Abstract
Solid organ transplant recipients (SOTRs) are on lifelong immunosuppression, which may interfere with adaptive immunity to COVID-19. The data on dynamics and duration of antibody response in SOTRs are limited. This longitudinal study examined the longevity of both anti-spike (S)- and anti-nucleocapsid (N)-specific IgG antibodies after COVID-19 in SOTRs compared to matched immunocompetent persons. SOTRs (n = 65) were matched with controls (n = 65) for COVID-19 disease severity, age, and sex in order of priority. Serum-IgG antibodies against N and S antigens of SARS-CoV-2 were analyzed. At 1 and 9 months after COVID-19, anti-S-IgG detectability decreased from 91% to 82% in SOTRs versus 100% to 95% in controls, whereas the anti-N-IgG decreased from 63% to 29% in SOTRs versus 89% to 46% in controls. A matched paired analysis showed SOTRs having significantly lower levels of anti-N-IgG at all time points (1 month p = .007, 3 months p < .001, 6 months p = .019, and 9 months p = .021) but not anti-S-IgG at any time points. A mixed-model analysis confirmed these findings except for anti-S-IgG at 1 month (p = .005) and identified severity score as the most important predictor of antibody response. SOTRs mount comparable S-specific, but not N-specific, antibody responses to SARS-CoV-2 infection compared to immunocompetent controls.
KEYWORDS: antibody biology, clinical research/practice, immunosuppression/immune modulation, infection and infectious agents—viral, infectious disease, organ transplantation in general
Abbreviations: Anti-N, anti-nucleocapsid; Anti-S, anti-spike; AZA, azathioprine; COVID-19, coronavirus disease 2019; MMF, mycophenolate mofetil; MMRM, mixed model for repeated measurements; mTOR, mechanistic/mammalian target of rapamycin; N, nucleocapsid protein; RBD, receptor binding domain; RT-PCR, real-time polymerase chain reaction; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOTRs, solid organ transplant recipients
1. INTRODUCTION
Almost 2 years into the COVID-19 pandemic, several studies indicate that solid organ transplant recipients (SOTRs) with COVID-19 may have an increased risk of mortality.1, 2, 3 Studies from the general population have reported seroconversion in most subjects after SARS-CoV-2 infection.4 , 5 Previous reports have shown a waning of antibody levels over time after natural infection, both in the general population6 and in SOTRs.7 Increased disease severity is associated with higher antibody levels,5 , 8 and the risk for severe COVID-19 is related to factors such as age, male sex, and several comorbidities, including the immunocompromised state.9 The primary viral antigens studied for seroconversion are the spike (S) and nucleocapsid (N) proteins. Neutralizing antibodies, which correlate with IgG antibodies specific for the receptor-binding domain (RBD) of the S-protein,10 are considered most important for protective immunity,11 and remain detectable in serum for up to a year in immunocompetent patients.12 N-specific antibodies are more short-lived in both the general population13 and in SOTRs,14 and their role in providing protective immunity against SARS-CoV-2 is presently unclear. A recent matched study found rapidly waning N-specific responses in liver transplant recipients compared to controls 6 months after COVID-19.15 Data on the long- and mid-term dynamics of N- and S-specific antibody responses to SARS-CoV-2 after COVID-19 in the immunocompromised population remain limited, and comparing results between studies is often problematic due to the different antibody assays used.
Transplant recipients usually require lifelong treatment with a combination of immunosuppressive agents to reduce the risk of rejection. These agents primarily affect T cell–mediated immunity, a vital component in the pathway to protective immunity following infection. Previous reports in SOTRs have shown a high level of seroconversion and stable anti-S-IgG levels for 6 months post-COVID-1914 , 16 , 17 but a low level of N-specific seroconversion with rapid waning.14 , 15 , 18 Currently, to our knowledge, no studies are available comparing the durability and magnitude of S- and N-specific antibody responses between immunosuppressed SOTRs and matched immunocompetent patients. The determination of dynamic changes in antibody response has important implications for long-term management of SARS-CoV-2-infected SOTRs and delineating prudent vaccination strategies in this population. This longitudinal study examines seroprevalence and duration of both S- and N-specific IgG antibodies up to 9 months after COVID-19 of varying severities in SOTRs and compares them to a matched cohort from the general population.
2. PATIENTS AND METHODS
2.1. Patients
The present study included COVID-19 patients enrolled in two prospective observational studies: one recruiting adult SOTRs via The Transplant Institute, Sahlgrenska University Hospital, Gothenburg, Sweden, ongoing since July 2020 (with biobank samples available from March 2020), and one recruiting adult patients via the Department of Infectious Diseases, Sahlgrenska University Hospital, Gothenburg, Sweden, ongoing since March 2020.5 Sixty-five SOTR cases were matched in a 1:1 ratio to non-SOTR controls as closely as possible for, in order of priority, COVID-19 disease severity, sex, and age, with no predefined limitations. Disease severity was classified as defined by the COVID-19 Treatment Guidelines Panel of the National Institutes of Health (NIH) as follows: 1, mild; 2, moderate; 3, severe; and 4, critical .19
COVID-19 was diagnosed whenever a patient had typical symptoms and was positive for SARS-CoV-2 RNA with RT-PCR using a throat or nasal swab. Both cohorts were followed for up to 9 months with sampling planned every third month, and serum samples collected between March 2020 and March 2021 were included in the analysis. The majority of patients had samples from multiple time points: median two times (range 1–4) for both SOTRs and controls. Blood samples were collected during hospitalization (when applicable) and follow-ups. The electronic medical records of all patients were reviewed. Data on patient characteristics, medical history, disease course, comorbidities, and outcomes were collected and analyzed. None of the participants were vaccinated before or during the study period.
The study was conducted in accordance with the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects, approved by the Swedish Ethical Review Authority (#2020-02153 and 2020-01771), and patients were included after written informed consent. The SOTR study was registered with ClinicalTrials.gov (NCT04407221).
2.2. Anti-SARS-CoV-2 antibody assays
Serum-IgG antibodies against SARS-CoV-2 were analyzed using two commercially available serological assays. Anti-S-IgG RBD antibodies were analyzed using the quantitative Architect SARS-CoV-2 IgG II Quant antibody test (Abbott Laboratories) (positive ≥7.1 BAU/ml, converted from AU/ml using the first WHO International Standard for anti-SARS-CoV-2 immunoglobulin [human] [NIBSC Code 20-136]). This assay has a stated sensitivity of 99.37% at ≥15 days post-symptom onset and a specificity of 99.55%. Since not all assays were run simultaneously, reproducibility of the SARS-CoV-2 IgG II Quant assay was tested, including two positive controls and three panels representing low, medium, and high reactivity tested at 60 different occasions. The coefficient of variance (%CV) varied within 3.3–5.0.
Anti-N-IgG antibodies were analyzed using the semiquantitative Architect chemiluminescent microparticle immunoassay (Abbott Laboratories), measuring IgG against SARS-CoV-2 N-protein (positive ≥1.4 index). This assay has a stated sensitivity of 100% at ≥14 days post-symptom onset and a specificity of 99.60%. Within-laboratory precision was evaluated. A negative and a positive sample were analyzed 10 times on 5 different days. The %CV for the negative sample was 5.9 and for the positive sample 1.2.
2.3. Statistical analysis
Statistical analyses were performed with JMP 10 and SAS 9.4 statistical software (SAS Institute, Cary, NC). Data are presented as means and standard deviations, medians and ranges, or numbers and percentages, as appropriate. Comparisons between N- and S-antibody levels in SOTRs and controls were performed at each time point.
The chi-squared test or Fisher’s exact test was employed for analyses of contingency tables. Log-transformed antibody levels after COVID-19 were analyzed using a mixed model for repeated measurements (MMRM) with an unstructured covariance matrix. The model included age group (below or above 50), sex, COVID-19 severity score, and group (SOTRs or controls). The effects on antibody levels of the use of antimetabolite (mycophenolate mofetil [MMF]/azathioprine [AZA]) were investigated in a separate analysis. Effects were nested within time points, thereby allowing for different effects across time points. Anti-S- and anti-N-antibody levels were further compared between SOTRs and controls at each time point by applying a t-test to the differences of log-transformed data within each matched pair. Fisher’s exact test was used to examine the positivity rate between groups. All statistical tests were two-tailed, and p-values <.05 were considered significant.
3. RESULTS
3.1. Patient characteristics
Sixty-five SOTRs and an equal number of controls underwent anti-SARS-CoV-2 antibody testing after RT-PCR confirmed symptomatic infection. The mean (SD) patient age in SOTRs and controls was 51.3 (11.8) and 49.6 (12.3) years, respectively. There were 50.8% females in the SOTR group and 53.8% in the control group. COVID-19 severity scores were exactly matched, and no statistically significant differences were seen regarding age or sex. The clinical characteristics of both study cohorts are detailed in Table 1 and Table S1.
TABLE 1.
Clinical characteristics of solid organ transplant recipients and controls
| SOTRs (n = 65) | Controls (n = 65) | |
|---|---|---|
| Age (SD) | 51.3 (11.8) | 49.6 (12.3) |
| Sex | ||
| Female (%) | 50.8 | 53.8 |
| COVID-19 severity score | n = 65 | n = 65 |
| Mild | 40 (61.5%) | 40 (61.5%) |
| Moderate | 7 (10.8%) | 7 (10.8%) |
| Severe | 15 (23.1%) | 15 (23.1%) |
| Critical | 3 (4.6%) | 3 (4.6%) |
| Comorbidities | n = 65 | n = 53a |
| Hypertension | 52.3% | 26.4% |
| Diabetes | 23.1% | 9.4% |
| Liver disease | 7.7% | 0% |
| Cardiovascular disease | 6.2% | 7.5% |
| Asthma | 1.5% | 5.7% |
| Chronic obstructive pulmonary disease | 0% | 1.9% |
| Malignancy | 1.5% | 1.9% |
| Chronic kidney disease stage 4 or 5 | 15.4% | 0% |
| Transplant type | ||
| Kidney TXb | 42 | — |
| Liver TX | 12 | — |
| Heart TX | 7 | — |
| Lung Tx | 5 | — |
| Pancreas TXb | 4 | — |
| Median time since most recent transplant in months (range) | 74 (−2b to 332) | — |
| Immunosuppressantsc | n = 65 | n = 54 |
| Tacrolimus | 84.6% | 0% |
| Cyclosporine | 12.3% | 0% |
| Mycophenolate mofetil | 66.2% | 0% |
| Prednisolone | 81.5% | 3.7% |
| Azathioprine | 12.3% | 0% |
| mTOR inhibitor | 3.1% | 0% |
| Methotrexate | 1.5% | 0% |
| Triple immunosuppressive therapy | 66.2% | — |
| Double immunosuppressive therapy | 27.7% | — |
| Mono immunosuppressive therapy | 6.2% | — |
Abbreviations: mTOR, mechanistic/mammalian target of rapamycin.
Data concerning comorbidities were available for all 65 SOTRs but only 53 controls. The missing data represent 12 patients with few previous journal entries.
Retransplantation kidney 2 months after COVID.
Data concerning medication use were available for all 65 SOTRs but only 54 controls.
All the controls and 75% of the SOTRs were infected during the first pandemic wave; 16 of 65 SOTRs were infected during a period of low COVID-19 incidence or during the second wave (Figure S1).
3.2. Serology
3.2.1. Proportion with detectable antibodies
At 1 and 9 months after COVID-19, the detectable SARS-CoV-2-specific anti-S-IgG decreased from 96% to 82% in SOTRs and from 100% to 96% in controls, whereas anti-N-IgG decreased from 68% to 30% in SOTRs and from 87% to 49% in controls ( Table 2).
TABLE 2.
Comparison of antibody positivity rate between matched SOTRs and controls
| Anti-N-IgG |
Anti-S-IgG |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOTRs |
Controls |
SOTRs |
Controls |
|||||||
| Months | Positive (%) | n | Positive (%) | n | p | Positive (%) | N | Positive (%) | N | P |
| 1 | 68.2 | 15/22 | 87.0 | 20/23 | .165 | 95.5 | 21/22 | 100 | 21/21 | 1.000 |
| 3 | 52.4 | 22/42 | 87.3 | 48/55 | <.001 | 80.4 | 37/46 | 98.2 | 54/55 | .005 |
| 6 | 34.2 | 14/41 | 58.6 | 17/29 | .053 | 84.4 | 38/45 | 96.3 | 26/27 | .244 |
| 9 | 30.0 | 6/20 | 48.8 | 20/41 | .182 | 82.1 | 23/28 | 95.5 | 21/22 | .111 |
Note: Cutoff value for anti-N-IgG > 1.4 and for anti-S-IgG > 7.1 BAU/ml. p-values from Fisher’s exact test.
Values in bold are considered statistically significant with a p value <.05.
There was a significant difference in the proportions of IgG-positive subjects between the SOTR group and the control group at 3 months only, for both anti-N-IgG (p < .001) and anti-S-IgG (p = .005).
3.2.2. Magnitudes and durability of antibody responses
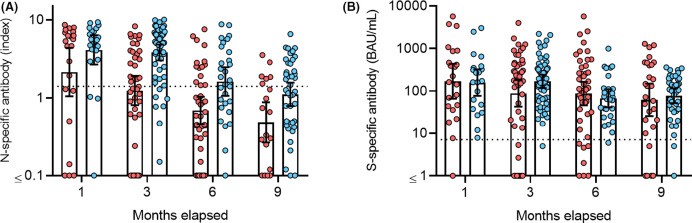
In the paired sample analysis, SOTRs had significantly lower levels of anti-N-IgG at all time points but not lower levels of anti-S-IgG at any time point ( Table 3). Notably, SOTRs had a much greater interindividual antibody level variability than the controls, particularly for anti-S-IgG ( Figure 1).
TABLE 3.
Comparison of antibody levels between SOTRs and controls utilizing only matched paired samples
| Anti-N-IgG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | SOTRs |
Controls |
n diff | G-mean ratio | Lower 95% CL for ratio | Upper 95% CL for ratio | p | ||||
| n | G-mean | CV (%) | n | G-mean | CV (%) | ||||||
| 1 | 22 | 1.77 | 748.5 | 23 | 4.05 | 144.2 | 12 | 0.16 | 0.04 | 0.54 | .007 |
| 3 | 42 | 0.98 | 568.2 | 55 | 3.80 | 104.6 | 40 | 0.27 | 0.15 | 0.51 | .0001 |
| 6 | 41 | 0.54 | 433.4 | 29 | 1.55 | 187.9 | 23 | 0.30 | 0.11 | 0.80 | .019 |
| 9 | 20 | 0.39 | 359.6 | 41 | 1.06 | 184.3 | 18 | 0.31 | 0.11 | 0.82 | .021 |
| Anti-S-IgG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Months | SOTRs |
Controls |
n diff | G-mean ratio | Lower 95% CL for ratio | Upper 95% CL for ratio | p | ||||
| n | G-mean | CV (%) | n | G-mean | CV (%) | ||||||
| 1 | 22 | 164.7 | 1151.6 | 21 | 158.1 | 331.6 | 11 | 0.51 | 0.04 | 6.65 | .570 |
| 3 | 46 | 71.7 | 5705.8 | 55 | 169.2 | 218.0 | 42 | 0.42 | 0.17 | 1.02 | .055 |
| 6 | 45 | 78.9 | 1642.5 | 27 | 67.8 | 189.3 | 21 | 0.61 | 0.19 | 1.99 | .393 |
| 9 | 28 | 46.9 | 5543.3 | 41 | 74.0 | 269.4 | 24 | 0.71 | 0.16 | 3.25 | .650 |
Note: Comparison between groups at each time point by applying a t-test to the differences of log-transformed data within each matched pair. The analysis only uses serological data points that have a corresponding data point available in the matched control.
Values in bold are considered statistically significant with a p value <.05.
FIGURE 1.
Antibody levels in SOTRs (red) and controls (blue). (A) Comparison between anti-N-IgG and (B) anti-S-IgG levels in SOTRs and controls. Positivity threshold level for anti-S-IgG ≥ 7.1 BAU/ml and anti-N-IgG ≥ 1.4 index (dotted lines). Bars indicate geometric mean with 95% confidence interval
Based on results from the MMRM, which utilizes all serological data points, the COVID-19 severity score was the most important and consistent predictor of antibody responses against both S- and N-proteins ( Tables 4 and 5). The use of antimetabolites (MMF or AZA) was only found to be associated with lower anti-N-IgG at 1-month follow-up. Similar analyses of immunosuppression did not uncover any consistent influence of type or number of drugs used (data not shown).
TABLE 4.
Antibody levels in SOTRs
| Months | Comparison | Anti-N-IgG |
Anti-S-IgG |
||||
|---|---|---|---|---|---|---|---|
| Estimated ratio (%) | 95% CI | p | Estimated ratio (%) | 95% CI | p | ||
| 1 | Age <50 vs. ≥50 | 126.4 | 33.5–477.4 | .726 | 398.9 | 69.0–2306 | .120 |
| 3 | Age <50 vs. ≥50 | 194.0 | 69.1–544.1 | .204 | 248.0 | 62.0–991.0 | .195 |
| 6 | Age <50 vs. ≥50 | 38.9 | 13.2–114.4 | .085 | 116.7 | 30.5–445.7 | .819 |
| 9 | Age <50 vs. ≥50 | 97.9 | 26.8–357.3 | .974 | 300.3 | 57.4–1570 | .189 |
| 1 | Sex female vs. male | 15.1 | 4.0–57.1 | .006 | 19.0 | 3.4–106.7 | .059 |
| 3 | Sex female vs. male | 85.7 | 33.2–221.2 | .746 | 61.0 | 16.6–223.8 | .450 |
| 6 | Sex female vs. male | 65.1 | 24.2–175.2 | .390 | 134.4 | 38.3–472.1 | .640 |
| 9 | Sex female vs. male | 121.8 | 37.0–401.5 | .742 | 130.3 | 28.2–601.8 | .731 |
| 1 | Severity score 1 vs. 2–4 | 19.2 | 5.1–71.7 | .015 | 8.9 | 1.6–50.4 | .007 |
| 3 | Severity score 1 vs. 2–4 | 17.5 | 6.4–47.4 | .001 | 9.5 | 2.5–36.8 | .001 |
| 6 | Severity score 1 vs. 2–4 | 58.6 | 21.7–157.8 | .285 | 24.2 | 6.7–87.3 | .031 |
| 9 | Severity score 1 vs. 2–4 | 38.5 | 10.6–139.8 | .144 | 13.6 | 2.8–66.7 | .015 |
| 1 | MMF/AZA 0 vs. 1 | 9.0 | 2.1–38.9 | .002 | 110.6 | 17.4–705.1 | .914 |
| 3 | MMF/AZA 0 vs. 1 | 186.4 | 55.9–621.3 | .305 | 82.6 | 16.6–410.4 | .812 |
| 6 | MMF/AZA 0 vs. 1 | 31.2 | 9.2–105.5 | .061 | 27.6 | 5.7–133.0 | .107 |
| 9 | MMF/AZA 0 vs. 1 | 262.3 | 65.9–1044 | .168 | 26.8 | 4.0–178.7 | .171 |
Note: An analysis of clinical factors predicting antibody response utilizing a mixed model for repeated measures (MMRM). Estimated ratios refer to difference in antibody levels between first and second comparison groups. COVID-19 severity score was defined according to the NIH criteria (1: mild, 2: moderate, 3: severe, and 4: critical).
Values in bold are considered statistically significant with a p value <.05.
Abbreviations: AZA, azathioprine; MMF, mycophenolate mofetil.
TABLE 5.
Antibody levels in SOTRs and controls
| Months | Comparison | Anti-N-IgG |
Anti- S IgG |
||||
|---|---|---|---|---|---|---|---|
| Estimated ratio (%) | 95% CI | p | Estimated ratio (%) | 95% CI | p | ||
| 1 | Age <50 vs. ≥50 | 116.8 | 49.6–274.7 | .720 | 250.3 | 86.0–728.3 | .092 |
| 3 | Age <50 vs. ≥50 | 101.9 | 60.9–170.4 | .943 | 112.1 | 56.4–222.8 | .743 |
| 6 | Age <50 vs. ≥50 | 70.3 | 35.2–140.3 | .315 | 83.9 | 40.8–172.6 | .630 |
| 9 | Age <50 vs. ≥50 | 87.3 | 49.8–153.2 | .634 | 90.6 | 39.8–206.3 | .814 |
| 1 | Sex female vs. male | 41.9 | 17.8–98.2 | .045 | 41.7 | 14.2–122.0 | .109 |
| 3 | Sex female vs. male | 95.0 | 56.3–160.1 | .845 | 69.3 | 34.3–139.9 | .304 |
| 6 | Sex female vs. male | 61.3 | 30.0–125.4 | .178 | 110.7 | 53.1–230.9 | .785 |
| 9 | Sex female vs. male | 100.6 | 57.4–176.1 | .984 | 83.8 | 36.5–192.4 | .675 |
| 1 | Severity score 1 vs. 2–4 | 38.3 | 14.5–101.2 | .053 | 7.9 | 2.3–27.4 | <.001 |
| 3 | Severity score 1 vs. 2–4 | 37.9 | 22.1–64.9 | .001 | 16.3 | 7.9–33.8 | <.001 |
| 6 | Severity score 1 vs. 2–4 | 79.4 | 38.3–164.8 | .534 | 32.9 | 15.4–70.5 | .005 |
| 9 | Severity score 1 vs. 2–4 | 40.8 | 22.6–73.8 | .003 | 29.0 | 12.1–69.5 | .006 |
| 1 | SOTRs vs. controls | 20.7 | 8.7–49.0 | <.001 | 20.3 | 6.7–61.6 | .005 |
| 3 | SOTRs vs. controls | 28.9 | 17.4–48.1 | <.001 | 59.3 | 30.0–117.1 | .131 |
| 6 | SOTRs vs. controls | 30.7 | 15.6–60.5 | <.001 | 91.6 | 44.6–188.1 | .809 |
| 9 | SOTRs vs. controls | 27.6 | 15.5–49.2 | <.001 | 56.5 | 25.1–127.0 | .165 |
Note: An analysis of clinical factors predicting antibody response utilizing a mixed model for repeated measures (MMRM). The model utilizes all serological data points regardless of a corresponding data point being available in the matched control. SOTRs vs. controls adjusted for age, sex, and COVID-19 severity score. Estimated ratio refers to the difference in antibody levels between the first and the second comparison groups. Severity score was defined according to the NIH criteria (1: mild, 2: moderate, 3: severe, 4: critical).
Values in bold are considered statistically significant with a p value <.05.
Abbreviations: AZA, azathioprine; MMF, mycophenolate mofetil.
The MMRM also showed lower anti-N-IgG levels in SOTRs than controls at all time points. Similar differences in levels of anti-S-IgG were not as evident, only reaching statistical significance at 1-month follow-up (Table 5).
3.3. Reinfections
No RT-PCR-confirmed reinfections were identified in the SOTR or control groups during the study period. One SOTR patient had a clinically suspected reinfection with typical symptoms 5 months after the initial infection but was RT-PCR negative. This patient was initially negative in serology for both anti-N- and anti-S-IgG antibodies at 3 months of follow-up (anti-N-IgG = 0.03 and anti-IgG = 0.4 BAU/ml) and developed positive serology after the suspected reinfection at 6 months of follow-up (anti-N-IgG = 7.54 and anti-S-IgG = 53 BAU/ml).
4. DISCUSSION
To our knowledge, this is the first longitudinal study of humoral immune responses (both anti-S- and anti-N-IgG antibodies) to SARS-CoV-2 in SOTRs with matched immunocompetent controls, up to 9 months after COVID-19 of all severities. We found that SOTRs had consistently lower N-specific IgG levels than matched controls, but the differences were less pronounced for S-specific IgG responses. Aside from belonging to the SOTR or control group, the COVID-19 severity score was the most important and consistent predictor of N-specific antibody responses. Interestingly, the use of antimetabolites appeared to have negligible additive effects on the ability to mount an antibody response after COVID-19.
In SOTRs, antibody responses directed toward the N-protein were more diminished than those directed toward the S-protein at all time points, both in the paired analysis and in the MMRM utilizing all serological data points, confirming the findings of a previous uncontrolled study of SOTRs20 and a small controlled study.17 While an absolute correlate of protection against reinfection with SARS-CoV-2 remains to be defined, neutralizing antibodies are likely the most relevant measure of protective immunity, and antibodies specific for the RBD of the S-protein have been shown to correlate well with neutralizing antibodies.21 , 22 The fact that SOTRs appear to mount broadly comparable magnitudes of such antibodies as controls over 9 months of follow-up is encouraging, with the caveat that the interindividual variation was more prominent in SOTRs than in the controls.
In our study, we believe that missing data regarding antibody levels occurred randomly. Hence a MMRM was applied to be able to use all patients in the analysis at all time points as a complement to the paired analysis. The MMRM is unbiased under the missing at random (MAR) assumption and can be thought of as aiming to estimate the difference between groups that would have been observed if all patients had contributed to measurements at all time points. Using MMRM, we show that increased disease severity was associated with greater antibody responses in SOTRs, as has been previously shown in several populations.5 , 23 , 24
Calcineurin inhibitors, such as tacrolimus, interfere with CD4+ T helper cell signaling. T follicular helper cells, in particular, are imperative for aiding B cells to mount efficient antibody responses and memory B cell development via germinal center reactions and have been shown to be suppressed by tacrolimus.25 MMF and mTOR inhibitors, such as sirolimus and everolimus, inhibit B cell immunoglobulin production directly,26 while AZA causes B cell depletion. As a consequence, SOTRs have been found to have impaired immune responses to several vaccinations compared to controls.27 In recent COVID-19 vaccine studies, SOTRs treated with MMF-free immunosuppressive regimens are more likely to develop antibodies against SARS-CoV-2.28 Using MMRM analysis, treatment with antimetabolites such as MMF and AZA was only found to be associated with significantly lower anti-N-IgG at 1-month follow-up. Therefore, somewhat surprisingly, antimetabolites did not appear to have substantial additional detrimental effects on antibody responses compared to other immunosuppressants in the present study. Germinal center reactions are required for affinity maturation of antibody responses, and we cannot exclude the antimetabolites interfered with the avidity development that is associated with better neutralization after COVID-19 disease.29
The major strengths of this study are the long follow-up period of 9 months, careful matching for age, sex, and disease severity between SOTRs and immunocompetent controls, and assessment of both anti-S- and anti-N-IgG antibodies, enabling an accurate estimate of the effect of SOT on the magnitudes and durability of humoral immune responses to SARS-CoV-2 infection. However, the SOTRs had more comorbidities, such as hypertension and diabetes, than the controls, which may be a source of bias.30 A major weaknesses of the study is a lack of samples from all patients at all time points, a consequence of either late inclusion or short follow-up time before COVID-19 vaccines became available. This could be a potential source of bias since COVID-19 severity scores are not perfectly balanced at every time point between groups (Table S1). This has been addressed by including a paired analysis that only uses serological data points with a corresponding data point available in the matched control (Table 3). This analysis confirmed significant differences in anti-N-IgG but not in anti-S-IgG between SOTRs and controls. The time of sampling had some variability in both groups (Table S1), albeit with good agreement in the median times. Furthermore, the timing of enrollment differed slightly between the SOTRs and controls. As all the controls were infected during the first pandemic wave compared to 75% of the SOTRs, the controls potentially had a longer time frame to be reexposed to the virus and boost antibody levels. Nevertheless, none of the controls suffered reinfection, whereas only one SOTR was suspected of having done so.
In conclusion, SOTRs mount comparable magnitudes of S-specific, but not N-specific, IgG antibody responses to SARS-CoV-2 infection compared to immunocompetent controls. These data provide an encouraging view that natural infection may elicit a relatively robust immune response in most SOTRs, with possible implications for the management and vaccination planning of SOTRs with previous COVID-19 infection. However, further studies are needed to elucidate the factors predisposing a minority of patients to a lack of response and the significance of N-specific antibodies.
ACKNOWLEDGMENTS
The study was financed by grants from Njurstiftelsen, The Healthcare Board, Region Västra Götaland (Hälso- och sjukvårdsstyrelsen) (941182), Transplantationsstiftelsen, the SciLifeLab National COVID-19 Research Program, financed by the Knut and Alice Wallenberg Foundation (KAW 2020-0015, 2020.0182, and 2020.0241), Göteborgs läkaresällskap (960901), SU-fonderna (961094), and by the Swedish state, under an agreement between the Swedish government and the county councils (ALFGBG-717531 and 679621).
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information SU-fonderna, Grant/Award Number: 961094; Swedish state, under an agreement between the Swedish government and the county councils, Grant/Award Number: ALFGBG-717531; Njurstiftelsen; SciLifeLab/KAW, Grant/Award Number: 2020.0182, 2020.0241 and 2020-0015; Göteborgs läkaresällskap, Grant/Award Number: 960901; Transplantationsstiftelsen; The Healthcare Board, Region Västra Götaland, Grant/Award Number: 941182
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Supplementary Material
REFERENCES
- 1.Felldin M, Softeland JM, Magnusson J, et al. Initial report from a swedish high-volume transplant center after the first wave of the COVID-19 pandemic. Transplantation. 2021;105(1):108–114. doi: 10.1097/TP.0000000000003436. [DOI] [PubMed] [Google Scholar]
- 2.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2020;35(1):100588. doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ao G, Wang Y, Qi X, et al. The association between severe or death COVID-19 and solid organ transplantation: A systematic review and meta-analysis. Transplant Rev (Orlando). 2021;35(3):100628. doi: 10.1016/j.trre.2021.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marklund E, Leach S, Axelsson H, et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS One. 2020;15(10):e0241104. doi: 10.1371/journal.pone.0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavarot N, Leruez-Ville M, Scemla A, et al. Decline and loss of anti-SARS-CoV-2 antibodies in kidney transplant recipients in the 6 months following SARS-CoV-2 infection. Kidney Int. 2021;99(2):486–488. doi: 10.1016/j.kint.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen CB, Jarlhelt I, Perez-Alos L, et al. SARS-CoV-2 antibody responses are correlated to disease severity in COVID-19 convalescent individuals. J Immunol. 2021;206(1):109–117. doi: 10.4049/jimmunol.2000898. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Science brief: evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html#anchor_1616780486662. Published 2021. Accessed June 8, 2021.
- 10.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021. Online ahead of print. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed]
- 11.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolotin S, Tran V, Osman S, et al. SARS-CoV-2 seroprevalence survey estimates are affected by anti-nucleocapsid antibody decline. J Infect Dis. 2021;223(8):1334–1338. doi: 10.1093/infdis/jiaa796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Softeland JM, Friman G, von Zur-Muhlen B, et al. COVID-19 in solid organ transplant recipients: a national cohort study from Sweden. Am J Transplant. 2021;21(8):2762–2773. doi: 10.1111/ajt.16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caballero-Marcos A, Salcedo M, Alonso-Fernandez R, et al. Changes in humoral immune response after SARS-CoV-2 infection in liver transplant recipients compared to immunocompetent patients. Am J Transplant. 2021;21(8):2876–2884. doi: 10.1111/ajt.16599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benotmane I, Gautier Vargas G, Velay A, et al. Persistence of SARS-CoV-2 antibodies in kidney transplant recipients. Am J Transplant. 2021;21(6):2307–2310. doi: 10.1111/ajt.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CC, Vlad G, Vasilescu ER, et al. Disparity between levels of anti-RBD IgG and anti-nucleocapsid protein IgG antibodies in COVID-19-recovered patients who received a kidney transplant. Kidney Int. 2021;100(1):240–241. doi: 10.1016/j.kint.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed December 20, 2020. [PubMed]
- 20.Burack D, Pereira MR, Tsapepas DS, et al. Prevalence and predictors of SARS-CoV-2 antibodies among solid organ transplant recipients with confirmed infection. Am J Transplant. 2021;21(6):2254–2261. doi: 10.1111/ajt.16541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5(12):1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 23.Chen W, Zhang J, Qin X, et al. SARS-CoV-2 neutralizing antibody levels are correlated with severity of COVID-19 pneumonia. Biomed Pharmacother. 2020;130:110629. doi: 10.1016/j.biopha.2020.110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5(54) doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallin EF, Hill DL, Linterman MA, Wood KJ. The calcineurin inhibitor tacrolimus specifically suppresses human T follicular helper cells. Front Immunol. 2018;9:1184. doi: 10.3389/fimmu.2018.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidt S, Roelen DL, Eijsink C, van Kooten C, Claas FH, Mulder A. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation. 2008;86(9):1292–1300. doi: 10.1097/TP.0b013e3181874a36. [DOI] [PubMed] [Google Scholar]
- 27.Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974. doi: 10.1371/journal.pone.0056974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantauskaite M, Muller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2021. Online ahead of print. 10.1111/ajt.16851 [DOI] [PMC free article] [PubMed]
- 29.Pichler D, Baumgartner M, Kimpel J, et al. Marked increase in avidity of SARS-CoV-2 antibodies 7–8 months after infection is not diminished in old age. J Infect Dis. 2021;224(5):764–770. doi: 10.1093/infdis/jiab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu KK, Fischinger S, Smith MT, et al. Comorbid illnesses are associated with altered adaptive immune responses to SARS-CoV-2. JCI Insight. 2021;6(6) doi: 10.1172/jci.insight.146242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.