Abstract
Background
Laparoendoscopic single-site (LESS) surgery is performed to further narrow the incisions and reduce tissue injury. It has been more than10 years since the surgery was first described. However, there is still no report on the results of 10-year follow-up. This study evaluated the use of long-term oncology and the renal outcomes of LESS radical nephrectomy (LESS-RN) in the treatment of localized renal cancer.
Methods
We retrospectively analyzed the clinical data of patients treated with LESS-RN at Changhai Hospital from 2009 to 2012. Patients with localized kidney cancer who were followed-up for at least 10 years were included in the study. The baseline data and major perioperative outcome variables were analyzed. Overall survival (OS) and cancer-specific survival (CSS) were calculated using the Kaplan-Meier method.
Results
A total of 48 patients were included in the study, which had a median follow-up of 11 years (interquartile range, 10.7–11.8 years). The 10-year OS and CSS rates were 87.5% [42/48; 95% confidence interval (CI): 0.778–0.972] and 97.9% (47/48; 95% CI: 0.937–1.021), respectively. At the most recent follow-up, there were 5 patients with a chronic kidney disease stage ≥3. Among these 5 patients, 3 developed uremia and required continuous dialysis.
Conclusions
For localized renal cancer, LESS-RN is safe and effective with excellent long-term oncology controllability and good functional outcomes. Prospective studies with large sample sizes need to be conducted to validate our results.
Keywords: Laparoendoscopic single-site surgery (LESS surgery), renal cancer, radical nephrectomy, laparoscopy, outcomes
Highlight box.
Key findings
• The 10-year overall survival and cancer-free survival rates of laparoendoscopic single-site radical nephrectomy (LESS-RN) were 87.5% [42/48; 95% confidence interval (CI): 0.778–0.972] and 97.9% (47/48; 95% CI: 0.937–1.021), respectively;
• A total of 5 patients had a chronic kidney disease stage ≥3. Among these 5 patients, 3 developed uremia and required continuous dialysis before death.
What is known and what is new?
• The 3–5-year follow-up showed that the prognosis of LESS-RN patients was comparable to that of conventional laparoscopy patients;
• We reported the oncology and renal outcomes of LESS-RN with a 10-year follow-up.
What is the implication, and what should change now?
• The long-term oncology controllability and functional outcomes of LESS were comparable to those of conventional laparoscopic nephrectomy.
Introduction
Renal cell carcinoma (RCC) is a common urological malignancy and is the 10th most frequently diagnosed cancer worldwide (1). Radical nephrectomy is the gold standard for the treatment of localized RCC when a partial nephrectomy is not appropriate (2,3). Since the laparoscopic nephrectomy was first reported by Clayman, the use of open nephrectomy in the treatment of RCC has now largely been replaced by conventional laparoscopic nephrectomy (CL-N), as CL-N provides comparable outcomes and has an improved cosmesis compared to that of open radical nephrectomy (4-6).
Laparoendoscopic single-site (LESS) surgery was first described for salpingectomy by Wheeless in 1969 (7). After that, more and more surgeries were performed with LESS (8-10). It has several advantages compared with CL-N including simplicity, expediency, lower cost, avoidance of potential complications associated with CL-N, improved cosmetic results, and flexibility to be converted when indicated (8). In 2007, Rane et al. reported the first case of single-port laparoscopic nephrectomy at the 25th World Congress of Endourology (11). Since 2007, much research has focused on the perioperative and oncologic outcomes of LESS radical nephrectomy (LESS-RN) and found that it is safe and has excellent cosmetic results (12,13). A recent meta-analysis compared CL-N and LESS-RN and found that patients who underwent LESS-RN have a longer operation time but a shorter length of hospital stay (LOS) and suffered less pain (14). However, the research was limited due to its small sample size and short follow-up period. In this study, we report the oncologic and functional outcomes of LESS-RN with a follow-up of 10 years to provide long-term evidence and further evaluate the effect of LESS-RN. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-863/rc).
Methods
Study population
Patients with localized RCC (cT1–T2N0M0) who received LESS-RN at Changhai Hospital between 2009 and 2012 were enrolled in the study. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) have a stage T1–T2 tumor for which RN was indicated; (II) have a lower body mass index (BMI) ≤30 kg/m2; and (III) have T1a tumors that were not suitable for partial nephrectomy (e.g., tumors localized in the hilum with significant involvement of the pelvicalyceal system), or the patient elected to undergo the radical procedure. Patients who were lost to follow-up were excluded from the study. The final decision to proceed with the LESS-RN was made after the patients were advised of all their surgical options. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Changhai Hospital Ethics Committee (No. 2021-140) and informed consent was taken from all the patients. All the patients provided their written informed consent to undergo the LESS-RN and an auxiliary incision or, if necessary, intraoperative open surgery. We described the details of the surgical procedure in an earlier publication (15).
Baseline and perioperative data
Age, gender, BMI, American Society of Anesthesiologists (ASA) classification, age-weighted Charlson comorbidity index (CCI), and complexity (R.E.N.A.L. nephrometry system) were used to evaluate the baseline situation (16). The perioperative data included the operative time, estimated blood loss (EBL), perioperative transfusions and complications, surgical conversions, LOS, and pathologic outcomes. The postoperative complications were graded according to the Clavien scale (17). The surgical terms were defined as follows: (I) reduced port laparoscopy: the addition of an extra trocar ≥5 mm; (II) conventional laparoscopic surgery: the unplanned installation of >1 trocar; (III) open surgery: the creation of an unplanned abdominal incision.
Follow-up assessment
Independent investigators performed the 10-year follow-up visits. It has been recommended that 5 years after surgery, patients undergo abdominal computed tomography (CT) or magnetic resonance imaging (MRI) every 2 years (2). Details of the first 5 years of follow-up were reported in our previously published article (18). Patients who had stopped follow-up at our institution were contacted by telephone to enquire about their latest imaging results and serum creatinine (SCr) levels. Some patients were only able to report that their SCr values were slightly higher than normal and could not provide the exact values. Reports of slightly elevated SCr levels did not concern the examining physician and were considered normal. The chronic kidney disease (CKD) stage of each patient was defined according to the National Kidney Foundation Kidney Disease Outcome Quality Initiative classification (19). The estimated glomerular filtration rate (eGFR)was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) (20).
Statistical analysis
All the statistical analyses were performed using SPSS (IBM Corp., Somers, NY, USA). A survival curve was generated using R 4.2.0 (http://cran.r-project.org). For all the statistical analyses, a two-sided P value <0.05 was considered statistically significant. The categorical variables are presented as the frequency and percentage, and the continuous variables are presented as the median and interquartile range (IQR). The Kaplan-Meier (KM) method was used to calculate the overall survival (OS) and cancer-specific survival (CSS) rates. Missing values are listed in Table 1, and patients with missing values were excluded from the KM analysis.
Table 1. Baseline and perioperative data.
| Variable | Median [IQR] or n (%) |
|---|---|
| Patients (n) | 48 |
| Gender (M/F) | 34 (70.8)/14 (29.2) |
| Age (years) | 57.5 [53.3–65.0] |
| BMI (kg/m2) | 23.88 [22.31–26.14] |
| ASA | 2 [2–2] |
| Symptomatic | 12 (25.0) |
| History of abdominal surgery | 7 (14.6) |
| Comorbidity status | |
| Hypertension | 26 (54.2) |
| Diabetes mellitus | 10 (20.8) |
| Age-adjusted CCI | 1 [0–2] |
| Tumor laterality (L/R) | 32 (66.7)/16 (33.3) |
| Tumor size (cm) | 4.8 [4.0–5.2] |
| Solitary kidney | 0 (0) |
| Multiple ipsilateral tumors | 0 (0) |
| Bilateral kidney tumors | 0 (0) |
| RENAL sum score† | 9 [9–10] |
| RENAL score complexity† | |
| Low [4–6] | 0 (0) |
| Moderate [7–9] | 27 (56.3) |
| High [10–12] | 19 (39.6) |
| Operative time (min) | 183 [161–210] |
| EBL (mL) | 100 [50–200] |
| Length of skin incision‡ (cm) | 6 [5–6] |
| Conversions | |
| To auxiliary hole | 4 (8.3) |
| To open surgery | 2 (4.2) |
| LOS‡ (days) | 6 [6–7] |
| Intraoperative complications | 3 (6.3) |
| Postoperative complications | 2 (4.2) |
| Clavien-grade 1 | 1 (2.1) |
| Clavien-grade 3b | 1 (2.1) |
| Transfusions | 4 (8.3) |
| Pathologic size (cm) | 4.0 [3.5–5.0] |
| Tumor histology | |
| Clear-cell RCC | 44 (91.7) |
| Chromophobe RCC | 2 (4.2) |
| Unclassified RCC | 1 (2.1) |
| Other malignancy | 1 (2.1) |
| Fuhrman grade | |
| I–II | 38 (79.2) |
| III–IV | 5 (10.4) |
| Undetermined | 5 (10.4) |
†, the RENAL scores of 2 patients were unknown; ‡, converted patients were excluded from the analysis. IQR, interquartile range; M, male; F, female; BMI, body mass index; ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; L, left; R, right; RENAL, (R)adius (tumor size as maximal diameter), (E)xophytic/endophytic properties of the tumor, (N)earness of tumor deepest portion to the collecting system or sinus, (A)nterior (a)/posterior (p) descriptor and the (L)ocation relative to the polar line; EBL, estimated blood loss; LOS, length of stay; RCC, renal cell carcinoma.
Results
Baseline and perioperative data
In total, 55 patients were included in this study, of whom 8 patients were lost to follow-up and 48 (87.3%) were followed-up for at least 10 years. Of the patients, 34 (70.8%) were male. The patients had a median age of 57.5 years (IQR, 53.3–65.0 years), a median BMI of 23.88 kg/m2 (IQR, 22.31–26.14 kg/m2), a median ASA score of 2 (IQR, 2–2), and a median CCI of 1 (IQR, 0–2). In total, 12 (25.0%) patients were diagnosed because of their symptoms (i.e., hematuria, flank comfort, or both). Additionally, 26 (54.2%) patients had hypertension, and 10 (20.8%) had diabetes mellitus.
A total of 32 (66.7%) patients’ tumors were on the left and 16 (33.3%) were on the right. No patient had bilateral kidney tumors. The median size of the tumors was 4.8 cm (IQR, 4.0–5.2 cm) on CT or MRI, and the median pathological size was 4.0 cm (IQR, 3.5–5.0 cm). The complexity of the tumors ranged from moderate to high and had a median RENAL score of 9 (IQR, 9–10). Specifically, 27 (56.3%) tumors had moderate complexity, 19 (39.6%) had high complexity, and none had low complexity. RENAL scores could not be calculated for 2 patients because some of the necessary details were not available.
The median operative time was 183 min (IQR, 161–210 min) with an EBL of 100 mL (IQR, 50–200 mL). A total of 6 patients (12.5%) experienced surgical conversions, of whom, 4 (8.3%) had an auxiliary hole, and 2 (4.2%) were converted to open surgery because of severe intraoperative hemorrhage. The median LOS were 6 days (IQR, 6–7 days). The intraoperative complications were all hemorrhages in 3 (6.3%) patients with a blood transfusion. Additionally, 2 (4.2%) patients experienced complications after the surgery, including 1 patient who received a blood transfusion, and 1 who had a fever. The total transfusion rate was 8.3% (4/48). Most of the tumors were clear-cell RCCs (91.7%, 44/48) with a Fuhrman grade of I–II (79.2%, 38/48) (Table 1).
Follow-up data
The median length of the follow-up in the 48 patients was 11 years (IQR, 10.7–11.8 years). A total of 6 patients died during the 10 years. Only 1 death was associated with renal cancer. The other 3 patients experienced a uremia and died due to their poor physical condition, while the remaining 2 patients’ deaths were not related to their tumors or poor kidney function. Notably, the family of 1 patient refused to inform us of the specific time of death or provide any other details. Thus, the OS and CSS rates at 10 years were 87.5% [42/48; confidence interval (CI): 0.778–0.972] and 97.9% (47/48; 95% CI: 0.937–1.021), respectively (Figure 1).
Figure 1.
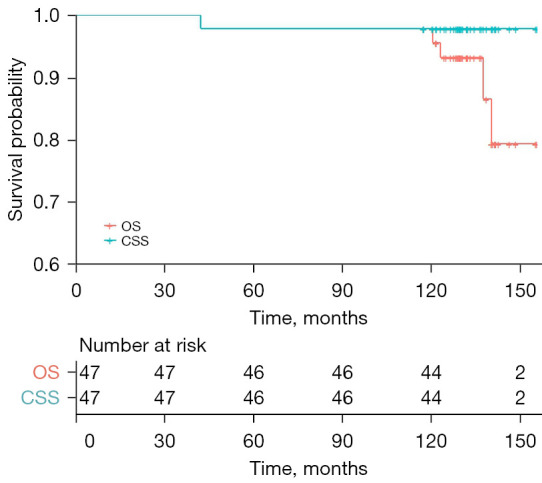
Survival curve with 10-year follow-up. OS, overall survival; CSS, cancer-specific survival.
As for the other 42 patients, 40 had normal or higher SCr levels, but no interventions were required; 1 patient with SCr levels of 160–170 reported feeling weak sometimes; another patient had SCr levels of 180–190 but had no symptoms. The 2 patients had not received any intervention measures and were advised to consult a nearby hospital. Therefore, a total of 5 patients (10.4%, 3 uremia and 2 with elevated SCr) had CKD stage ≥3. Recurrence and metastasis of the tumor only occurred in a patient with stage T1b 22 months after the surgery, who died because of the local recurrence and pulmonary metastasis 20 months later.
Discussion
It has been >10 years since LESS was first reported. Fewer incisions not only improve cosmesis but also cause less tissue injury and blood loss. Major surgical trauma will affect tumor outcomes through the abnormal immune system (21,22). Debate continues as to whether it is worthwhile to perform a more difficult surgery to derive some potential benefits. However, the long-term data on tumor outcomes in LESS-RN patients are limited. We reported the oncological and functional outcomes of 48 patients with pathologically confirmed renal cancer who underwent LESS-RN surgery, each of whom was followed-up for at least 10 years. To our knowledge, this is the first series with a 10-year follow-up period for LESS-RN to date. Previously, a single-institutional study with a 5-year follow-up period was published in 2013 (12). In the 5-year study, 24 LESS-RN and 48 CL-RN patients with malignant tumors were included. The mean follow-up times of the LESS-RN and CL-RN patients were 21 and 38 months, respectively. There was no difference between the patients in terms of OS (91.7% vs. 95.8%; P=0.123) and CSS (95.8% vs. 87.5%; P=0.384). We had better outcomes for OS (100%) compared to that reported in our previous publication (18). The new deaths occurred in the last 2 years. Among the patients who died, 3 (50%) had uremia before their deaths, which suggested that poor physical condition caused by reduced kidney function was the main cause of death after LESS-RN. Only 1 patient with stage T1b experienced tumor recurrence and metastasis. That patient developed local recurrence and pulmonary metastasis 22 months after surgery and died some 20 months later. Recurrence and metastasis has not been reported in many other studies (1,23-25). Taking these data together, we concluded that LESS-RN performs well in long-term cancer control for localized renal cancer at the tertiary care level.
The latest eGFR was not available, as most of the patients could not provide their precise SCr levels during the follow-up. However, the patients reported that their SCr levels were normal or slightly high, and based on these reports, the doctor was not overly concerned. Thus, the SCr levels appeared to be largely normal in the patients included in this study.
These results represent an improvement compared to those reported in our previous publication (18). In the previous study, the CKD stage in 38 cases (74.5%) were upstaging, and 22 of these 38 patients (57.9%, 22/38) increased from a low stage (CKD stage 1 or 2) to a high stage (CKD ≥3) 3–5 years after the surgery. However, in the 10-year follow-up study, only 5 (10.4%) patients had high stage CKD, of whom 3 had uremia and 2 had increased SCr levels for which they had received no treatment at the time of the last follow-up. Lau et al. performed a matched study of patients who underwent partial and radical resections and were followed-up for 10 years. The cumulative incidence rates of CKD were 11.6% and 22.4%, respectively (26). Our results were slightly better than those of other studies in which radical nephrectomies have been performed. The recovery of renal function means that the normal contralateral kidney must provide continuous functional compensation in the intermediate period after surgery, but such compensation will disappear gradually as time goes by. Thus, careful attention should be paid to renal function during the early and middle follow-up periods.
There are still some problems in LESS, such as the disappearance of the operating triangle of the main instruments, and the mutual interference between the speculum and other surgical instruments. These limitations have hindered the promotion of LESS. These problems can be solved by the improvement of surgical instruments. In 2009, Kaouk et al. reported 2 cases of single-port robotic partial nephrectomy (27). The emergence of the latest generation of Da Vinci platform, Da Vinci SP (Intuitive Surgical Corporation, Sunnyvale, CA, USA), better solves the problem of mutual interference between instruments. In 2014, Kaouk et al. reported four cases of partial nephrectomy and two cases of radical nephrectomy using Da Vinci SP with good postoperative renal function and no recurrence (28). Therefore, driven by science and technology, LESS has a good development prospect.
This study had some limitations. First, as a retrospective study with a small sample size, selection bias was unavoidable. Second, in most cases, we did not have exact values for the follow-up SCr levels; thus, the true prevalence of CKD may have been underestimated.
Conclusions
For localized renal cancer, LESS-RN is safe and effective with excellent long-term oncology controllability and has good functional outcomes. Prospective studies with large sample sizes need to be conducted to validate our results.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (Nos. 82072825 and 81874093 to Zhenjie Wu), Program of Shanghai Municipal Health Bureau (Hospital New Star Program of Shanghai to Zhenjie Wu), the Shuguang Program of Shanghai Education Development Foundation and Shanghai Municipal Education Commission (to Zhenjie Wu), and Excellent Ph.D. Talents Program of Changhai Hospital (to Zhenjie Wu).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Shanghai Changhai Hospital Ethics Committee (No. 2021-140), and informed consent was taken from all the patients.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-863/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-863/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-863/coif). The authors have no conflicts of interest to declare.
(English Language Editor: L. Huleatt)
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur Urol 2022;82:399-410. 10.1016/j.eururo.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Campbell SC, Clark PE, Chang SS, et al. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J Urol 2021;206:199-208. 10.1097/JU.0000000000001911 [DOI] [PubMed] [Google Scholar]
- 4.Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: initial case report. J Urol 1991;146:278-82. 10.1016/S0022-5347(17)37770-4 [DOI] [PubMed] [Google Scholar]
- 5.Colombo JR, Jr, Haber GP, Aron M, et al. Oncological outcomes of laparoscopic radical nephrectomy for renal cancer. Clinics (Sao Paulo) 2007;62:251-6. 10.1590/S1807-59322007000300008 [DOI] [PubMed] [Google Scholar]
- 6.Hemal AK, Kumar A, Kumar R, et al. Laparoscopic versus open radical nephrectomy for large renal tumors: a long-term prospective comparison. J Urol 2007;177:862-6. 10.1016/j.juro.2006.10.053 [DOI] [PubMed] [Google Scholar]
- 7.Wheeless CR. A rapid inexpensive and effective method of surgical sterilization by laparoscopy. J Reprod Med 1969;3:65-9. [Google Scholar]
- 8.Wolenski M, Markus E, Pelosi MA. Laparoscopic appendectomy incidental to gynecologic procedures. Todays OR Nurse 1991;13:12-8. [PubMed] [Google Scholar]
- 9.Navarra G, Pozza E, Occhionorelli S, et al. One-wound laparoscopic cholecystectomy. Br J Surg 1997;84:695. [PubMed] [Google Scholar]
- 10.Dou Y, Wang Y, Tang S, et al. Learning curve for laparoendoscopic single-site radical hysterectomy using the "chopstick" technique: a retrospective cohort study. Ann Transl Med 2022;10:1165. 10.21037/atm-22-4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rane A, Kommu S, Eddy B, et al. Clinical evaluation of a novel laparoscopic port (R-port) and evolution of the single laparoscopic port procedure (SLiPP). J Endourol 2007;21:A22-A23. [Google Scholar]
- 12.Antonelli JA, Bagrodia A, Odom C, et al. Laparoendoscopic single-site nephrectomy compared with conventional laparoscopic nephrectomy: a 5-year, single-surgeon experience. Eur Urol 2013;64:412-8. 10.1016/j.eururo.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 13.Greco F, Veneziano D, Wagner S, et al. Laparoendoscopic single-site radical nephrectomy for renal cancer: technique and surgical outcomes. Eur Urol 2012;62:168-74. 10.1016/j.eururo.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 14.Feng D, Cong R, Cheng H, et al. Laparoendoscopic single-site nephrectomy versus conventional laparoendoscopic nephrectomy for kidney tumor: a systematic review and meta-analysis. Biosci Rep 2019;39:BSR20190014. 10.1042/BSR20190014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Liu B, Wu Z, et al. Transumbilical laparoendoscopic single-site surgery: more than 1-year experience in radical nephrectomy and its learning curve study. J Endourol 2011;25:1859-65. 10.1089/end.2011.0015 [DOI] [PubMed] [Google Scholar]
- 16.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844-53. 10.1016/j.juro.2009.05.035 [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng J, Chen J, Sheng X, et al. Oncologic and Functional Outcomes of Laparoendoscopic Single-Site Radical Nephrectomy for Localized Kidney Cancer: A Single Surgeon's Series with a Minimum of 3-Year Follow-Up. J Endourol 2015;29:1242-7. 10.1089/end.2015.0350 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137-47. 10.7326/0003-4819-139-2-200307150-00013 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greco F, Hoda MR, Wagner S, et al. Adipocytokine: a new family of inflammatory and immunologic markers of invasiveness in major urologic surgery. Eur Urol 2010;58:781-7. 10.1016/j.eururo.2010.07.022 [DOI] [PubMed] [Google Scholar]
- 22.Fornara P, Doehn C, Seyfarth M, et al. Why is urological laparoscopy minimally invasive? Eur Urol 2000;37:241-50. 10.1159/000052351 [DOI] [PubMed] [Google Scholar]
- 23.Greco F, Hoda MR, Mohammed N, et al. Laparoendoscopic single-site and conventional laparoscopic radical nephrectomy result in equivalent surgical trauma: preliminary results of a single-centre retrospective controlled study. Eur Urol 2012;61:1048-53. 10.1016/j.eururo.2012.01.043 [DOI] [PubMed] [Google Scholar]
- 24.Springer C, Inferrera A, Kawan F, et al. Laparoendoscopic single-site versus conventional laparoscopic radical nephrectomy for renal cell cancer in patients with increased comorbidities and previous abdominal surgery: preliminary results of a single-centre retrospective study. World J Urol 2013;31:213-8. 10.1007/s00345-012-1005-z [DOI] [PubMed] [Google Scholar]
- 25.Rosoff JS, Fine RG, Velez MC, et al. Laparoendoscopic single-site radical nephrectomy for large renal masses. J Endourol 2013;27:34-9. 10.1089/end.2012.0115 [DOI] [PubMed] [Google Scholar]
- 26.Lau WK, Blute ML, Weaver AL, et al. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 2000;75:1236-42. 10.4065/75.12.1236 [DOI] [PubMed] [Google Scholar]
- 27.Kaouk JH, Goel RK. Single-port laparoscopic and robotic partial nephrectomy. Eur Urol 2009;55:1163-9. 10.1016/j.eururo.2008.12.029 [DOI] [PubMed] [Google Scholar]
- 28.Kaouk JH, Haber GP, Autorino R, et al. A novel robotic system for single-port urologic surgery: first clinical investigation. Eur Urol 2014;66:1033-43. 10.1016/j.eururo.2014.06.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


