One of the most prominent hematologic effects of infection with coronavirus disease 2019 (COVID‐19) is increased thrombotic risk.1., 2. The mechanisms of this hypercoagulable state, which defies even therapeutic anticoagulation, are under intense investigation. Autopsy studies have confirmed thrombotic disease not only at the macrovascular scale (pulmonary emboli, venous thromboses) but also with microthrombi in alveolar capillaries and severe endothelial injury.3., 4. Several studies have reported elevated levels of von Willebrand factor (VWF) activity, VWF antigen, and factor VIII levels in COVID‐19, and incremental increases are reported with severity of disease.5., 6., 7.
Most recently, Mancini et al. have added to this data by evaluating the VWF–A Disintegrin And Metalloprotease with ThromboSpondin 1‐domain (ADAMTS‐13) axis.8 This cross‐sectional analysis of 50 COVID‐19 patients included patients on high‐flow nasal cannula (low intensity, n = 14), non‐invasive positive pressure (intermediate, n = 17), and invasive ventilation (high, n = 19) compared to historical controls. They found median VWF levels markedly elevated and increasing with intensity of care, and there was a relative increase of intermediate and low molecular weight VWF multimers in severe cases. ADAMTS‐13 activity was mild to moderately reduced, and this inversely correlated with severity. The authors concluded that an elevated VWF antigen (VWF:Ag) to ADAMTS‐13 activity ratio was strongly associated with disease severity. They hypothesize that these findings may be explained by massive release of VWF multimers by the activated endothelium with subsequent increased VWF proteolysis by ADAMTS‐13, leading to consumptive reduction in its activity. In this article we wish to discuss these findings as related to a case of thrombotic thrombocytopenic purpura (TTP) with severe COVID‐19 infection.
A 69‐year‐old African American female with a history of immune TTP presented in early 2020 with thrombocytopenia. She was initially diagnosed in 2003 and had a severe phenotype with multiple relapses treated by plasmapheresis and prednisone. Testing by next generation sequencing confirmed there were no genetic variants associated with thrombotic microangiopathy (TMA) syndromes. On diagnosis she was treated with rituximab but developed intolerance at second infusion, leading to trials of alternative immunosuppression including vincristine, cyclosporine, and cyclophosphamide, which had some effect but were also intolerable. In 2013 she developed pulmonary embolism and started anticoagulation using fondaparinux with good response, but due to patient preference she attempted to come off after 6 months. Shortly after she had a cerebral infarct in the left temporoparietal lobe and resumed fondaparinux. Four years later she again tried stopping fondaparinux but had an acute left occipital infarct and resumed anticoagulation. In 2018 she decided to continue with surveillance (no immunosuppression) and anticoagulation using fondaparinux 7.5 mg daily.
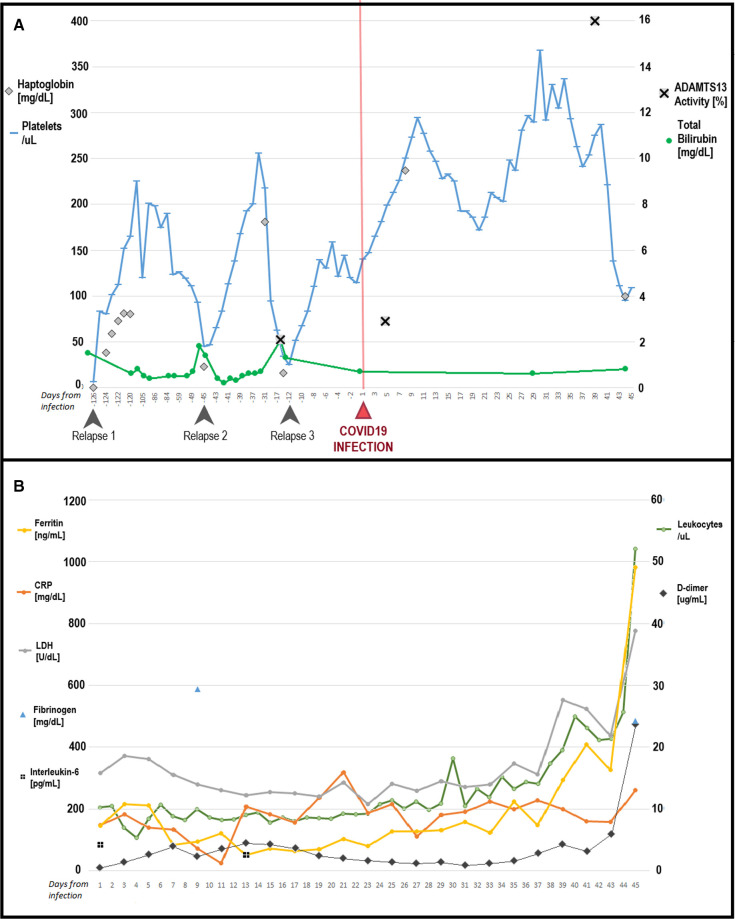
The patient had no symptoms suggestive of COVID‐19, with negative testing, and was admitted for relapsed TTP (Figure 1A ‐ Relapse 1). She responded to four sessions of plasmapheresis and prednisone, which was continued on discharge. In clinic she continued to decline other immunosuppression and tapered prednisone over a month. Five weeks later she presented with relapsed TTP again and was admitted, treated with seven sessions of plasmapheresis and steroids (Figure 1A ‐ Relapse 2). Given relapsing disease, an inpatient trial of rituximab was recommended, but the patient developed hypotension and dyspnea during the infusion and declined further infusions. She was discharged but 3 weeks later was readmitted (Figure 1A ‐ Relapse 3). ADAMTS‐13 activity was 2.1% at this relapse; she was treated with plasmapheresis and steroids, after 3 days of steroids refused any more and asked to be discharged with outpatient surveillance and plasmapheresis as needed.
Figure 1.
A, Response in platelets and hemolytic indices leading up to and following infection with COVID‐19. B, Trends in inflammatory markers after infection
Unfortunately, 2 days after, she returned with acute hypoxic respiratory failure and was found to be COVID‐19 positive on nasopharyngeal polymerase chain reaction (PCR; SARS‐CoV‐2 RT‐PCR Assay, Roche 6800 platform). She received high flow oxygen by nasal cannula, remdesivir for 5 days, and dexamethasone 6 mg daily for 10 days and was continued on fondaparinux 7.5 mg daily. Surprisingly her platelet count increased without further immunosuppression or plasmapheresis (Figure 1A) and maintained >150 × 109/L several weeks out from last treatment. ADAMTS‐13 activity was 2.9% at COVID‐19 diagnosis. Four weeks later ADAMTS‐13 activity was 16% with an inhibitor titer of 1.7 (<0.4). At this time VWF:Ag and activity (RCo:F) were checked and both were unreadably elevated (>597%); factor VIII activity was 414% (56–140). The patient's respiratory status did not improve; PCR for COVID‐19 was persistently positive and inflammatory markers worsened over time (Figure 1B). She was intubated 2 weeks after diagnosis. At the same time she was found to have deep venous thrombosis at the site of a left femoral central venous catheter. The catheter was removed and anticoagulation switched to heparin. After 5 weeks the patient had hypoxia refractory to all management and severe multi‐organ dysfunction syndrome; this continued for 1 more week and she died 6 weeks following COVID‐19 infection.
It has been suggested that TTP, cerebral malaria, and severe COVID‐19 all share similar TMA pathophysiology.9 Recent research suggests that the VWF–ADAMTS‐13 axis has a central role in COVID‐19 vasculopathy. These studies seem consistent but there are still unexplained in vivo hematologic aspects of severe COVID‐19. Highlighted by our recent case is the question of why despite having markedly increased VWF:Ag to ADAMTS‐13 ratio there was improvement in platelet count and no evident hemolysis or TMA progression. Interestingly, looking at the study by Mancini et al., there was no thrombocytopenia, and in fact patients at intermediate and high intensity care (severe COVID‐19) who had reduced ADAMTS‐13 and elevated VWF had increasingly elevated platelet counts.8 These observations combined with the case above lead us to think that several pathways in severe COVID‐19 interact with the VWF–ADAMTS‐13 axis and modify the classical TTP/TMA response. These interactions mitigate some effects that would lead to platelet consumption and result in fulminant TMA, but still drive a hypercoagulable state. It would be interesting to see reports of cases from other centers and as iterated by all, ongoing research to define the mechanisms of COVID‐19 hypercoagulability need to be supported.
CONFLICTS OF INTEREST
All authors certify there are no conflicts of interest or disclosures. No sources of funding apply to this article.
AUTHOR CONTRIBUTIONS
SM, RX, and AR were involved in direct care of the patient. All authors conceptualized the idea for the study and contributed to data collection. SM wrote the manuscript. RX and AR contributed to and edited the manuscript. All authors approved the final version.
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 23 December 2020
REFERENCES
- 1.Lodigiani C., Iapichino G., Carenzo L., et al. Humanitas COVID‐19 Task Force. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middeldorp S., Coppens M., van Happs T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menter T., Haslbauer J.D., Nienhold R., et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID‐19‐associated coagulopathy: evidence from a single‐centre, cross‐sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyvandi F., Artoni A., Novembrino C., et al. Hemostatic alterations in COVID‐19. Haematologica. 2020 doi: 10.3324/haematol.2020.262634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch A., Labreuche J., Lassalle F., et al. Coagulation biomarkers are independent predictors of increased oxygen requirements in COVID‐19. J Thromb Haemost. 2020;18(11):2942–2953. doi: 10.1111/jth.15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancini I., Baronciani L., Artoni A., et al. The ADAMTS13‐von Willebrand factor axis in COVID‐19 patients. Journal of Thrombosis and Haemostasis. 2020 doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Sullivan J.M., Gonagle D.M., Ward S.E., Preston R.J.S., O'Donnell J.S. Endothelial cells orchestrate COVID‐19 coagulopathy. Lancet Haematol. 2020;7(8):e553–e555. doi: 10.1016/S2352-3026(20)30215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]