c.768_769delCT, p.Leu258GlufsTer7 | LoF | Mutant Loss | ER+/HER2unknown | 5 | — | MS | na | na | Negative | |
| 462 | c.1624delG, p.Asp542ThrfsTer2 | LoF | Mutant Loss | TN | 44 | LoF | — | na | na | Negative | |
| 3093 | c.2695C>T, p.Arg899Ter | LoF | Het | ER+/HER2- | 5 | — | MS | na | na | na | |
| 2287 | c.2875C>T, p.Arg959Ter | LoF | Het | ER+/HER2- | 11 | — | — | na | na | Negative | |
| 2083 | c.3210 + 2delT | LoF | Het | ER+/HER2- | 6 | — | — | na | na | Failed | |
| 35 | c.3558 + 1G>T | LoF | Het | ER+/HER2unknown | 17 | — | MS | na | na | Negative | |
| 1245 | CDK9 | c.130delA, p.Lys44ArgfsTer4 | LoF | Het | ER+/HER2- | 13 | MS | — | na | na | Failed |
| 2345 | c.274delT, p.Tyr92IlefsTer23 | LoF | Het | ER+/HER2+ | 4 | LoF | — | na | na | Negative | |
| 3075 | c.620_621insC, p.Ile210HisfsTer2 | LoF | Het | TN | 56 | LoF | — | na | na | Failed | |
| 2723 | c.689_690insG, p.Asn232GlnfsTer20 | LoF | Het | ER+/HER2+ | 0 | — | — | na | na | na | |
| 2045 | CTH | c.465G>A, p.Trp155Ter | LoF | Het | ER+/HER2- | 19 | — | — | na | na | Failed |
| 1322 | c.465G>A, p.Trp155Ter | LoF | Het | ER+/HER2+ | 20 | MS | — | na | na | Failed | |
| 3119 | c.465G>A, p.Trp155Ter | LoF | Het | ER+/HER2+ | 0 | — | — | na | na | na | |
| 1865 | c.1064delC, p.Thr355IlefsTer19 | LoF | Het | ER+/HER2- | 36 | MS | — | na | na | Failed | |
| 1092 | c.230C>T, p.Ala77Val | MS | Het | ER+/HER2- | 20 | ESS | — | No | 5, 12 | Failed | |
| 307 | c.323T>C, p.Ile108Thr | MS | Het | TN | 24 | LoF | — | na | na | Failed | |
| 2593 | c.620T>C, p.Met207Thr | MS | Het | na | 9 | — | — | na | na | na | |
| 1065 | c.718C>G, p.Gln240Glu | MS | Het | ER+/HER2- | 0 | — | — | na | na | na | |
| 4142 | c.794G>A, p.Arg265Gln | MS | Het | ER+/HER2- | 10 | LoF | — | na | na | Failed | |
| 3197 | c.1124G>A, p.Arg375Gln | MS | Het | ER+/HER2- | 19 | — | — | Weak | 1, 12 | na | |
| 2260 | ERCC5 | c.589delC, p.Pro198LeufsTer3 | LoF | Mutant Loss | TN | 41 | — | — | Weak | 15 | Failed |
| 901 | c.1774_1775insAAGCA, p.Val592GlufsTer8 | LoF | Mutant Loss | TN | 83 | — | MS | No | 11 | na | |
| 1367 | FANCM | c.2267G>A, p.Arg756His | LoF | Het | ER+/HER2- | 24 | — | MS | No | 6 | Failed |
| 1709 | c.3589delG, p.Asp1197MetfsTer18 | LoF | Mutant Loss | TN | 26 | MS | — | Weak | 6 | Failed | |
| 3147 | c.5101C>T, p.Gln1701Ter | LoF | Het | ER+/HER2- | 6 | — | — | Weak | 3, 6 | Failed | |
| 691 | c.5791C>T, p.Arg1931Ter | LoF | Het | ER+/HER2- | 2 | MS | MS | No | 6 | na | |
| 1172 | c.5791C>T, p.Arg1931Ter | LoF | Het | ER+/HER2+ | 0 | — | — | No | 1 | Failed | |
| 2771 | c.5791C>T, p.Arg1931Ter | LoF | WT Loss | TN | 18 | LoF | — | No | 1, 26 | DNT | |
| 1127 | c.163G>A, p.Asp55Asn | MS | Mutant Loss | TN | 51 | — | — | Weak | 3 | Failed | |
| 2094 | c.2267G>A, p.Arg756His | MS | Het | TN | 40 | MS | — | Strong | 1, 3 | Failed | |
| 1879 | c.2267G>A, p.Arg756His | MS cpd | Het | ER+/HER2- | 72 | LoF | — | Weak | 1, 19 | DNT | |
| 1222 | c.3998A>C, p.Gln1333Pro | MS | WT Loss | TN | 48 | LoF | — | Weak | 5 | DNT | |
| 901 | c.5108A>G, p.His1703Arg | MS | Het | TN | 83 | — | MS | No | 5 | na | |
| 2743 | MUTYH | c.925-2A>G | LoF | Het | TN | 10 | MS | — | na | na | Failed |
| 2727‡ | c.925-2A>G | LoF | Het | TN | 36 | LoF | — | na | na | Failed | |
| 1253 | c.384G>A, p.Trp128Ter | Biallelic LoF | Germline homozygous | TN | na | — | — | na | na | na | |
| 1474 | PARP2 | c.979_980insTT, p.Ser328CysfsTer8 | LoF | Mutant Loss | ER+/HER2- | 67 | — | MS | na | na | DNT |
| 2294 | c.985_986insA, p.Ile331AsnfsTer11 | LoF | Het | ER-/HER2+ | 81 | — | — | na | na | Failed | |
| 333 | c.1109_1110insT, p.Leu372ProfsTer2 | LoF | Mutant Loss | ER+/HER2- | na | — | — | na | na | na | |
| 1185 | c.1304delG, p.Val436TrpfsTer4 | LoF | Het | ER-/HER2+ | 30 | MS | MS | na | na | Failed | |
| 1327 | c.965G>A, p.Arg322Gln | MS | WT Loss | ER+/HER2- | 28 | — | — | na | na | DNT | |
| 2883 | RAD50 | c.1291_1297delGAGATAA, p.Asp434LysfsTer7 | LoF | Het | ER+/HER2- | 47 | — | — | No | 16 | Failed |
| 2193 | c.1958C>A, p.Ser653Ter | LoF | Het | ER+/HER2- | 23 | — | — | Strong | 3 | Failed | |
| 2251 | c.2467C>T, p.Arg823Ter | LoF | Het | ER+/HER2- | 24 | — | MS | Strong | 3, 5 | na | |
| 1031 | c.3207delA, p.Asn1070IlefsTer6 | LoF | Het | ER+/HER2+ | 8 | — | MS | No | 25 | Failed | |
| 2923 | RAD51B | c.103C>T, p.Pro35Ser | MS | Het | TN | 32 | MS | — | na | na | na |
| 1932 | c.277G>A, p.Ala93Thr | MS | Het | TN | 2 | — | — | na | na | Failed | |
| 1795 | c.436G>A, p.Ala146Thr | MS | Mutant Loss | TN | 30 | LoF | — | na | na | na | |
| 3024 | c.553T>G, p.Cys185Gly | MS | Mutant Loss | TN | 76 | — | — | na | na | DNT | |
| 3054 | WRN | c.171C>A, p.Tyr57Ter | LoF | Het | ER+/HER2- | 24 | — | MS | Weak | 5 | Failed |
| 2963 | c.944_948delTAAAC, p.Leu315PhefsTer5 | LoF | Het | ER+/HER2- | 24 | — | — | Weak | 5 | Failed | |
| 1115 | c.3961C>T, p.Arg1321Ter | LoF | Het | ER+/HER2- | 40 | MS | MS | No | 5, 6 | Failed | |
| 1847 | c.4216C>T, p.Arg1406Ter | LoF | WT Loss | ER+/HER2- | 53 | — | MS | Strong | 3 | DNT | |
| 2562 | c.4216C>T, p.Arg1406Ter | LoF | Het | ER+/HER2- | 29 | — | — | Strong | 3, 11 | Failed | |
| 3093 | c.4216C>T, p.Arg1406Ter | LoF | WT Loss | ER+/HER2- | 5 | — | MS | na | na | na | |
| 1349 | c.4216C>T, p.Arg1406Ter | LoF | Het | TN | 43 | — | — | Strong | 3 | Failed | |
| 863 | XRCC2 | c.39 + 1G>A | LoF | Het | ER-/HER2+ | 12 | LoF | — | Strong | 3 | Failed |
| 3062 | c.794T>A, p.Leu265Ter | LoF | WT Loss | TN | 44 | LoF | — | Strong | 3 | DNT |
Subject 2727 also carried a germline ATM variant that had biallelic loss in tumor. Subject 3054 carried a germline RAD51C that had biallelic loss in tumor. Subject 3093 carried variants of interest in BLM and WRN. Subject 901 carried variants in both ERCC5 and FANCM. “—” signifies feature not present. BC = breast cancer; DNT = did not test; na = not available; ER+ = estrogen receptor–positive breast cancer; HER2- = HER2 negative; HER2+ = HER2 positive; HRD = homologous recombination deficiency score; LoF = loss of function; MS = missense; TN = triple-negative; WT = wild type.
WT loss, somatic loss of the wild-type allele; Mutant loss, somatic loss of the allele carrying the known germline variant, Het, heterozygous. Case 425 showed loss of heterozygosity across the gene regions but unable to determine which allele had been lost.
Proportion of mutational signature 3 (COSMIC v2, assessed on whole-exome sequenced tumors only) above 25% is classified as “strong,” below 25% as “weak.”
LoF mutations in FANCM have previously been reported to be associated with a small increase in BC risk (24-26), but 4 of 6 tumors from individuals with FANCM LoF variants remained heterozygous with only 1 having lost the WT allele and did not show a high HRD score. Similarly, there was no consistent loss of the WT allele in tumors associated with germline FANCM MS variants (Supplementary Table 3, available online). BLM has previously been implicated in BC predisposition (27-29) and in the BEACCON data (Supplementary Figure 1, available online), but 5 of the BCs with LoF variants remained heterozygous with no evidence of promoter hypermethylation, whereas 2 BCs lost the LoF allele.
For the candidate genes, loss of the WT allele was not observed for the majority. The BCs carrying LoF or MS variants in the candidate genes CDK9 and CTH (Supplementary Table 4, available online) remained heterozygous although promoter methylation assessment was not successful for these genes. Of the 4 PARP2 BCs with LoF mutations, 2 showed loss of the LoF allele with the other 2 remaining heterozygous. The 2 ERCC5 BCs showed loss of the LoF allele. In contrast, the WRN gene, which was found to have a statistcally significant association with ER-positive BC in the BEACCON study (unadjusted P = .003), showed loss of the WT allele in 2 of 6 ER-positive cases. A WRN-heterozygous BC also carried an ATM germline variant that had experienced biallelic loss, suggesting that the ATM variant was instead responsible for the tumor. Overall, evidence of loss of the WT allele was rare among candidate genes despite evidence in case-control frequencies.
Discussion
The frequent observation of loss of the WT allele in BCs carrying germline pathogenic mutations in BRCA1 and BRCA2 supports the model of biallelic inactivation being required for BC predisposition, at least in some high penetrance genes. Recent studies indicate that biallelic inactivation is also common in BCs carrying pathogenic mutations in PALB2 (12,30,31) and ATM (13) and has been used as a biomarker to support the role of RAD51C (11) as a TNBC predisposition gene. These examples suggest tumor sequencing can provide a useful orthogonal approach to validate new BC genes and rare MS variants in known genes.
In this study, tumor sequencing demonstrated that TN tumors from BARD1 LoF mutation carriers frequently exhibit biallelic inactivation consistent with data from case-control studies (3-5) that indicate that BARD1 pathogenic variants are associated with predisposition to TNBC. Recent case-control studies have also provided support for the role of RAD51D in TNBC predisposition (4). Although 1 of 2 LoF BCs studied here showed loss of the WT allele, the rarity of its variants precluded any confident conclusion to be drawn. RAD51D MS variants as a group showed an excess in the BEACCON case-control analysis, but most of the tumors from rare MS variant carriers showed no evidence of a second hit, and the 2 cases with loss of the WT allele had low HRD scores suggesting they are benign variants.
The role of BRIP1 in BC predisposition is debated with most, but not all, published case-control studies failing to identify a statistically significant excess of LoF mutations in cases (4,5). Tumor sequencing did not find evidence to support a role for BRIP1 in BC predisposition with most BCs remaining heterozygous and, importantly, an equivalent number of cases losing the WT and LoF alleles. Previous tumor sequencing studies on BRIP1 BCs are limited, but our findings are consistent with a previous report on 3 BRIP1 BC where only 1 was found to have biallelic inactivation (10). Overall, our results for BARD1, RAD51D, and BRIP1 are consistent with the findings of 2 recent large case-control studies cited previously (4,5) where BARD1 and RAD51D are associated with BC, specifically TNBC, whereas no causative link was identified for BRIP1.
Based on the data for BARD1 and the other previously studied BC predisposing genes BRCA1, BRCA2, PALB2, and ATM, it might be extrapolated that biallelic inactivation is a typical feature for all BC predisposition genes. However, the data for CHEK2, which has highly robust case-control evidence supporting its role as a moderate penetrance BC gene, suggest that this is not true in all cases. Of the 17 BCs with germline CHEK2 LoF mutations, only 6 showed loss of the WT allele, and the majority (53%) showed no evidence of biallelic loss. The established low penetrance CHEK2 variant p.Ile157Thr was also not detected with WT allele loss. This is consistent with previous studies that found that LOH across CHEK2 in BCs from LoF mutation carriers was infrequent (32 of 93, 34%) (32-37) and occurs at a similar rate in sporadic BCs (40%, n = 560) (38). A recent sequencing-based study reported that 13 of 16 (81%) BC from CHEK2 LoF carriers had biallelic inactivation (32), however, only 5 of the 8 (63%) CHEK2-null tumors were of ER-positive and HER2-negative ductal histological subtype that are known to be associated with CHEK2 predisposition. Our data based on 17 LoF and 20 MS CHEK2 variant-carrying tumors suggested that although CHEK2 displayed a selective predisposition to ER-positive ductal BC, there was no consistent biallelic inactivation, and the BCs had low mutational burden and were not consistently associated with a characteristic mutational signature or somatic driver mutations. These data suggest that the effect of pathogenic variants in CHEK2 is possibly mediated by haploinsufficiency (39), which has implications for the reliability of using biallelic inactivation as an indicator of disease association.
Candidate genes analyzed in this study were identified in the BEACCON case-control and included BLM, PARP2, and WRN, which showed statistically significant association with BC with odds ratios of 2.5, 5.0, and 2.0, respectively, whereas other candidate genes CDK9, CTH, and XRCC2 also showed relatively high odds ratios despite the small number of cases. The BEACCON study included more than 11 500 subjects with enrichment for high-risk familial cases, however, LoF variants in candidate genes were still too rare to confidently assert a genuine association with BC predisposition. Inclusion of tumor sequencing from 57 cases did not provide definitive evidence for their roles in BC predisposition: 1 of 2 XRCC2 and 2 of 7 WRN BCs were among the minority that showed loss of the WT allele. Despite multiple studies reporting an association with BLM, no instance of biallelic inactivation has been found in BLM-carrying tumors to date, including our study of 7 tumors and the previous evaluation of 22 cases across 3 studies (28,34,40). Despite early studies and inclusion of RAD50 in many HBC gene panels (41,42), recent large studies have demonstrated that it is not a BC predisposition gene (4,5), consistent with the findings in the 4 RAD50 tumors analyzed here, which all remained heterozygous.
Findings from this study have demonstrated that tumor sequencing is useful in validating BC predisposing genes that operate in carcinogenesis through a mechanism of biallelic inactivation, such as BARD1. However, based on the evidence from CHEK2-associated tumors, the absence of biallelic inactivation does not appear to preclude a role in BC predisposition. It is interesting to note that most of the established HBC genes that have been shown to undergo frequent biallelic inactivation in breast tumors, such as BRCA1, BRCA2, PALB2, and RAD51C, are highly penetrant and/or predispose selectively to TNBC, currently the sole exception being ATM, which is a moderate penetrance and ER-positive BC-associated gene. Interestingly, similar to CHEK2, the lower penetrance BRCA2 variant p.Lys3326Ter does not appear to require biallelic activation. A recent study of 26 BRCA2 p.Lys3326Ter-associated breast tumors found no instance of LOH (43).
Despite the ready availability of archival formalin-fixed, paraffin-embedded tumor blocks from BEACCON study participants, the main limitation to this study, was the small sample size for rare genes. In addition, the quality of formalin-fixed, paraffin-embedded tumor samples may cause potential errors in the determination of LOH. Tumor purity, especially in tumors that have high levels of infiltrating lymphocytes, may also introduce further complexity to the interpretation of allele frequency and copy number status, as addressed in the methods. Lastly, because of the greater requirements in terms of tumor DNA quality and quantity, methylation sequencing was not able to be carried out for all samples, therefore promoter hypermethylation cannot be ruled out for those samples, although in this study, no such instance was found.
In summary, this study demonstrates the utility of inclusion of tumor sequencing in HBC gene discovery and validation, but the absence of consistent biallelic inactivation in CHEK2 suggests this approach might not be reliable for lower penetrance genes.
Supplementary Material
Contributor Information
Belle W X Lim, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Drug Discovery Biology, Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, Victoria, Australia.
Na Li, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, Victoria, Australia; Parkville Familial Cancer Centre, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, Melbourne, Victoria, Australia.
Sakshi Mahale, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Simone McInerny, Parkville Familial Cancer Centre, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, Melbourne, Victoria, Australia.
Magnus Zethoven, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Bioinformatics Core Facility, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Simone M Rowley, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Joanne Huynh, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Theresa Wang, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Parkville Familial Cancer Centre, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, Melbourne, Victoria, Australia.
Jue Er Amanda Lee, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, Victoria, Australia; Molecular Genomics Core, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Mia Friedman, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Parkville Familial Cancer Centre, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, Melbourne, Victoria, Australia.
Lisa Devereux, Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, Victoria, Australia; Lifepool, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Rodney J Scott, Discipline of Medical Genetics and The Centre for Cancer Detection and Therapy, The University of Newcastle and Hunter Medical Research Institute, Newcastle, New South Wales, Australia; Division of Molecular Medicine, New South Wales Health Pathology North, Newcastle, New South Wales, Australia.
Erica K Sloan, Drug Discovery Biology, Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, Victoria, Australia; Division of Cancer Surgery, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Paul A James, Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, Victoria, Australia; Parkville Familial Cancer Centre, Peter MacCallum Cancer Centre and Royal Melbourne Hospital, Melbourne, Victoria, Australia.
Ian G Campbell, Cancer Genetics Laboratory, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia; Sir Peter MacCallum Department of Oncology, The University of Melbourne, Melbourne, Victoria, Australia; Lifepool, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
Funding
This work was supported by the National Breast Cancer Foundation (IF-15-004, IGC and PAJ), Cancer Australia/National Breast Cancer Foundation (PdCCRS_1107870, IGC and PAJ), Cancer Australia/National Breast Cancer Foundation (PdCCRS_1188547, IGC and PAJ), the Victorian Cancer Agency (Tumor Stream Grant, PAJ), the National Health and Medical Research Council of Australia (GNT1023698, PAJ; GNT1041975, IGC) and Cancer Australia (PdCCRS_1188547, IGC, PAJ, and EKS). EKS is supported by the National Health and Medical Research Council GNT114749, the National Breast Cancer Foundation IIRS-20-025 and Cancer Council Victoria Grants-in-Aid. NL is supported by Cancer Council Victoria.
Notes
Role of the funder: The funding sources did not participate in the design and conduct of the study, the collection, analysis, and interpretation of the data, the preparation and writing of the manuscript, and the decision to submit the manuscript for publication.
Disclosures: The authors declare that there are no competing interests.
Author contributions: Conceptualization: IGC, PAJ. Data curation: BWXL, NL, SMR, MZ. Formal analysis: BWXL, IGC, PAJ. Funding acquisition: IGC, PAJ. Investigation: BWXL, NL, SM, SM, MZ, SMR, JH, TW, MF, AJEL, LD, RJS, EKS, PAJ, IGC. Methodology: BWXL, NL, MZ, PAJ, IGC. Resources: IGC, PAJ, RJS. Supervision: IGC, PAJ. Visualization: BWXL, NL, MZ. Writing—original draft: BWXL, IGC, PAJ, EKS. Writing—review & editing: BWXL, NL, SM, MZ, LD, EKS, RJS, PAJ, IGC.
Acknowledgements: The authors thank all the participants of the Variants in Practice and Lifepool studies for donating their DNA samples and clinical information. We also thank Norah Grewal, the Variants in Practice study site principal investigators Geoffrey Lindeman, Marion Harris, Lucinda Salmon, Ingrid Winship, and Yoland Antill and the staff at the Peter MacCallum Cancer Centre, Royal Melbourne Hospital, Monash Health, Cabrini Health and Barwon Health Familial Cancer Centres, and the Austin and Tasmanian Clinical Genetics Services, who enrolled participants and provided clinical data. We thank the following staff from Peter MacCallum Cancer Centre; Kaushalya Amarasinghe, Niko Thio, and Richard Lupat from Bioinformatics core facility for helping with the bioinformatic analysis and Steven Macaskill from Pathology Lab for Sanger sequencing services.
Prior presentations: Part of this study was presented at BRCA Symposium 2021, Montreal, Canada and Familial Aspects of Cancer Conference 2021, Melbourne, Australia.
Data availability
All sequencing data has been deposited to the European Genome-phenome Archive under accession numbers Study: EGAS00001006532 and Dataset: EGAD00001009299. Standard R codes were used. Code requests should be addressed to Prof. Ian Campbell.
References
- 1. Narod SA, Foulkes WD.. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665-676. [DOI] [PubMed] [Google Scholar]
- 2. Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371(6):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li N, Lim BWX, Thompson ER, et al. Investigation of monogenic causes of familial breast cancer: data from the BEACCON case-control study. NPJ Breast Cancer. 2021;7(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breast Cancer Association Consortium. Breast cancer risk genes—association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu C, Hart SN, Gnanaolivu R, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384(5):440-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maxwell KN, Wubbenhorst B, Wenz BM, et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun. 2017;8(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sokol ES, Pavlick D, Khiabanian H, et al. Pan-cancer analysis of BRCA1 and BRCA2 genomic alterations and their association with genomic instability as measured by genome-wide loss of heterozygosity. J Clin Oncol Precis Oncol. 2020;4:442-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tung N, Miron A, Schnitt SJ, et al. Prevalence and predictors of loss of wild type BRCA1 in estrogen receptor positive and negative BRCA1-associated breast cancers. Breast Cancer Res. 2010;12(6):R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osorio A, de la Hoya M, Rodriguez-Lopez R, et al. Loss of heterozygosity analysis at the BRCA loci in tumor samples from patients with familial breast cancer. Int J Cancer. 2002;99(2):305-309. [DOI] [PubMed] [Google Scholar]
- 10. Polak P, Kim J, Braunstein LZ, et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat Genet. 2017;49(10):1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li N, McInerny S, Zethoven M, et al. Combined tumor sequencing and case/control analyses of RAD51C in breast cancer. J Natl Cancer Inst. 2019;111(12):1332-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee JEA, Li N, Rowley SM, et al. Molecular analysis of PALB2-associated breast cancers. J Pathol. 2018;245(1):53-60. [DOI] [PubMed] [Google Scholar]
- 13. Weigelt B, Bi R, Kumar R, et al. ; for the kConFab Investigators. The landscape of somatic genetic alterations in breast cancers from ATM germline mutation carriers. J Natl Cancer Inst. 2018;110(9):1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Telli ML, Timms KM, Reid J, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22(15):3764-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lanchbury J, Timms K, Reid J, et al. 3-biomarker HRD score versus individual biomarker (LOH, TAI, LST) scores in platinum treated serous ovarian cancer (SOC). Ann Oncol. 2016;27(Suppl 6):vi34. [Google Scholar]
- 16. Rosenthal R, McGranahan N, Herrero J, Taylor BS, Swanton C.. DeconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol. 2016;17:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 18.Thompson ER, Rowley SM, Li N, et al. Panel Testing for Familial Breast Cancer: Calibrating the Tension Between Research and Clinical Care. J Clin Oncol. 2016;34(13):1455-1459. [DOI] [PubMed] [Google Scholar]
- 19.Rowley SM, Mascarenhas L, Devereux L, et al. Population-based genetic testing of asymptomatic women for breast and ovarian cancer susceptibility. Genet Med. 2019;21(4):913-922. [DOI] [PubMed] [Google Scholar]
- 20. Weischer M, Bojesen SE, Ellervik C, Tybjærg-Hansen A, Nordestgaard BG.. CHEK21100delC genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol. 2008;26(4):542-548. [DOI] [PubMed] [Google Scholar]
- 21. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3(9):1190-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hilbers FS, Wijnen JT, Hoogerbrugge N, et al. Rare variants in XRCC2 as breast cancer susceptibility alleles. J Med Genet. 2012;49(10):618-620. [DOI] [PubMed] [Google Scholar]
- 23. Park DJ, Lesueur F, Nguyen-Dumont T, et al. ; for the Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer. Rare mutations in XRCC2 increase the risk of breast cancer. Am J Hum Genet. 2012;90(4):734-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Figlioli G, Bogliolo M, Catucci I, et al. ; for kConFab. The FANCM:p.Arg658 truncating variant is associated with risk of triple-negative breast cancer. NPJ Breast Cancer. 2019;5(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kiiski JI, Pelttari LM, Khan S, et al. Exome sequencing identifies FANCM as a susceptibility gene for triple-negative breast cancer. Proc Natl Acad Sci USA. 2014;111(42):15172-15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neidhardt G, Hauke J, Ramser J, et al. Association between loss-of-function mutations within the FANCM gene and early-onset familial breast cancer. JAMA Oncol. 2017;3(9):1245-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prokofyeva D, Bogdanova N, Dubrowinskaja N, et al. Nonsense mutation p.Q548X in BLM, the gene mutated in Bloom’s syndrome, is associated with breast cancer in Slavic populations. Breast Cancer Res Treat. 2013;137(2):533-539. [DOI] [PubMed] [Google Scholar]
- 28. Sokolenko AP, Iyevleva AG, Preobrazhenskaya EV, et al. High prevalence and breast cancer predisposing role of the BLM c.1642 C>T (Q548X) mutation in Russia. Int J Cancer. 2012;130(12):2867-2873. [DOI] [PubMed] [Google Scholar]
- 29. Thompson ER, Doyle MA, Ryland GL, et al. ; for kConFab. Exome sequencing identifies rare deleterious mutations in DNA repair genes FANCC and BLM as potential breast cancer susceptibility alleles. PLoS Genet. 2012;8(9):e1002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li A, Geyer FC, Blecua P, et al. ; for kConFab Investigators. Homologous recombination DNA repair defects in PALB2-associated breast cancers. NPJ Breast Cancer. 2019;5(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ng PS, Pan JW, Ahmad Zabidi MM, et al. Characterisation of PALB2 tumours through whole-exome and whole-transcriptomic analyses. NPJ Breast Cancer. 2021;7(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mandelker D, Kumar R, Pei X, et al. The landscape of somatic genetic alterations in breast cancers from CHEK2 germline mutation carriers. JNCI Cancer Spectr. 2019;3(2):pkz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Massink MPG, Kooi IE, Martens JWM, Waisfisz Q, Meijers-Heijboer H.. Genomic profiling of CHEK2*1100delC-mutated breast carcinomas. BMC Cancer. 2015;15:877- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suspitsin EN, Yanus GA, Sokolenko AP, et al. Development of breast tumors in CHEK2, NBN/NBS1 and BLM mutation carriers does not commonly involve somatic inactivation of the wild-type allele. Med Oncol. 2014;31(2):828. [DOI] [PubMed] [Google Scholar]
- 35. Muranen TA, Greco D, Fagerholm R, et al. Breast tumors from CHEK2 1100delC-mutation carriers: genomic landscape and clinical implications. Breast Cancer Res. 2011;13(5):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oldenburg RA, Kroeze-Jansema K, Kraan J, et al. The CHEK21100delC variant acts as a breast cancer risk modifier in non-BRCA1/BRCA2 multiple-case families. Cancer Res. 2003;63(23):8153-8157. [PubMed] [Google Scholar]
- 37. Sodha N, Bullock S, Taylor R, et al. CHEK2 variants in susceptibility to breast cancer and evidence of retention of the wild type allele in tumours. Br J Cancer. 2002;87(12):1445-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nik-Zainal S, Davies H, Staaf J, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534(7605):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Teugels E, De Brakeleer S.. An alternative model for (breast) cancer predisposition. NPJ Breast Cancer. 2017;3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kluźniak W, Wokołorczyk D, Rusak B, et al. Inherited variants in BLM and the risk and clinical characteristics of breast cancer. Cancers (Basel). 2019;11(10):1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heikkinen K, Rapakko K, Karppinen S-M, et al. RAD50 and NBS1 are breast cancer susceptibility genes associated with genomic instability. Carcinogenesis. 2006;27(8):1593-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tommiska J, Seal S, Renwick A, et al. Evaluation of RAD50 in familial breast cancer predisposition. Int J Cancer. 2006;118(11):2911-2916. [DOI] [PubMed] [Google Scholar]
- 43. Butz H, Papp J, Bozsik A, et al. Application of multilayer evidence for annotation of c-terminal BRCA2 variants. Cancers (Basel). 2021;13(4):881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sequencing data has been deposited to the European Genome-phenome Archive under accession numbers Study: EGAS00001006532 and Dataset: EGAD00001009299. Standard R codes were used. Code requests should be addressed to Prof. Ian Campbell.