1. INTRODUCTION
The novel corona virus infection (now classified as COVID‐19), first identified in December 2019 in Wuhan, China, has contributed to significant mortality in several countries with the number of infected cases increasing exponentially worldwide.1 The majority of the most severely ill patients initially present with single organ failure (ie, respiratory insufficiency) but some of them progress to more systemic disease and multiple organ dysfunction. One of the most significant poor prognostic features in those patients is the development of coagulopathy.2 In patients who develop sepsis from various infectious agents, development of coagulopathy is one of the key and persistent features which is associated with poor outcomes.3 In this context, the role of International Society of Thrombosis and Haemostasis (ISTH) will be crucial in guiding health‐care professionals in how to manage the coagulopathy of COVID‐19. A simple and easily followable algorithm for the management of COVID‐19 coagulopathy would currently be useful in both the well‐resourced and less‐resourced settings as a guide in managing this complication. This pragmatic statement should clearly be considered as an interim guidance because the clinical experience of managing this pandemic is increasing. The authors are certain that this statement will be modified with developing knowledge and therapeutics in managing COVID‐19. The aim of this guidance document is to provide a risk stratification at admission for a COVID‐19 patient and management of coagulopathy which may develop in some of these patients, based on easily available laboratory parameters.
2. COAGULATION MARKERS AT ADMISSION
One of the key issues recognized with the management of COVID‐19 infection has been the very high volume of patients presenting to health centers or hospitals. It clearly overwhelms the human and mechanistic capacities available, in particular the need for critical care support. As such, risk stratification measures would clearly be helpful. Currently, this is based on the clinical characteristics of severe pneumonia and a consistent finding of lymphopenia in most patients.4
In addition, one of the most common laboratory findings noted in COVID‐19 patients requiring hospitalization has been the increase in D‐dimers.3 It is already well established that older individuals and those who have co‐morbidities (both groups tend to have higher D‐dimer) are more likely to die from COVID‐19 infection. In the largest analysis of clinical cases published to date, which included data regarding 1099 patients with laboratory‐confirmed COVID‐19 from more than 550 hospitals in China, a D‐dimer ≥0.5 mg/L was noted in 260/560 (46.4%) patients tested with only 43% having raised D‐dimer if the disease was non‐severe, and about 60% had severe illness.2 In a study specifically looking at abnormal coagulation parameters, Tang and colleagues have identified markedly elevated D‐dimers as one of the predictors of mortality.3 They noted a presentation D‐dimer of 2.12 μg/mL (range 0.77‐5.27 μg/mL) in the non‐survivors while it was 0.61 μg/mL (range 0.35‐1.29 μg/mL) in the survivors with the lab normal ranges of <0.50 μg/mL.3 Similarly, Huang and colleagues showed D‐dimer level on admission were higher in patients needing critical care support (median D‐dimer level 2.4 mg/L [0.6‐14.4]) than those patients who did not require it (median D‐dimer level 0.5 mg/L [0.3‐0.8], P = .0042).1 For these reasons, the patients who have markedly raised D‐dimers (which may be arbitrarily defined as three‐ to four‐fold increase), admission to hospital should be considered even in the absence of other severe symptoms because this clearly signifies increased thrombin generation.
The other diagnostic tests commonly performed in any sick patient are the prothrombin time (PT), and the platelet count. In the Tang study, PT was also prolonged in the non‐survivors at admission but only rather modestly (15.5 seconds [range 14.4–16.3 seconds] in non‐survivors versus 13.6 seconds [13.0‐14.3 seconds] in survivors; normal range [11.5−14.5 seconds]).3 The PT was also mildly prolonged at admission in those who needed critical care support versus the non‐ICU cohort (12.2 seconds [range 11.2–13.4] versus 10.7 seconds [range 9.8–12.1], respectively).1 Of note, it is likely that such subtle changes will not be picked up if the prothrombin time is reported as international normalized ratio (INR), which occurs in many centers (INR is not the same as PT ratio).
Thrombocytopenia is often considered an indicator of sepsis mortality.5 Interestingly, this is not the case at admission in many of the COVID‐19 patients. In the study of 41 patients published in the Lancet, a platelet count less than 100 × 109/L was only seen in 2/40 (5%) patients with one each in ICU and non‐ICU category while <150 × 109/L was seen in 38/40 (95%) with similar numbers needing or not needing critical care support.1 On admission, thrombocytopenia determined as 150 × 109/L was also noted in 36.2% in the largest series but further subclassification was not given although while 31.6% of the patients had non‐severe illness, 57.7% had severe illness.2 A meta‐analysis of nine studies including COVID‐19 patients with nearly 400 with severe disease identified that the platelet count was significantly lower in patients with more severe COVID‐19 (weighted mean difference −31 × 109/L; 95% confidence interval [CI], from −35 to −29 × 109/L).6 Subgroup analysis comparing patients by survival noted lower platelet count correlated with mortality. Thrombocytopenia was also associated with over five‐fold increased risk of severe COVID‐19 illness (odds ratio [OR], 5.1; 95% CI, 1.8‐14.6). This suggests thrombocytopenia at the time of admission may be but not consistent prognosticator.
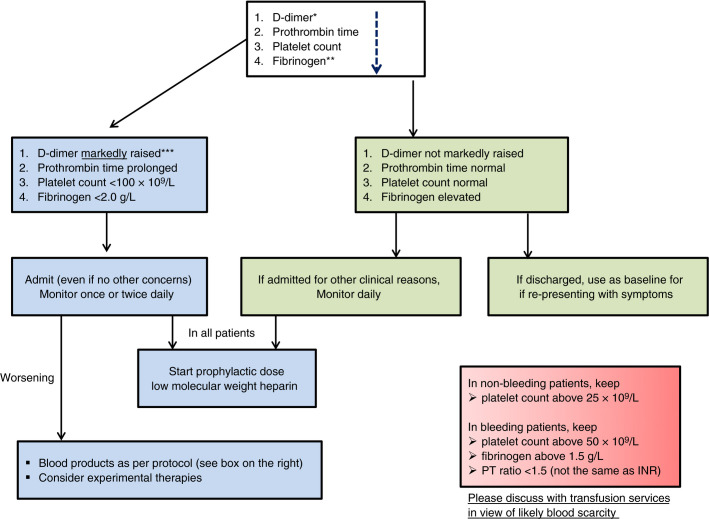
Based on the currently available literature, we would recommend measuring D‐dimers, prothrombin time, and platelet count (in decreasing order of importance) in all patients who present with COVID‐19 infection. This may help in stratifying patients who may need admission and close monitoring or not (see Figure 1 ). Any underlying condition (eg, liver disease) or medication (eg, anticoagulants) which may alter the parameters should be accounted for while using the algorithm.
Figure 1.
Algorithm for the management of coagulopathy in COVID‐19 based on simple laboratory markers. * The list of markers is given in decreasing order of importance. ** Performing fibrinogen assays may not be feasible in many laboratories but monitoring the levels can be helpful after patient admission. *** Although a specific cut‐off cannot be defined, a three‐ to four‐fold increase in D‐dimer values may be considered significant. Any one of the values in this table may be considered significant
3. MONITORING COAGULATION MARKERS
It is common practice in most critical care units to monitor hemostatic markers to identify worsening coagulopathy. In addition to the platelet count, PT, and D‐dimers, it may be useful to measure fibrinogen in this scenario, as recommended by the ISTH guidance on disseminated intravascular coagulation (DIC).7 With respect to COVID‐19 infection, this is an area which has not yet been well studied except by Tang et al.3 They noted the development of DIC on day 4 in the 71.4% of patients who did not survive the infection compared to DIC in just one patient (0.6%) who survived. The researchers also noted a statistically significant increase in D‐dimer levels, and PT, and decrease in fibrinogen levels in non‐survivors at days 10 and 14. This establishes the huge importance of regular laboratory monitoring in these patients. They also observed a decrease in antithrombin levels in the non‐survivors although this test cannot be undertaken easily in many laboratories.
Based on this study and the experience from published literature on septic coagulopathy, monitoring PT, D‐dimer, platelet count, and fibrinogen can be helpful in determining prognosis in COVID‐19 patients requiring hospital admission.8., 9., 10. If there is worsening of these parameters, more aggressive critical care support is warranted and consideration should be given for more “experimental” therapies and blood product support as appropriate. If these markers are stable or improving, it gives the added confidence for stepdown of treatment if corroborating with the clinical condition.
4. MANAGEMENT OF COVID‐19 COAGULOPATHY
This section is based on the only currently available evidence that markedly increased D‐dimer is associated with high mortality in COVID‐19 patients. It is also based on the evidence that multi‐organ failure is more likely in patients with sepsis if they develop coagulopathy and inhibiting thrombin generation may have benefit in reducing mortality.11., 12. The only widely available treatment in this respect is prophylactic dose low molecular weight heparin (LMWH), which should be considered in all patients (including non‐critically ill) who require hospital admission for COVID‐19 infection, in the absence of any contraindications (active bleeding and platelet count less than 25 × 109/L; monitoring advised in severe renal impairment; abnormal PT or activated partial thromboplastin time [APTT] is not a contraindication). The benefit of this approach has recently been submitted as an abstract by Dr Ning Tang. The study included 449 patients with severe COVID‐19, of which 99 received heparin (mainly with LMWH) at prophylactic doses. Although no difference was observed in the 28‐day mortality in those who received heparin compared to those who did not, if a SIC (sepsis‐induced coagulopathy) score ≥4 were to be applied to the patients, anticoagulant therapy with LMWH appears to be associated with better prognosis in relation to mortality (40.0% versus 64.2%, P = .029).13 A similar benefit was noted in those with D‐dimer >six‐fold of upper limit of normal (32.8% versus 52.4%, P = .017). LMWH will also protect critically ill patients against venous thromboembolism. In addition, LMWH has been shown to have anti‐inflammatory properties which may be an added benefit in COVID infection where pro‐inflammatory cytokines are markedly raised.1., 2., 14.
Bleeding is rare in the setting of COVID‐19. If bleeding does develop, similar principles to septic coagulopathy as per the harmonized ISTH guidelines with respect to blood transfusions may be followed7 (see Figure 1). There are several other therapies for COVID‐19 which can only be considered experimental at the moment including antithrombin supplementation, recombinant thrombomodulin, and hydroxychloroquine based on mitigating the excess thrombin generation hypothesis and immunosuppressive agents including inhalational therapies which may put a check on the “immunothrombosis” model (bidirectional relationship between inflammation and thrombosis).
CONFLICTS OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
JT conceived and wrote the initial draft of the paper. NT, SG, MC, AF, ML, CC, and TI made comments. All authors approved the final submission.
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 21 March 2020
REFERENCES
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J., Zhou L., Yang Y., Peng W. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet. 2019 doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson D.R., Albert M., Heels‐Ansdell D., et al. Thrombocytopenia in critically ill patients receiving thromboprophylaxis. Chest. 2013;144(4):1207–1215. doi: 10.1378/chest.13-0121. [DOI] [PubMed] [Google Scholar]
- 6.Lippi G., Plebani M., Henry B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: A meta‐analysis. Clin Chim Acta. 2020;8981(20):145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada H., Thachil J., Di Nisio M., et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. The Scientific Standardization Committee on DIC of the International Society on Thrombosis Haemostasis. J Thromb Haemost. 2013 doi: 10.1111/jth.12155. [DOI] [PubMed] [Google Scholar]
- 8.Taylor F.B., Toh C.H., Hoots W.K., Wada H., Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. [PubMed] [Google Scholar]
- 9.Levi M., Toh C.H., Thachil J., Watson H.G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 10.di Nisio M., Baudo F., Cosmi B., et al. Diagnosis and treatment of disseminated intravascular coagulation: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb Res. 2012;129(5):e177–e184. doi: 10.1016/j.thromres.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Shankar‐Hari M., Phillips G.S., Levy M.L., et al. Sepsis definitions task force. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis‐3) JAMA. 2016;315(8):775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iba T., Levy J.H., Warkentin T.E., et al. Diagnosis and management of sepsis‐induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 13.Iba T., Nisio M.D., Levy J.H., Kitamura N., Thachil J. New criteria for sepsis‐induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poterucha T.J., Libby P., Goldhaber S.Z. More than an anticoagulant: do heparins have direct anti‐inflammatory effects? Thromb Haemost. 2017;117(3):437–444. doi: 10.1160/TH16-08-0620. [DOI] [PubMed] [Google Scholar]