Abstract
Objective
Describe characteristics, treatment patterns and clinical outcomes of patients with small-cell lung cancer (SCLC).
Design
Retrospective chart review study defining several cohorts: (1) limited-stage disease (LD) SCLC initiating 1L therapy (1 L LD-SCLC), (2) extensive-stage disease (ED) SCLC initiating 1L therapy (1L ED-SCLC) and (3) patients initiating 2L therapy.
Setting
39 physicians (medical oncologists, thoracic oncologists and/or pulmonologists) from France, Italy and the UK.
Participants
Patients >18 years of age with a confirmed diagnosis of LD-SCLC or ED-SCLC and a full oncology medical history. Patients included initiated a 1L (2013–2015) or 2L (2013–2016) treatment (chemotherapy and/or radiotherapy—RT).
Primary and secondary outcome measures
Overall survival (OS) and progression-free survival (PFS).
Results
231 patients in 1L LD-SCLC, 308 in 1L ED-SCLC and 225 with relapse/refractory SCLC initiating 2L treatment were included. The proportion of men was higher across all groups (56.8% to 68.5%) and mean age at time of diagnosis was 66.0 and 65.4 years in 1L LD-SCLC and 2L ED-SCLC cohorts. The majority of patients in LD-SCLC 1L group received chemotherapy with RT (76.2%). Patients initiating 2L therapy predominantly received chemotherapy alone (79.6%).
Median OS in 1 L patients was 17.3 months in LD-SCLC and 8.8 months in ED-SCLC. Median PFS was 11.6 months in LD-SCLC and 6.1 months in ED-SCLC patients. Median OS in patients initiating 2L treatment was 6.6 months. OS from start of 2L treatment was lower in patients initially diagnosed with ED (5.1 months) than in patients initially diagnosed with LD (9.3 months) (p<0.0001). OS and PFS were assessed from the start of 1L or 2L therapy, depending on the cohort.
Conclusions
Despite the availability of a high number of treatments and combinations, the prognosis of SCLC is still unsatisfactory, especially for those patients diagnosed with ED-SCLC, indicating high unmet need in this patient population.
Keywords: respiratory tract tumours, chemotherapy, radiotherapy, epidemiology
Strengths and limitations of this study.
The main strengths of the study lie in the multicountry retrospective cohort study conducted in 39 sites with a representative sample of patients per study cohort distributed across three countries (France, Italy and UK).
Investigators were asked to examine their medical records for patient eligibility and include cases into each cohort consecutively, until reaching the expected sample per cohort, to minimise potential selection bias.
The main limitations of the study include the limited sample size and the data source. The data are derived from medical records and are, therefore, limited to what is recorded as part of routine clinical care.
Introduction
Small-cell lung cancer (SCLC) accounts for 15% to 17% of all lung cancers.1 2 The disease develops predominantly in patients aged 60–70 years and is strongly associated with a history of smoking.3–5 SCLC metastasises commonly to the brain, liver or bone and is often asymptomatic until the cancer has progressed to a more advanced stage. Its rapid growth, combined with widespread metastasis early in the disease course, results in a 5-year mortality of 90% or more, which makes SCLC the most lethal lung cancer subtype.6 7
For treatment purposes, SCLC is typically classified into two stages; limited-stage disease (LD) and extensive-stage disease (ED),8 although the tumour, node, metastasis (TNM) classification is recommended. The TNM staging system helps in evaluating the extent of spread of cancer and represents a global recognised standard.9 Moreover, although stratification of patients with SCLC in LD and ED is generally satisfactory, the TNM classification is recommended for more detailed prognostic information and treatment evaluation in these patients.10 LD is confined to an area within the thorax that can be encompassed within a radiation port. ED, however, may include malignant pleural or pericardial effusions or metastases consistent with haematogenous spread.11 Most SCLC cases (70%) present as ED at diagnosis (ED-SCLC) while the remaining 30% of patients present with LD (LD-SCLC).12 13 The overall prognosis for patients with SCLC is poor, with a median overall survival (OS) of 15–20 months for LD-SCLC and 8–13 months for ED-SCLC.14 15 The prognosis for relapsed or refractory SCLC is worse with a median survival of 4–5 months.4 16
The clinical management of SCLC is difficult due to the aggressive nature of the disease; survival time is 2–4 months after diagnosis when left untreated.14 Patients with LD have been shown to benefit from surgery or thoracic radiotherapy (TRT) in combination with chemotherapy; however, patients with ED rely historically solely on the systemically delivered chemotherapy to target the disseminated tumour cells.
The optimal first-line (1L) treatment for LD-SCLC is, therefore, concurrent chemotherapy and radiation.17 18 The preferred chemotherapy backbone for the treatment of LD-SCLC is cisplatin and etoposide.8 For patients who achieve a complete response, partial response or stable disease with chemoradiotherapy, subsequent prophylactic cranial irradiation (PCI) is generally recommended.
Until recently the standard of 1L treatment for ED-SCLC was platinum based (cisplatin-etoposide or cisplatin-irinotecan or cisplatin-topotecan) with or without consolidation thoracic and/or PCI.19 Novel treatment options in the form of immunotherapies have also emerged.20 Atezolizumab (Tecentriq, Genentech), a programmed death-ligand 1 (PD-L1) blocking antibody, was shown to improve OS in patients with ED-SCLC and was recently approved by both the Food and Drug Administration (FDA) and the European Medicine Agency as 1L treatment for those patients, in combination with etoposide and carboplatin.21 Also, durvalumab (Imfinzi, AstraZeneca), another PD-L1 inhibitor, has been recently approved by the FDA as 1L treatment of patients with ED-SCLC, in combination with etoposide and either carboplatin or cisplatin.22
The specific selection of cytotoxic second-line (2L) and subsequent regimens is currently defined by the timeframe between 1L treatment initiation and relapse. Both US (American Society of Clinical Oncology) and European (European Society for Medical Oncology) guidelines suggest that patients who relapse more than 6 months after 1L are recommended to be retreated with the original regimen, whereas the preferred options for patients with early relapse (< 6 weeks) are mostly treated with topotecan therapy.17 Platinum rechallenge can also represent a useful 2L option for sensitive-relapsed patients with SCLC.17
Although third-line (3L) treatments are rare due to rapid disease progression and poor performance status, approximately 10%–20% of patients who receive 1L therapy are also willing to receive 3L regimens.20 In this setting, the PD-L1 inhibitors nivolumab and pembrolizumab have been approved by the FDA as 3L treatments of SCLC population.23 24
Last June, the FDA granted approval to lurbinectedin (Zepzelca, Pharma Mar S.A.) for adult patients with metastatic SCLC with disease progression on or after platinum-based chemotherapy.25
To understand how novel therapies may shift the treatment landscape and potentially improve patient outcomes, it is necessary to understand disease management prior to novel therapies and unmet need in SCLC. Equally, as new medicines emerge, these data will help to provide a preimmunotherapy baseline from which changes in patient outcomes that arise as a result of changes in standard of care (SOC) can be assessed. In Europe, there was limited evidence acquired from routine clinical practice that describes the characteristics of ED and LD patients as well as the effectiveness of the treatments used as SOC at the time of our study. Therefore, the purpose of the study was to describe the baseline characteristics, treatment patterns and clinical outcomes of patients diagnosed with LD-SCLC or ED-SCLC receiving 1L treatment and those patients with relapsed/refractory disease receiving 2L treatment across three European countries: France, Italy and the United Kingdom (UK).
Methods
Study design
A non-interventional, retrospective cohort study of advanced SCLC patients was conducted in three European countries (France, Italy and the UK) using a chart review approach. Data from the patients’ medical records were extracted by participant sites and recorded in an electronic case report form specifically designed for the study. Medical records of each patient constituted the primary data source. The study investigators were physicians who manage patients in routine clinical practice (eg, medical oncologists, thoracic oncologists and/or pulmonologists), as applicable per country. The patient identification period covered 2 years starting from October 2013 to October 2015. There were three patient groups: (1) patients with confirmed diagnosis of LD-SCLC within the patient identification period and initiating 1L therapy for SCLC (referred as 1L LD-SCLC), (2) patients with confirmed diagnosis of ED-SCLC within the patient identification period and initiating 1L therapy for SCLC (referred as 1L ED-SCLC) and (3) patients with confirmed diagnosis of SCLC no earlier than October 2013 and initiating 2L treatment due to relapse after 1L therapy no later than August 2016 (2L). This 2L cohort of patients was further classified according to SCLC stage at diagnosis (2L LD-SCLC or 2L ED-SCLC). Study investigators were asked to examine their medical records for patient eligibility and include cases in each cohort consecutively, until reaching the expected sample size per cohort, to minimise potential selection bias. Depending on the disease progression and therapy prescribed after 1L therapy (excluding investigational drugs), patients who were initially part of one of the two 1L treatment groups could be included in the 2L cohort. Index date was defined as date of 1L treatment initiation in the first two cohorts, and as date of 2L treatment initiation for the last cohort of patients. Patients’ data were collected from medical charts from the index date to the point of chart abstraction or death, whichever occurred first.
This study involves human participants and was approved by Ethics Committee(s) and Institutional Board(s) as required by local regulations from each of the participant countries and sites.
Study patients
Patients >18 years of age with a clinician confirmed diagnosis of LD-SCLC or ED-SCLC within the identification period, and with a full oncology medical history for mandatory variables were eligible for inclusion in the study. In addition, patients included in 1L groups were required to have initiated a 1L treatment (chemotherapy and/or radiotherapy—RT) for their SCLC, and patients included in 2L were required to have initiated 2L therapy due to relapse after 1L therapy, no later than August 2016. Patients with prior or concomitant malignancy other than SCLC were excluded. Signed informed consent was obtained as required by local regulations.
Patient and public involvement
None, patients were not involved in the study design, definition of outcomes or plans to disseminate the study results.
Study variables
Study variables included patient characteristics (age, gender, ethnicity, family history and smoking status) and clinical variables (including laboratory parameters, Veterans Administration Lung Study Group and TNM, staging, Eastern Cooperative Oncology Group or Karnofsky scales of performance status and comorbidities in several organ systems: cardiovascular, respiratory, renal, hepatic, neurological, infections and others). Treatments for SCLC were recorded including chemotherapy, RT (eg, TRT, PCI and RT other locations) and surgery in each treatment line. Where applicable, disease progression was collected for all treatment lines, including criteria used to define progression and date of progression.
Statistical analyses
Given the descriptive nature of the study, the sample size required for each cohort was estimated to detect a population percentage of around 50% (requiring the highest sample size) with a 95% CI and a level of precision of 0.1 in each country. For each cohort and country, the study population size was used for the calculation considering a replacement rate of 15%. A target sample for each country required for the study was calculated as France:321, Italy;320, UK: 316.
Data analysis was carried out using SAS statistics software V.9.2. All patients who met eligibility criteria were included in the study population. Missing values were described, but imputation methods were not used. Descriptive analysis was performed stratifying by patient subgroups, including the two subgroups of patients receiving 1L therapy (1L LD-SCLC and 1L ED-SCLC) and the subgroup of patients receiving 2L therapy stratified according to disease stage at diagnosis (2L LD-SCLC or 2L ED-SCLC). Statistical tests were not used to compare baseline characteristics and treatment patterns between study cohorts. OS and progression-free survival (PFS) were analysed using the Kaplan-Meier method. OS was calculated from treatment start date (date of 1L treatment initiation for patients from groups 1L LD-SCLC and 1L ED-SCLC; and date of 2L treatment initiation for patients from 2L group) up to the point of chart abstraction or death, whichever occurred first. PFS was calculated from treatment start date until date of disease progression, date of initiation of a subsequent treatment line or date of death, whichever occurred first. Patients without death or disease progression at date of clinical chart review or patients who were lost-to-follow-up during the observation period were censored. OS and PFS were only analysed in those patients receiving systemic active treatment for SCLC, excluding those patients receiving only RT. OS for patients initiating 2L treatments was compared according to disease stage at diagnosis (2L LD-SCLC vs 2L ED-SCLC).
Results
A total of 39 investigators from 39 sites participated in the study (16 from France, 11 from Italy and 12 from the UK). Eleven of the participant sites were specialised oncology hospitals, 6 were university hospitals, 20 were general hospital with an oncology clinic and 2 hospitals were described as other hospital type. Participating investigators had prior experience in the management of SCLC; 61.5% had more than 15 years of experience and 89.7% had at least 6 years of experience. The sample of patients was planned to be equally distributed between participant sites, but it was adjusted for feasibility reasons, obtaining a spread distribution of patients between participant sites. Twenty-five sites included between 10 and 21 patients, 10 sites included less than 10 patients and only 4 sites included more than 21 patients.
A total of 231 patients with LD-SCLC and 308 patients with ED-SCLC who initiated 1L treatment were included in the study. Overall, 225 patients with relapsed/refractory SCLC who initiated 2L treatment were also considered for the analyses. Out of the 225 patients in the 2L cohort, 198 patients were initially part of one of the two 1L treatment groups. The total number of unique patients included in the study (considering that one patient could contribute data for one or two lines of treatment) was 292 from France, 199 from Italy and 273 from the UK (figure 1).
Figure 1.
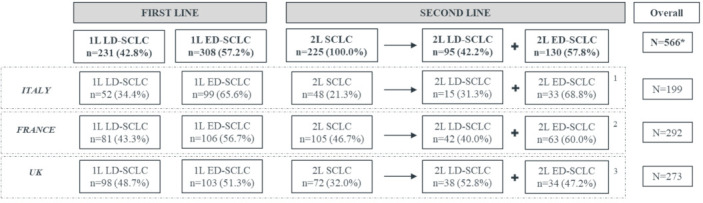
Patients disposition. 1L LD-SCLCL, patients with limited-stage disease at diagnosis receiving first-line (1L) treatment; 1L ED-SCLC, patients with extensive-stage disease at diagnosis receiving 1L treatment; 2L LD-SCLC, patients with limited-stage disease at diagnosis receiving second-line (2L) treatment; 2L ED-SCLC, patients with extensive-stage disease at diagnosis receiving 2L treatment. *Most of the patients included in the 2L treatment cohort were also included in one of the two 1L cohorts. Therefore, the overall sample reported is smaller than the addition of all cohorts. 1All the n=15 patients included in the 2L LD-SCLC group in Italy were initially part of the correspondent 1L cohort; all the n=33 patients included in the 2L ED-SCLC group in Italy were initially part of the correspondent 1L cohort. 2All the n=42 patients included in the 2L LD-SCLC group in France were initially part of the correspondent 1L cohort; n=60 patients included in the 2L ED-SCLC group in France were initially part of the correspondent 1L cohort while n=3 patients were directly included at 2L treatment. 3Out of the 38 patients included in the 2L LD-SCLC group in UK, n=26 were initially part of the 1L LD-SCLC group. Out of the 34 patients included in the 2L ED-SCLC cohort, n=22 were initially part of the 1L ED-SCLC group. The remaining 2L patients (n=12 in both 2L LD and ED groups) were directly included at 2L treatment.
The proportion of men was higher across all groups, ranging from 56.8% to 68.5% (table 1), although in the UK female patients accounted for 61.1% of the total sample (online supplemental table 1). Patient’s mean age (SD) at the time of the diagnosis was 66.0 years (9.2) and 65.4 years (9.2) in LD-SCLC and ED-SCLC patients receiving 1L treatment, respectively. 2L SCLC patients had a mean age of 63.7 (8.7) years (table 1). More than half of the patients were current smokers at the time of the diagnosis in all treatment groups, with percentages ranging from 54.1% to 60.0% (table 1), although the French sample reported a high proportion of ex-smokers (online supplemental table 2). Most of the patients had at least one comorbidity (ranging from 68.2% to 72.6%) (table 1). In terms of number of organ systems affected (including cardiovascular, respiratory, hepatic, neurological systems, infections, diabetes), patients showed a mean higher than one in all groups, indicating that, on average, most patients had comorbidities affecting one or more organ systems (table 1).
Table 1.
Patient characteristics at the time of SCLC diagnosis and treatment initiation
| First-line | Second-line | |||||
| 1L LD-SCLC | 1L ED-SCLC | Diagnosed as 2L LD-SCLC | Diagnosed as 2L ED SCLC | Overall | ||
| (N=231) | (N=308) | (N=95) | (N=130) | (N=225) | ||
| Gender | N | 231 | 308 | 95 | 130 | 225 |
| Male | 133 (57.6%) | 211 (68.5%) | 54 (56.8%) | 87 (66.9%) | 141 (62.7%) | |
| Age at SCLC diagnosis | N | 231 | 308 | 95 | 130 | 225 |
| Mean (SD) | 66.0 (9.2) | 65.4 (9.2) | 63.2 (9.0) | 64.1 (8.6) | 63.7 (8.7) | |
| Smoking status at the time of SCLC diagnosis | N | 231 | 308 | 95 | 130 | 225 |
| Never smoked | 4 (1.7%) | 9 (2.9%) | 2 (2.1%) | 5 (3.9%) | 7 (3.1%) | |
| Current smoker | 125 (54.1%) | 168 (54.6%) | 54 (56.8%) | 78 (60.0%) | 132 (58.7%) | |
| Ex-smoker* | 87 (37.7%) | 114 (37.0%) | 35 (36.8%) | 44 (33.9%) | 79 (35.1%) | |
| Unknown | 15 (6.5%) | 17 (5.5%) | 4 (4.21%) | 3 (2.3%) | 7 (3.1%) | |
| At least one comorbidity at the time of SCLC diagnosis† | N | 231 | 308 | 95 | 130 | 225 |
| Yes | 163 (70.6%) | 210 (68.2%) | 69 (72.6%) | 89 (68.5%) | 158 (70.2%) | |
| # of organ systems affected at the time of SCLC diagnosis† | N | 231 | 308 | 95 | 130 | 225 |
| Mean (SD) | 1.1 (1.0) | 1.2 (1.1) | 1.2 (1.0) | 1.2 (1.0) | 1.2 (1.0) | |
| Stage based on TNM at the time of SCLC diagnosis | Occult Carcinoma | 2 (0.9%) | 0 (0.0%) | 2 (2.1%) | 0 (0.0%) | 2 (0.9%) |
| Stage 0 – IIB | 31 (13.4%) | 0 (0.0%) | 10 (10.5%) | 0 (0.0%) | 10 (4.5%) | |
| Stage IIIA | 70 (30.3%) | 2 (0.6%) | 26 (27.4%) | 1 (0.8%) | 27 (12.0%) | |
| Stage IIIB | 80 (34.6%) | 7 (2.3%) | 33 (34.7%) | 3 (2.3%) | 36 (16.0%) | |
| Stage IV | 0 (0.0%) | 231 (75.0%) | 1 (1.1%) | 101 (77.7%) | 102 (45.3%) | |
| Missing | 48 (20.8%) | 68 (22.1%) | 23 (24.2%) | 25 (19.2%) | 48 (21.3%) | |
| ECOG at initiation of first- or second-line treatment | Unknown | 90 (39.0%) | 128 (41.6%) | 32 (33.7%) | 53 (40.8%) | 85 (37.8%) |
| 0 | 32 (13.9%) | 21 (6.8%) | 8 (8.4%) | 7 (5.4%) | 15 (6.7%) | |
| 1 | 69 (29.9%) | 92 (29.9%) | 33 (34.7%) | 34 (26.2%) | 67 (29.8%) | |
| 2 | 31 (13.4%) | 46 (14.9%) | 18 (19.0%) | 28 (21.5%) | 46 (20.4%) | |
| 3 | 7 (3.0%) | 17 (5.5%) | 3 (3.2%) | 8 (6.2%) | 11 (4.9%) | |
| 4 | 2 (0.9%) | 4 (1.3%) | 1 (1.1%) | 0 (0.0%) | 1 (0.4%) | |
| Presence of brain metastasis at the time of SCLC diagnosis | Yes | 0 (0.0%) | 55 (17.9%) | 0 (0.0%) | 29 (22.3%) | 29 (12.9%) |
1L LD-SCLC: patients with limited-stage disease at diagnosis receiving first-line (1L) treatment; 1L ED-SCLC: patients with extensive-stage disease at diagnosis receiving 1L treatment; 2L LD-SCLC: patients with limited-stage disease at diagnosis receiving second-line (2L) treatment; 2L ED-SCLC: patients with extensive-stage disease at diagnosis receiving 2L treatment. Out of the 225 patients in the 2L cohort, 198 patients were initially part of one of the two 1L treatment groups.
*Ex-smoker: quit smoking at least 6 months ago.
†Collected using the following list of organ systems and comorbidities: cardiovascular (myocardial infarction, congestive cardiac failure, peripheral vascular disease, other cardiovascular disease), respiratory (COPD, other respiratory diseases), renal (moderate-severe kidney disease, other renal disease), hepatic (mild liver disease, moderate-severe liver diseases, other hepatic disease), neurological (cerebrovascular disease, dementia, hemiplegia, other neurological disease), infections (AIDS, other infection), diabetes (with or without end-organ damage) and other (ulcers, connective tissue disease). ECOG 0: fully active, able to carry on all pre-disease performance without restriction; ECOG 1: restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature, for example, light house work, office work; ECOG 2: ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours; ECOG 3: capable of only limited self-care; confined to bed or chair more than 50% of waking hours; ECOG 4: completely disabled; cannot carry on any self-care; totally confined to bed or chair.
ECOG, Eastern Cooperative Oncology Group; TNM, tumour, node, metastasis staging system.
bmjopen-2021-052556supp001.pdf (143.3KB, pdf)
With respect to clinical characteristics, patients with LD-SCLC initiating 1L therapy presented predominantly with TNM stage IIIA (30.3%) or stage IIIB (34.6%), while patients with ED-SCLC initiating 1L therapy mainly presented with TNM stage IV (75.0%) (table 1). These characteristics were generally similar across the three countries (online supplemental tables 1-3). At 2L therapy, LD-SCLC patients predominantly presented with stage IIIB (34.7%) while ED-SCLC patients with stage IV (77.7%%).
Although a high proportion of patients had missing data on ECOG at index date (table 1), available ECOG values indicated similar performance status at initiation of both 1L and 2L therapies, even though differences between study groups were not compared using statistical tests. Brain metastases were only present in ED groups (17.9% of patients initiating 1L therapy and 22.3% of 2L ED-SCLC patients) (table 1).
Most of the patients in 1L LD-SCLC group (76.2%) received chemotherapy with some type of RT (table 2). The majority of the patients (72.7%) received chemotherapy and TRT. Only 2.6% of patients in the 1L LD-SCLC group had surgery and 2.2% received RT alone. Most patients within the 1L ED-SCLC group (58.4%) received chemotherapy alone, 39.3% patients received chemotherapy plus RT, and among these patients, TRT was the type of RT most frequently used (21.4%). No patients in the 1L ED-SCLC cohort had surgery and 1.3% received RT alone (table 2). PCI was essentially used in combination with chemotherapy and TRT in 1L LD-SCLC patients (16.9% and 16.0% of patients received concurrent or sequential chemotherapy, TRT and PCI, respectively), while for 1L ED-SCLC patients, sequential use of PCI was most common over its concurrent use, for both chemotherapy/TRT/PCI and chemotherapy/PCI combinations (table 2).
Table 2.
Treatment patterns used as first-line (1L) and second-line (2L) therapies for LD-SCLC and ED-SCLC patients
| Treatment received | First-line | Second-line | |||
| 1L LD-SCLC | 1L ED-SCLC | Diagnosed as 2L LD-SCLC | Diagnosed as 2L ED SCLC | Overall | |
| (N=231) | (N=308) | (N=95) | (N=130) | (N=225) | |
| Chemotherapy alone | 43 (18.6%) | 180 (58.4%) | 70 (73.7%) | 109 (83.8%) | 179 (79.6%) |
| Chemotherapy+RT | 176 (76.2%) | 121 (39.3%) | 9 (9.5%) | 10 (7.7%) | 19 (8.4%) |
| Chemotherapy+TRT | 168 (72.7%) | 66 (21.4%) | 4 (4.2%) | 5 (3.8%) | 9 (4.0%) |
| Concurrent TRT alone | 42 (18.2%) | 5 (1.6%) | 1 (1.1%) | 1 (0.8%) | 2 (0.9%) |
| Concurrent TRT+PCI | 39 (16.9%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Concurrent TRT+other | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | 1 (0.4%) |
| Sequential TRT | 50 (21.6%) | 24 (7.8%) | 3 (3.2%) | 2 (1.5%) | 5 (2.2%) |
| Sequential TRT+PCI | 37 (16.0%) | 32 (10.4%) | 0 (0.0%) | 1 (0.8%) | 1 (0.4%) |
| Sequential TRT+other | 0 (0.0%) | 4 (1.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Chemotherapy+other (non-TRT) | 0 (0.0%) | 32 (10.4%) | 4 (4.2%) | 1 (0.8%) | 5 (2.2%) |
| Concurrent other alone | 0 (0.0%) | 19 (6.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Concurrent other+PCI | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Sequential other | 0 (0.0%) | 11 (3.6%) | 4 (4.2%) | 1 (0.8%) | 5 (2.2%) |
| Sequential other+PCI | 0 (0.0%) | 2 (0.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Chemotherapy+PCI alone | 8 (3.5%) | 23 (7.5%) | 1 (1.1%) | 4 (3.1%) | 5 (2.2%) |
| Concurrent PCI | 0 (0.0%) | 6 (1.9%) | 0 (0.0%) | 3 (2.3%) | 3 (1.3%) |
| Sequential PCI | 8 (3.5%) | 17 (5.5%) | 1 (1.1%) | 1 (0.8%) | 2 (0.9%) |
| Radiotherapy alone | 5 (2.2%) | 4 (1.3%) | 16 (16.8%) | 11 (8.5%) | 27 (12.0%) |
| Surgery | 6 (2.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Surgery alone | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Surgery+chemotherapy alone | 4 (1.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Surgery+chemotherapy+radiotherapy | 2 (0.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Surgery+radiotherapy alone | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unknown | 1 (0.4%) | 3 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
If initial treatment used is 'Unknown', it means that physician cannot specify any category of treatment used.
1L ED-SCLC, patients with extensive-stage disease at diagnosis receiving 1L treatment; 2L ED-SCLC, patients with extensive-stage disease at diagnosis receiving 2L treatment; 1L LD-SCLC, patients with limited-stage disease at diagnosis receiving 1L treatment; 2L LD-SCLC, patients with limited-stage disease at diagnosis receiving 2L treatment; PCI, prophylactic cranial irradiation; RT, radiotherapy; TRT, thoracic radiotherapy.
Patients initiating a 2L therapy mostly received chemotherapy alone (79.6%). This percentage was slightly higher in patients with ED-SCLC at diagnosis than in patients with LD-SCLC at diagnosis (83.8% vs 73.7%), but statistical comparison was not performed (table 2). The second most adopted treatment was RT alone, especially in 2L LD-SCLC group (16.8%), while this percentage was lower in 2L ED-SCLC group (8.5%). A minor proportion of patients received both chemotherapy and RT (9.5% in 2L LD-SCLC group and 7.7% in 2L ED-SCLC group). Among the patients who received chemotherapy and RT, 4.2% in the LD-SCLC group and 3.8% in the ED-SCLC group received chemotherapy and TRT as their 2L treatment. None of the patients had surgery as part of 2L therapy. Very few patients (N=6) at 2L were administered with PCI: out of the six patients, one received sequential chemotherapy, TRT and PCI; three concurrent chemotherapy and PCI and two sequential chemotherapy and PCI (table 2).
The median OS in patients initiating 1L treatment was 17.3 months in LD-SCLC (95% CI 13.8 to 19.6 months) and 8.8 months in ED-SCLC (95% CI 8.2 to 9.3 months) as shown in figure 2. The median PFS was 11.6 months in LD-SCLC (95% CI 10.1 to 12.9 months) and 6.1 months (95% CI 5.4 to 6.6 months) in ED-SCLC patients (figure 3). The median OS in patients from 2L treatment was 6.6 months (95% CI 5.3 to 7.6 months) from initiation of 2L therapy. OS after 2L treatment initiation was also lower in patients initially diagnosed with ED (5.1 months, 95% CI 4.4 to 5.5) than in patients initially diagnosed with LD (9.3 months, 95% CI 8.3 to 11.5) (p<0.0001), as shown in figure 2.
Figure 2.
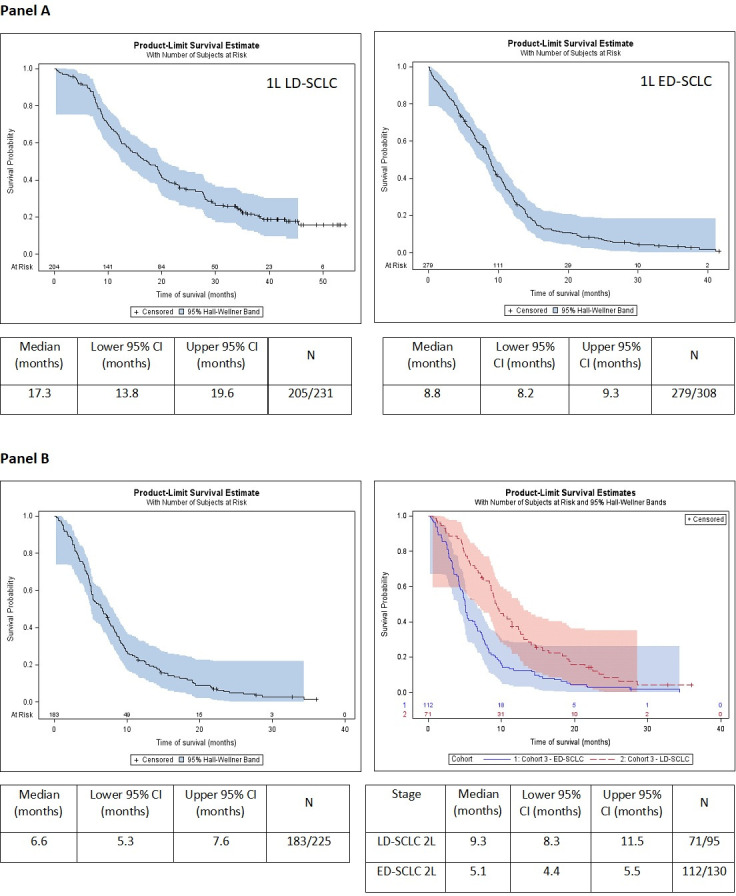
Overall survival (OS) from first-line (1L) treatment initiation in LD-SCLC and ED-SCLC patients (A) and OS from second-line treatment initiation in relapse/refractory patients for overall samples (figure 1B) and according to the stage at diagnosis (figure 2B). 1L LD-SCLC, patients with limited-stage disease at diagnosis receiving 1L treatment; 1L ED-SCLC, patients with extensive-stage disease at diagnosis receiving 1L treatment; 2L LD-SCLC, patients with limited-stage disease at diagnosis receiving second-line (2L) treatment; 2L ED-SCLC, patients with extensive-stage disease at diagnosis receiving 2L treatment; CI: confidence interval; L: lower; U: upper.
Figure 3.
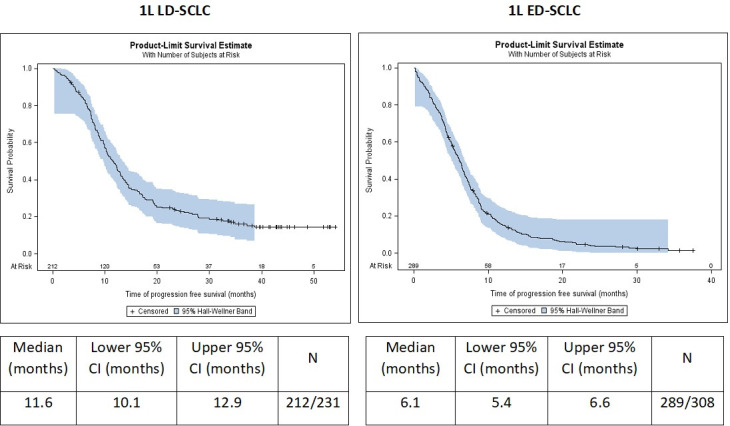
Progression-free survival (PFS) from first-line (1L) treatment initiation in LD-SCLC and ED-SCLC patients. 1L LD-SCLC, patients with limited-stage disease at diagnosis receiving 1L treatment; 1L ED-SCLC, patients with extensive-stage disease at diagnosis receiving 1L treatment.
Discussion
This study showed that combined chemotherapy and RT were the most frequently used treatments at 1L for patients presenting with LD in three European countries. Chemotherapy alone was the most common therapy in both 1L ED-SCLC and overall 2L SCLC groups, which is in line with clinical guidelines.3 Longer OS was observed in patients with LD at diagnosis both at 1L and 2L (17.3 and 9.3 months, respectively) compared with patients with ED (8.8 months at 1L treatment and 5.1 months at 2L). The OS observed among patients included in this study is consistent with prior studies, and the results demonstrate significant unmet need in SCLC patients.
Characteristics of patients included in this study were similar to those reported in other observational studies in SCLC.4 26–28 We observed a median OS of 17.3 months in LD-SCLC, which was substantially longer than the 8.8 months observed in the ED-SCLC cohort initiating 1L treatment. These findings are aligned with those reported in other recent studies and summarised in systematic literature reviews, where a median survival time estimated at 15–20 months for LD-SCLC14 15 29–31 and 8–13 months for ED-SCLC14 15 29 30 is reported.
The median OS from 2L treatment initiation in relapse/refractory patients was 6.6 months, in line with data obtained from other studies that showed median OS ranging from 4.4 to 7.6 months.32–35 When stratifying according to the patients’ initial diagnosis, OS was shorter in patients initially diagnosed with ED-SCLC (5.1 months in relapsed/refractory patients initially diagnosed with ED-SCLC vs 9.3 months in relapsed/refractory patients initially diagnosed with LD-SCLC). Disease at diagnosis was, therefore, not only related to treatment response at 1L but also impacted patient survival at 2L. The limited survival observed within the SCLC population underlines the unmet need in terms of treatments, especially for those patients with extensive disease stage at diagnosis (ED-SCLC).
The study has some limitations, which should be considered. The use of a retrospective chart review approach limits the data collection to only information reported as part of routine clinical practice and as such key clinical variables may be missing (in this study, ECOG data were unknown for ~40% of patients). In addition, data retrospectively collected from clinical charts can depend on country-specific clinical practice and there might be a lack of standardisation of procedures followed for the assessment of study outcomes between participant sites. Disease progression was assessed by each physician following the clinical practice in the site and outcomes may not necessarily be defined as accurately as in a clinical trial.36 Nevertheless, results collected are all aligned with information reported in previous interventional studies.
In terms of patient characteristics, approximately 70% of patients had an ED-SCLC diagnosis and about 30% had an LD-SCLC diagnosis. In this study, the sample size was calculated to include a minimum number of patients in each stage group and in each country. Therefore, the overall 1L sample does not reflect the real-world incidence and prevalence across the two disease stages in terms of SCLC classification. However, some selection bias is likely; sites participating in the study were selected at country level and most relevant centres for SCLC treatment were included. Finally, the number of patients included in this study was lower than expected and this impacted the precision levels. Despite the presentation of some exploratory comparisons of outcomes between disease stages, the study objectives were purely descriptive in order to describe clinical practice at the time of first-line and second-line treatment initiation in SCLC patients in Europe, and, therefore, statistical comparisons between groups were not made.
In conclusion, despite the availability of a high number of treatments and combinations, the prognosis of SCLC remains poor, particularly for those patients diagnosed with ED-SCLC. Given the poor outcomes observed with chemotherapy regimens in this patient population, the introduction of novel therapies reflects an important step-change in the treatment paradigm for patients with SCLC. Future research should explore the effectiveness of these new treatments in the real world. The results of this study will provide a benchmark for the assessment of these new therapies to improve survival outcomes in the real world.
Supplementary Material
Footnotes
Contributors: FB, NG and AL made a substantial contribution to the design of the study and the interpretation of data, provided a critical review of the manuscript and approved the final version to be published. LM and AJG made a substantial contribution to the design of the study, and acquisition and interpretation of data, worked with the preparation of the manuscript and approved the final version to be published. AJG acts as guarantor for the study. MR and NL made a substantial contribution to the design of the study, and acquisition, analysis and interpretation of data, worked with the preparation of the manuscript and approved the final version to be published.
Funding: This work was supported by BMS. Award/Grant number is not applicable.
Competing interests: FB reports personal fees from Abbvie, Amgen, Bayer, Cellgene, CellMedica, Ipsen, Mediation, Pfizer, Regeneron, Roche, and Takeda; grants from Amgen, Pfizer, and Novartis; and non-financial support from Amgen, and AstraZeneca; outside the submitted work. NG reports personal fees from Lilly, Pharmamar and BMS; and grants from Lilly, and BMS; during the conduct of the study. AL does not report personal fees, grants or non-financial support during the conduct of the study. LM and AJG report BMS employment. MR and NL report IQVIA employment.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Data collected for the study are not available for public domain based on data collection and privacy aspects described during regulatory process.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by Ethics Committee(s) and Institutional Board(s) as required by local regulations from each of the participant countries and sites: Sheffield Research Ethics Committee, ID Number: 17/YH/0368; Comitato Etico Lazio I—Ref number: 2276/CE Lazio 1; Comitato Etico Regione Marche - Ref number: CERM—2017 567; Comitato Etico Unico 11egionale per la Basilicata—Ref number: TS/CEUR n. 564, del 09.10.2017; Comitato Etico Regione Marche - Ref number: CERM - 2017 630; Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana—Ref number: Prot n 57219; Regione Veneto Comitato Etico per La Sperimentazione Clinica Delle Province Di Treviso E Belluno—Ref number: DDG 1215/2017; Comitato Etico di Brescia - Ref number: NO 2906—Studio CA209913; Comitato Etico 'Lazio 2' - Ref number: 0205801/2017; Comitato Etico Provinciale Di Reggio Emilia—Ref number: 2017/0111780; Comitato Etico Seconda Universita degli Studi 'Luigi Venvitelli' - Azienda Ospedaliera Universitaria 'Luigi Venvitelli'—AORN 'Ospedali dei Colli' Prot. 694/2017; Comitato Etico Area Pavia—P-20170031769; Comitato Etico Interaziendale A.O.U. Citta' Della Salute e Della Scienza Di Torino Prot. N. 0023548.
References
- 1.Gazdar AF, Bunn PA, Minna JD. Small-Cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017;17:725–37. 10.1038/nrc.2017.87 [DOI] [PubMed] [Google Scholar]
- 2.Globocan. 2018. Available: https://gco.iarc.fr/today/data/factsheets/populations/724-spain-fact-sheets.pdf
- 3.Bernhardt EB, Jalal SI. Small cell lung cancer. Cancer Treat Res 2016;170:301–22. 10.1007/978-3-319-40389-2_14 [DOI] [PubMed] [Google Scholar]
- 4.Coutinho AD, Shah M, Lunacsek OE, et al. Real-world treatment patterns and outcomes of patients with small cell lung cancer progressing after 2 lines of therapy. Lung Cancer 2019;127:53–8. 10.1016/j.lungcan.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 5.van de Kamp HJ, Molder MT, Schulkes KJG, et al. Impact of lung cancer treatment on cognitive functioning. Clin Lung Cancer 2020;21:114–26. 10.1016/j.cllc.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 6.George J, Lim JS, Jang SJ, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524:47–53. 10.1038/nature14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahnert K, Kauffmann-Guerrero D, Huber RM. SCLC-state of the art and what does the future have in store? Clin Lung Cancer 2016;17:325–33. 10.1016/j.cllc.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 8.Alvarado-Luna G, Morales-Espinosa D. Treatment for small cell lung cancer, where are we now? -A review. Transl Lung Cancer Res 2016;5:26–38. 10.3978/j.issn.2218-6751.2016.01.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim W, Ridge CA, Nicholson AG, et al. The 8th lung cancer TNM classification and clinical staging system: review of the changes and clinical implications. Quant Imaging Med Surg 2018;8:709–18. 10.21037/qims.2018.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarogoulidis K, Latsios D, Porpodis K, et al. New dilemmas in small-cell lung cancer TNM clinical staging. Onco Targets Ther 2013;6:539–47. 10.2147/OTT.S44201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farago AF, Keane FK. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res 2018;7:69–79. 10.21037/tlcr.2018.01.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almquist D, Mosalpuria K, Ganti AK. Multimodality therapy for limited-stage small-cell lung cancer. J Oncol Pract 2016;12:111–7. 10.1200/JOP.2015.009068 [DOI] [PubMed] [Google Scholar]
- 13.Hermes A, Waschki B, Gatzemeier U, et al. Characteristics, treatment patterns and outcomes of patients with small cell lung cancer -- a retrospective single institution analysis. Lung Cancer 2011;71:363–6. 10.1016/j.lungcan.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 14.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664–72. 10.1002/cncr.29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 2017;7:1339. 10.1038/s41598-017-01571-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong J, Salgia R. Managing patients with relapsed small-cell lung cancer. J Oncol Pract 2018;14:359–66. 10.1200/JOP.18.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Früh M, De Ruysscher D, Popat S, et al. Small-Cell lung cancer (SCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi99–105. 10.1093/annonc/mdt178 [DOI] [PubMed] [Google Scholar]
- 18.Osterlind K, Andersen PK. Prognostic factors in small cell lung cancer: multivariate model based on 778 patients treated with chemotherapy with or without irradiation. Cancer Res 1986;46:4189–94. [PubMed] [Google Scholar]
- 19.Waqar SN, Morgensztern D. Treatment advances in small cell lung cancer (SCLC). Pharmacol Ther 2017;180:16–23. 10.1016/j.pharmthera.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol 2019;12:47. 10.1186/s13045-019-0736-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horn L, Mansfield AS, Szczęsna A, et al. First-Line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 22.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (caspian): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929–39. 10.1016/S0140-6736(19)32222-6 [DOI] [PubMed] [Google Scholar]
- 23.FDA grants nivolumab accelerated approval for third-line treatment of metastatic small cell lung cancer. 2018. Available: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-third-line-treatment-metastatic-small-cell-lung-cancer
- 24.US Food and Drug Administration . FDA approves pembrolizumab for metastatic small cell lung cancer. 2019. Available: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-metastatic-small-cell-lung-cancer
- 25.Kepp O, Zitvogel L, Kroemer G. Lurbinectedin: an FDA-approved inducer of immunogenic cell death for the treatment of small-cell lung cancer. Oncoimmunology 2020;9:1795995. 10.1080/2162402X.2020.1795995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacheco J, Bunn PA. Advancements in small-cell lung cancer: the changing landscape following impower-133. Clin Lung Cancer 2019;20:148–60. 10.1016/j.cllc.2018.12.019 [DOI] [PubMed] [Google Scholar]
- 27.Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic sclc: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol 2020;15:618–27. 10.1016/j.jtho.2019.12.109 [DOI] [PubMed] [Google Scholar]
- 28.Simeone E, Grimaldi AM, Festino L, et al. Nivolumab for the treatment of small cell lung cancer. Expert Rev Respir Med 2020;14:5–13. 10.1080/17476348.2020.1681977 [DOI] [PubMed] [Google Scholar]
- 29.Pezzi TA, Schwartz DL, Mohamed ASR, et al. Barriers to combined-modality therapy for limited-stage small cell lung cancer. JAMA Oncol 2018;4:e174504. 10.1001/jamaoncol.2017.4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun A, Durocher-Allen LD, Ellis PM, et al. Initial management of small-cell lung cancer (limited- and extensive-stage) and the role of thoracic radiotherapy and first-line chemotherapy: a systematic review. Curr Oncol 2019;26:e372–84. 10.3747/co.26.4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu L, Zhang S, Xu X, et al. Increased biological effective dose of radiation correlates with prolonged survival of patients with limited-stage small cell lung cancer: A systematic review. PLoS One 2016;11:e0156494. 10.1371/journal.pone.0156494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagmann R, Hess V, Zippelius A, et al. Second-Line therapy of small-cell lung cancer: topotecan compared to a combination treatment with adriamycin, cyclophosphamide and vincristine (ACO)-a single center experience. J Cancer 2015;6:1148–54. 10.7150/jca.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Igawa S, Shirasawa M, Fukui T, et al. Efficacy of platinum-based chemotherapy for relapsed small-cell lung cancer after amrubicin monotherapy in elderly patients and patients with poor performance status. Oncology 2018;94:207–14. 10.1159/000486038 [DOI] [PubMed] [Google Scholar]
- 34.Imai H, Sugiyama T, Tamura T, et al. A retrospective study of amrubicin monotherapy for the treatment of relapsed small cell lung cancer in elderly patients. Cancer Chemother Pharmacol 2017;80:615–22. 10.1007/s00280-017-3403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugiyama K, Kogure Y, Torii A, et al. Solvent-based paclitaxel or nab-paclitaxel for heavily treated relapsed/refractory small cell lung cancer: retrospective single-institution observational study. Medicine (Baltimore) 2019;98:e14758. 10.1097/MD.0000000000014758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soni PD, Hartman HE, Dess RT, et al. Comparison of population-based observational studies with randomized trials in oncology. J Clin Oncol 2019;37:1209–16. 10.1200/JCO.18.01074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-052556supp001.pdf (143.3KB, pdf)
Data Availability Statement
No data are available. Data collected for the study are not available for public domain based on data collection and privacy aspects described during regulatory process.


