Abstract
Background
Vaccine‐induced immune thrombotic thrombocytopenia (VITT) has so far only been reported after adenovirus vector severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vaccines.
Objective
We report findings in a 25‐year‐old woman who presented with thrombocytopenia, venous thrombosis, elevated D‐dimer levels, and high levels of platelet‐activating antibodies to platelet factor 4‐polyanion complexes 10 days after Gardasil 9 vaccination for human papillomavirus (HPV). The patient exhibited clinical and laboratory features in line with the recently defined VITT syndrome, described after adenoviral vector vaccination to prevent coronavirus disease 2019.
Conclusion
We report a case of VITT following HPV vaccination. This should raise awareness of the possibility of VITT also occurring after other vaccines, not exclusively adenoviral vector‐based SARS‐CoV‐2 vaccines.
Keywords: antibodies, hematology, thrombocytopenia, thrombosis, vaccines
Essentials
-
•
Vaccine‐induced immune thrombotic thrombocytopenia (VITT).
-
•
Thrombocytopenia, thrombosis and platelet‐activating antibodies to PF4‐complexes after HPV vaccination.
-
•
Our findings were very similar to the recently described VITT syndrome.
-
•
We suggest; VITT can be triggered after vaccination with other than adenoviral SARS‐CoV‐2 vaccines.
Alt-text: Unlabelled Box
1. INTRODUCTION
Human papillomavirus (HPV) is associated with cervical intraepithelial neoplasia and cervical cancer. Vaccination against major oncogenic types of HPV has proven benefit in preventing these conditions.1 HPV vaccines have been used for more than a decade, and their use is increasing worldwide.2
Recently, a syndrome of thrombosis and thrombocytopenia occurring 5–24 days after vaccination with ChAdOx1 nCoV‐19 (AstraZeneca) and Ad26.COV2.S (Johnson & Johnson), both recombinant adenoviral vector vaccines encoding the spike protein antigen of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was described.3., 4., 5., 6. This syndrome has been named vaccine‐induced immune thrombotic thrombocytopenia (VITT) or thrombosis with thrombocytopenia syndrome. To our knowledge, this syndrome has never been described after other vaccines. We assessed data for a patient presenting with a clinical picture resembling this syndrome, occurring within 10 days after Gardasil 9 vaccination.
2. CASE PRESENTATION
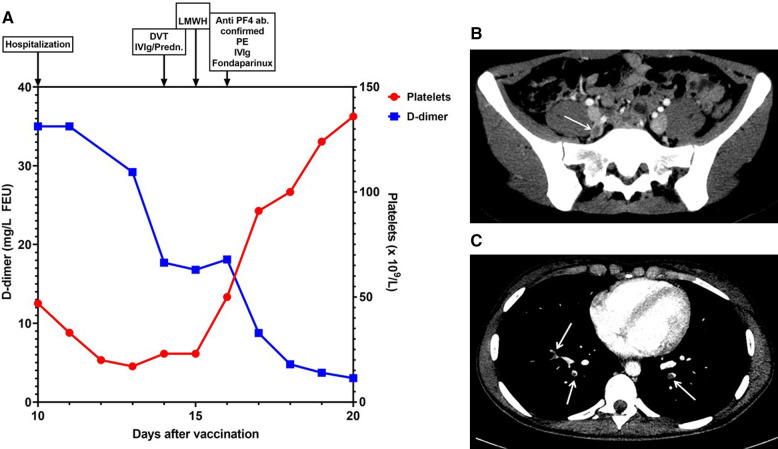
We present a 25‐year‐old healthy woman, without any regular medication except hormonal intrauterine device for contraception. She had not received any SARS‐CoV‐2 vaccine and had no history of thrombosis, previous exposure to heparin, or family history of thromboembolism. She had received three doses of the bivalent HPV vaccine, Cervarix, 3 years previously without side effects. She was admitted to hospital 10 days after Gardasil 9 HPV vaccination following recent cervical intraepithelial neoplasia grade III diagnosis, while awaiting cervical conization. Hematology parameters on the day of vaccination were within normal range. Two days before hospital admission, she experienced malaise, a slight fever (<38°C), lower abdominal and back pain, nausea, and headache. SARS‐CoV‐2 polymerase chain reaction was negative. Initial hematology and coagulation parameters are shown in Table 1 . Examination with abdominal ultrasound, magnetic resonance of the brain, gynecological examination including ultrasound of her uterus and ovaries showed no signs of pathology. Blood tests revealed increased D‐dimer and low platelet count (Figure 1A ). Her abdominal complaints aggravated, and contrast‐enhanced abdominal computed tomography confirmed a 3‐cm‐long thrombus in her right internal iliac vein (Figure 1B). Because of clinical similarity to recent reports of VITT after SARS‐CoV‐2 vaccination, analysis of antibodies to platelet factor 4 (PF4) complexes and platelet activation were performed (see Results). Having confirmed thrombosis and thrombocytopenia, she received treatment with intravenous immunoglobulins (IVIg) 1 g/kg for 2 days, prednisone 1 mg/kg (Figure 1A), and one dose low molecular weight heparin. The following day, her abdominal symptoms resolved and platelet count increased (Figure 1A). As a heparin‐induced thrombocytopenia‐related mechanism was suspected, anticoagulant treatment was switched to fondaparinux.7
TABLE 1.
Laboratory parameters of the patient
| Parameter | Admission | High/low peak | Reference range |
|---|---|---|---|
| Hemoglobin, g/dl | 14.6 | 11.3 | 11.7–15.3 |
| Leukocytes, ×109/L | 11.4 | 14.4 | 3.5–11.0 |
| Platelets, ×109/L | 47 | 17 | 165–387 |
| APTT, s | 29 | 29 | 22–30 |
| INR | 1.2 | 1.2 | 0.9–1.2 |
| D‐dimer, mg/L | 35 | 35 | <0.50 |
| Fibrinogen, g/L | 2.9 | 1.0 | 1.9−4.0 |
| CRP, mg/L | 89 | 89 | <5 |
| PF4‐ ELISA ODa | 3.827b | 2.950c | <0.400 |
| HemosIL AcuStar HIT‐IgG, U/ml | ‐ | 2.52c | <1.00 |
Note
The table demonstrated the main hematological and coagulation parameters for the patient at admission and the highest or lowest levels during hospitalization. The normal references ranges are given in the right column.
Abbreviations: CRP, C‐reactive protein; INR, International Normalized Ratio; OD, optical density.
Pre‐ and post‐IVIg ELISA values.
In pre‐IVIg sample drawn 4 days after admission.
In post‐IVIg sample drawn 5 days after admission.
FIGURE 1.
Patient laboratory and radiological results. (A) Platelet count and D‐dimer levels for the patient after admission. The x‐axis indicates days after vaccination, and the D‐dimer levels on the left y‐axis and platelet count on the right y‐axis. Diagnostic and therapeutic interventions are indicated at the top of the figure. Abbreviations: Anti‐PF4 ab, anti‐platelet factor 4 antibodies; DVT, deep vein thrombosis; IVIg, intravenous immunoglobulin; LMWH, low molecular weight heparin; PE, pulmonary embolism; Predn; prednisolone. (B) Radiological examination with contrast‐enhanced axial abdominal computed tomography (CT) scan showing right internal iliac vein thrombosis (arrow). (C) Radiological examination with contrast‐enhanced axial chest CT scan showing bilateral segmental pulmonary emboli (arrows)
A contrast‐enhanced chest computed tomography scan revealed bilateral pulmonary segmental and subsegmental emboli (Figure 1C). Diagnostic workup regarding hereditary thrombophilia, microangiopathy, including thrombotic thrombocytopenic purpura, immune thrombocytopenia, underlying solid or hematological malignancies, and autoimmune conditions including systemic lupus erythematosus were negative. The patient was discharged from the hospital after 12 days, with no signs of sequelae. Anticoagulation was switched to apixaban, and prednisone was prescribed in tapering doses. Five months after discharge, there are no signs of recurrence of the disease, and she is closely monitored as an outpatient.
3. METHODS AND RESULTS
Antibodies to PF4/polyanionic polyvinyl sulfonate were tested for by ELISA using LIFECODES PF4 IgG (Immucor), dilution 1:50, with an optical density (OD) cutoff value ≥0.400, including a high‐dose heparin inhibition step in three samples; sample derived from the patient at (a) blood donation four months prior to vaccination, (b) before IVIg administration, and (c) after IVIg administration. The prevaccination sample was negative; however, both diagnostic samples showed remarkably high OD values; 3.827 in the initial sample and 2.950 in the sample drawn after the first IVIg dose, both fully inhibited by high‐dose heparin. Additionally, patient serum was positive (2.52 U/ml) in the AcuStar HIT‐IgG test (Instrumentation Laboratory), cutoff value ≥ 1.0 U/ml.
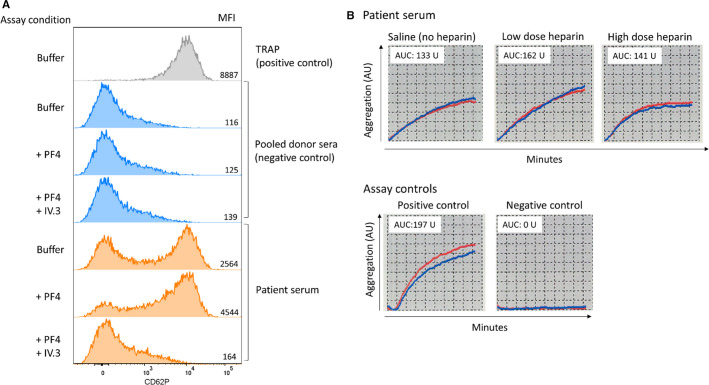
Two functional platelet activation assays were performed. Heparin‐induced multiple electrode aggregometry (HIMEA) run on the multiplate analyzer (Dynabyte Medical),8 as described in Schultz et al.,3 and a PF4‐dependent P‐selectin expression assay, modified from Samuelson Bannow et al.9 Both platelet activation assays were positive. In the P‐selectin expression assay, patient serum activated donor test platelets in buffer. Addition of PF4 enhanced platelet activation, while blocking of FcɣRIIA with mAb IV.3, completely inhibited activation (Figure 2A ). Corresponding heparin‐independent aggregation of whole blood donor platelets were obtained in the HIMEA assay. The aggregation was only slightly affected by heparin (Figure 2B).
FIGURE 2.
Functional platelet activation and aggregation testing. Patient serum activates and aggregates donor platelets in functional tests. Expression of P‐selectin on donor test platelets (pooled platelets from two donors) was measured by flow cytometry (anti‐CD62P mAb, PE) after incubation with patient serum both under buffer conditions and with addition of PF4 (10 μg/ml). The FcɣRIIA‐blocking mAb IV.3 (10 μg/ml) efficiently inhibited the activation. Pooled serum from four healthy donors served as negative control, and TRAP stimulation of donor test platelets was used as positive control (A). Aggregation of donor test platelets was measured by whole‐blood impedance aggregometry in heparin‐induced multiple electrode aggregometry, in presence of low or high heparin concentrations and in the absence of added heparin (saline). The red and blue lines represent duplicate measurements. AU denotes arbitrary units, and the area under the curve (B)
4. DISCUSSION
Our case demonstrates a young woman presenting with thrombocytopenia, thrombosis, high levels of D‐dimer, and antibodies to PF4 complexes within 10 days after nine‐valent HPV vaccination. A combination of these features has not previously been reported after this vaccine.
Recent publications have presented patients with a similar symptom constellation of thrombosis combined with low platelets 5–24 days postvaccination with the ChAdOx1 nCoV‐19 and Ad26.COV2.S vaccines.3., 4., 5., 6. Laboratory results in these patients show a consistent pattern of antibodies to PF4 complexes with unusually high OD values in ELISA, as well as heparin‐independent platelet activation in a variety of functional platelet activation assays.
Our patient exhibited laboratory features indistinguishable from those of the previously reported VITT cases; high anti‐PF4 antibody levels in ELISA and positive functional platelet activation tests. Addition of PF4 enhanced platelet activation, whereas FcɣRIIA‐blocking abolished activation, demonstrating a FcɣR‐dependent mechanism. Noteworthy, the HemosIL HIT IgG assay was positive. This assay does not reliably identify antibodies to PF4 complexes in VITT patient sera,10 suggesting that our patient's antibodies differ slightly from the majority of previously described VITT patients, at least in vitro.
The clinical course with increasing platelet counts rapidly following anticoagulant and immunosuppressive treatment is consistent with reported VITT cases. We propose this to be the first documented case of VITT occurring after HPV vaccination.
Spontaneous HIT is an autoimmune condition triggered by recent inflammatory stimuli such as surgery or infections.11 VITT may represent spontaneous HIT, with the recent vaccine as the triggering event. However, the mechanism from inflammation to formation of pathological platelet‐activating antibodies leading to thrombosis and thrombocytopenia remains unknown.12 Why some individuals are more vulnerable to these vaccine side effects also remains elusive.7
Our patient had previously received three doses of a bivalent HPV vaccine without any side effects. A negative anti‐PF4 antibody test 4 months before vaccination further implicates the nine‐valent vaccine as an initial triggering event, as opposed to a recall response. The bivalent and nine‐valent vaccine are very similar, but the production cell lines and the alum adjuvant formulations are different.13 These vaccines are based on a virus‐like particle, composed of the major L1 capsid protein for HPV types and an aluminum‐containing adjuvant, whereas ChAdOx1 nCoV‐19 and Ad26.COV2.S are adenoviral vector vaccines.2 Thus, theories centered on the SARS‐CoV2 spike protein and adenoviral proteins as factors contributing to antigenic PF4 complex formation, might need reassessment.
Concerns regarding an increase in thromboembolic events following HPV vaccination have been raised,2., 14. but follow‐up studies adjusting for preexisting VTE risk factors found no increased risk.15., 16. Because VITT was just recently recognized, cases of anti‐PF4‐driven thromboembolism following HPV vaccination might not have been identified.
However, our patient is not directly comparable to the usual demographic receiving this vaccine (i.e., she was slightly older and previously HPV vaccinated). In addition, she had an active HPV infection, thus the use of a fourth dose was off‐label. Whether her age, the previous vaccine, and/or the underlying infection contributed to the VITT development are unknown. VITT has only been linked to recombinant adenoviral vector coronavirus disease 2019 vaccines, no other risk factors have been identified and the age‐adjusted incidence is not yet determined.17
Mass vaccination campaigns allow for uncovering rare complications. The current attention to VITT made a timely diagnosis and appropriate treatment possible for our patient, who showed clear and rapid clinical improvement. That VITT potentially is a previously underdiagnosed condition, not restricted to recombinant adenoviral vector or coronavirus disease 2019 vaccines, is conceivable. We suggest that physicians be aware of signs of thrombocytopenia and thrombosis after any vaccination, and that this be monitored in upcoming register vaccine studies.
CONFLICT OF INTEREST
All the authors declare no conflicts of interest related to the manuscript.
AUTHOR CONTRIBUTIONS
Silje Johansen: Patient treatment; writing the initial manuscript, case, and discussion; and revision. Ingvild Jenssen Lægreid: Laboratory analyses, writing the initial manuscript, methods, and discussion; and revision. Siw Leiknes Ernstsen: Laboratory analysis; contribution to initial manuscript, methods, and discussion; and revision. Nessar Ahmad Azrakhsh: Patient treatment; contribution to initial manuscript, case, and discussion; and revision. Astrid Olsnes Kittang: Contribution to initial manuscript, case, and discussion, and revision. Roald Lindås: Contribution to initial manuscript, case, and discussion, and revision. Bjørn Tore Gjertsen: Contribution to initial manuscript, case, and discussion, and revision. Nils Vetti: Contribution to initial manuscript, Figure 1, radiology, and text; and revision. Trude Victoria Mørtberg: Contribution to initial manuscript, methods, and discussion; and revision. Ingvild Hausberg Sørvoll: Contribution to initial manuscript, Figure 2, and revision. Pål André Holme: Involved in patient treatment, contribution to initial manuscript, and revision. Maria Therese Ahlen: Contribution to initial manuscript, Figure 2, and revision. Håkon Reikvam: Patient treatment; contribution to initial manuscript, case, and discussion; Figure 1 and Table 1; and revision.
ETICHAL CONSIDERATIONS
Written informed consent for publication was obtained from the patient.
ACKNOWLEDGMENTS
The authors thank laboratory technicians Svetlana Lund, Renathe Henriksen Grønli, and Marthe Pedersen at The Norwegian National Unit for Platelet Immunology, University Hospital of North Norway, for routine ELISA, HIMEA, and HIT‐IgG Acustar analysis.
Footnotes
Silje Johansen and Ingvild Jenssen Lægreid contributed equally to this work.
Manuscript handled by: Andreas Greinacher
REFERENCES
- 1.Lei J., Ploner A., Elfstrom K.M., et al. HPV Vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 2.Gee J., Naleway A., Shui I., et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29:8279–8284. doi: 10.1016/j.vaccine.2011.08.106. [DOI] [PubMed] [Google Scholar]
- 3.Schultz N.H., Sorvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muir K.L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021;384:1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.See I., Su J.R., Lale A., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arepally G.M., Ortel T.L. Vaccine‐induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 2021;138:293–298. doi: 10.1182/blood.2021012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galea V., Khaterchi A., Robert F., Gerotziafas G., Hatmi M., Elalamy I. Heparin‐induced multiple electrode aggregometry is a promising and useful functional tool for heparin‐induced thrombocytopenia diagnosis: confirmation in a prospective study. Platelets. 2013;24:441–447. doi: 10.3109/09537104.2012.724736. [DOI] [PubMed] [Google Scholar]
- 9.Samuelson Bannow B., Warad D.M., Jones C.G., et al. A prospective, blinded study of a PF4‐dependent assay for HIT diagnosis. Blood. 2021;137:1082–1089. doi: 10.1182/blood.2020008195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platton S., Bartlett A., MacCallum P., et al. Evaluation of laboratory assays for anti‐platelet factor 4 antibodies after ChAdOx1 nCOV‐19 vaccination. J Thromb Haemost. 2021;19:2007–2013. doi: 10.1111/jth.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greinacher A., Selleng K., Warkentin T.E. Autoimmune heparin‐induced thrombocytopenia. J Thromb Haemost. 2017;15:2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 12.Scully M., Singh D., Lown R., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vincenzo R., Conte C., Ricci C., Scambia G., Capelli G. Long‐term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999–1010. doi: 10.2147/IJWH.S50365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slade B.A., Leidel L., Vellozzi C., et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750–757. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 15.Scheller N.M., Pasternak B., Svanstrom H., Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA. 2014;312:187–188. doi: 10.1001/jama.2014.2198. [DOI] [PubMed] [Google Scholar]
- 16.Arnheim‐Dahlstrom L., Pasternak B., Svanstrom H., Sparen P., Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013;347 doi: 10.1136/bmj.f5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karron R.A., Key N.S., Sharfstein J.M. Assessing a rare and serious adverse event following administration of the Ad26.COV2.S vaccine. JAMA. 2021;325:2445–2447. doi: 10.1001/jama.2021.7637. [DOI] [PubMed] [Google Scholar]