Abstract
Diabetic nephropathy causes cardiovascular complications among individuals with diabetes which results in decreased kidney function and overall physical decline. The objective of this systematic review was to determine effects of exercise on various renal function parameters amond individuals with type 2 diabetes and nephropathy. It was registered with PROSPERO (CRD42020198754). Total 6 databases (PubMed/Medline, Scopus, Web of Science, CINAHL, ProQuest, and Cochrane) were searched. Among 1734 records, only four randomized controlled trials were included. The review included a total of 203 participants (103 in the intervention group and 100 in the control/standard group) with type 2 diabetic nephropathy or stage 2,3, or 4 of chronic kidney disease. The meta-analysis showed no effects of exercise on serum creatinine, serum cystatin c and varied eGFR equations. However, exercise decreased urinary albumin to creatinine ratio, urinary protein to creatinine ratio, serum urea nitrogen, creatinine clearance, and urinary protein excretion while increasing urea clearance. Limited evidence on the reno-protective role of exercise demands future research in this direction.
Keywords: Chronic kidney disease, Diabetes mellitus, Diabetic kidney disease, Exercise, Kidney function, Nephropathy
Abbreviations: CI, confidence interval; eGFR, estimated glomerular filtration rate; RCT, randomized controlled trial
الملخص
أهداف البحث
يسبب اعتلال الكلية السكري مضاعفات في القلب والأوعية الدموية لدى الأفراد المصابين بداء السكري، مما يؤدي إلى انخفاض وظائف الكلى والتدهور الجسدي العام. كان الهدف من هذه المراجعة المنهجية هو تحديد تأثير التمرين على متغيرات وظائف الكلى المختلفة بين الأفراد المصابين بداء السكري من النوع الثاني واعتلال الكلية.
طرق البحث
تم تسجيل المراجعة المنهجية في قاعدة بيانات "بروسبيرو"، وتم البحث في قواعد البيانات التالية: ميدلاين، وسكوبس، وشبكة العلوم، وسيناهل، وبروكويست، وكوكران. من بين 1734 سجل وجدت فقط أربع تجارب معشاة ذات شواهد، وتم تضمينها في التوليف النوعي والكمي بعد فحص أهلية الدراسة في مرحلة العنوان والملخص والنص الكامل.
النتائج
تضمنت المراجعة ما مجموعه 203 شخصا (103 في مجموعة التدخل و 100 في المجموعة الضابطة / القياسية) مصابين باعتلال الكلية السكري من النوع 2 أو مرض الكلى المزمن في المراحل 2 و 3 و 4. استخدمت ثلاث من الدراسات الأربع مبدأ "فيت" (التكرار، والشدة، والوقت، والنوع) في تدريب التمرينات والوصفات الطبية. تلقت مجموعة التدخل تمارين هوائية ومقاومة، منزلية، خاضعة للإشراف، ومنظمة. أظهر التحليل التلوي عدم وجود تأثير للتمرين على كرياتينين المصل، وسيستاتين المصل، ومعادلة الكرياتينين، ومعادلة سيستاتين، ومعادلة الكرياتينين-سيستاتين. ومع ذلك ، فقد أدت التمارين الرياضية إلى خفض نسبة الألبومين البولي إلى نسبة الكرياتينين، ونسبة البروتين البولي إلى نسبة الكرياتينين، ونتروجين اليوريا في الدم، وإزالة الكرياتينين، وإفراز البروتين في البول مع زيادة تصفية اليوريا.
الاستنتاجات
هناك دليل محدودة على دور التمارين في وقاية الكلى وتغيير معايير وظائف الكلى بين مرضى السكري من النوع الثاني المصابين باعتلال الكلية.
الكلمات المفتاحية: أمراض الكلى المزمنة, أمراض الكلى السكري, داء السكري, التمارين الرياضية, وظائف الكلى, اعتلال الكلية, إعادة التأهيل
Introduction
The global increase in type 2 diabetes mellitus increases cardiovascular risk and has health implications.1 Diabetic nephropathy is a microvascular complication of type 2 diabetes mellitus indicated by a loss of urinary protein2; it damages the small blood vessels of the kidneys, thus leading to deterioration of renal function.3 Renal dysfunction gradually progresses from a slight functional decline to mild, moderate, or severe nephron loss among these individuals.4 The primary management comprises pharmacological interventions, whereas haemodialysis, peritoneal dialysis, renal transplantation, or renal replacement therapies are best suited to address severe renal dysfunction.
The potential risk of cardiovascular disease is high among adults with youth-onset type 2 diabetes, diabetic nephropathy (DN), diabetic kidney disease (DKD), or chronic kidney disease (CKD), even within the normoalbuminuria range.5,6 These changes are associated with metabolic compensation as a consequence of inadequate glycaemic control, hypertension, and dyslipidaemia due to impaired arterial blood pressure among individuals with DKD/CKD.
Early screening is essential, because microalbuminuria is found in approximately 7% of individuals with type 2 diabetes mellitus at the time of diagnosis.7,8 Moreover, 30% of individuals with diabetes and normoalbuminuria show a diminished glomerular filtration rate (GFR).9,10 Beyond albuminuria and GFR, assessment of serum creatinine levels has recently been recommended.11
The burden of diabetes and nephropathy has become an emerging challenge for healthcare members and caregivers of affected individuals.12,13 Identified risk factors for DN and its progression include age, male sex, long duration of diabetes, estimated GFR (eGFR) above 90 mL/min/1.73 m2 (early hyperfiltration), systolic blood pressure above 130 mm Hg, and persistent proteinuria and concomitant retinopathy.14 These risk factors affect the quality of life, physical function/fitness, and performance of individuals, owing to the development of fatigue, pain, dyspnoea, sarcopenia, frailty, and renal anaemia.
Individuals with CKD have approximately 50%–80% poorer physical function than their healthy counterparts, owing to protein-energy wasting through protein catabolism, and mitochondrial dysfunction.15,16 The chronic inflammation and hyperglycaemic state in DN triggers various molecular pathways.17 A combination of exercise and caloric restriction has been reported to delay renal failure by postponing renal fibrotic changes, in a study by Dong et al.18 The role of rehabilitation for individuals with kidney dysfunction has gained traction in recent years.19 Given that exercise may help improve health related outcomes among individuals with DN, its reno-protective action must be clarified in future research. A strong need exists to focus on preventive strategies and measures for early identification and screening, patient education, promotion of lifestyle modification, and incorporation of exercise among individuals with type 2 diabetes and nephropathy. This systematic review was aimed at determining the effects of exercise on renal function among patients with type 2 diabetes mellitus with nephropathy.
Materials and Methods
Review protocol registration
The systematic review was registered with PROSPERO (CRD42020198754) and is reported in accordance with the PRISMA checklists.20
Literature search strategy
A detailed electronic search was performed in the following databases: PubMed/Medline, Scopus, Web of Science, CINAHL, ProQuest, and Cochrane. A comprehensive search was conducted through selection of keywords and subject heading terms for DN, DKD, type 2 diabetes mellitus, diabetes mellitus, non-insulin dependent diabetes mellitus, exercise, rehabilitation, blood urea nitrogen, azotaemia, creatine, uraemia, creatinine clearance, serum creatinine, and creatinine. The alternative search terminologies used truncations. The search words were thereafter combined with the Boolean operators “AND” and “OR,” and articles from the database inception until 31st December 2021 were searched. In addition to the database search, articles associated with exercise, renal function, DN, or CKD were manually searched in the reference lists of the reviewed records.
Eligibility criteria
Detailed inclusion and exclusion criteria were defined for the selection of studies to this systematic review.
Population (P)
-
•
The operational definition for study participants included in the review comprised studies in individuals diagnosed with type 2 diabetes mellitus in the presence of nephropathy, on the basis of: 1) microalbuminuria in the range of 30–300 mg/24 h or 20–200 μg/min, macroalbuminuria above ≥300 mg/24 h or ≥200 μg/min, or both; 2) impaired GFR between 15 and 90 mL/min/1.73 m2; or 3) both 1) and 2).
-
•
Studies in populations of patients with DKD or CKD with type 2 diabetes mellitus who met the operational definition of DN as stated above were also included.
-
•
Studies in populations 18 years of age or above of either sex were included.
Intervention (I)
-
•
Studies that administered an exercise intervention strictly associated with physiotherapy practices (for e.g., aerobic, resistance, strength, endurance, balance, supervised, structured, individualized, home-based exercise, centre-based exercise, or group therapy) to participants were included.
-
•
Studies that administered an exercise intervention in combination with diet or drug therapy were also included.
Comparison (C)
-
•
Studies in which participants in an exercise intervention group were compared with either a control or standard care group were included.
Outcome (O)
-
•
Studies that assessed renal function parameters such as blood urea nitrogen, albuminuria, serum creatinine, GFR, or any reported measure of creatine clearance, urea clearance, urinary albumin-creatinine ratio, urinary protein-creatinine ratio, and urinary protein excretion as their primary or secondary outcome measures were included.
-
•
The standard unit of measurement for reporting for blood urea nitrogen was considered to be serum creatinine level in mmol/L, creatinine and urea clearance in mL/min, and measured GFR or eGFR in mL/min/1.73 m2, according to the equations or formulae. The urinary albumin creatinine ratio and urinary protein creatinine ratio were measured in mg/g. Urinary protein excretion was measured in mg/24 h.
Study Design (S): Only randomized controlled trials (RCTs) were included.
Exclusion criteria
-
•
Studies in participants who had a low GFR below 15 mL/min/1.73 m2 or renal failure; who were receiving haemodialysis, peritoneal dialysis, or ongoing renal replacement therapy; or who were diagnosed with other renal or renal tract disorders were excluded.
-
•
Study designs other than RCTs, such as observational studies, non-RCTs, systematic reviews, meta-analysis, conference papers, poster presentations, book chapters, animal studies, and in vitro studies were excluded.
-
•
Studies reporting exercise induced albuminuria or proteinuria were excluded, because they involve acute exercise bouts of maximal or submaximal intensity, which causes structural damage to tissues, with long duration of impaired functioning.
-
•
Studies providing alternative medicine therapies not associated with physiotherapy practice, such as tai chi, dance therapy, acupuncture, acupressure, or use of iso-kinetic machines and whole body vibratory devices were excluded from the systematic review.
-
•
Studies reporting mixed or alternative participant groups, such as those with hypertension and metabolic syndrome, or studies without any control group or non-diabetic healthy control group, were excluded.
Study selection
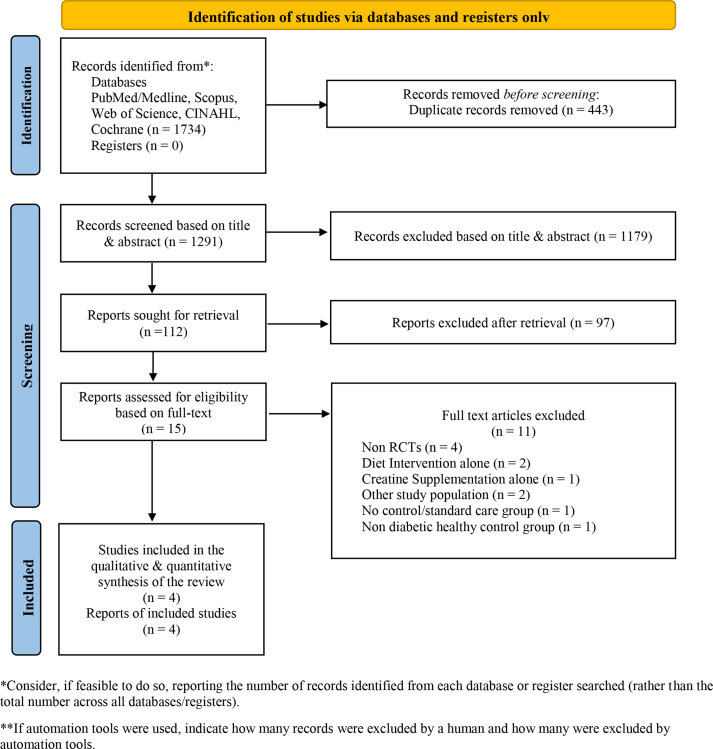
The records obtained from the database search were exported to Rayyan. Two authors independently reviewed the studies, on the basis of the pre-specified eligibility criteria for the review.21 Disagreements were clarified after mutual discussion and agreement with the other study authors. The detailed study selection procedure is presented in a PRISMA 2020 flow diagram (Figure 1).
Figure 1.
PRISMA 2020 flow diagram of the study selection process.
Data extraction
A data collection checklist was designed. The following details were extracted from individual studies: author details; publication year; study location; study design; population characteristics; total sample size; number of exercise/intervention participants; number of control/standard participants; intervention duration; and exercise intervention details in terms of frequency, intensity, time (duration), exercise type/mode, and number of repetitions. The renal function outcome measures were all continuous variables. The sample sizes were recorded as mean and standard deviation, standard error of the mean, mean and 95% confidence interval (CI), or median and interquartile range. Because different units of measurements for renal function outcomes were obtained across the selected studies, the units were converted to the SI unit system and are reported accordingly in this review.
Risk of bias assessment
The Revised Cochrane risk-of-bias tool for randomized trials (ROB 2) criteria were used to assess bias among the included studies.22 The criteria comprise five domains for assessment and are scored under three categories of low, some concerns, or high risk for each domain. Two independent reviewers performed the risk assessment, and their cumulative response was scored. Any disagreements in reporting were resolved by a third reviewer.
Data synthesis
Review Manager Software (RevMan V.5.4) was used to statistically analyse, compute, and calculate the required effect sizes across the outcome measures, on the basis of the guidelines from the Cochrane Handbook of Systematic Reviews in Interventions. Quantitative synthesis of renal outcomes was performed for statistically and clinically homogeneous data. Heterogeneity in data was tested with chi-square (χ2) and I2 statistical tests only if I2 > 50% or P < 0.01. Thereafter, meta-analysis was performed if the data were indicated to be appropriate. All continuous renal outcome measures were analysed with the mean difference or standard mean difference with 95% CI.
GRADE assessment
GRADEproGDT software (gdt.gradepro.org/app/) was used to evaluate the included studies’ level of evidence and certainty.23 The GRADE working group comprises four levels of certainty ratings: very low, low, moderate, and high. These levels indicate how close the true effect is to the effect estimate.
Therapeutic quality of exercise program
The quality of exercise interventions used for study participants in the included RCTs were assessed with the International Consensus on Therapeutic Exercise aNd Training (i-CONTENT) tool.24 This tool enables researchers and clinicians to easily identify and interpret heterogeneity, which is often reported across exercise intervention trials. Two independent reviewers performed quality assessment of the exercise intervention program delivered to study participants under the seven categories of the i-CONTENT Tool: 1) patient selection, 2) dosage of exercise intervention, 3) type of exercise intervention, 4) presence of a qualified therapist, 5) exercise type and time when outcomes were assessed, 6) safety of the exercise intervention program, and 7) participants’ adherence to the exercise program. The third reviewer verified the cumulative responses of the previous two reviewers and resolved any disagreements. The results of i-CONTENT tool scoring are presented in Table 4.
Table 4.
Scoring of i-CONTENT Tool.
| No. | i-CONTENT Tool Categorization for Assessment of Therapeutic Quality of Exercise Program | Author and Year of Included Studies |
|||
|---|---|---|---|---|---|
| Leehey et al., 200925 | Greenwood et al., 201526 | Leehey et al., 201627 | Beetham et al., 201828 | ||
| 1. | Patient selection | High risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness |
| 2. | Dosage of the exercise program | High risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness |
| 3. | Type of the exercise program | High risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness |
| 4. | Qualified supervisor (if applicable) | Low risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness |
| 5. | Type and timing of outcome assessment | High risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness | Low risk of ineffectiveness |
| 6. | Safety of the exercise program | Probably not determined | Probably not determined | Probably not determined | Probably not determined |
| 7. | Adherence to the exercise program | Probably not determined | Probably not determined | Probably not determined | Probably not determined |
Results
Search results
A total of 1734 records were obtained from searching six databases. Rayyan software was used for study selection. A total of 443 duplicate records were identified and eliminated with Rayyan. Thereafter, 1291 records were screened on the basis of their study titles alone, thus resulting in elimination of 1179 records. The remaining 112 records were screened on the basis of their abstracts, thus resulting in the elimination of 97 records. Finally, 15 full text records were considered eligible for this systematic review. Of these, 11 records were eliminated, and four RCTs25, 26, 27, 28 were included in the qualitative and quantitative synthesis of this systematic review, as presented in Figure 1.
Study population characteristics
A total of 218 individuals diagnosed with type 2 diabetes mellitus and nephropathy or CKD stages 2, 3, or 4, on the basis of the level of renal function, proteinuria, different measures of GFR, the urinary albumin/protein ratio, or creatinine clearance, were included in the review (Table 1). A total of 15 individuals from the four studies were not included in the final analysis because of discontinuation/loss to follow-up. Hence, the results of 203 individuals (103 in the exercise or intervention group, and 100 in the control or standard care group) were included in our systematic review. The overall age of the study participants was 18–80 years. Participants with obesity (body mass index ≥30 kg/m2) were included in studies by Leehey et al., in 200925 and 201627; overweight individuals (body mass index ≥25 kg/m2) were included by Beetham et al.28 The study by Greenwood et al. did not report details of body mass index for participants.26
Table 1.
Included studies and their population characteristics.
| Author, Year | Study Location | Study Design | Study Population | Total Sample Size | Exercise | Controls | Study Duration |
|---|---|---|---|---|---|---|---|
| Leehey et al., 200925 | USA | RCT (pilot) | CKD stages 2–4, BMI ≥30 kg/m2, UACR >200 mg/g for ≥3 months | 20 | 7 | 4 | 24 weeks |
| Greenwood et al., 201526 | UK | RCT (pilot) | CKD stages 3 or 4, GFR 20–60 mL/min/1.73 m2, with progressive decline ≥2.9 mL/min/1.73 m2, age 18–80 years | 20 | 8 | 10 | 52 weeks |
| Leehey et al., 201627 | USA | RCT | CKD stages 2–4, obesity with BMI ≥30 kg/m2, eGFR 15–90 mL/min/1.73 m2, persistent proteinuria, UACR >200 mg/g for at least 3 months, age 49–81 years | 36 | 14 (exercise + diet) | 18 (diet alone) | 52 weeks |
| Beetham et al., 201828 | Australia | RCT | CKD stages 3 or 4, eGFR 25–60 mL/min/1.73 m2, overweight (BMI ≥25 kg/m2), poor glycaemic control with HbA1c >7%, age 18–75 years | 142 | 74 | 68 | 52 weeks |
Study intervention characteristics
The exercise intervention duration in all studies ranged from 24 weeks to 52 weeks. Overall, both supervised and home-based exercises were prescribed to study participants. The supervised exercise training was conducted in research laboratories or gym-based settings from 6 weeks to 12 weeks. The home-based exercise programme ranged from 18 to 40 weeks, until study completion. A combination of aerobic and resistance exercises were prescribed in three studies except the study by Leehey et al., in 2009, which delivered an aerobic programme alone.25 Most studies used the “FITT principle” for exercise prescription and progression among participants, with the exception of the study by Leehey et al., in 2009.25 The details of the exercise intervention are presented in Table 2.
Table 2.
Details of the exercise interventions used by included studies.
| Author, Year | Study Duration | Details of Exercise Intervention |
|---|---|---|
| Leehey et al., 200925 | 24 weeks |
|
| Greenwood et al., 201526 | 52 weeks | Combination aerobic and resistance (supervised and home-based) Aerobic exercise FITT description:
|
| Leehey et al., 201627 | 52 weeks | 12 weeks of supervised exercise followed by 40 weeks of a home-based exercise program Aerobic exercise FITT description (supervised):
|
| Beetham et al., 201828 | 52 weeks | Aerobic and resistance exercise combined in a supervised program (8 weeks) plus home-based exercise program (10 months) Exercise FITT description (supervised):
|
Control group characteristics
The control group participants received standard nephrological care for management of diabetes and CKD, diabetes education based on individual hospital guidelines, and telephone follow-up. However, diet and nutritional counselling was provided only to control group participants in the study by Leehey et al., in 2016.27
Renal function outcomes and units of measurement
The renal function outcomes and units of measurements among the included studies were as follows: serum creatinine (mg/dL or mmol), eGFR (on the basis of the CKD-EPI creatinine-based equation, CKD-EPI cystatin-based equation, CKD-EPI creatine-cystatin-based equation, or MDRD equation), GFR (mL/min), serum cystatin C (mg/L), urine albumin-creatinine ratio (mg/g), urine protein-creatinine ratio (mg/g), serum urea nitrogen (mg/dL), creatinine clearance (mL/min), urea clearance (mL/min), and urine protein excretion (mg/24 h). Differences were observed in the units of measurement used for reporting of serum creatinine. Hence, the SI system of measurement was used for quantitative synthesis and analysis.
Risk of bias in the included studies
Assessment of the risk of bias was performed by two independent authors (Table 3). Both authors rated three studies25,27,28 as high risk, whereas only one study26 was rated as having some concerns.
Table 3.
Risk of bias assessment (ROB2) of the included studies, according to the Cochrane Tool Kit.
| No. | Domains of ROB2 | Author and Year of Included Studies |
|||
|---|---|---|---|---|---|
| Leehey et al., 200925 | Greenwood et al., 201526 | Leehey et al., 201627 | Beetham et al., 201828 | ||
| 1. | Bias due to the randomization process | High risk | Low risk | Some concerns | High risk |
| 2. | Bias due to deviations from intended intervention | High risk | Some concerns | Some concerns | Some concerns |
| 3. | Bias due to missing outcome data | High risk | Some concerns | Some concerns | High risk |
| 4. | Bias in measurement of the outcome | High risk | Low risk | High risk | High risk |
| 5. | Bias in selection of the reported result | Some concerns | Low risk | Low risk | Low risk |
| Overall bias | High risk | Some concerns | High risk | High risk | |
Effects of exercise interventions on renal function outcomes
Exercise and serum creatinine
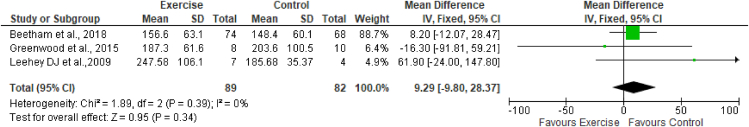
Three studies (Leehey et al., 200925; Greenwood et al., 201526; Beetham et al., 201828) assessed the effects of exercise on serum creatinine, with 89 participants in the exercise group and 82 participants in the control group. The results of meta-analysis indicated no effects of exercise on serum creatinine (MD 9.29 mmol; 95% CI -9.80 to 28.37; I2 = 0%) (Figure 2).
Figure 2.
Forest plot showing the effects of exercise on serum creatinine levels.
Exercise and eGFR and GFR
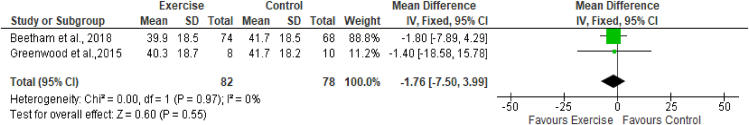
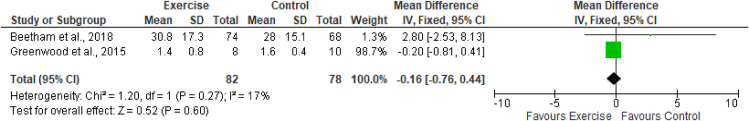
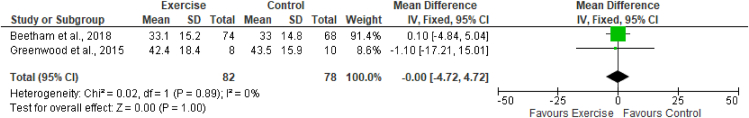
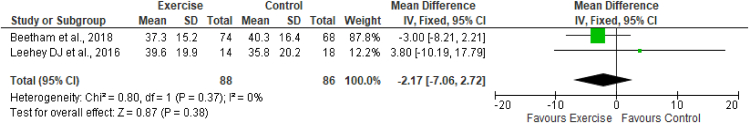
The estimated equation-based method and actual measurement method of GFR were used across the studies included in this review. Two studies (Greenwood et al., 201526; Beetham et al., 201828) assessed the effects of exercise on eGFR by using CKD-EPI creatinine, CKD-EPI cystatin, and CKD-EPI creatinine-cystatin-based equations. These studies included a total of 82 participants in the exercise group and 78 in the control group. The results of meta-analysis indicated no effects of exercise on any equations measuring the eGFR. The results (Figures 3, 4, and 5) were as follows: eGFR CKD-EPI creatinine equation (MD −1.76 mL/min/1.73 m2; 95% CI −7.50 to 3.99; I2 = 0%), eGFR CKD-EPI cystatin equation (MD −0.16 mL/min/1.73 m2; 95% CI −0.76 to 0.44; I2 = 0%), and eGFR CKD-EPI creatinine–cystatin equation (MD −0.00 mL/min/1.73 m2; 95% CI −4.72 to 4.72; I2 = 0%). However, the MDRD creatinine-based equation was used by two studies (Leehey et al., 201627; Beetham et al., 201828) to assess the effects of exercise on eGFR. These studies included a total of 88 participants in the exercise group and 86 participants in the control group. The results of meta-analysis indicated no effects of exercise on the eGFR MDRD creatinine equation (MD −2.17 mL/min/1.73 m2; 95% CI −7.06, 2.72; I2 = 0%). One study, by Leehey et al., in 2009,25 measured GFR (mL/min) by using the sum of creatinine and urea clearance divided by 2. That study indicated no significant difference in GFR in the exercise group participants after 6 weeks of intervention but observed a lower level than that in the control group after 24 weeks (Figures 3, 4, 5, and 6.)
Figure 3.
Forest plot showing the effects of exercise on eGFR, according to the CKD-EPI creatinine-based equation.
Figure 4.
Forest plot showing the effects of exercise on eGFR, according to the CKD-EPI cystatin C-based equation.
Figure 5.
Forest plot showing the effects of exercise on eGFR, according to the CKD-EPI creatinine-cystatin C-based equation.
Figure 6.
Forest plot showing the effects of exercise on eGFR, according to the MDRD creatinine-based equation.
Exercise and serum cystatin C
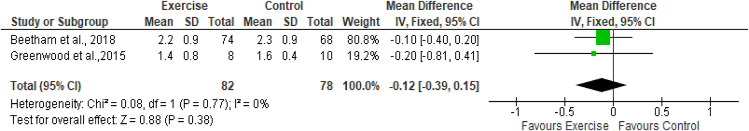
Two studies (Greenwood et al., 201526; Beetham et al., 201828) assessed the effects of exercise on serum cystatin C levels, with 82 participants in the exercise group and 78 participants in the control group. The results of meta-analysis indicated no effects of exercise on serum cystatin C (MD -0.12 mg/L; 95% CI -0.39 to 0.15; I2 = 0%) (Figure 7).
Figure 7.
Forest plot showing the effects of exercise on serum cystatin C levels.
Exercise and UACR
The two studies by Leehey et al., in 200925 and 201627 assessed the effects of exercise on the urine albumin-creatinine ratio (mg/g). The baseline values of UACR, reported as mean ± SD by Leehey et al., in 2009,25 were 327 ± 385 in exercise participants and 156 ± 148 in the control group. After 24 weeks of intervention, the UACR decreased to 305 ± 456 in exercise participants and 221 ± 304 in the control group. However, the baseline values of UACR, reported as median [interquartile range] by Leehey et al., in 2016,27 were 329 [94–1307] in exercise participants and 428 [161–119] in the control group. After 52 weeks of intervention, the UACR decreased to 253 [81–619] in the exercise group and 323 [187–894] in the control group.
Exercise and UPCR
The two studies by Leehey et al., in 200925 and 201627 assessed the effects of exercise on the urine protein-creatinine ratio (mg/g). The baseline values of UPCR, reported as mean ± SD by Leehey et al., in 2009,25 were 565 ± 600 in exercise participants and 347 ± 178 in the control group. After 24 weeks of intervention, the UPCR decreased to 493 ± 544 in exercise participants and 387 ± 374 in the control group. However, the baseline values of UPCR, reported as median [interquartile range] by Leehey et al., in 2016,27 were 626 [275–1619] in exercise participants and 626 [413–1563] in the control group. After 52 weeks of intervention, the UPCR decreased to 405 [225–1038] in the exercise group and 618 [323–1155] in the control group.
Exercise and serum urea nitrogen
The study by Leehey et al., in 2009 assessed the effects of exercise on serum urea nitrogen (mg/dL).25 The baseline values of serum urea nitrogen, reported as mean ± SD, were 64 ± 40 in the exercise group and 45 ± 11 in the control group. After 24 weeks of intervention, the serum urea nitrogen levels decreased to 57 ± 34 in the exercise group and increased to 50 ± 18 in the control group.
Exercise and creatinine clearance
The study by Leehey et al., in 2009 assessed the effects of exercise on creatinine clearance (mL/min).25 The baseline values of creatinine clearance, reported as mean ± SD, were 64 ± 54 in the exercise group and 66 ± 19 in the control group. After 24 weeks of intervention, the creatinine clearance decreased to 51 ± 26 in the exercise group and 64 ± 10 in the control group.
Exercise and urea clearance
The study by Leehey et al., in 2009 assessed the effects of exercise on urea clearance (mL/min).25 The baseline values of urea clearance, reported as mean ± SD, were 24 ± 20 in the exercise group and 28 ± 0.2 in the control group. After 24 weeks of intervention, the urea clearance increased to 26 ± 19 in the exercise group and decreased to 19 ± 0.5 in the control group.
Exercise and urine protein excretion
The study by Leehey et al., in 2009 assessed the effects of exercise on urine protein excretion (mg/24 h).25 The baseline values of urine protein excretion, reported as mean ± SD, were 1020 ± 1081 in the exercise group and 542 ± 258 in the control group. After 24 weeks of intervention, the urine protein excretion decreased to 821 ± 1010 in the exercise group and decreased to 490 ± 237 in the control group.
GRADE assessment
RCTs provide a high certainty of evidence. In this review, two authors assessed the quality of the included studies and indicated moderate certainty regarding serum creatinine and the eGFR CKD-EPI creatinine-based equation as renal function outcome measures. The other renal outcomes of eGFR CKD-EPI cystatin C, eGFR MDRD, and eGFR CKD-EPI creatine-cystatin C and serum cystatin C had low certainty.
Therapeutic quality of exercise program
The study by Leehey et al., in 2009 is the first RCT in this field that revealed a high risk of ineffectiveness with relation to patient selection, dosage, type of exercise programme, and timing of outcome assessment.25 All studies used an exercise programme that was provided under close supervision by a qualified therapist.25, 26, 27, 28 However, safety and programme adherence with exercise were not described Table 4.
Discussion
The review aimed to determine the effects of an exercise-based intervention programme on renal function parameters among individuals with type 2 diabetes mellitus and nephropathy.
Stage of renal dysfunction
According to the review's operational definition, the study participants in the four RCTS had stage 2, 3, or 4 DN/CKD.25, 26, 27, 28 Interestingly, none of the studies included participants with stage 1 nephropathy, thus implying a lack of understanding of when renal dysfunction accelerates and progresses to stages 2 or higher. This transition is important because it marks the onset of a sudden increase in GFR due to glomerular hyper-filtration, which, if left untreated, can worsen kidney function. Renal function changes may be reversible if detected early (in stages 1–3).29, 30, 31 When changes are detected in stage 3 or higher, however, the risk of irreversible damage remains high because structural damage outweighs functional nephron loss.
Obesity as a precursor for renal damage
Three of four RCTs (except that by Greenwood et al., 2015) included overweight or obese participants, thereby indicating a possible link between adiposity and renal dysfunction. Thus, in the management of patients with DKD, addressing the complex relationships among obesity, peripheral insulin resistance, underlying hypertension, low-grade systemic inflammation, and dyslipidaemia is critical. Obesity causes renal lipotoxicity, which results in structural and functional impairment of mesangial cells, podocytes, and proximal tubules of the kidney, owing to the accumulation of ectopic lipids in renal tissues.32,33 Increased glomerular permeability, hyper-filtration, albuminuria, glomerulomegaly, and complete nephron injury are all consequences of these factors.34 The Macula densa feedback mechanism, in contrast, mediates glomerular hyperfiltration, which is indicative of an early rise in GFR.35
Exercise intervention
The aerobic and resistance activities were performed at home or at a centre for a period of 24–52 weeks during the exercise intervention. The FITT model was used for exercise prescription in three of the four RCTS (except that by Leehey et al., in 2009). The use of FITT eliminates misinterpretation by having exercise physiologists/trained physiotherapists or exercise scientists pre-define the frequency, intensity, type, and time (duration) of exercise sessions. This method ensures that participants perform exercises to the best of their abilities while also maintaining health benefits. Interestingly, the therapeutic quality of the exercise intervention delivered to study participants had low risk of ineffectiveness in three studies25,27,28 and high risk of ineffectiveness in one study.26 Other than discontinuation or loss to follow-up, none of the studies reported any serious adverse events among study participants. However, these three studies provided no information on safety and the adherence of participants to the exercise therapy.
Muscle loss in patients with CKD and haemodialysis is multifactorial. Muscles affect lipid and glucose metabolism, and the state of inflammation; hence, the maintenance of healthy muscle mass remains critical for patients with DN.36,37 Studies on other kidney conditions such as haemodialysis, peritoneal dialysis, and renal transplants have indicated that sedentary behaviour or total physical inactivity predominates among this group, because of overprotection by family members or caregivers. Overall physical function can be gained by encouraging individuals to participate in physical activity or exercise programmes. Those with diabetes/CKD stages 1–3, in contrast, experience a decline in physical function due to fatigue, muscle loss, dyspnoea, swelling of the face or feet/ankles, diminished energy levels, water retention, and volume overload. Uptake of clinical services appears to be slow, owing to a lack of consensus regarding effective therapeutic exercise training programmes for patients with diabetes or CKD, as well as the scarcity of RCTs with positive results in this population. Among patients with diabetes/CKD, improving diabetes education and health literacy regarding kidney disease, as well as selecting appropriate dietary and lifestyle modifications, may be effective tools for decreasing renal dysfunction.
Exercise and renal function
Serum creatinine, GFR, urinary albumin creatinine ratio, the urine protein creatinine ratio, and serum cystatin C were unaffected by exercise, according to our findings. A recent meta-analysis of 14 non-RCTs and four RCTs has examined the protective effects of physical activity on DN progression and reported similar results.38 With exercise, GFR increases (SMD = 0.9; 95% CI 0.02 to 0.17), UACR decreases (SMD = −0.53; 95% CI -0.72 to −0.34), the rate of microalbuminuria decreases (OR = 0.61; 95% CI 0.46 to 0.81), and serum creatinine levels do not change (SMD = −0.02; 95% CI −0.09 to 0.06), according to a prior study.38 Zhang et al. have conducted a meta-analysis of 421 patients with CKD and indicated that in the exercise group, eGFR increased by +2.6 mL/min.39 Resistance training combined with a low protein diet has beneficial effects on longitudinal changes in eGFR in patients with CKD after 12 weeks, according to Castaneda et al.40 The training group's eGFR increased by 1.2 mL/min, whereas the control group's decreased by 1.6 mL/min. A statistically significant difference was observed between the groups (P = 0.048). Nakamura et al. have investigated the effects of exercise on physical and kidney function in patients CKD without dialysis.41 No effects of exercise on eGFR have been observed in nine trials with 459 participants (MD = −0.34; 95% CI −1.91 to 1.22; I2 = 0%). The results of five trials with a total of 231 participants were also examined, and exercise had no effects on serum creatinine (MD = 1.48; 95% CI −7.50 to 1.31; I2 = 0%). According to the study, regular moderate-intensity exercise performed for 8 weeks to 1.5 years may be considered safe for those with non-dialysis CKD. Patients with kidney disease have a higher rate of functional decline and accelerated ageing than the general population, owing to uremic muscle dysfunction, shared cardio-metabolic risk factors, and disease-associated complications. Randomized controlled trials are needed to establish the effects of exercise on renal function.
Several factors might have contributed to the results of our study:
First, very few clinical trials in nephrology have assessed the effects of an exercise program on individuals with DKD/DN. Most nephrology trials have a relatively short duration and involve clinically meaningful endpoints for ESRD/RRT, etc.
Second, existing trials have been conducted on small samples, possibly because of the high cardio-metabolic risk associated among DKD/DN stages 2 and above. Consequently, the window for early identification, management, and reversal of renal dysfunction is limited. In the present review, three of four RCTs included overweight and obese participants with DKD stages 2–4. A possibility exists that the participants in the included trials might have developed irreversible renal dysfunction.
Third, the conventional trajectory of hyperfiltration, microalbuminuria, proteinuria, and a decrease in GFR is notably not observed in a substantial portion of patients.42 Even in the absence of proteinuria, an accelerated GFR decline has been observed in patients with type 2 diabetes mellitus.43
Fourth, the existing trials have been conducted in the UK, USA, and Australia. Clarity regarding how DKD/DN presents in the rest of the world is urgently needed to alleviate the public health burden of CKD and implement health care policy change.
Strengths of the study
-
➢
This is the first systematic review of RCTs conducted solely to assess the therapeutic effects of an exercise-based rehabilitation program on various renal function outcome measures in patients with DN or diabetes/CKD.
Limitations of the study
-
➢
Evidence is lacking regarding renal outcomes, such as urinary albumin/protein creatinine ratio, serum cystatin c, serum urea nitrogen, urea clearance, and creatinine clearance, owing to a paucity of RCTs addressing these measurements.
Conclusion
The level of evidence regarding the effects of exercise on renal function outcome measures ranges from low to moderate, according to the current systematic review. In the future, RCTs with large sample sizes are required to establish concrete evidence on this topic.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This manuscript did not require any ethical approval, because it is a systematic review with meta-analysis. However, it was registered with PROSPERO (CRD42020198754) on 14/07/2020.
Authors contributions
MN, GAM, SPN, BAS, and SKN conceptualized and designed the entire protocol for this systematic review. MN and GAM performed the preliminary database literature search and screened articles in RAYYAN software. SPN, BAS, and SKN contributed to selection of the studies included in this review. MN and GAM contributed to the data extraction and data collection. The data were analysed, and findings were interpreted by, MN, GAM, SPN, BAS, and SKN. The preliminary draft of the manuscript was prepared by MN. The manuscript was critically curated for its scientific content and novelty by GAM, SPN, BAS, and SKN. The final version of the manuscript was read and approved by all authors before journal submission. All authors have met the recommended criteria by ICMJE for authorship and effectively contributed to the manuscript. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
We thank the Centre for Diabetic Foot Care and Research (CDFCR), Department of Physiotherapy, Manipal College of Health Professions (MCHP), Manipal Academy of Higher Education, India, for supporting us.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Footnotes
Peer review under responsibility of Taibah University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtumed.2022.11.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Pálsson R., Patel U.D. Cardiovascular complications of diabetic kidney disease. Adv Chron Kidney Dis. 2014;21(3):273–280. doi: 10.1053/j.ackd.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross J.L., de Azevedo M.J., Silveiro S.P., Canani L.H., Caramori M.L., Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 3.diabetes A. Idf.org; 2020. International diabetes federation - complications [Internet]https://www.idf.org/aboutdiabetes/complications.html [cited 22 January 2020]. Available from. [Google Scholar]
- 4.Greenberg A. Elsevier Health Sciences; 2009. Primer on kidney diseases E-book. [Google Scholar]
- 5.Amatruda M., Gembillo G., Giuffrida A.E., Santoro D., Conti G. The aggressive diabetic kidney disease in youth-onset type 2 diabetes: pathogenetic mechanisms and potential therapies. Medicina. 2021;57:868. doi: 10.3390/medicina57090868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ene-Iordache B., Perico N., Bikbov B., et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Global Health. 2016;4(5):e307–e319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- 7.Adler A.I., Stevens R.J., Manley S.E., et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein H.C., Mann J.F., Yi Q., et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 9.MacIsaac R.J., Tsalamandris C., Panagiotopoulos S., Smith T.J., McNeil K.J., Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care. 2004;27(1):195–200. doi: 10.2337/diacare.27.1.195. [DOI] [PubMed] [Google Scholar]
- 10.Kramer H.J., Nguyen Q.D., Curhan G., Hsu C.Y. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289(24):3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 11.Norris K.C., Smoyer K.E., Rolland C., Van der Vaart J., Grubb E.B. Albuminuria, serum creatinine, and estimated glomerular filtration rate as predictors of cardio-renal outcomes in patients with type 2 diabetes mellitus and kidney disease: a systematic literature review. BMC Nephrol. 2018;19(1):36. doi: 10.1186/s12882-018-0821-9. Published 2018 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z., Chaudhari P., Yang H., et al. Healthcare resource use, costs, and disease progression associated with diabetic nephropathy in adults with type 2 diabetes: a retrospective observational study. Diabetes Ther. 2017;8(3):555–571. doi: 10.1007/s13300-017-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elshahat S., Cockwell P., Maxwell A.P., Griffin M., O'Brien T., O'Neill C. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS One. 2020;15(3) doi: 10.1371/journal.pone.0230512. Published 2020 Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alwakeel J.S., Isnani A.C., Alsuwaida A., et al. Factors affecting the progression of diabetic nephropathy and its complications: a single-center experience in KSA. Ann Saudi Med. 2011;31(3):236–242. doi: 10.4103/0256-4947.81528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamaki M., Miyashita K., Wakino S., Mitsuishi M., Hayashi K., Itoh H. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase. Kidney Int. 2014;85(6):1330–1339. doi: 10.1038/ki.2013.473. [DOI] [PubMed] [Google Scholar]
- 16.Tamaki M., Hagiwara A., Miyashita K., et al. Improvement of physical decline through combined effects of muscle enhancement and mitochondrial activation by a gastric hormone ghrelin in male 5/6Nx CKD model mice. Endocrinology. 2015;156(10):3638–3648. doi: 10.1210/en.2015-1353. [DOI] [PubMed] [Google Scholar]
- 17.Yaribeygi H., Butler A.E., Sahebkar A. Aerobic exercise can modulate the underlying mechanisms involved in the development of diabetic complications. J Cell Physiol. 2019;234(8):12508–12515. doi: 10.1002/jcp.28110. [DOI] [PubMed] [Google Scholar]
- 18.Dong L., Li J., Lian Y., et al. Long-term intensive lifestyle intervention promotes improvement of stage III diabetic nephropathy. Med Sci Monit. 2019;25:3061–3068. doi: 10.12659/MSM.913512. Published 2019 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Japanese Society of Nephrology Essential points from evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin Exp Nephrol. 2019;23(1):1–15. doi: 10.1007/s10157-018-1648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. Published 2016 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cumpston M., Li T., Page M.J., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GRADEpro guideline development tool [Software] McMaster University; 2015. [GRADEpro GDT. gradepro.org] [Google Scholar]
- 24.Hoogeboom T.J., Kousemaker M.C., van Meeteren N.L., et al. i-CONTENT tool for assessing therapeutic quality of exercise programs employed in randomised clinical trials. Br J Sports Med. 2021;55(20):1153–1160. doi: 10.1136/bjsports-2019-101630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leehey D.J., Moinuddin I., Bast J.P., et al. Aerobic exercise in obese diabetic patients with chronic kidney disease: a randomized and controlled pilot study. Cardiovasc Diabetol. 2009;8:62. doi: 10.1186/1475-2840-8-62. Published 2009 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenwood S.A., Koufaki P., Mercer T.H., et al. Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: a pilot randomized controlled trial. Am J Kidney Dis. 2015;65(3):425–434. doi: 10.1053/j.ajkd.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Leehey D.J., Collins E., Kramer H.J., et al. Structured exercise in obese diabetic patients with chronic kidney disease: a randomized controlled trial. Am J Nephrol. 2016;44(1):54–62. doi: 10.1159/000447703. [DOI] [PubMed] [Google Scholar]
- 28.Beetham K.S., Howden E.J., Isbel N.M., Coombes J.S. Agreement between cystatin-C and creatinine based eGFR estimates after a 12-month exercise intervention in patients with chronic kidney disease. BMC Nephrol. 2018;19(1):366. doi: 10.1186/s12882-018-1146-4. Published 2018 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onyenwenyi C., Ricardo A.C. Impact of lifestyle modification on diabetic kidney disease. Curr Diabetes Rep. 2015;15(9):60. doi: 10.1007/s11892-015-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tufescu A., Kanazawa M., Ishida A., et al. Combination of exercise and losartan enhances renoprotective and peripheral effects in spontaneously type 2 diabetes mellitus rats with nephropathy. J Hypertens. 2008;26(2):312–321. doi: 10.1097/HJH.0b013e3282f2450b. [DOI] [PubMed] [Google Scholar]
- 31.Dunkler D., Kohl M., Heinze G., et al. Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus. Kidney Int. 2015;87(4):784–791. doi: 10.1038/ki.2014.370. [DOI] [PubMed] [Google Scholar]
- 32.Tonelli M., Moyé L., Sacks F.M., Cole T., Curhan G.C. Cholesterol and Recurrent Events Trial Investigators. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14(6):1605–1613. doi: 10.1097/01.asn.0000068461.45784.2f. [DOI] [PubMed] [Google Scholar]
- 33.Unger R.H., Scherer P.E. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab. 2010;21(6):345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch V.H. The effects of obesity on kidney function: a challenge for nephrologists. J Bras Nefrol. 2019;41(2):162–165. doi: 10.1590/2175-8239-JBN-2019-0064. Published 2019 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall M.E., do Carmo J.M., da Silva A.A., Juncos L.A., Wang Z., Hall J.E. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovascular Dis. 2014;7:75–88. doi: 10.2147/IJNRD.S39739. Published 2014 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith A.C., Burton J.O. Exercise in kidney disease and diabetes: time for action. J Ren Care. 2012;38(Suppl 1):52–58. doi: 10.1111/j.1755-6686.2012.00279.x. [DOI] [PubMed] [Google Scholar]
- 37.Ohkawa S., Odamaki M., Ikegaya N., Hibi I., Miyaji K., Kumagai H. Association of age with muscle mass, fat mass and fat distribution in non-diabetic haemodialysis patients. Nephrol Dial Transplant. 2005;20(5):945–951. doi: 10.1093/ndt/gfh643. [DOI] [PubMed] [Google Scholar]
- 38.Cai Z., Yang Y., Zhang J. Effects of physical activity on the progression of diabetic nephropathy: a meta-analysis. Biosci Rep. 2021;41(1) doi: 10.1042/BSR20203624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L., Wang Y., Xiong L., Luo Y., Huang Z., Yi B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: evidence from a meta-analysis. BMC Nephrol. 2019;20(1):398. doi: 10.1186/s12882-019-1586-5. Published 2019 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castaneda C., Gordon P.L., Uhlin K.L., et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med. 2001;135(11):965–976. doi: 10.7326/0003-4819-135-11-200112040-00008. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K., Sasaki T., Yamamoto S., Hayashi H., Ako S., Tanaka Y. Effects of exercise on kidney and physical function in patients with non-dialysis chronic kidney disease: a systematic review and meta-analysis. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-75405-x. Published 2020 Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekinci E.I., Jerums G., Skene A., Crammer P., Power D., Cheong K.Y., et al. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care. 2013;36:3620–3626. doi: 10.2337/dc12-2572. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimizu M., Furuichi K., Yokoyama H., Toyama T., Iwata Y., Sakai N., et al. Kidney lesions in diabetic patients with normoalbuminuric renal insufficiency. Clin Exp Nephrol. 2014;18:305–312. doi: 10.1007/s10157-013-0870-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.