Abstract
We examined ice-nucleating particles (INPs) in the plumes of the Tocantins and Amazon rivers, which drain watersheds with different proportions of degraded land. The concentration of INPs active at −15°C (INP−15) was an order of magnitude lower in the Tocantins (mean = 13.2 ml−1; s.d. = 7.8 ml−1), draining the more degraded watershed, compared with the Amazon (mean = 175.8 ml−1; s.d. = 11.2 ml−1), where the concentration was also significantly higher than in Atlantic surface waters (mean = 3.2 ml−1; s.d. = 2.3 ml−1). Differences in heat tolerance suggest that INPs emitted by the Amazon rainforest to the atmosphere or washed into the river might originate from contrasting sources on top of and below the rainforest canopy, respectively. For the Amazon River, we estimate a daily discharge of 1018 INP−15 to Atlantic waters. Rivers in cooler climate zones tend to have much higher concentrations of INPs and could, despite a smaller water volume discharged, transfer even larger absolute numbers of INP−15 to shelf waters than does the Amazon. To what extent these terrestrial INPs become aerosolized by breaking waves and bubble-bursting remains an open question.
Keywords: ice-nucleating particles, river plume, Amazon, Tocantins, watershed, atmosphere
1. Introduction
Ice-nucleating particles (INPs) are a prerequisite for initial ice formation in clouds at temperatures warmer than −38°C [1,2]. Ice is the starting point for most precipitation above continents [3]. Land and ocean both emit INPs, but ice-nucleation active site densities in sea-surface aerosols are a few orders of magnitude lower than in continental boundary layer aerosols [4]. Some rivers have been reported to carry high concentrations of INPs that have been produced on land rather than in the river itself [5–9]. When discharged to the ocean, they probably enhance INP concentration at the surface of shelf waters, possibly creating source areas from where INPs of marine and terrestrial origins are aerosolized in parallel by wave-breaking and bubble-bursting. Earlier work on the number of biological INPs produced by decaying leaves has suggested that biological INP production on land may increase by orders of magnitude successively from tropical to temperate to cold climate zones [10,11]. Further, different INP densities are observed on contrasting types of vegetation, where land-use change is suspected to modify INP sources and thereby the formation of precipitation [12].
Perhaps, the study of INPs in rivers draining watersheds with different land cover can reveal effects of land-use change on INP production and, subsequently, on the atmospheric hydrological cycle [12,13]. Here, we report on INPs in the plumes of the Amazon and Tocantins rivers. The Amazon River drains the largest watershed worldwide and has an annual average flow rate at the Óbidos station of 150 500 m3 s−1 [14]. The watershed of the Tocantins River is about six times smaller and its average discharge at the Tucurui station is about 13 times smaller [15] than the Amazon. Seasonal changes in rainfall [15] are reflected in discharge, which in the Amazon ranges from approximately 100 200 to 240 000 m3 s−1 [14].
A much larger fraction of the Tocantins watershed has deteriorated due to deforestation and drought over the past decades than is the case for the Amazon watershed [16]. Unlike the Amazon, the Tocantins watershed also includes dryer climate types. Thus, effects of land-use change cannot be separated from climate effects. However, both climate change and deforestation are expected to lead to drier conditions in the Amazon region [17]. Therefore, INPs in the Tocantins watershed today might be a proxy for what they might become in the Amazon watershed in future. We expected differences in land cover between both watersheds to result in different INP populations in the plumes of the Amazon and Tocantins rivers. These differences should emerge primarily in INPs active at −15°C or above (INP−15), because most INP−15 are of biological origin and are ultimately derived from plants and associated microorganisms [18–20].
2. Material and methods
2.1. Land cover in the Amazon and Tocantins watersheds
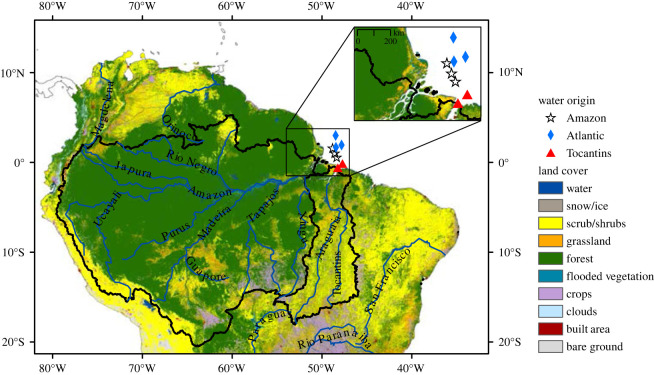
Currently, the majority of the Amazon River watershed is covered by forest (81%), followed by scrub and shrubs (10%) (figure 1). Grassland (4%) and crops (3%) cover a smaller proportion than in the Tocantins River watershed (9% and 7%, respectively), where the share of forest is only 36%, and scrub and shrubs are more abundant (45%) (figure 1).
Figure 1.
Land cover (100 × 100 m resolution) in the watersheds of the Amazon River (large, western area bordered by black line) and the Tocantins River (smaller, eastern area bordered by black line). Sampling locations in the Amazon (white stars) and Tocantins (red triangles) river plumes, and in the Atlantic (blue diamonds). Sources: Land cover: Sentinel-2, 2020, resampled at 100 m resolution (https://www.arcgis.com/apps/instant/media/index.html?appid=fc92d38533d440078f17678ebc20e8e2); watersheds: Agência Nacional de Águas e Saneamento Básico (ANA) (https://metadados.snirh.gov.br/geonetwork/srv/search?keyword=Ottobacia).
2.2. Field sampling
Water was sampled towards the end of the rainy season from the plumes of the Amazon River and the Tocantins River during the cruise M174 of the research vessel R/V Meteor [21]. The focus of the cruise was on exploring the fate of nitrogen in the Amazon River plume and the western tropical North Atlantic [22]. Surface water pumped from the moon pool, an opening in the base of the hull 5.70 m below the water line, was collected in sterile polypropylene tubes (40 ml). To prevent eventual contamination with residues in the pumping system, the water was kept running for several minutes beforehand. Samples were immediately put into a −20°C freezer and remained frozen until analysis. High-resolution data on water quality was collected with the CTD-system ‘SBE 911plus’ (SN-0603, SEABIRD-ELECTRONICS, USA), with added sensors to measure pressure, temperature (2× SBE 3), conductivity (2× SBE 4), oxygen concentration (2× SBE 43), chlorophyll-a fluorescence (683 nm), turbidity, PAR, SPAR and nitrate (SUNA sensor).
Salinity data confirms that samples collected at the mouths of the Amazon and Tocantins rivers were indeed river water with little or no seawater (table 1). The fluorescence values were practically the same in both river plumes. Turbidity was lower by a factor of two or three in one sample from the Amazon River and one from the Tocantins River plume. Both of these samples also had a slightly enhanced salinity.
Table 1.
Origin of samples analysed for their content of INPs and properties of the sampled waters.
| origin of water | sample no. | date | time (UTC) | latitude | longitude | conductivity (mS cm−1) | salinity (PSU) | fluorescence (mg m−3) | turbidity (NTU) |
|---|---|---|---|---|---|---|---|---|---|
| Tocantins | 1 | 23.04.2021 | 07:23 | 00°07.30' S | 047°43.87' W | 7.5 | 3.9 | 1.1 | 5.3 |
| 2 | 23.04.2021 | 15:54 | 00°35.85' S | 048°14.63' W | 2.0 | 0.9 | 1.1 | 9.7 | |
| Amazon | 3 | 24.04.2021 | 12:57 | 00°35.83' N | 048°22.40' W | 1.1 | 0.5 | 1.0 | 9.7 |
| 4 | 24.04.2021 | 16:07 | 01°02.91' N | 048°35.18' W | 1.6 | 0.7 | 1.1 | 9.7 | |
| 5 | 24.04.2021 | 00:48 | 01°36.73' N | 048°50.29' W | 6.4 | 3.2 | 1.2 | 3.2 | |
| Atlantic | 6 | 29.04.2021 | 07:40 | 03°00.15' N | 048°28.91' W | 57.3 | 35.1 | 0.09 | 0.08 |
| 7 | 30.04.2021 | 16:09 | 04°24.35' N | 049°07.29' W | 57.4 | 36.0 | 0.22 | 0.08 | |
| 8 | 03.05.2021 | 20:00 | 05°02.01' N | 050°21.12' W | 57.5 | 35.6 | 0.08 | 0.08 |
2.3. Analysis of INPs
Immediately after melting a sample at room temperature, we transferred a subsample of 100 µl in each of 52 Eppendorf safe-lock tubes (0.5 ml) for analysis on the ice-nucleation detection apparatus described in Stopelli et al. [23]. Tubes were cooled at a rate of 0.3°C min−1 from 0 to −20°C. The freezing temperature of the subsample in each tube was detected automatically and the cumulative INP concentration at every 0.5°C step was calculated according to Vali [24]. When all 52 droplets had frozen before reaching −12°C, we reanalysed samples in a 1:10 dilution with ultrapure water (Sigma-Aldrich, W4502-1L) to extend the measurement range to −15°C. Duplicate blank tests with only ultrapure water confirmed it was free of INP−15. For characterizing INPs in terms of their heat sensitivity, we performed the same freezing assay two more times: after treating the same array of subsamples in their Eppendorf tubes for 10 min in a water bath at 60°C and after a second such treatment at 95°C. In the Atlantic water samples, we shifted measured values by 2°C towards the warmer end of the temperature scale to account for freezing depression due to high salt concentration. We derived the 2°C value from the difference between freezing spectra with Snomax in pure water and with Snomax in pure water to which we added an equivalent of 35 g l−1 NaCl.
3. Results and discussion
Cruise M174 gave us the opportunity to take a snapshot of INPs in a region we otherwise could not have accessed. The sampled river plumes integrate diversity and variation of innumerous watercourses that supply the Tocantins and Amazon rivers before they reach the Atlantic. Successive integration of distant tributaries with asynchronous variation within a large watershed confers a relative stability in terms of biotic and abiotic properties to the water discharged to the ocean [25]. Therefore, we expect our investigation to afford meaningful interpretation despite its snapshot character and the small number of samples.
3.1. Concentration of INPs in river plumes
The onset of freezing in 1 ml of water was observed at −6.5°C in the Amazon River plume and at −12.5°C in the Tocantins River plume. Differences between samples taken from within the same river plume were small (figure 2). The number of INP−15 in 1 ml of water was one order of magnitude higher in the Amazon River plume (mean = 175.8; s.d. = 11.2) than the Tocantins River plume (mean = 13.2; s.d. = 7.8). While this difference was statistically significant (p < 0.001), the difference between the Tocantins River plume and Atlantic surface water (mean = 3.2; s.d. = 2.3) was much smaller and statistically insignificant (p = 0.31). From the annual average flow rate of both rivers and the concentration difference between their plumes and Atlantic surface water, we estimate a net daily discharge in the order of 1018 INP−15 for the Amazon and 1016 INP−15 for the Tocantins.
Figure 2.
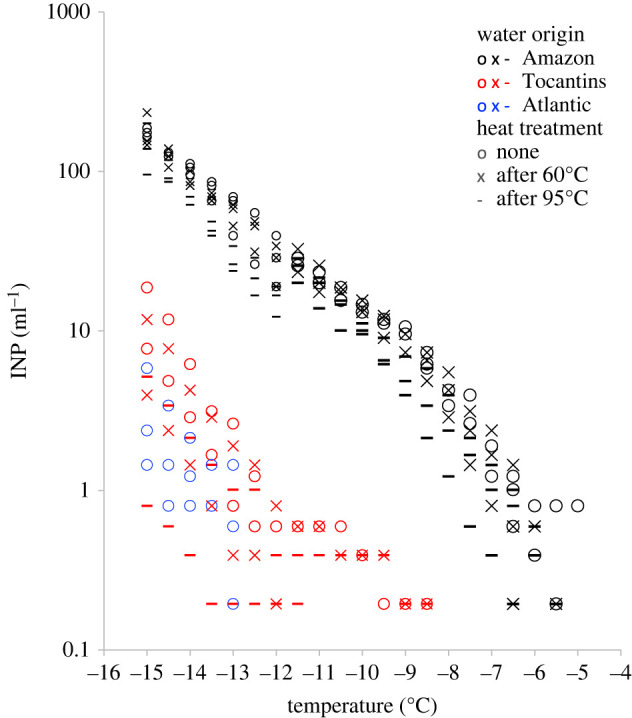
Concentration of INPs active between −5 and −15°C in three samples of the Amazon River plume (black), two samples of the Tocantins River plume (red) and three samples of Atlantic surface water (blue). The Atlantic samples, all containing more than 35 g salt l−1, were adjusted for freezing point depression. We analysed the same sample material three times: first, without any treatment (circles); second, after heating to 60°C (cross symbols); and third, after heating to 95°C (dash symbols). In addition, we analysed Amazon River plume samples in a 10-fold dilution with pure water (smaller symbols) to determine INP concentration at lower temperatures than was possible with undiluted samples (data in electronic supplementary material).
A substantial fraction of INPs found in river water pass through a 0.22 µm filter [5–8]. In a limnological context, these small INPs would be referred to as dissolved organic matter (DOM). The quality of DOM in rivers clearly depends on land cover in the watershed area [26], which might explain some of the difference in INPs between the Tocantins and Amazon. The 101 and 102 INP−15 ml−1 in the Tocantins and the Amazon rivers, respectively, were three and two orders of magnitude lower than what has been reported from the lower reaches of the Mississippi River [7], the largest river in North America. Interestingly, the difference in INP−15 on decaying leaves from tropical and temperate regions is of the same direction and order of magnitude [10]. Therefore, despite an about tenfold smaller discharge, the Mississippi River might still transfer an order of magnitude more INPs from land to ocean than does the Amazon. Mississippi freshwater influences substantial areas in the northern Gulf of Mexico [27], where discharged INPs could become aerosolized by wave-breaking and bubble-bursting [28,29], as are INPs of marine origin in the Gulf of Mexico [30], perhaps with atmospheric implications. For example, at their coastal observatory in Yucatan, Ladino et al. [31] found an enhanced atmospheric concentration of INP−15 in marine air masses arriving with two cold fronts from northerly directions. Also in other regions, shelf waters enriched with INPs from large rivers could be an atmospherically relevant source of INPs to marine air masses. Furthermore, the origin of highly active INPs in the atmosphere above the North Pole has been traced back to the region of shallow waters over the Russian continental shelf [32]. Unfortunately, the INP concentration in Siberian rivers feeding these shelf waters is still unknown, but the analyses by Schnell & Vali [10,11] on decaying leaves from different climatic zones suggest even higher INP concentrations in the Siberian rivers than in rivers in temperate regions. In addition, thawing permafrost in Siberian river catchments probably adds further INPs that are discharged into shelf waters of the Arctic Ocean [33].
3.2. River water and the atmosphere can have different sources of INPs
The concentration of INPs was different between the two river plumes, as was the pattern of heat sensitivity in these INPs, although other parameters known to drive INP concentration—such as turbidity [8] and humic-like substances [34], of which fluorescence is a measure—were similar in both river plumes (table 1).
Typically, heat tolerance of INPs decreases markedly with increasing activation temperature, whether in soil [18,35], air [36–38], precipitation [37,39] or seawater [40,41]. Therefore, in the following we focus on a temperature window from −13 to −15°C and average all measurements made in 0.5°C steps within this window.
In the Tocantins water, 39% of all INPs were heat sensitive and only 25% were heat tolerant (table 2). In the Amazon water, few INPs were heat sensitive but 66% were heat tolerant. This fraction is nearly four times as large as the heat-tolerant fraction of INPs in airborne particles above the middle of the Amazon rainforest (18%, s.d. = 15%; derived from values in fig. 6 in [38]). An explanation for this discrepancy could be that INPs in the air above dense canopies and INPs in water below them are from separate and contrasting sources. Seven-day back trajectories indicate that air masses above the canopy had arrived from the Atlantic and crossed the coastline near the mouths of the Amazon and Tocantins rivers before reaching the air-sampling location about 1200 km further inland [38]. If the air masses contained INPs from the Amazon River plume, their signature in terms of heat sensitivity could have been unrecognizably diluted by further INPs taken up during the 1200 km journey westward over the Amazon rainforest.
Table 2.
Fraction of INPs active between −13 and −15°C by category of heat sensitivity. The fraction of INPs lost in the 60°C treatment is termed heat sensitive; the fraction tolerating 60°C but deactivated by 95°C is termed moderately heat tolerant; and the fraction still active after both treatments is called heat tolerant. Mean values are for five measurement points on three and two samples from the Amazon and Tocantins river plumes, respectively (s.d. = standard deviation).
| category of INPs | Amazon |
Tocantins |
||
|---|---|---|---|---|
| mean | s.d. | mean | s.d. | |
| heat sensitive | 0.06 | 0.16 | 0.39 | 0.14 |
| moderately heat tolerant | 0.29 | 0.14 | 0.36 | 0.05 |
| heat tolerant | 0.66 | 0.18 | 0.25 | 0.13 |
During the rainy season, when samples were taken, much of the DOM in river water probably originates from compounds washed from litter and soil into the river [42], whereas aerosol particles above the canopy more likely are emitted from the canopy itself. When raindrops impinge on leaves and branches, they generate aerosols containing microorganisms from these surfaces. Driving mechanisms can involve bubble-bursting at the surface of water films by which tiny droplets (10–100 µm) and particles are aerosolized [43]. Other possibilities of aerosol generation by rain impact on canopies include micro-splashing [44]. Droplets thus produced can be smaller than 50 µm [45] and get lofted above a canopy [46], where they evaporate in the drier air [47] and enrich it with the INPs [48,49] that were initially contained in the splash droplets. Whatever the mechanism, the most likely source of INPs aerosolized by raindrops impinging on the canopy would be ice-nucleation active epiphytic microorganisms, most of which are heat sensitive and only some are moderately heat tolerant [20]. Heat-sensitive INPs washed by rain from canopies will mix on the ground with a probably much larger pool of INPs that themselves are very unlikely to be transported through and above the canopy of the tropical rainforest. At night, the air below the canopy is persistently decoupled from that above it [50,51]. During the daytime, larger turbulent structures may occasionally penetrate the dense foliage of the canopy and result in an occasional exchange of air and particles. However, observations of fluorescent biological aerosol particles above and below a rainforest canopy in Borneo have suggested only a weak coupling of larger aerosol particles between these layers [52]. Known heat-tolerant INPs that could be washed from litter and soil into the river are mineral particles [53], lignin [54] and other heat-stable organics [9,36], such as macromolecules of pollen [55]. A much stronger source of such heat-tolerant INPs below the canopy in the Amazon compared with the Tocantins watershed would explain (i) the greater abundance of INPs in the Amazon, (ii) the larger fraction of heat-tolerant INPs in the Amazon compared with the Tocantins River plume, and (iii) the difference in heat-tolerant fraction between INPs in the river plume and INPs in the air above the Amazon rainforest.
4. Conclusion
To conclude, land-use change probably affects the abundance and composition of INPs washed into watercourses. Although INPs might be produced at higher rates where rainforests are less degraded, the dense canopies of intact rainforests probably obstruct the stronger sources from emitting to the atmosphere. Therefore, the difference in INP concentration and heat-tolerance in the two investigated river plumes is unlikely to translate to similar differences in INPs emitted directly from their watersheds to the atmosphere above. Nevertheless, INPs discharged by the Amazon River into the Atlantic significantly increase the INP concentration in water above the continental shelf and could affect the INP concentration and composition in marine air masses. Although the Amazon is the largest river in terms of discharge, smaller rivers in temperate or cold regions might transfer even more INPs to shelf waters, because decaying leaves are a stronger source of INPs in colder climates than in a tropical climate. Variations in atmospheric INP concentration reported from the Gulf of Mexico and the Arctic Ocean lend initial support to the idea that INPs transported by large rivers from land to shelf waters could have a discernible impact on the atmospheric INP concentration in marine air masses.
Data accessibility
The data are provided in electronic supplementary material [56].
Acknowledgements
We thank the captain, crew and all scientists on R/V Meteor during the cruise M174. We thank the principle investigator Maren Voss for organizing the cruise. We are grateful to Pedro Batista for his support in the analysis of land cover in the Amazon and Tocantins watersheds. This article has greatly benefited from comments and suggestions made by three referees.
Authors' contributions
A.E.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—review and editing; E.B.: conceptualization, data curation, investigation, methodology, validation, writing—review and editing; C.F.: conceptualization, formal analysis, funding acquisition, methodology, project administration, supervision, validation, writing—review and editing; F.C.: conceptualization, data curation, formal analysis, investigation, project administration, supervision, validation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The cruise was funded by the German Science Foundation under GPF 19-1-13.
References
- 1.Findeisen W. 1938. Die kolloidmeteorologischen Vorgänge bei der Niederschlagsbildung (Colloidal meteorological processes in the formation of precipitation). Meteorol. Z. 55, 121-133. (translated and edited by Volken E, Giesche AM, Brönnimann S. 2015 Meteorol. Z., 24 ( 10.1127/metz/2015/0675) [DOI] [Google Scholar]
- 2.Kanji ZA, Ladino LA, Wex H, Boose Y, Burkert-Kohn M, Cziczo DJ, Krämer M. 2017. Overview of ice nucleating particles. Meteorol. Monogr. 58, 1.1-1.33. ( 10.1175/AMSMONOGRAPHS-D-16-0006.1) [DOI] [Google Scholar]
- 3.Mülmenstädt J, Sourdeval O, Delanoë J, Quaas J. 2015. Frequency of occurrence of rain from liquid-, mixed-, and ice-phase clouds derived from A-Train satellite retrievals. Geophys. Res. Lett. 42, 6502-6509. ( 10.1002/2015GL064604) [DOI] [Google Scholar]
- 4.DeMott PJ, et al. 2015. Sea spray aerosol as a unique source of ice nucleating particles. Proc. Natl Acad. Sci. USA 113, 5797-5803. ( 10.1073/pnas.1514034112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moffett BF. 2016. Fresh water ice nuclei. Fundam. Appl. Limnol. 188, 19-23. ( 10.1127/fal/2016/0851) [DOI] [Google Scholar]
- 6.Larsen JA, Conen F, Alewell C. 2017. Export of ice nucleating particles from a watershed. R. Soc. Open Sci. 4, 170213. ( 10.1098/rsos.170213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moffett BF, Hill TCJ, DeMott PJ. 2018. Abundance of biological ice nucleating particles in the Mississippi and its major tributaries. Atmosphere 9, 307. ( 10.3390/atmos9080307) [DOI] [Google Scholar]
- 8.Knackstedt A, et al. 2018. Terrestrial origin for abundant riverine nanoscale ice-nucleating particles. Environ. Sci. Technol. 52, 12 358-12 367. ( 10.1021/acs.est.8b03881) [DOI] [PubMed] [Google Scholar]
- 9.Borduas-Dedekind N, Ossola R, David RO, Boynton LS, Weichlinger V, Kanji ZA, McNeill K. 2019. Photomineralization mechanism changes the ability of dissolved organic matter to activate cloud droplets and to nucleate ice crystals. Atmos. Chem. Phys. 19, 12 397-12 412. ( 10.5194/acp-19-12397-2019) [DOI] [Google Scholar]
- 10.Schnell RC, Vali G. 1973. Worldwide souce of leaf derived freezing nuclei. Nature 246, 212-213. ( 10.1038/246212a0) [DOI] [Google Scholar]
- 11.Schnell R, Vali G. 1976. Biogenic ice nuclei: part I. Terrestrial and marine sources. J. Atmos. Sci. 33, 1554-1564. () [DOI] [Google Scholar]
- 12.Morris CE, Conen F, Huffman JA, Phillips V, Pöschl U, Sands DC. 2014. Bioprecipitation: a feedback cycle linking earth history, ecosystem dynamics and land use through biological ice nucleators in the atmosphere. Glob. Change Biol. 20, 341-351. ( 10.1111/gcb.12447) [DOI] [PubMed] [Google Scholar]
- 13.Sheil D. 2018. Forests, atmospheric water and an uncertain future: the new biology of the global water cycle. For. Ecosyst. 5, 19. ( 10.1186/s40663-018-0138-y) [DOI] [Google Scholar]
- 14.Espinoza Villar JC, et al. 2009. Contrasting regional discharge evolutions in the Amazon basin (1974–2004). J. Hydrol. 375, 297-311. ( 10.1016/j.jhydrol.2009.03.004) [DOI] [Google Scholar]
- 15.García NO, Mechoso CR. 2005. Variability in the discharge of South American rivers and in climate / Variabilité des débits de rivières d'Amérique du Sud et du climat. Hydrol. Sci. J. 50, null-478. ( 10.1623/hysj.50.3.459.65030) [DOI] [Google Scholar]
- 16.Bullock EL, Woodcock CE, Souza C Jr, Olofsson P. 2020. Satellite-based estimates reveal widespread forest degradation in the Amazon. Glob. Chang. Biol. 26, 2956-2969. ( 10.1111/gcb.15029) [DOI] [PubMed] [Google Scholar]
- 17.Swann LS, Longo M, Knox RG, Lee E, Moorcroft PR. 2015. Future deforestation in the Amazon and consequences for South American climate. Agric. For. Meteorol. 214–215, 12-24. ( 10.1016/j.agrformet.2015.07.006) [DOI] [Google Scholar]
- 18.Hill TCJ, DeMott PJ, Tobo Y, Fröhlich-Nowoisky J, Moffett BF, Franc GD, Kreidenweis SM. 2016. Sources of organic ice nucleating particles in soils. Atmos. Chem. Phys. 16, 7195-7211. ( 10.5194/acp-16-7195-2016) [DOI] [Google Scholar]
- 19.Testa B, et al. 2021. Ice nucleating particle connections to regional Argentinian land surface emissions and weather during the Cloud, Aerosol, and Complex Terrain Interactions experiment. J. Geophys. Res Atmos. 126, e2021JD035186. ( 10.1029/2021JD035186) [DOI] [Google Scholar]
- 20.Conen F, Einbock A, Mignani C, Hüglin C. 2022. Measurement report: ice nucleating particles active ≥−15°C in free tropospheric air over western Europe. Atmos. Chem. Phys. 22, 3433-3444. ( 10.5194/acp-22-3433-2022) [DOI] [Google Scholar]
- 21.Voss M. 2021. Master tracks in different resolutions of METEOR cruise M174, Las Palmas - Emden, 2021-04-12 - 2021-05-29. PANGAEA Digital Repository. ( 10.1594/PANGAEA.935041) [DOI]
- 22.Voss M, et al. 2021. Nitrogen cycling and physical processes in the Amazon River plume, Cruise No. M174, 12/04/2021–30/05/2021, Las Palmas (Spain) - Emden (Germany). METEOR-Berichte M174, 1-40. ( 10.48433/cr_m174) [DOI] [Google Scholar]
- 23.Stopelli E, Conen F, Zimmermann L, Alewell C, Morris CE. 2014. Freezing nucleation apparatus puts new slant on study of biological ice nucleators in precipitation. Atmos. Meas. Tech. 7, 129-134. ( 10.5194/amt-7-129-2014) [DOI] [Google Scholar]
- 24.Vali G. 1971. Quantitative evaluation of experimental results on the heterogeneous freezing nucleation of supercooled liquids. J. Atmos. Sci. 28, 402-409. (). [DOI] [Google Scholar]
- 25.Moore JW, et al. 2015. Emergent stability in a large, free-flowing watershed. Ecology 96, 340-347. ( 10.1890/14-0326.1) [DOI] [PubMed] [Google Scholar]
- 26.Wagner S, Riedel T, Niggemann J, Vähätalo AV, Dittmar T, Jaffé R. 2015. Linking the molecular signature of heteroatomic dissolved organic matter to watershed characteristics in World Rivers. Environ. Sci. Technol. 49, 13 798-13 806. ( 10.1021/acs.est.5b00525) [DOI] [PubMed] [Google Scholar]
- 27.Dzwonkowski B, et al. 2018. Tracking sea surface salinity and dissolved oxygen on a river-influenced, seasonally stratified shelf, Mississippi Bight, northern Gulf of Mexico. Cont. Shelf Res. 169, 25-33. ( 10.1016/j.csr.2018.09.009) [DOI] [Google Scholar]
- 28.Axson JL, May NW, Colón-Bernal ID, Pratt KA, Ault AP. 2016. Lake spray aerosol: a chemical signature from individual ambient particles. Environ. Sci. Technol. 50, 9835-9845. ( 10.1021/acs.est.6b01661) [DOI] [PubMed] [Google Scholar]
- 29.McCluskey CS, et al. 2018. Marine and terrestrial organic ice-nucleating particles inpristine marine to continentally influenced Northeast Atlantic air masses. J. Geophys. Res. Atmos. 123, 6196-6212. ( 10.1029/2017JD028033) [DOI] [Google Scholar]
- 30.Rosinski J, Haagenson PL, Nagamoto CT, Quintana B, Parungo F, Hoyt SD. 1988. Ice-forming nuclei in air masses over the Gulf of Mexico. J. Aerosol. Sci. 19, 539-551. ( 10.1016/0021-8502(88)90206-6) [DOI] [Google Scholar]
- 31.Ladino LA, et al. 2019. Ice-nucleating particles in a coastal tropical site. Atmos. Chem. Phys. 19, 6147-6165. ( 10.5194/acp-19-6147-2019) [DOI] [Google Scholar]
- 32.Porter GCE, et al. 2022. Highly active ice-nucleating particles at the summer North Pole. J. Geophys. Res. 127, e2021JD036059. ( 10.1029/2021JD036059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creamean JM, Hill TCJ, DeMott PJ, Uetake J, Kreidenweis S, Douglas TA. 2020. Thawing permafrost: an overlooked source of seeds for Arctic cloud formation. Environ. Res. Lett. 15, 084022. ( 10.1088/1748-9326/ab87d3) [DOI] [Google Scholar]
- 34.Chen J, et al. 2021. Atmospheric humic-like substances (HULIS) act as ice active entities. Geophys. Res. Lett. 48, e2021GL092443. ( 10.1029/2021GL092443) [DOI] [Google Scholar]
- 35.O'Sullivan D, et al. 2014. Ice nucleation by fertile soil dusts: relative importance of mineral and biogenic components. Atmos. Chem. Phys. 14, 1853-1867. ( 10.5194/acp-14-1853-2014) [DOI] [Google Scholar]
- 36.Suski KJ, Hill TCJ, Levin EJT, Miller A, DeMott PJ, Kreidenweis SM. 2018. Agricultural harvesting emissions of ice-nucleating particles. Atmos. Chem. Phys. 18, 13755-13 771. ( 10.5194/acp-18-13755-2018) [DOI] [Google Scholar]
- 37.Šantl-Temkiv T, Lange R, Beddows D, Rauter U, Pilgaard S, Dall'Osto M, Gunde-Cimerman N, Massling A, Wex H. 2019. Biogenic sources of ice nucleating particles at the high Arctic site Villum Research Station. Environ. Sci. Technol. 53, 10 580-10 590. ( 10.1021/acs.est.9b00991) [DOI] [PubMed] [Google Scholar]
- 38.Patade S, et al. 2021. Empirical formulation for multiple groups of primary biological ice nucleating particles from field observations over Amazonia. J. Atmos. Sci. 78, 2195-2220. ( 10.1175/JASD-20-0096.1) [DOI] [Google Scholar]
- 39.Chen J, Wu Z, Chen J, Reicher N, Fang X, Rudich Y, Hu M. 2021. Size-resolved atmospheric ice-nucleating particles during East Asian dust events. Atmos. Chem. Phys. 21, 3491-3506. ( 10.5194/acp-21-3491-2021) [DOI] [Google Scholar]
- 40.Irish VE, et al. 2017. Ice-nucleating particles in Canadian Arctic sea-surface microlayer and bulk seawater. Atmos. Chem. Phys. 17, 10 583-10 595. ( 10.5194/acp-17-10583-2017) [DOI] [Google Scholar]
- 41.Irish VE, et al. 2019. Revisiting properties and concentrations of ice-nucleating particles in the sea surface microlayer and bulk seawater in the Canadian Arctic during summer. Atmos. Chem. Phys. 19, 7775-7787. ( 10.5194/acp-19-7775-2019) [DOI] [Google Scholar]
- 42.Seidel M, Dittmar T, Ward ND, Krusche AV, Jeffrey ER, Yager PL, Medeiros PM. 2016. Seasonal and spatial variability of dissolved organic matter composition in the lower Amazon River. Biogeochemistry 131, 281-302. ( 10.1007/s10533-016-0279-4) [DOI] [Google Scholar]
- 43.Joung YS, Ge Z, Buie CR. 2017. Bioaerosol generation by raindrops on soil. Nat. Commun. 8, 14668. ( 10.1038/ncomms14668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thoroddsen S, Takehara K, Etoh T. 2012. Micro-splashing by drop impacts. J. Fluid Mech. 706, 560-570. ( 10.1017/jfm.2012.281) [DOI] [Google Scholar]
- 45.Dunkerley D. 2009. Evaporation of impact water droplets in interception processes: historical precedence of the hypothesis and a brief literature overview. J. Hydrol. 376, 599-604. ( 10.1016/j.jhydrol.2009.08.004) [DOI] [Google Scholar]
- 46.Dunin FX, O'Loughlin EM, Reyenga W. 1988. Interception loss from eucalypt forest: lysimeter determination of hourly rates for long term evaluation. Hydrol. Process. 2, 315-329. ( 10.1002/hyp.3360020403) [DOI] [Google Scholar]
- 47.Murakami S. 2006. A proposal for a new forest canopy interception mechanism: splash droplet evaporation. J. Hydrol. 319, 72-82. ( 10.1016/j.jhydrol.2005.07.002) [DOI] [Google Scholar]
- 48.Huffman JA, et al. 2013. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos. Chem. Phys. 13, 6151-6164. ( 10.5194/acp-13-6151-2013) [DOI] [Google Scholar]
- 49.Mignani C, Wieder J, Sprenger MA, Kanji ZA, Henneberger J, Alewell C, Conen F. 2021. Towards parameterising atmospheric concentrations of ice-nucleating particles active at moderate supercooling. Atmos. Chem. Phys. 21, 657-664. ( 10.5194/acp-21-657-2021) [DOI] [Google Scholar]
- 50.Martens CS, et al. 2004. Radon fluxes in tropical forest ecosystems of Brazilian Amazonia: night-time CO2 net ecosystem exchange derived from radon and eddy covariance methods. Glob. Chang. Biol. 10, 618-629. ( 10.1111/j.1365-2486.2004.00764.x) [DOI] [Google Scholar]
- 51.Whitehead JD, et al. 2010. Aerosol fluxes and dynamics within and above a tropical rainforest in South-East Asia. Atmos. Chem. Phys. 10, 9369-9382. ( 10.5194/acp-10-9369-2010) [DOI] [Google Scholar]
- 52.Gabey AM, Gallagher MW, Whitehead J, Dorsey JR, Kaye PH, Stanley WR. 2010. Measurements and comparison of primary biological aerosol above and below a tropical forest canopy using a dual channel fluorescence spectrometer. Atmos. Chem. Phys. 10, 4453-4466. ( 10.5194/acp-10-4453-2010) [DOI] [Google Scholar]
- 53.Daily MI, Tarn MD, Whale TF, Murray BJ. 2021. The sensitivity of the ice-nucleating ability of minerals to heat and the implications for the heat test for biological ice nucleators. Atmos. Meas. Tech. Discuss. 15, 2635–2665. ( 10.5194/amt-15-2635-2022) [DOI] [Google Scholar]
- 54.Bogler S, Borduas-Dedekind N. 2020. Lignin's ability to nucleate ice via immersion freezing and its stability towards physicochemical treatments and atmospheric processing. Atmos. Chem. Phys. 20, 14 509-14 522. ( 10.5194/acp-20-14509-2020) [DOI] [Google Scholar]
- 55.Pummer BG, Bauer H, Bernardi J, Bleicher S, Grothe H. 2012. Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen. Atmos. Chem. Phys. 12, 2541-2550. [Google Scholar]
- 56.Einbock A, Burtscher E, Frey C, Conen F. 2023. Export of ice-nucleating particles from watersheds: results from the Amazon and Tocantins river plumes. Figshare. ( 10.6084/m9.figshare.c.6405756) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Voss M. 2021. Master tracks in different resolutions of METEOR cruise M174, Las Palmas - Emden, 2021-04-12 - 2021-05-29. PANGAEA Digital Repository. ( 10.1594/PANGAEA.935041) [DOI]
- Einbock A, Burtscher E, Frey C, Conen F. 2023. Export of ice-nucleating particles from watersheds: results from the Amazon and Tocantins river plumes. Figshare. ( 10.6084/m9.figshare.c.6405756) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in electronic supplementary material [56].