Abstract
Background
In many jurisdictions, cancer patients were prioritized for COVID-19 vaccination because of increased risk of infection and death. To understand sociodemographic disparities that affected timely receipt of COVID-19 vaccination among cancer patients, we undertook a population-based study in Ontario, Canada.
Methods
Patients older than 18 years and diagnosed with cancer January 2010 to September 2020 were identified using administrative data; vaccination administration was captured between approval (December 2020) up to February 2022. Factors associated with time to vaccination were evaluated using multivariable Cox proportional hazards regression.
Results
The cohort consisted of 356 535 patients, the majority of whom had solid tumor cancers (85.9%) and were not on active treatment (74.1%); 86.8% had received at least 2 doses. The rate of vaccination was 25% lower in recent (hazard ratio [HR] = 0.74, 95% confidence interval [CI] = 0.72 to 0.76) and nonrecent immigrants (HR = 0.80, 95% CI = 0.79 to 0.81). A greater proportion of unvaccinated patients were from neighborhoods with a high concentration of new immigrants or self-reported members of racialized groups (26.0% vs 21.3%, standardized difference = 0.111, P < .001), residential instability (27.1% vs 23.0%, standardized difference = 0.094, P < .001), or material deprivation (22.1% vs 16.8%, standardized difference = 0.134, P < .001) and low socioeconomic status (20.9% vs 16.0%, standardized difference = 0.041, P < .001). The rate of vaccination was 20% lower in patients from neighborhoods with the lowest socioeconomic status (HR = 0.82, 95% CI = 0.81 to 0.84) and highest material deprivation (HR = 0.80, 95% CI = 0.78 to 0.81) relative to those in more advantaged neighborhoods.
Conclusions
Despite funding of vaccines and prioritization of high-risk populations, marginalized patients were less likely to be vaccinated. Differences are likely due to the interplay between systemic barriers to access and cultural or social influences affecting uptake.
COVID-19 presents a substantial threat to the health of people with cancer. Reports published to date demonstrate higher infection rates (1), greater risk for severe disease (2), and higher rates of death compared with age-matched controls (3,4). As a result, patients with cancer, particularly those with newly diagnosed hematological malignancies, or those receiving treatment with hematopoietic stem cell transplant or immunosuppressive agents were prioritized for vaccination against COVID-19 in many jurisdictions (5,6). Although patients in the survivorship or remission phase of the cancer trajectory have not been shown to be at higher risk of COVID-19 infection, they have been shown to be at a higher risk of developing serious complications and requiring hospitalization compared with matched noncancer controls (7). Emerging studies demonstrate disparities in COVID-19 vaccine access and uptake across the globe within the general population, with vaccination rates varying from 28% to 86.1% (8); however, it remains unclear what disparities persisted among the cancer patient population.
There are both system-level and individual-level factors that influence both access to and uptake of vaccinations. Sociodemographic determinants of health that have long limited equitable access to care—such as being older, having greater material deprivation, having a disability, being a member of a racialized group, living alone or in multi-generational households (9), and being an immigrant, particularly from a low- or middle-income country (10)—are also associated with statistically significantly lower odds of being vaccinated. Furthermore, operational and supply chain challenges have further impeded timely access to COVID-19 vaccines in many jurisdictions (11,12). Many jurisdictions globally have adopted policies (13) prioritizing high-risk and marginalized populations (14,15) for receipt of vaccines. In Ontario, Canada, in addition to clinically defined high-risk groups including patients with cancer, Indigenous people, those living in congregate settings, and those residing in neighborhoods in areas with high COVID-19 positivity rates or with high proportions of essential workers were also prioritized for vaccination (5). However, it is unknown to what degree vaccine inequities persisted among patients with cancer.
As more immune escape variants of the virus emerge, it is increasingly important to ensure that these high-risk patients receive third and even fourth doses of the vaccine (16). We undertook a population-based study to evaluate the extent of COVID-19 vaccine uptake and factors associated with time to receipt of full vaccination (2 doses) and third doses among patients diagnosed with cancer, with a focus on equity to inform better planning moving forward.
Methods
Cohort identification
Ontario, Canada has a population of 14.8 million individuals and a single-payer, universal health-care system whereby COVID-19 vaccines were directly funded by the government. The study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board before initiation. The cohort was identified using the Ontario Cancer Registry deterministically linked to other administrative data holdings at ICES (formerly the Institute for Clinical Evaluative Sciences), which consistently and completely captures care episodes for Ontario residents (17). Patients were included in the cohort if they were 18 years and older, residents of Ontario, and were diagnosed with cancer between January 1, 2010, and September 30, 2020. Patients were excluded if they had unspecified or benign disease or if their date of death was before vaccine approval in Canada (December 9, 2020). Patients were considered to be vaccinated if they had received 2 doses. For third doses, the cohort was restricted to patients who were alive when they became eligible to receive their doses, defined as 56 days having elapsed since receipt of their second doses as recommended by Public Health Ontario. Information on vaccination administration from vaccine approval (December 9, 2020) to January 31, 2022, was obtained from the Ontario COVID-19 Vaccine database.
Variable creation
Demographic information (age, sex, postal code) and date of death were obtained from the Registered Persons Database. Information on immigration status was obtained from the Immigration, Refugees and Citizenship Canada database, which captures information on permanent and temporary residents of Canada as well as on citizenship and immigration programs. Patients were categorized as being recent (immigrated in the last 10 years) or nonrecent (immigrated since 1985) immigrants or were considered long-term residents/Canadian born. Based on postal code, patients were categorized as living in an urban or a rural location. Socioeconomic quintile for those residing in urban areas was assigned based on census data based on postal code. Comorbidity burden was assessed using the Johns Hopkins’ Aggregated Diagnosis Group (ADG) system (18) as a measure of non-cancer health-care resource use. Patients were categorized as having a low (1-4 ADGs), moderate (5-9 ADGs), or high (10+ ADGs) comorbidity burden. Patients were considered to be a resident of a long-term care home if they had a record for a long-term care facility in the Home Care database. Receipt of recent (within 6 months of vaccine approval date of December 9, 2020) systemic therapy was obtained from Ontario Health Insurance Plan physician billing data. Patients were considered to have had a positive COVID-19 test before vaccination if they had a record for a positive test in the Ontario Laboratory Services COVID-19 database before the date of their first vaccine dose. Patients were assigned to 1 of 10 regional public health units based on the postal code of their residence.
Marginalization
Quintiles of each of the dimensions of the Ontario Marginalization Index (Residential Instability, Material Deprivation, Dependency, Ethnic Concentration) were derived based on postal code (19). Each dimension of marginalization is a composite measure assembled from census data at the dissemination area level. Residential Instability is a measure of family and housing instability based on the type and density of residential accommodations and family characteristics. Material Deprivation is a measure of poverty based on income, housing quality, education, and family structure. Dependency measures the proportion of residents without employment income, including seniors and those receiving disability benefits. Ethnic Concentration is a measure of the proportion of residents who are recent immigrants and/or self-report belonging to a racialized group.
Statistical analysis
Demographic and clinical characteristics were summarized using descriptive statistics; vaccinated and unvaccinated patients were compared using χ2, 1-way ANOVA, and Kruskal-Wallis tests as appropriate; tests were 2-sided. Cumulative incidence plots were generated estimating probability against time to vaccination in days from December 9, 2020, for full vaccination (receipt of 2 doses) or from the date individual patients became eligible for third doses, as appropriate.
Adjusted associations between patient characteristics and time to vaccination were evaluated using Cox proportional hazards regression. Because residents of long-term care facilities were prioritized for early access to first and second doses of the vaccine, second-dose models were stratified by this covariate to address issues with effect modification. Additionally, because associations between time since diagnosis and time to vaccination were found to be affected by whether the patient had a solid vs a hematological malignancy as well as if they received recent treatment with systemic therapy, interaction terms between these variables were included in the models. Results are reported as hazard ratios (HR) with 95% confidence intervals (CIs). Variables included time since cancer diagnosis to index, age (cubic spline), sex, immigration status, COVID-19 infection before vaccination, 3 composite measures of marginalization, recent systemic therapy, comorbidities, socioeconomic status, long-term care residency, and regional public health authority. Because time to third dose was likely affected by the omicron variant surge and how quickly patients received their second dose, these were included as time-varying covariates in the booster model. Satisfaction of the proportional hazards assumption was confirmed by examining scaled Shoenfeld residuals. All analyses were completed using SAS EG (version: 7.1; Toronto, Canada); P < .05 was considered statistically significant.
Results
Cohort description
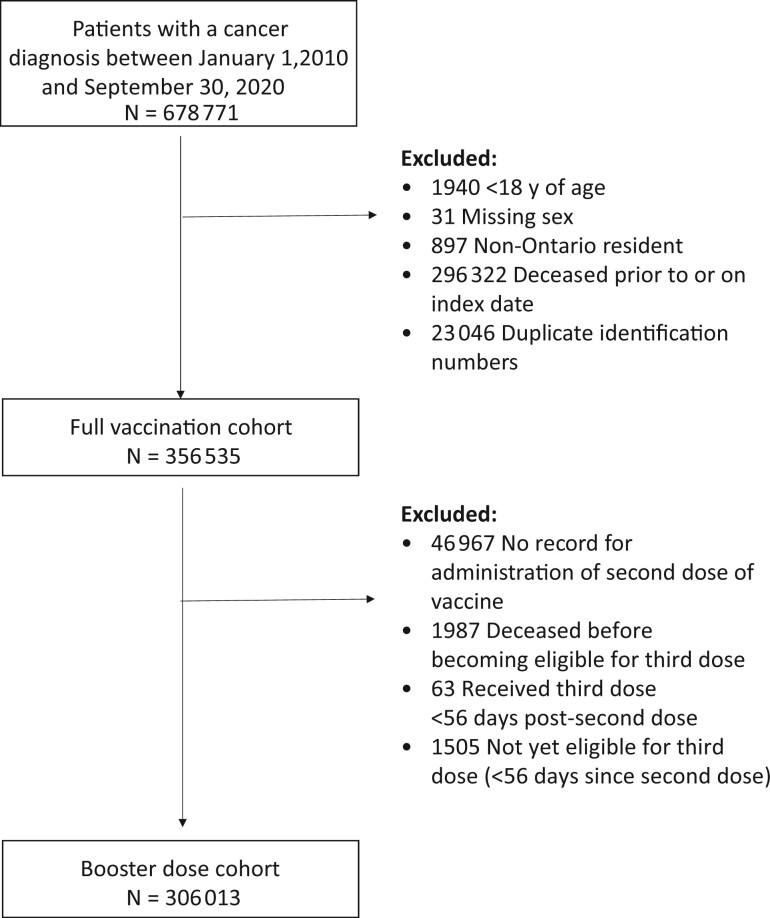
The full cohort consisted of 356 535 patients (Figure 1), of which 11.5% (40 971) were unvaccinated; 86.8% of patients were fully vaccinated and 66.8% had received a third dose. The mean age was 66 years (standard deviation: 14.4; Table 1); most of the patients were female (64.2%), diagnosed with solid tumors (85.9%), Canadian-born or long-time residents (90.8%), and had a moderate comorbidity burden (45.0%).
Figure 1.
Cohort creation.
Table 1.
Demographics and clinical characteristics of cohorta
| Variable | Full-vaccination cohort (n = 356 535) |
Third-dose cohort (n = 306 212) | ||||
|---|---|---|---|---|---|---|
| Overall (n = 356 535) | Vaccinated (n = 315 564) | Unvaccinated (n = 40 971) | Standardized difference | P | ||
| Age | ||||||
| Mean (SD) | 66 (14.4) | 66 (14.2) | 65 (15.8) | 0.072 | <.001 | 66 (14.1) |
| Median (IQR) | 67 (57,76) | 67 (57,76) | 66 (55,76) | 0.063 | <.001 | 67 (57,76) |
| Sex, no. (%) | <.001 | |||||
| Male | 127 769 (35.8) | 111 585 (35.4) | 16 184 (39.5) | 0.086 | 107 624 (35.1) | |
| Female | 228 766 (64.2) | 203 979 (64.6) | 24 787 (60.5) | 0.086 | 198 588 (64.9) | |
| Cancer type, No. (%) | <.001 | |||||
| Solid tumor | 306 337 (85.9) | 271 304 (86.0) | 35 033 (85.5) | 0.013 | 263 387 (86.0) | |
| Hematological | 50 198 (14.1) | 44 260 (14.0) | 5938 (14.5) | 0.013 | 42 825 (14.0) | |
| Time since diagnosis, No. (%) | <.001 | |||||
| Less than 1 y | 35 828 (10.0) | 30 948 (9.8) | 4880 (11.9) | 0.066 | 29 497 (9.6) | |
| 1-5 y | 162 899 (45.7) | 145 371 (46.1) | 17 528 (42.8) | 0.024 | 140 925 (46.0) | |
| 5-10 y | 157 808 (44.3) | 139 245 (44.1) | 18 563 (45.3) | 0.068 | 135 790 (44.3) | |
| Recent systemic therapy, No. (%) | 92 177 (25.9) | 81 257 (25.7) | 10 920 (26.7) | 0.021 | <.001 | 77 934 (25.5) |
| Immigration status, No. (%) | <.001 | |||||
| Recent | 7646 (2.1) | 6105 (1.9) | 1541 (3.8) | 0.11 | 5890 (1.9) | |
| Nonrecent | 25 008 (7.0) | 20 865 (6.6) | 4143 (10.1) | 0.127 | 20 176 (6.6) | |
| Canadian/long-time resident | 323 881 (90.8) | 288 594 (91.5) | 35 287 (81.0) | 0.169 | 280 146 (91.5) | |
| Socioeconomic status, No. (%) | <.001 | |||||
| Q1 (lowest) | 58 951 (16.5) | 50 388 (16.0) | 8563 (20.9) | 0.041 | 48 423 (15.8) | |
| Q2 | 62 447 (17.5) | 54 697 (17.3) | 7750 (18.9) | 0.013 | 52 939 (17.3) | |
| Q3 | 61 864 (17.4) | 54 937 (17.4) | 6927 (16.9) | 0.056 | 53 377 (17.4) | |
| Q4 | 62 058 (17.4) | 55 677 (17.6) | 6381 (15.6) | 0.101 | 54 194 (17.7) | |
| Q5 (highest) | 67 482 (18.9) | 61 122 (19.4) | 6360 (15.5) | 0.023 | 59 718 (19.5) | |
| Rural | 42 929 (12.0) | 38 073 (12.1) | 4855 (11.8) | 0.127 | 36 913 (12.1) | |
| Missing | 805 (0.2) | 670 (0.2) | 135 (0.3) | 0.007 | 648 (0.2) | |
| Comorbidities, No. (%) | <.001 | |||||
| 0-4 ADGs | 86 140 (24.2) | 70 664 (22.4) | 15 476 (37.8) | 0.34 | 69 011 (22.5) | |
| 5-9 ADGs | 160 478 (45.0) | 146 913 (46.6) | 13 565 (33.1) | 0.042 | 143 280 (46.8) | |
| 10+ ADGs | 109 917 (30.8) | 97 987 (31.1) | 11 930 (29.1) | 0.277 | 93 921 (30.7) | |
| Long-term care resident, No. (%) | 5220 (1.5) | 4724 (1.5) | 496 (1.2) | 6.247 | <.001 | 4305 (1.4) |
| Residential Instability quintile, No. (%) | <.001 | |||||
| Q1 (highest) | 66 252 (18.6) | 59 113 (18.7) | 7139 (17.4) | 0.034 | 57 691 (18.8) | |
| Q2 | 67 611 (19.0) | 60 475 (19.2) | 7136 (17.4) | 0.045 | 59 825 (19.2) | |
| Q3 | 68 848 (19.3) | 61 479 (19.5) | 7369 (18.0) | 0.038 | 59 707 (19.5) | |
| Q4 | 67 327 (18.9) | 59 524 (18.9) | 7803 (19.0) | 0.005 | 57 694 (18.8) | |
| Q5 (lowest) | 83 755 (23.5) | 72 651 (23.0) | 11 104 (27.1) | 0.094 | 70 058 (22.9) | |
| Missing | 2742 (0.8) | 2322 (0.7) | 420 (1.0) | 0.031 | 2237 (0.7) | |
| Material Deprivation quintile, No. (%) | <.001 | |||||
| Q1 (highest) | 81 287 (22.8) | 73 510 (23.3) | 7777 (19.0) | 0.106 | 71 701 (23.4) | |
| Q2 | 75 679 (21.2) | 67 802 (21.5) | 7877 (19.2) | 0.056 | 66 031 (21.6) | |
| Q3 | 69 055 (19.4) | 61 310 (19.4) | 7745 (18.9) | 0.013 | 59 514 (19.4) | |
| Q4 | 65 709 (18.4) | 57 605 (18.3) | 8104 (19.8) | 0.039 | 55 758 (18.2) | |
| Q5 (lowest) | 62 063 (17.4) | 53 015 (16.8) | 9048 (22.1) | 0.134 | 50 971 (16.6) | |
| Missing | 2742 (0.8) | 2322 (0.7) | 420 (1.0) | 0.031 | 2237 (0.7) | |
| Dependency quintile, No. (%) | <.001 | |||||
| Q1 (highest) | 68 608 (19.2) | 60 011 (19.0) | 8597 (21.0) | 0.052 | 58 268 (19.0) | |
| Q2 | 63 608 (17.9) | 56 253 (17.8) | 7670 (18.7) | 0.046 | 54 630 (17.8) | |
| Q3 | 63 002 (17.7) | 55 573 (17.7) | 7249 (17.7) | 0.033 | 54 163 (17.7) | |
| Q4 | 66 692 (18.7) | 59 231 (18.8) | 7461 (18.2) | 0.003 | 57 503 (18.8) | |
| Q5 (lowest) | 91 568 (25.7) | 81 994 (26.0) | 9574 (23.4) | 0.111 | 79 411 (25.9) | |
| Missing | 2742 (0.8) | 2322 (0.7) | 420 (1.0 | 0.031 | 2237 (0.7) | |
| Ethnic Concentration quintile, No. (%) | <.001 | |||||
| Q1 (highest) | 71 699 (20.1) | 64 211 (20.3) | 7488 (18.3) | 0.052 | 62 304 (20.3) | |
| Q2 | 68 388 (19.2) | 61 181 (19.4) | 7207 (17.6) | 0.046 | 59 442 (19.4) | |
| Q3 | 66 520 (18.7) | 59 333 (18.8) | 7187 (17.5) | 0.033 | 57 632 (18.8) | |
| Q4 | 69 484 (19.5) | 61 455 (19.5) | 8029 (19.6) | 0.003 | 59 681 (19.5) | |
| Q5 (lowest) | 77 702 (21.8) | 67 062 (21.3) | 10 640 (26.0) | 0.111 | 64 916 (21.2) | |
| Missing | 2742 (0.8) | 2322 (0.7) | 420 (1.0) | 0.031 | 2237 (0.7) | |
| COVID-19 infection before vaccination, No. (%) | 6658 (1.9) | 6658 (1.9) | — | — | — | 6292 (2.1) |
| Regional Public Health Authority, No. (%) | <.001 | |||||
| Central East | 28 345 (8.0) | 25 347 (8.0) | 2998 (7.3) | 0.027 | 24 551 (8.0) | |
| Central West | 69 665 (19.5) | 61 921 (19.6) | 7744 (18.9) | 0.018 | 60 098 (19.6) | |
| Durham | 16 467 (4.6) | 14 724 (4.7) | 1743 (4.3) | 0.020 | 14 336 (4.7) | |
| Eastern | 26 082 (7.3) | 23 396 (7.4) | 2686 (6.6) | 0.034 | 22 728 (7.4) | |
| North | 23 362 (6.6) | 20 791 (6.6) | 2571 (6.3) | 0.013 | 20 079 (6.6) | |
| Ottawa | 22 774 (6.4) | 20 555 (6.5) | 2219 (5.4) | 0.046 | 20 006 (6.5) | |
| Peel | 28 625 (8.0) | 24 724 (7.8) | 3901 (9.5) | 0.060 | 23 971 (7.8) | |
| South West | 46 234 (13.0) | 40 978 (13.0) | 5256 (12.8) | 0.005 | 39 711 (13.0) | |
| Toronto | 66 712 (18.7) | 58 048 (18.4) | 8664 (21.1) | 0.069 | 56 329 (18.4) | |
| York | 28 269 (7.9) | 25 080 (7.9) | 3189 (7.8) | 0.006 | 24 403 (8.0) | |
| Receipt of vaccinations, No. (%) | — | |||||
| One dose | 5996 (1.7) | 5996 (1.9) | — | — | — | |
| Two doses | 71 464 (20.0) | 71 464 (22.6) | — | — | 68 170 (22.3) | |
| Three doses | 238 104 (66.8) | 238 104 (75.5) | — | — | 238 042 (77.7) | |
| Unvaccinated | 40 971 (11.5) | — | 40 971 (100) | — | — | |
ADG = aggregated diagnosis groups; IQR= interquartile range.
Characteristics of unvaccinated patients
Compared with those who had received at least 1 vaccine dose (Table 1), a greater proportion of unvaccinated patients were male (39.5% vs 35.4%, standardized difference = 0.086, P < .001), recent (3.8% vs 1.9%; standardized difference = 0.110, P < .001) or nonrecent immigrants (10.1% vs 6.6%, standardized difference = 0.127, P < .001), and had a lower comorbidity burden (0-4 aggregated diagnosis groups = 37.8% vs 22.4%, standardized difference = 0.34, P < .001). Additionally, a greater proportion of unvaccinated patients were from neighborhoods with high Ethnic Concentration (26.0% vs 21.3%, standardized difference = 0.111, P < .001), low socioeconomic status (20.9% vs 16.0%, standardized difference = 0.041, P < .001), and high Residential Instability (27.1% vs 23.0%, standardized difference = 0.094, P < .001) or Material Deprivation (22.1% vs 16.8%, standardized difference = 0.134, P < .001) compared with those who had received at least 1 dose of the vaccine.
Associations between clinical characteristics and rate of vaccination
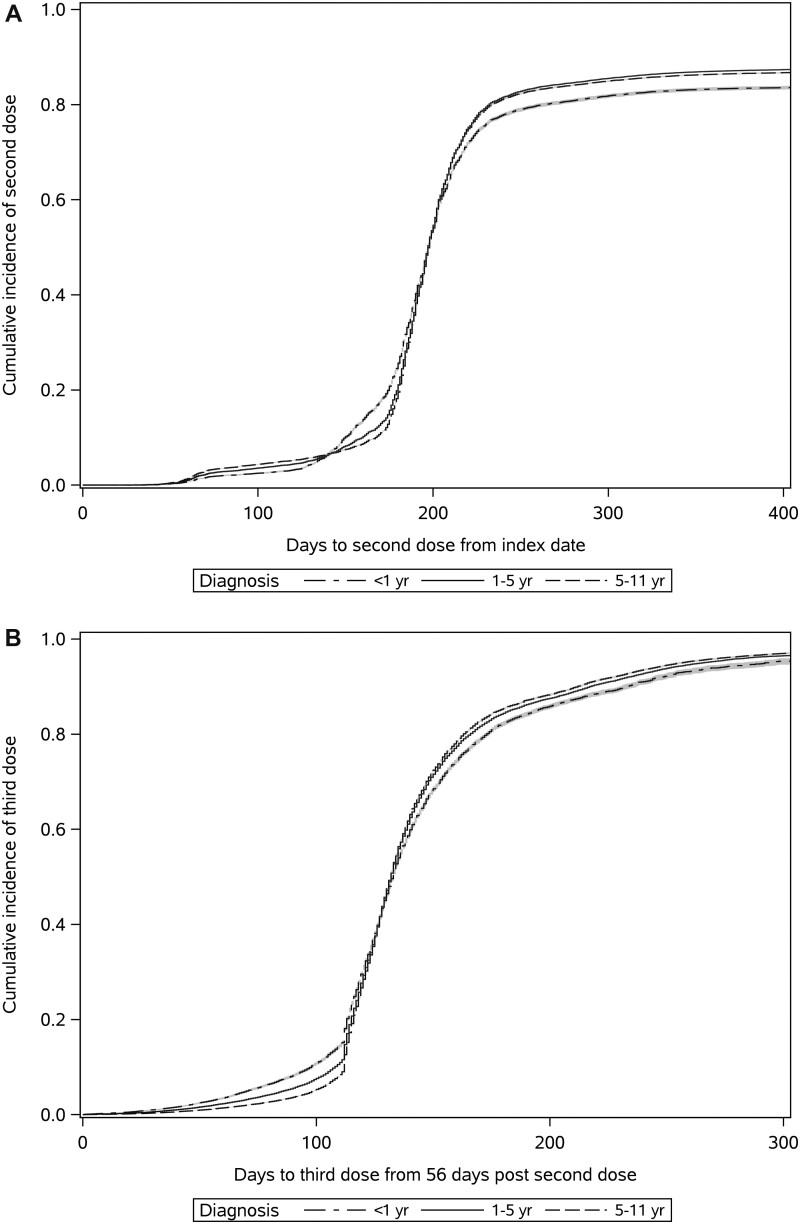
A greater proportion of patients with more remote cancer diagnoses received both full vaccination and third doses compared with recently diagnosed patients (diagnosed in the last year; Figure 2). Patients with hematological cancers took less time to get vaccinated than patients with solid tumor cancers; for patients diagnosed in the last year, those with hematological malignancies received full vaccination 20% faster than those with solid tumors (HR = 1.20, 95% CI = 1.16 to 1.24; Table 2) and third doses 37% faster (HR = 1.37, 95% CI = 1.32 to 1.43). There was little difference in the rate of receipt of full vaccination or third doses between those recently treated with systemic therapy and those who were not, except in patients with latent cancer diagnoses (diagnosed 5-10 years prior), where patients recently treated with systemic therapy received their vaccinations 11%-12% faster than those without recent treatment (full vaccination: HR = 1.12, 95% CI = 1.12 to 1.14; third dose: HR = 1.11, 95% CI = 1.09 to 1.13). Having a moderate (HR = 1.36, 95% CI = 1.35 to 1.38) or high (HR = 1.30, 95% CI = 1.29 to 1.32) comorbidity burden or having a COVID-19 infection before first dose (HR = 1.12, 95% CI = 1.10 to 1.15) was associated with a higher rate of receipt of full vaccination; similar findings were observed for third doses.
Figure 2.
Cumulative incidence plots estimating probability against time to receipt of full vaccination from the date of vaccine approval (A), and third dose from when the patient became eligible (56 days following receipt of dose 2; B).
Table 2.
Factors associated with receipt of full vaccination and booster doses based on multivariable Cox proportional hazards models with interaction terms for time since diagnosis with cancer type and receipt of recent systemic therapya
| Variables | Full vaccination (n = 356 535) | Booster doses (n = 306 212) |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Age | ||
| Point estimate at 40 y | 1.06 (1.05 to 1.06) | 1.09 (1.08 to 1.09) |
| Point estimate at 80 y | 1.00 (0.99 to 1.00) | 0.95 (0.94 to 0.95) |
| Sex | ||
| Female | 1.08 (1.08 to 1.09) | 1.01 (1.00 to 1.01) |
| Male | Referent | Referent |
| Cancer type | ||
| Hematologic vs solid cancer (diagnosed in the last year) | 1.20 (1.16 to 1.24) | 1.37 (1.32 to 1.43) |
| Hematologic vs solid cancer (diagnosed 1-5 y prior) | 1.09 (1.07 to 1.11) | 1.22 (1.20 to 1.24) |
| Hematologic vs solid cancer (diagnosed 5-10 y prior) | 1.03 (1.01 to 1.05) | 1.12 (1.10 to 1.14) |
| Recent systemic therapy | ||
| Recent systemic therapy vs none (diagnosed in last year) | 1.02 (1.00 to 1.04) | 1.01 (0.98 to 1.03) |
| Recent systemic therapy vs none (diagnosed 1-5 y prior) | 1.00 (0.99 to 1.01) | 1.07 (1.06 to 1.08) |
| Recent systemic therapy vs none (diagnosed 5-10 y prior) | 1.12 (1.12 to 1.14) | 1.11 (1.09 to 1.13) |
| Immigration status | ||
| Recent | 0.74 (0.72 to 0.76) | 0.67 (0.65 to 0.70) |
| Nonrecent | 0.80 (0.79 to 0.81) | 0.71 (0.70 to 0.73) |
| Long-term resident/Canadian | Referent | Referent |
| Socioeconomic status | ||
| Rural | 0.87 (0.86 to 0.89) | 0.88 (0.86 to 0.90) |
| Q1-low | 0.82 (0.81 to 0.84) | 0.86 (0.84 to 0.88) |
| Q2 | 0.87 (0.85 to 0.88) | 0.89 (0.88 to 0.91) |
| Q3 | 0.90 (0.89 to 0.91) | 0.91 (0.89 to 0.92) |
| Q4 | 0.94 (0.93 to 0.95) | 0.93 (0.92 to 0.94) |
| Q5-high | Referent | Referent |
| Aggregated clinical groups | ||
| 0-4 ADGs | Referent | Referent |
| 5-9 ADGs | 1.36 (1.35 to 1.38) | 1.06 (1.05 to 1.07) |
| 10+ ADGs | 1.30 (1.29 to 1.32) | 1.04 (1.03 to 1.05) |
| Residential Instability quintile | ||
| Q1- low | Referent | Referent |
| Q2 | 0.99 (0.98 to 1.00) | 1.02 (1.00 to 1.03) |
| Q3 | 1.00 (0.99 to 1.01) | 1.04 (1.03 to 1.06) |
| Q4 | 1.04 (1.02 to 1.05) | 1.09 (1.07 to 1.11) |
| Q5- high | 1.02 (1.00 to 1.03) | 1.12 (1.10 to 1.14) |
| Material Deprivation quintile | ||
| Q1-low | Referent | Referent |
| Q2 | 0.95 (0.94 to 0.96) | 0.96 (0.95 to 0.97) |
| Q3 | 0.91 (0.90 to 0.92) | 0.93 (0.92 to 0.95) |
| Q4 | 0.86 (0.85 to 0.88) | 0.89 (0.87 to 0.90) |
| Q5-high | 0.80 (0.78 to 0.81) | 0.81 (0.80 to 0.83) |
| Dependency quintile | ||
| Q1- low | Referent | Referent |
| Q2 | 1.04 (1.02 to 1.05) | 1.02 (1.01 to 1.04) |
| Q3 | 1.05 (1.04 to 1.07) | 1.04 (1.02 to 1.05) |
| Q4 | 1.07 (1.06 to 1.08) | 1.05 (1.04 to 1.07) |
| Q5- high | 1.13 (1.12 to 1.15) | 1.07 (1.06 to 1.09) |
| Ethnic Concentration quintile | ||
| Q1-low | Referent | Referent |
| Q2 | 1.00 (1.00 to 1.02) | 1.02 (1.01 to 1.04) |
| Q3 | 1.01 (1.00 to 1.02) | 1.03 (1.01 to 1.04) |
| Q4 | 1.03 (1.01 to 1.04) | 1.01 (1.00 to 1.03) |
| Q5- high | 1.00 (0.99 to 1.02) | 0.97 (0.95 to 0.99) |
| Resident of a long-term care facility | ||
| Yes | — | 1.05 (1.02 to 1.09) |
| No | — | Referent |
| COVID-19 infection before first dose | ||
| Yes | 1.12 (1.10 to 1.15) | 0.87 (0.84 to 0.90) |
| No | Referent | Referent |
| Regional Public Health Authority | ||
| Central East | 0.83 (0.81 to 0.84) | 1.15 (1.13 to 1.18) |
| Central West | 0.86 (0.85 to 0.87) | 1.05 (1.04 to 1.07) |
| Durham | 0.95 (0.93 to 0.97) | 0.98 (0.95 to 1.00) |
| Eastern | 0.83 (0.81 to 0.84) | 1.22 (1.20 to 1.25) |
| North | 0.90 (0.88 to 0.91) | 1.08 (1.05 to 1.10) |
| Ottawa | 0.93 (0.91 to 0.94) | 1.28 (1.24 to 1.30) |
| Peel | 0.93 (0.91 to 0.94) | 0.85 (0.84 to 0.87) |
| South West | 0.87 (0.86 to 0.88) | 1.15 (1.13 to 1.17) |
| York | 0.98 (0.97 to 1.00) | 0.96 (0.94 to 0.98) |
| Toronto | Referent | Referent |
| Time to second dose | ||
| Increment: week | — | 1.07 (1.07 to 1.07) |
| Omicron | ||
| After Omicron | — | 1.05 (1.03 to 1.06) |
| Before Omicron | — | Referent |
HR = hazard ratio; CI = confidence interval; ADG = aggregated diagnosis groups.
Associations between sociodemographic determinants of health and rate of vaccination
Compared with patients who were Canadian born, the rate of full vaccination was found to be approximately 20%-25% lower for recent (HR = 0.74, 95% CI = 0.72 to 0.76) or nonrecent immigrants (HR = 0.80, 95% CI = 0.79 to 0.81); similar findings were observed for third doses (recent: HR = 0.67, 95% CI = 0.65 to 0.70; nonrecent: HR = 0.71, 95% CI = 0.70 to 0.73). Additionally, relative to patients in the most advantaged quintiles, the rate of full vaccination was approximately 20% lower in those patients in the lowest socioeconomic status quintile (HR = 0.82, 95% CI = 0.81 to 0.84) and those residing in neighborhoods with highest Material Deprivation (HR = 0.80, 95% CI = 0.78 to 0.82). Residential Instability was not associated with the rate of receipt of full vaccination (HR = 1.02, 95% CI = 1.00 to 1.03) but did affect receipt of third doses, whereby rate of vaccination was approximately 10% higher in those residing in the most Residentially Instable quintile than those in the most advantaged quintile (HR = 1.12, 95% CI = 1.10 to 1.14). Compared with the most advantaged quintile, those experiencing the most Material Deprivation had rates of vaccination approximately 20% lower for both full vaccination (HR = 0.80, 95% CI = 0.78 to 0.81) and third doses (HR = 0.81, 95% CI = 0.80 to 0.83). Conversely, those in the highest Dependency quintile had a higher rate of vaccination that those in the most advantaged quintile for both full vaccination (HR = 1.13, 95% CI = 1.12 to 1.15) and third doses (HR = 1.07, 95% CI = 1.06 to 1.09). Neighborhood Ethnic Concentration had no impact on rate of full vaccination (HR = 1.00, 95% CI = 0.99 to 1.02) and only a marginal impact on receipt of third doses (HR = 0.97, 95% CI = 0.95 to 0.99). Rate of receipt of vaccinations differed by geographical location, particularly for third doses (HR range = 0.85-1.28).
Discussion
Our findings suggest that the provincial prioritization policy did achieve some of its intended aims because those identified as high risk of COVID-19 infection or mortality at the outset of the pandemic (20), particularly those of advanced age, diagnosed with hematological malignancies, having a high comorbidity burden, or living in long-term care facility, had higher rates of receipt of vaccination. Compared with patients with solid tumors, rate of receipt of vaccination of patients with hematological cancers was fastest in patients with newly diagnosed malignancies. This difference likely reflects clinical guidance to vaccinate patients before initiating treatment with lympho-depleting therapies (21) due to increased risk of severe infection and death (22), and concerns with decreased immunogenicity and vaccine efficacy within this patient population after treatment (23). However, despite prioritization of patients receiving systemic therapy for vaccination, there was little difference in the rate of receipt of full vaccination or third doses between those recently treated with systemic therapy and those who were not, except in patients with latent cancer diagnoses (diagnosed 5-10 years prior). This may reflect clinical guidance to time COVID-19 vaccinations so they are between cycles of treatment or to delay vaccination until 1 month following completion of systemic therapy in those receiving treatment (21).
Even within the prioritized cancer population, disparities in receipt of COVID-19 vaccination in marginalized populations persisted. Similarly to other jurisdictions (24), we found that despite having an increased risk of exposure and infection (25-27), higher proportions of recent immigrants; those from neighborhoods with high Ethnic Concentrations, Residential Instability, and Material Deprivation; or those of low socioeconomic status were unvaccinated. It took longer for immigrants to receive full vaccination and booster doses than long-time residents. Additionally, it took longer for patients in the lowest socioeconomic strata or highest Material Deprivation quintiles to receive full vaccination and booster doses than those who were most advantaged. Despite direct government funding of COVID-19 vaccines and distribution policies aimed at prioritizing high-risk populations, marginalized patients with cancer were less likely to be vaccinated than other patients with cancer. To our knowledge, this is the first article to examine COVID-19 vaccine equity within the cancer patient population.
Observed disparities in receipt of vaccination are likely due to an inter-play between systemic barriers to access (physical barriers to accessible care, transportation challenges, long work hours, barriers in navigating the health-care system) as well as socio-cultural influences limiting uptake (inability of the health-care system to demonstrate trustworthiness, lack of access to professional translator services and lack of linguistic and cultural diversity among the health-care force, religion, education, preferences for alternative medicine, exposure to information or misinformation on vaccine-related adverse effects and deaths) (28-31). Particularly during the current pandemic, exposure to online medical misinformation regarding vaccine efficacy and side effects and through social media has been widespread and associated with increased vaccine hesitancy and vaccine refusal (32).
Reliance on current, area-based datasets rather than routinely collected, individual-level sociodemographic data makes it challenging to effectively identify high-risk populations, design appropriate interventions, or test their impact (33). As such, there is a paucity of concrete examples from the literature of interventions that effectively overcome barriers to accessing health care. In future health emergencies, and should additional booster doses be warranted, marginalized populations and those at highest risk should continue to be prioritized and allocation processes must be transparent (34). Community partnerships should be leveraged to develop policies and interventions through participatory design that are sensitive to community needs, unique barriers, and cultural characteristics (35). Use of mobile clinics in neighborhoods with high risk of exposure (36,37) and community-based models that engage leaders and health-care providers that are representative of the racialized populations they serve have been successful strategies to improve rates of COVID-19 vaccination (38). To better meet patient needs, immunizations should be offered at a greater variety of times and points of contact within the health-care system (39). However, these strategies should be embedded within a more comprehensive plan that addresses the underlying inequities that put marginalized groups at higher risk of infection in the first place and through programs aimed at improving employment conditions, housing security, and social service systems (40). Additionally, formal evaluations grounded in equity frameworks (41-43) are needed to ascertain the impacts of interventions to improve inequities and inform future policy and interventions (43).
Our study must be taken in the context of its limitations. We used routinely collected, administrative data holdings to examine vaccination across Ontario residents who are enrolled in the provincial health insurance plan. With the exception of immigration status, which is collected at the individual level, other characteristics of marginalization that we examined are based on census data for dissemination area, which may affect the validity of our findings. Some sociodemographic factors associated with inequities in accessing health care, such as gender identity, sexuality, race, and Indigenous status (44), are either poorly captured or not readily available within the administrative data. Additionally, administrative data do not allow for the inference of causality, so it is difficult to ascertain whether observed differences in vaccination rates are related to issues of access to care or willingness to be vaccinated against COVID-19. We undertook multivariable analyses examining associations between individual clinical and demographic characteristics and rate of vaccination; however, future work should examine issues of intersectionality affecting vaccine access and uptake by evaluating social patterning and the interaction between different sociodemographic characteristics (45). Finally, we report on differences in rate and receipt of COVID-19 vaccination in marginalized groups relative to those who are more advantaged; however, future work should focus on understanding drivers of lower rates of vaccination of these groups to inform future policies and interventions to address these inequities.
Despite direct government funding of COVID-19 vaccines and distribution policies aimed at prioritizing high-risk populations, marginalized patients with history of cancer were less likely to be vaccinated than other patients with cancer. Differences in receipt of vaccination are likely due to the interplay between systemic barriers to access (lack of trustworthiness of the health-care system, transportation barriers, work schedules), and cultural/social influences affecting uptake. Future efforts should work directly with members of high-risk communities to understand how to improve vaccine delivery among these communities.
Contributor Information
Melanie Powis, Cancer Quality Lab (CQuaL), Princess Margaret Cancer Centre—University Health Network, Toronto, ON, Canada; Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre—University Health Network, Toronto, ON, Canada.
Rinku Sutradhar, Institute for Clinical Evaluative Sciences, Toronto, ON, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada.
Aditi Patrikar, Institute for Clinical Evaluative Sciences, Toronto, ON, Canada.
Matthew Cheung, Institute for Clinical Evaluative Sciences, Toronto, ON, Canada; Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; Division of Medical Oncology and Hematology, Department of Medicine, University of Toronto, Toronto, ON, Canada.
Inna Gong, Division of Medical Oncology and Hematology, Department of Medicine, University of Toronto, Toronto, ON, Canada.
Abi Vijenthira, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre—University Health Network, Toronto, ON, Canada; Division of Medical Oncology and Hematology, Department of Medicine, University of Toronto, Toronto, ON, Canada.
Lisa K Hicks, Division of Medical Oncology and Hematology, Department of Medicine, University of Toronto, Toronto, ON, Canada; Division of Hematology/Oncology, St. Michael’s Hospital—Unity Health, Toronto, ON, Canada.
Drew Wilton, Institute for Clinical Evaluative Sciences, Toronto, ON, Canada.
Monika K Krzyzanowska, Cancer Quality Lab (CQuaL), Princess Margaret Cancer Centre—University Health Network, Toronto, ON, Canada; Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre—University Health Network, Toronto, ON, Canada; Institute for Clinical Evaluative Sciences, Toronto, ON, Canada; Division of Medical Oncology and Hematology, Department of Medicine, University of Toronto, Toronto, ON, Canada.
Simron Singh, Institute for Clinical Evaluative Sciences, Toronto, ON, Canada; Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, ON, Canada; Division of Medical Oncology and Hematology, Department of Medicine, University of Toronto, Toronto, ON, Canada.
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). This work is supported by the Ontario Health Data Platform (OHDP), a Province of Ontario initiative to support Ontario’s ongoing response to COVID-19 and its related impacts. OHDP supported the project by providing ICES analyst hours inkind. Additionally, this work was funded by grants through the Canadian Institute for Health Research (CIHR).
Notes
Role of the funder: The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors declare no conflict of interest.
Author contributions: MP: conceptualization, writing-original draft, funding acquisition, formal analysis, project administration; RS: formal analysis, methodology, writing- review and editing; AP: data curation, formal analysis, visualization; MC: conceptualization, formal analysis, writing- review and editing, resources; IG: conceptualization, formal analysis, project administration, writing- review and editing; AV: conceptualization, formal analysis, writing-review and editing; LKH: conceptualization, formal analysis, writing- review and editing; DW: methodology, formal analysis, visualization, supervision; MKK: conceptualization, formal analysis, funding acquisition, resources, supervision, writing- review and editing; SS: conceptualization, formal analysis, funding acquisition, resources, supervision, writing- review and editing.
Acknowledgements: The study was approved by the institutional review board of the Sunnybrook Health Sciences Centre (REB# 4995).
Disclaimers: The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by the OHDP, its partners, or the Province of Ontario is intended or should be inferred.
Prior presentations: This study was presented at the 2022 American Society of Clinical Oncology (ASCO) Quality Care Symposium (Chicago, IL USA).
Data availability
The administrative data underlying this article were accessed from ICES. External researchers can access the dataset used in this analysis by requesting access through ICES Data and Analytic Services; details of this process and the required forms are available at: https://www.ices.on.ca/DAS/Public-Sector/Access-to-ICES-Data-Process.
References
- 1. Yu J, Ouyang W, Chua MLK, Xie C.. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Onder G, Rezza G, Brusaferro S.. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775-1776. [DOI] [PubMed] [Google Scholar]
- 4. Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ontario Ministry of Health. COVID-19: Guidance for prioritization of Phase 2 populations for COVID-19 vaccination. https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID-19_Phase_2_vaccination_prioritization.pdf. Accessed April 30, 2021.
- 6. Pergam S, Baden L, Abel G, et al. Preliminary Recommendations of the NCCN COVID-19 Vaccination Advisory Committee. https://www.nccn.org/covid-19/pdf/COVID-19_Vaccination_Guidance_V1.0.pdf. Accessed April 30, 2021.
- 7. Sun L, Surya S, Le AN, et al. Rates of COVID-19-related outcomes in cancer compared with noncancer patients. JNCI Cancer Spectr. 2021;5(1):pkaa120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biswas MR, Alzubaidi MS, Shah U, Abd-Alrazaq AA, Shah Z.. A scoping review to find out worldwide COVID-19 vaccine hesitancy and its underlying determinants. Vaccines (Basel). 2021;9(11):1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nafilyan V, Dolby T, Razieh C, et al. Sociodemographic inequality in COVID-19 vaccination coverage among elderly adults in England: a national linked data study. BMJ Open. 2021;11(7):e053402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spetz M, Lundberg L, Nwaru C, et al. The social patterning of Covid-19 vaccine uptake in older adults: a register-based cross-sectional study in Sweden. Lancet Reg Health Eur. 2022;15:100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ontario Ministry of Health. Extension of the second dose interval. https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID_19_vaccine_dose_intervals.pdf. Accessed April 30, 2021.
- 12. Chen Z, Zheng W, Wu Q, et al. Global diversity of policy, coverage, and demand of COVID-19 vaccines: a descriptive study. BMC Med. 2022;20(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Organization for Economic Cooperation and Development. Access to COVID-19 vaccines: global approaches in a global crisis. https://www.oecd.org/coronavirus/policy-responses/access-to-covid-19-vaccines-global-approaches-in-a-global-crisis-c6a18370/. Accessed June 3, 2022.
- 14. Guttmann A, Gandhi S, Wanigaratne S, et al. COVID-19 in immigrants, refugees and other newcomers in Ontario: characteristics of those tested and those confirmed positive, as of June 13, 2020. https://www.ices.on.ca/Publications/Atlases-and-Reports/2020/COVID-19-in-Immigrants-Refugees-and-Other-Newcomers-in-Ontario. Accessed November 1, 2021.
- 15. Ioannou GN, Green P, Locke ER, Berry K.. Factors associated with early receipt of COVID-19 vaccination and adherence to second dose in the Veterans Affairs healthcare system. PLoS One. 2021;16(12):e0259696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457-466.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams J, Young W.. A summary of studies on the quality of healthcare administrative databases in Canada. In: V Goel, JI Williams, GM Anderson, P Blackstien-Hirsch, C Fooks, CD Naylor, eds. Patterns of Healthcare in Ontario: The ICES Practice Atlas. Ottawa, Ontario: Canadian Medical Association; 1996:339-345. [Google Scholar]
- 18. Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM.. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49(10):932-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ontario Agency for Health Protection and Promotion (Public Health Ontario). 2011 Ontario Marginalization Index: user guide. https://www.publichealthontario.ca/-/media/documents/O/2017/on-marg-user-2011.pdf. Accessed November 1, 2021.
- 20. Akhtar-Danesh N, Baumann A, Crea-Arsenio M, Antonipillai V.. COVID-19 excess mortality among long-term care residents in Ontario, Canada. PLoS One. 2022;17(1):e0262807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Society of Hematology. General principles of COVID-19 vaccines for immunocompromised patients. https://www.hematology.org/covid-19/covid-19-and-vaccines. Accessed November 1, 2021.
- 22. Martinez JC, Sica RA, Stockerl-Goldstein K, Rubinstein SM.. COVID-19 in patients with hematologic malignancies: outcomes and options for treatments. Acta Haematol. 2022;145(3):244-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seebacher NA. The antibody response of haematological malignancies to COVID-19 infection and vaccination. Br J Cancer. 2022;126(5):691-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Oliveira BRB, da Penha Sobral AIG, Marinho MLM, et al. Determinants of access to the SARS-CoV-2 vaccine: a preliminary approach. Int J Equity Health. 2021;20(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML.. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ontario Health. Tracking COVID-19 through race-based data. https://www.ontariohealth.ca/sites/ontariohealth/files/2021-08/Tracking%20COVID%2019%20Through%20Race%20Based%20Data-EN.pdf. Accessed June 6, 2022.
- 27. Zimmermann R, Sarma N, Thieme-Thörel D, et al. COVID-19 outbreaks in settings with precarious housing conditions in Germany: challenges and lessons learned. Front Public Health. 2021;9:708694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balasuriya L, Santilli A, Morone J, et al. COVID-19 vaccine acceptance and access among Black and Latinx communities. JAMA Netw Open. 2021;4(10):e2128575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lavoie K, Gosselin-Boucher V, Stojanovic J, et al. ; iCARE Study Team. Understanding national trends in COVID-19 vaccine hesitancy in Canada: results from five sequential cross-sectional representative surveys spanning April 2020-March 2021. BMJ Open. 2022;12(4):e059411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Griffith J, Marani H, Monkman H.. COVID-19 vaccine hesitancy in Canada: content analysis of tweets using the theoretical domains framework. J Med Internet Res. 2021;23(4):e26874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burke PF, Masters D, Massey G.. Enablers and barriers to COVID-19 vaccine uptake: an international study of perceptions and intentions. Vaccine. 2021;39(36):5116-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loomba S, de Figueiredo A, Piatek SJ, et al. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat Hum Behav. 2021;5(3):337-348. [DOI] [PubMed] [Google Scholar]
- 33. McKenzie K. Socio-demographic data collection and equity in COVID-19 in Toronto. Lancet EClinicalMedicine. 2021;34:100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sekalala S, Perehudoff K, Parker M, et al. An intersectional human rights approach to prioritising access to COVID-19 vaccines. BMJ Glob Health. 2021;6(2):e004462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. AuYoung M, Rodriguez Espinosa P, Chen W, et al. ; the STOP COVID-19 C. A. Communications Working Group. Addressing racial/ethnic inequities in vaccine hesitancy and uptake: lessons learned from the California alliance against COVID-19. J Behav Med. 2022;1-11. doi: 10.1007/s10865-022-00284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leibowitz A, Livaditis L, Daftary G, et al. Using mobile clinics to deliver care to difficult-to-reach populations: a COVID-19 practice we should keep. Prev Med Rep. 2021;24:101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petrova E, Farinholt T, Joshi TP, et al. A community-based management of COVID-19 in a mobile container unit. Vaccines (Basel). 2021;9(11):1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown-DeVeaux DV, Richards BR, Gilles S, Johnson J.. The impact of African-American nurses: how the implementation of a community-based model increased vaccination rates in marginalized communities in NYC. J Natl Black Nurses Assoc. 2021;32(2):23-27. [PubMed] [Google Scholar]
- 39. MacDonald NE, Comeau JL, Dubé È, Bucci LM.. COVID-19 and missed routine immunizations: designing for effective catch-up in Canada. Can J Public Health. 2020;111(4):469-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Government of Canada. From risk to resilience: an equity approach to COVID-19. https://www.canada.ca/en/public-health/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/from-risk-resilience-equity-approach-covid-19.html#a3. Accessed September 12, 2022.
- 41. Pressman AR, Lockhart SH, Shen Z, Azar KMJ.. Measuring and promoting SARS-CoV-2 vaccine equity: development of a COVID-19 vaccine equity index. Health Equity. 2021;5(1):476-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dover DC, Belon AP.. The health equity measurement framework: a comprehensive model to measure social inequities in health. Int J Equity Health. 2019;18(1):36. doi: 10.1186/s12939-019-0935-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barsanti S, Salmi LR, Bourgueil Y, et al. Strategies and governance to reduce health inequalities: evidences from a cross-European survey. Glob Health Res Policy. 2017;2:18. doi: 10.1186/s41256-017-0038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Community-Based Solutions to Promote Health Equity in the United States. In: Baciu A, Negussie Y, Geller A, Weinstein JN, eds. Communities in Action: Pathways to Health Equity. Washington (DC: ): National Academies Press (US; ); 2017. [PubMed] [Google Scholar]
- 45. Rich JA, Miech EJ, Bilal U, Corbin TJ.. How education and racial segregation intersect in neighborhoods with persistently low COVID-19 vaccination rates in Philadelphia. BMC Public Health. 2022;22(1):1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The administrative data underlying this article were accessed from ICES. External researchers can access the dataset used in this analysis by requesting access through ICES Data and Analytic Services; details of this process and the required forms are available at: https://www.ices.on.ca/DAS/Public-Sector/Access-to-ICES-Data-Process.