Abstract
Teratomas in the neonatal age group are mostly benign at first, and the common site is the sacrococcygeal region. They are rarely associated with HIV infection. We report a case of an HIV-exposed newborn with a congenital teratoma at the post-auricular site who developed an infection. Early intervention by total surgical resection will prevent complications such as infections and malignant transformation. A term baby was delivered spontaneously by an HIV-positive mother who was on her regular medications. Prenatal ultrasound carried out in the third trimester showed a cyst swelling on the right post-auricular region. Radiological imaging and the histopathological result revealed a congenital teratoma. A wide major excision with preservation of the facial nerve was performed at the age of 8 weeks. Post-auricular teratomas are the rarest anatomical location and the prevalence of malignant transformation from benign is very low. If left untreated, this tumour is associated with high mortality and malignant transformation rates. An early complete surgical excision allows a good result with a low risk of complications and recurrence.
Keywords: HIV / AIDS, Oncology, Neonatal health
Background
Teratomas are composed of multiple tissues foreign to the organ in which they arise. A teratoma originates from aberrant germ cells during the early stage of embryology. It involves more than one germ cell layer (ectoderm, mesoderm and endoderm).1 2 Teratomas can be either gonadal or extragonadal lesions depending on their location, and they are classified as mature or immature tumours after histopathological evaluation.3
In the neonatal age group, most of these tumours are benign and the most common anatomical site is the sacrococcygeal region, which accounts for 40–70% of all teratomas.1–4 Teratomas in the head and neck region are rare and represent only 5% of all congenital teratomas, with the post-auricular area being the rarest region representing one in 10 000 births.2 We report a case of post-auricular teratoma in an HIV-exposed newborn complicated with a bacterial infection which posed a challenge in a country with limited resources.
Case presentation
Maternal history
A woman in her early 30s, Para 2, was HIV positive before conception and reported to have good adherence to antiretroviral drugs (tenofovir, lamivudine and dolutegravir). Prenatal screening ultrasound was carried out at 7 months of pregnancy which showed a cystic mass involving the right post-auricular area and a plan for delivery at the peripheral hospital was made.
Baby
A baby girl, delivered by spontaneous vaginal delivery at term, cried immediately and had APGAR scores of 7 and 8 at 1 and 5 min, respectively. She weighed 2.5 kg. A swelling was noted soon after delivery at the posterior aspect of the right ear which was slightly tender. The swelling was a variegated mass. It gradually increased in size and produced a foul-smelling pus-like discharge. It was not associated with fever or convulsions, and the infant was breastfeeding normally.
Referral
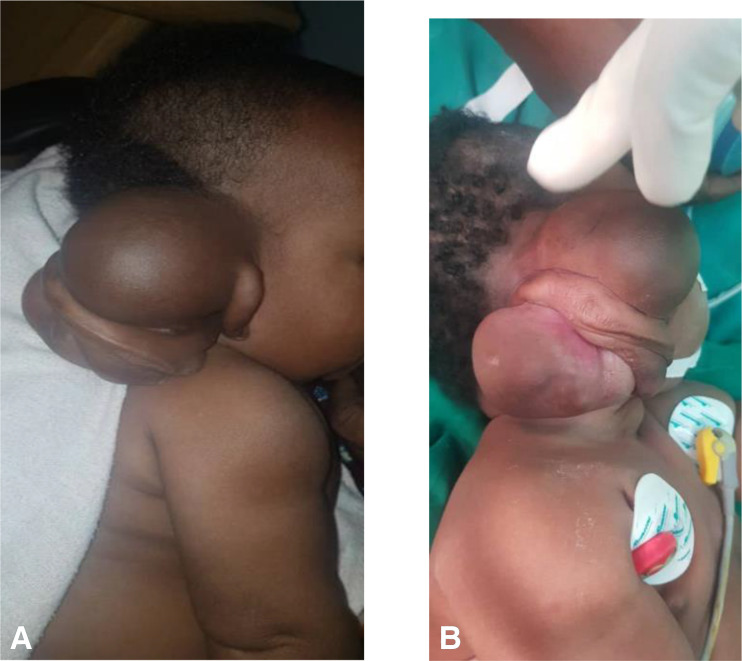
She was referred to our facility at the age of 15 days. Local examination revealed a mass on the right post-auricular region measuring 12 cm × 18 cm at the greatest dimensions, lobulated, cystic superiorly and firm inferiorly (figure 1A, B). The lesion was not ulcerative or tender. It was mobile with hyperpigmentation of the surrounding skin with the pinna, ear canal and tympanic membrane unaffected. There was no fever.
Figure 1.
(A, B) Mass on the right post-auricular region measuring 12 cm x 18 cm at the greatest dimensions, lobulated, cystic superiorly and firm inferiorly prior to surgery.
Investigations
Laboratory investigations
The complete blood count showed white blood cells of 20 000 /mL, predominantly neutrophilia (67%), haemoglobin 10 g/dL and C-reactive protein 194 mg/L. The alfa-fetoprotein level was very high at 7390.5 ng/L.
Cranial ultrasound showed Arnold–Chiari malformations grade 1. Echocardiography revealed a moderate atrial septal defect. Brain MRI and CT angiography were done at 30 days of age and showed a mixed type of teratoma. There was erosion of the parietal and temporal bones.
Differential diagnosis
The primary diagnosis of post-auricular teratoma was made with the differential of hygroma and haemangioma.
Histopathological analysis showed a tissue section covered by skin with a focal area of ulceration showing stroma with several components including cysts lined by cuboidal epithelium, epithelial glands and respiratory epithelium with mucus-secreting cells. Other areas showed mature adipose tissue, nerve tissue and blood vessels. There was a focus on lymphocytic infiltrates. A pathological diagnosis of post-auricular mature cystic teratoma was therefore made.
Treatment
The infant was transfused with 30 mL packed red blood cells and intravenous ceftriaxone 250 mg was given once daily for 7 days. She was also given oral nevirapine prophylaxis to prevent mother-to-child transmission. After 7 days her fever spiked again and she was then given intravenous meropenem 75 mg 8-hourly for 7 days and kept on antiretroviral therapy as per our HIV treatment national guidelines.
A wide major excision was made when the baby was 2 months old and a cystic and solid mass was noted after the elliptical skin flap incision. The mass was excised, the facial nerve was preserved and the skin sutured.
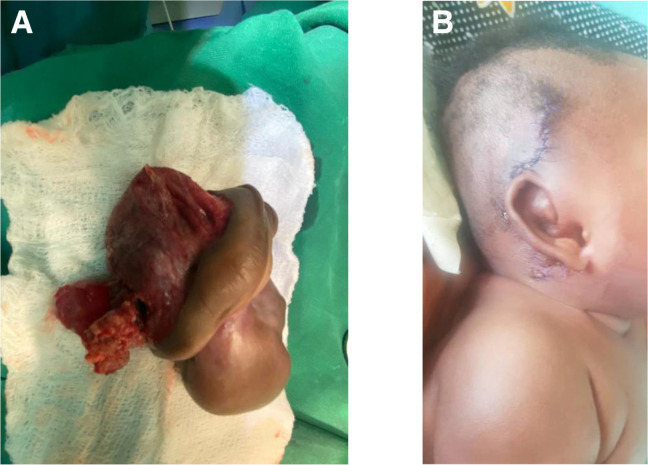
Postoperatively she was kept on intravenous antibiotics and wound dressing daily. The sutures were removed after 10 days and the wound healed completely (figure 2A, B).
Figure 2.
(A) Resected mass. (B) Photograph of the infant 1 hour post-surgery showing the intact pinna.
Outcome and follow-up
The infant was discharged home at the age of 2 months but, for socioeconomic reasons, she was unable to return to the clinic and was lost to follow-up at the age of 6 months. Until then she was reported to be progressing well.
Discussion
Diagnosis of congenital teratoma is usually established by prenatal ultrasound.3 Postnatal imaging with ultrasound, CT scan or MRI provides useful information for surgical intervention. Teratomatous swellings are benign at first, non-tender and can cause erosion in the surrounding bone area.4 Clinically, a cystic teratoma may be confused with lymphatic vascular malformation as some of the features such as the location, size and progression of the tumour are similar.2 In our case, the infant had an unusual appearance, lobulated, cystic superiorly and firm inferiorly. The differential diagnosis of cystic hygroma and haemangioma was made. It has been reported that complex heterogenous areas appearing in the mass raise suspicion for malignancy and should be evaluated sooner.4
Early confirmation of the teratoma is needed for proper management. A radiological investigation usually shows calcifications to confirm a diagnosis of congenital teratoma.2 Although a neonatal tumour may be histologically benign, these tumours may be life-threatening if they are large and compressing a vital organ. In our case, the cranial ultrasound revealed Arnold–Chiari malformation grade 1 due to the mass effect of the tumour.
Arteriovenous shunting and intratumoral haemorrhage due to a ruptured tumour can cause severe anaemia and infection.4 Our patient had a severe infection for which a blood culture was not done. Empirical antibiotics were given, and a major challenge was the appropriateness of antibiotics due to the unavailability of blood culture tests. Complete surgical excision is the treatment of choice for neonatal teratomas.2–4
Histologically, teratomas can be cystic, solid or both, and contain tissues of different origins. They may contain immature or mature tissues.1 Mature teratoma is the most common type of teratoma.2 4 Our patient had both cystic and solid components with mature tissue.
The recurrence rates of mature teratomas, immature teratomas and malignant teratomas are 0–26%, 12–55% and 0–36%, respectively. The prevalence of this malignant transformation (mature cystic teratoma) is estimated at 1–2% of cases. Left untreated, lesions are associated with a high mortality rate (80%) and a high malignant transformation rate.2 An early complete surgical approach allows a good result with low complication and recurrence rates. It has been reported that the prognosis of congenital teratoma is determined if the tumour is completely resected where the chance of recurrence is minimal.5 6
Alpha-fetoprotein is the tumour marker mainly found in liver cancer and germ cell tumours. It is used to assess the recurrence of the tumour.3 A newborn usually has an elevated serum alpha-fetoprotein level which decreases and reaches an adult level at around 1 year.2 4 5 Serial follow-up levels should be measured to properly interpret this tumour marker. Yearly alpha-fetoprotein levels and MRI examinations should be done up to the age of 3 years to ensure there is no recurrence.
Congenital malformation associated with congenital teratoma is very rare; the small number reported include imperforated anus, hypoplastic left heart syndrome and agenesis of the corpus callosum.6 Cleft palate malformation is usually associated with teratoma of the nasopharynx.7 8 In our case, the infant had a moderate atrial septal defect which did not require immediate intervention. The association of congenital teratoma with HIV exposure needs to be confirmed with further studies. Ghazi et al reported a case of rapid growth of a fetal teratoma in an HIV-infected woman and concluded that further research is needed in this area.9 HIV-associated tumours are recognised to be predominantly lymphocytic and include Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, Kaposi sarcoma, and others. Its association with teratoma is very rare.10
Patient’s perspective.
I knew my baby had this unusual swelling at the back of the right ear from the first ultrasound in the third trimester. I was referred to this institute because here there are doctors who can do operations. I am very grateful that my baby is fine now.
Learning points.
Post-auricular teratoma is the rarest anatomical location of congenital teratomas.
Early intervention prevents complications such as infections and transformation to malignancy.
Yearly alpha-fetoprotein levels and MRI examinations should be done up to the age of 3 years to ensure there is no recurrence.
Acknowledgments
We would like to thank the Neonatal Unit registrars and consultants as well as those from the Otorhinolarngology department. We acknowledge the support from the Muhuimbili National Hospital.
Footnotes
Contributors: All the authors contributed equally to the management, review of the literature, drafting the manuscript and final approval of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s)
References
- 1.Bailey NA. Cervical teratoma in a newborn. Consultant 2020;61:e31–4. 10.25270/con.2020.11.00012 [DOI] [Google Scholar]
- 2.El Ezzi O, Gengler C, de Buys Roessingh A. Cystic teratoma of the head: diagnosis pitfalls. Oral Maxillofac Surg Cases 2020;6:100135. 10.1016/j.omsc.2019.100135 [DOI] [Google Scholar]
- 3.Lakhoo K. Neonatal teratomas. Early Hum Dev 2010;86:643–7. 10.1016/j.earlhumdev.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 4.Yoon HM, Byeon S-J, Hwang J-Y, et al. Sacrococcygeal teratomas in newborns: a comprehensive review for the radiologists. Acta Radiol 2018;59:236–46. 10.1177/0284185117710680 [DOI] [PubMed] [Google Scholar]
- 5.Heerema-McKenney A, Harrison MR, Bratton B, et al. Congenital teratoma: a clinicopathologic study of 22 fetal and neonatal tumors. Am J Surg Pathol 2005;29:29–38. 10.1097/01.pas.0000146006.46468.ef [DOI] [PubMed] [Google Scholar]
- 6.Hochwald O, Gil Z, Gordin A, et al. Three-step management of a newborn with a giant, highly vascularized, cervical teratoma: a case report. J Med Case Rep 2019;13:73. 10.1186/s13256-019-1976-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo WX, Tan KK. Diagnosis and surgical management of congenital intranasal teratoma in a newborn: a rare case report. Case Rep Otolaryngol 2018;2018:1403912. 10.1155/2018/1403912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Mendalawi MD. Immature teratoma of the posterior fossa in an infant: case report. Turk Pediatri Ars 2020;55:333–4. 10.14744/TurkPediatriArs.2020.87847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghazi L, Ko F, Bathgate SL, et al. Rapid growth of a fetal sacrococcygeal teratoma in an HIV-infected woman: a case report. J Reprod Med 2006;51:431–4. [PubMed] [Google Scholar]
- 10.Potthoff A, Brockmeyer NH. HIV-associated tumors. Hautarzt 2006;57:988. 10.1007/s00105-006-1223-7 [DOI] [PubMed] [Google Scholar]