Abstract
Background
Respiratory syncytial virus (RSV) is the principal cause of acute lower respiratory infections (ALRI) among infants worldwide, and an important cause of morbidity, hospitalisation and mortality. While infants are universally exposed to RSV, most mortality occurs among normal term infants from low-income and middle-income countries. Breastfeeding has been suggested to have a protective effect against RSV infection. This study aims to determine the association of breastfeeding on the frequency and severity of RSV-associated ALRI among infants.
Methods
A systematic review was conducted using keywords and Medical Subject Headings on MEDLINE, PubMed, Google Scholar, EMBASE, MedRxiv and Cochrane Central Register of Controlled Trials. Full-text articles published in English from 2000 to 2021 that studied exclusively or partially breastfed infants who developed RSV-associated ALRI <12 months of age were included. Covidence software-based evidence extraction and Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol guidelines were followed. Quality of evidence was analysed using UK National Service Framework grading and the risk-of-bias assessment using Robvis.
Results
Among 1368 studies screened, 217 qualified full-text review and 198 were excluded based on pre-agreed criteria. Nineteen articles published from 12 countries that included 16 787 infants from 31 countries (of which 8 middle-income) were retained for analysis. Results indicate that non-breastfeeding practices pose a significant risk for severe RSV-associated ALRI and hospitalisation. Exclusive breastfeeding for >4–6 months significantly lowered hospitalisation, length of stay, supplemental oxygen demand and admission to intensive care units.
Conclusion
In the context of no effective or standardised treatment for established RSV-associated ALRI, available evidence suggest that breastfeeding is associated with lower frequency and severity of RSV-associated ALRI, based on observational studies of variable grades of evidence and risk-of-bias. With both exclusive and partial breastfeeding benefiting infants who develop RSV-associated ALRI, breastfeeding should be promoted globally as an adjunct primary prevention; in addition to emerging immunoprophylaxis and maternal immunisation strategies.
Keywords: systematic review; public health; infections, diseases, disorders, injuries; child health; paediatrics
WHAT IS ALREADY KNOWN ON THIS TOPIC
While infants are universally exposed to respiratory syncytial virus (RSV), most of the RSV-related mortality occurs among normal term infants from low-income and middle-income countries.
Previous systematic review, conducted nearly two decades ago, suggested beneficial effects of breastfeeding on RSV-associated acute lower respiratory infections.
Current immunoprophylaxis-based RSV preventive strategy is costly, highly selective and requires monthly intramuscular injection during winter months.
Since lifting the stringent public health mitigation measures against COVID-19 (lockdowns), there was an unprecedented and out-of-season incidence of RSV disease globally.
WHAT THIS STUDY ADDS
This systematic review, based predominantly on observational studies of variable levels of evidence and risk-of-bias, suggests that adherence to breastfeeding practices is associated with less severe RSV-associated acute lower respiratory infections and lower hospitalisation, length of stay, assisted respiratory support in intensive care units and supplemental oxygen usage among infants <12 months.
While exclusive breastfeeding offers better protection, value of partial breastfeeding in reducing RSV-related morbidity among infants is also suggested.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Exclusive breastfeeding as a universal, low-cost and sustainable primary prevention deserves optimal promotion while awaiting acceptable and affordable specific immunoprophylaxis or maternal immunisation measures against RSV, especially with the resurgence of severe RSV infections in the post-COVID-19 environment.
Even though the pipeline of products on RSV immunoprophylaxis seems promising, an adjunct role offered by exclusive breastfeeding for the first 6 months would potentially serve as ‘nature’s first vaccine’ for millions of infants during their most vulnerable period of contracting RSV infection.
Introduction
Breastfeeding strengthens infants’ immune system, offering protection against serious childhood infections.1 WHO and UNICEF recommend breastfeeding to be initiated within the first hour of birth and to continue exclusively for the first 6 months.2 Afterwards solids may be offered while continuing breastfeeding for up to 2 years and beyond. Mothers own milk (MOM) is a source of several bioactive components, such as leukocytes, cytokines, and growth factors.3 4 Breastfeeding provides passive immunity during neonatal period by influencing the development of infant microbiome, immune system metabolites and modulators such as IgA antibodies.5
Viral lower respiratory tract infections (LRTI) including bronchiolitis is the leading cause of respiratory illness among infants and young children worldwide.6 Risk factors recognised for serious disease are prematurity, low income, presence of school-age siblings, passive cigarette smoke exposure, lack of breastfeeding and early introduction of infants to crèche or childcare.7 Respiratory syncytial virus (RSV) is the most common cause of acute LRTI among infants and young children globally, contributing to over 50% of hospital admissions, with the highest incidence in low-income and middle-income countries (LMICs). Currently, no effective vaccine is available against childhood RSV infections. Millions of infants and young children are hospitalised annually with RSV-associated acute lower respiratory infections (ALRI), clinically manifesting mainly as bronchiolitis and pneumonia, resulting in high morbidity and mortality and posing considerable burden on healthcare systems worldwide.8 The onset, duration and severity of RSV epidemics vary among countries, often linked to seasonality, peaking in winter months.8 Modelling studies estimated that globally in 2019, 33.0 million episodes of RSV-ALRI occured.9 10 Furthermore, 3.6 million RSV-ALRI episodes, 1.4 million hospital admissions and 45 700 in-hospital deaths occurred in children under the age of 5 from RSV.9 10 Hospitalisation rates, duration of supplemental oxygen therapy and use of mechanical ventilation among infants increase as age declines, with highest incidence in the first few months of life.8 11
During the first wave of COVID-19 pandemic in 2020, lockdowns and isolation protocols prevented the spread of RSV, nearly eliminating cases worldwide during late winter.12 As countries reopened schools, crèche and day-care facilities, out-of-season increase of RSV infections emerged.12 There are limited preventative strategies to decrease RSV-associated ALRI in vulnerable infants. Current prophylaxis protocols advise the use of palivizumab, a monoclonal antibody (mAb) licensed in June 1998 by the Food and Drug Administration, to decrease serious infections among high-risk infants.13 It is a monthly intramuscular injection, offering passive immunity to infants during the RSV season. However, the treatment is expensive, with a unit cost of a 100 mg vial ranging from US$904 to US$1866 and mostly available in high-income countries where adequate supportive healthcare is accessible.13 Guidelines recommend palivizumab to preterm infants <29 weeks gestational age (GA).14 Other high-risk infants include haemodynamically significant congenital heart disease, chronic lung disease, cystic fibrosis (CF), Down syndrome, pulmonary or neuromuscular conditions of significant respiratory morbidity and immunocompromised.13–15 A modelling study estimated global preterm birth rate for 2014 at 10.6% (9.0–12.0) equating to 14.84 million live preterm births.16 Higher numbers of premature infants are born >30 weeks GA, in comparison to <28 weeks GA, which represented the lowest percentage of all births.16 Recent research appears promising regarding the effectiveness of candidate vaccines against RSV for maternal uptake during pregnancy.17 In addition, a new long-acting mAb, nirsevimab, has demonstrated high levels of efficacy after one injection and is proposed for all young infants, however, the product may not be available for wider public use immediately.18
Breastfeeding enhances immune response against RSV-derived inflammation in airway epithelial and peripheral blood mononuclear cells (PBMC).3 5 Disease severity in viral bronchiolitis is linked to innate immunity.19 PBMC interleukin (IL)-15 expression and serum IL-15 levels have been correlated with severity of illness in bronchiolitis.19 The reduction in neutrophilic airway infiltration among breastfed infants has been shown to reduce disease severity when compared with formula-fed (FF) infants.20 Breast milk (BM) increases interferon-α among the RSV infected and decreases concentration of immune modulators, such as airway chemokines and IL-8.20
A review from the USA nearly two decades ago reported reduced hospitalisation for infants breast-fed for 4 or more months.6 Another review concluded that FF infants showed a 3.6-fold increase of hospitalisation due to bronchiolitis, compared to exclusively breast-fed >4 months.21 Our review summarises available published evidence on the association of breastfeeding and RSV-associated ALRI during the past two decades (2000–2021). The primary aim is to analyse the available evidence of association of breastfeeding on the frequency and severity of RSV-associated ALRI among infants <12 months. Second, to determine the influence of breastfeeding on hospitalisation rates, length of stay (LOS), mechanical respiratory support and supplemental oxygen use among infants with RSV-associated ALRI.
Methods
Search strategy and selection criteria
This systematic review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol (PRISMA-P 2021)22 (figure 1). We developed and performed comprehensive searches in six English language health-related databases (MEDLINE, PubMed, Google Scholar, EMBASE, MedRxiv and Cochrane Central Register of Controlled Trials) to collect evidence that fits prespecified eligibility criteria. A preliminary literature search was conducted using keywords (breastfeeding, respiratory syncytial virus, RSV, RSV-LRTI, RSV-ALRI, RSV-bronchiolitis, RSV-pneumonia, infant, breast milk, donor milk) and Medical Subject Headings (table 1). No other filtering or restrictions were applied to the search strategy. Additionally, we manually reviewed reference lists from key articles that fulfilled our eligibility criteria and ‘related articles’ feature in PubMed was used. After screening for title and abstract, two researchers (GMM and RKP) independently reviewed full texts, excluding irrelevant papers (to this systematic review). Consensus was achieved through discussion among reviewers.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart summary of the methodology. RSV, respiratory syncytial virus.
Table 1.
Search strategy using keywords and Medical Subject Headings
| Query | Sources | Date first accessed | |
| S1 | breastfeeding AND respiratory AND syncytial AND virus AND bronchiolitis AND LRTI | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S2 | breastfeeding AND RSV AND burden AND pneumonia AND ALRI | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S3 | breastfeeding AND RSV AND burden | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S4 | infant AND LRTI AND bronchiolitis AND treatment AND breast AND milk | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S5 | breastfeeding AND RSV AND LRTI AND bronchiolitis | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S6 | breast AND milk AND RSV AND pneumonia AND infant | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S7 | RSV AND bronchiolitis AND LRTI AND infant AND outcomes | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S8 | RSV AND LRTI AND pneumonia AND bronchiolitis AND infant | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S9 | RSV AND LRTI AND ALRI bronchiolitis AND breastfeeding | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S10 | RSV AND bronchiolitis AND pneumonia AND management | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S11 | donor AND breast AND milk AND RSV AND LRTI AND bronchiolitis | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S12 | breastfeeding AND RSV AND bronchiolitis AND pneumonia | PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
| S13 | breastfeeding AND respiratory AND syncytial AND virus AND bronchiolitis AND LRTI AND ALRI |
PubMed, EMBASE, Cochrane Library, MedRxiv, Google Scholar | 1 May 2021 to 21 September 2021 |
Population, Intervention, Comparator, Outcome questions
This study aims to answer the following Population, Intervention, Comparator, Outcome questions:
Is breastfeeding, in comparison to FF, offers a level of protection against RSV-associated ALRI among infants <12 months of age?
Is the protective or preventive role of human milk (HM) limited to exclusive breastfeeding, or does partial (non-exclusive or combined feeding) also influence hospitalisation rates, LOS, need for mechanical ventilation or supplemental oxygen use among infants with RSV-associated ALRI?
Selection criteria
Studies on infants with RSV-associated ALRI (encompassing RSV bronchiolitis and RSV pneumonia) were selected, based on preset inclusion and exclusion criteria as outlined below.
Participants
Inclusion criteria
Infants <12 months, irrespective of GA at birth.
Infants who received exclusive or partial breastfeeding, irrespective of the duration.
Infants who received MOM (at breast or expressed) or banked donor human milk (DHM) or both.
Infants who developed clinical ALRI with laboratory confirmed RSV infection (RSV-associated ALRI).
Exclusion criteria
Infants >12 months.
Infants with lower respiratory symptoms due to other confirmed causes (as per study) such as asthma, bacterial LRTI or croup.
Studies limited to infants with pre-existing morbidity due to cardiac abnormalities, CF, Down syndrome and other genetic syndromes.
Studies limited to only one sex among the study participants (Sex and Gender Equity in Research (SAGER) guidelines).
Studies describing only clinical diagnosis of viral ALRI with no laboratory confirmation of RSV.
Studies limited to ALRI with other viral or bacterial aetiologies confirmed on laboratory studies.
Type of study
Inclusion criteria
Full-length articles, longitudinal/observational studies, cross-sectional studies, case-control studies, clinical trials, cohort studies, randomised controlled trials (RCTs) and epidemiological studies that evaluate development of RSV-associated ALRI (encompassing both RSV bronchiolitis and RSV pneumonia) among breastfed infants.
Prospective and retrospective studies.
Exclusion criteria
Case reports, case studies, in vitro studies, animal studies, letters to the editor, qualitative thematic analysis, narrative reviews, systematic reviews, literature reviews, expert opinions, abstract-only publications, conference proceedings, books and book chapters.
Comparison groups or comparator
Inclusion criteria
Studies with information on infants exposed to BM or FF.
Exclusive and partial breastfeeding.
Exclusion criteria
Studies limited to only FF infants.
Studies limited to other interventions for the prevention of RSV-associated ALRI.
Timeframe
Timeframe for outcome evaluation differs among selected studies. If the timeframe for listed outcomes are not specified or unclear, authors were contacted to provide specific data. If the authors are unwilling or unable to provide this information, additional outcomes were not included.
Language and publication time
Studies published between 2000 and 2021 (1 January 2000 to 21 September 2021), in English language only.
Outcomes
Primary outcome: effect of breastfeeding on the frequency and severity of RSV-associated ALRI among infants <12 months.
Secondary outcomes: hospital admission rates, LOS, invasive or non-invasive mechanical ventilation or need for supplemental oxygen.
Criteria
RSV confirmation is required by PCR or other equivalent laboratory methods, in the context of clinically diagnosed ALRI by a healthcare practitioner or by medical records. RSV-associated ALRI was defined as an acute LRTI with laboratory-confirmed RSV infection. This encompasses both RSV bronchiolitis and RSV pneumonia (as the accepted terminology used in most of the earlier publications).
Articles were assessed for titles and abstracts independently by two authors (GMM and RKP). First, the two independent reviewers screened article titles and abstracts in duplicate using initial screening criteria (level 1). Subsequently, full-text screening of all articles retained was conducted against eligibility criteria. A match between authors was reached before an article entered full-text review. Any disagreement was settled by consensus and when not possible an independent consultant paediatrician with research and subject expertise was contacted for resolution. Additional information from study authors was sought where necessary to resolve questions about eligibility. For level 2 screening, we recorded reasons for excluding studies. The review authors were not blinded to journal titles, study authors or institutions.
Patient and public involvement
While direct patient and public involvement was not applicable for conduct of systematic review of published literature, representation by the designated patient delegate of our Limerick Neonatal Charity (Irish Charity Registration No: 20073732) was ensured as an independent observer and offered parents’ views from the very outset.
Sex and Gender Equity in Research guidelines
Principles of sex and gender equity were applied to this systematic review by excluding publications limited to only one sex from the eligibility criteria for analysis.
Data extraction and management
Literature search results were uploaded to Covidence, an internet-based software program that facilitates study selection process. Screening questions for level 1 (title and abstract screening) and level 2 (full-text screening), based on the inclusion and exclusion criteria, were developed, and tested. For level 2 screening, full-text articles were uploaded and reviewed. Data extraction tables were developed a priori and pilot-tested by our team using a standardised extraction form on Microsoft Excel. Two independent reviewers (GMM and RKP) performed the data extraction using a single charting method. The extraction tables were piloted on five random studies and confirmed data charting remains consistent and in alignment with research questions and purpose. In case of disagreement between reviewers, a third independent reviewer (KM, as above) was consulted. Data charting tables were continuously updated in an iterative process to be inclusive of all selection criteria. The following information were extracted: lead author, year of publication, country of origin, the purpose of study/study objectives, study design, population and setting, sample size (total and per group), study outcome measures and study findings.
Quality of evidence appraisal
A systematic narrative synthesis of available evidence is presented to summarise the characteristics and findings of included studies. Methodological quality was assessed by two authors (GMM and RKP) independently using the UK National Service Framework (NSF) for cohort or case-control studies.23 This research assessment tool provides a simple method to appraise quality of evidence of both qualitative and quantitative studies. Quality of research was assessed based on five questions to reach a maximum score of 10. Research-based evidence was awarded a rating based on three categorisations: design—category of research design; quality rating—high, medium or low; applicability—population context of the study.23 A high-quality study was awarded a score of at least 7/10, medium-quality study scored 4–6/10 and poor-quality one scored 3/10 or less.23
Risk-of-bias assessment of eligible studies
A structured and validated internet-based assessment checklist app (Robvis) based on the Cochrane risk-of-bias tool was used to evaluate the inherent bias of the included studies on the basis of five domains for potential bias: (1) randomisation, (2) deviation from intended intervention, (3) missing data, (4) outcome measurements and (5) selection of reported results.24 An automated visualisation of the risk-of-bias judgements within each domain will be summarised in a generic ‘traffic light’ format.24
Results
General characteristics
This systematic review included 19 eligible publications (from 12 countries) that collectively recruited/analysed the data of infants from 31 countries: Japan, South Korea, Bahrain, Egypt, Jordan, Lebanon, Oman, Saudi Arabia, Bosnia and Herzegovina, Bulgaria, Czech Republic, Estonia, Latvia, Lithuania, Slovakia, Slovenia, Spain, Italy, France, Portugal, Sweden, Norway, Switzerland, Austria, The Netherlands, Denmark, Italy, the USA, Brazil, Mexico and Chile, offering a global relevance to the observations (table 2).
Table 2.
RSV confirmed acute lower respiratory infection summary and quality assessment of studies included
| Study | Country | Study aims/objectives | Study design | Population/Setting | Sample size | Study outcome measures | Study findings | NSF evidence rating |
| Dornelles et al30 | Brazil | To evaluate type of breastfeeding in acute viral bronchiolitis cases | Prospective cross-sectional study | Infants 0–6 months April 2004–April 2005 |
175 infants | Length of oxygen use, length of hospital stay | Breastfeeding <1 month increased incidence of RSV infection. As the duration of exclusive breastfeeding increased, length of oxygen use, and LOS decreased. | P3 High (8) Direct |
| Prinelli et al31 | Italy | To evaluate if breastfeeding influences RSV-LRTI incidence in infants <12 months | Multicentre, prospective study | >33 weeks of GA | 1814 newborns | Environmental risk conditions for RSV infection | LRTI incidence: never breast-fed infants 7% and ever breast-fed infants 3.9%. Crude RR for never breast-fed infants was 1.5 (95% CI 1.0 to 2.4). Risk increase was independent of type of breastfeeding (exclusive or partial). | P2 High (7) Direct |
| Linstow et al32 | Denmark | To identify risk factors for early RSV infection in otherwise healthy children | Prospective cohort | May 2004–May 2005 | 200 infants | Breastfeeding duration, RSV infection and RSV hospitalisation | Exclusive breastfeeding for the first 14 days of life protected against hospitalisation due to RSV bronchiolitis (OR 0.21; 95% CI 0.06 to 0.79). | P1 High (8) Direct |
| Lanari et al33 | Italy | To evaluate effect of breastfeeding on the risk of hospitalisation for bronchiolitis | Prospective cohort study | Newborns of 33+0 days to 34+6 days GA | 2154 newborns | Hospitalisation and/or death due to bronchiolitis | Infants receiving (exclusive or partial) maternal milk had lower risk of RSV bronchiolitis. Partial breastfeeding reduces risk of hospitalisation. | P3 High (9) Direct |
| Jang et al34 | South Korea | To investigate if breastfeeding has a beneficial effect against RSV bronchiolitis compared with formula-fed infants | Retrospective chart review | January 2016–February 2018 | 411 infants | Duration of fever, oxygen use, oxygen therapy, ICU admission and LOS | Oxygen use: higher in formula-fed group (aOR, 3.807; 95% CI 1.22 to 11.90; p=0.021). ICU admissions: breast-fed (1.1%), mixed-fed (3.5%) and formula-fed (4.5%) groups. Breastfeeding decreases incidence and severity of RSV disease. | P3 High (8) Direct |
| Bulkow et al37 | USA (Alaska) | To evaluate factors leading to hospitalisation due to RSV infection in high-risk population | Case-control study | Southwest Alaska | n/a | Hospitalisation incidence | Breastfeeding was associated with a lower risk of RSV hospitalisation (OR 0.34). | P1 High (8) Direct |
| Figueras-Alloy et al38 | Spain | To identify risk factors leading to RSV-LRTI and subsequent hospital admission | Prospective case-control study | October 2002–April 2003 | 186 cases, 371 controls | Hospital admission incidence | The risk of RSV-LRTI hospital admission in infants 33–35 weeks GA: breastfeeding for ≤2 months (OR 3.26; 95% CI 1.96 to 5.42) | P1 High (7) Direct |
| Roine et al39 | Chile | To explore breastfeeding and cytokine responses in RSV-infected infants: acute and recovery phase of primary infection | Cohort study | Term birth, no underlying illness, first 8 days of life | 349 infants | Breastfeeding, hospitalisation rates | Breastfeeding reduced the risk of hospitalisation (OR 0.47, 95% CI 0.25 to 0.89, p=0.021). It provided partial protection against severe RSV disease. | P1 High (7) Direct |
| Simones et al40 | USA | To develop validated risk factor-based model to predict RSV-related hospitalisation in infants 33–35 weeks GA | Predictive model | October 2002–April 2003 | 186 cases, 371 control | Risk factors for RSV hospitalised and non-hospitalised infants | Univariate analysis of FLIP data: hospitalised infants were significantly more likely to be breast-fed ≤2 months or not at all. | S2 High (7) Direct |
| Lanari et al41 | Italy | To predict the likelihood of RSV hospitalisation in newborns 33–35 weeks GA | Predictive model | PICNIC and FLIP: 33– 35 (+6 days) | PICNIC: 66 cases, FLIP: 186, VRS: 145 | PICNIC and FLIP: hospital admission | Independent risk factor for RSV-hospitalisation: breastfeeding <2 months. FLIP: breastfeeding <2 months aOR 3.26 (95% CI 1.96 to 5.42). | S2 Medium (6) Direct |
| Nishimura et al42 | Japan | To evaluate effects of breastfeeding on severity of RSV infection in early infancy | Multicentre prospective cohort | <100 days old 1 May 2002–30 April 2005 |
203 infants | Hospitalisation, duration of hospitalisation, required oxygen | Risk of oxygen use, and duration of hospitalisation was significantly reduced in full breastfeeding group when compared with token group. | P1 High (7) Direct |
| Carbonell- Estrany et al43 | Spain | To examine risk of RSV hospitalisation of infants <12 months old | Case-control study | Infants of 32–35 weeks GA (FLIP-2 study) | 190 cases/4566 controls | Risk of RSV hospitalisation | Duration of breastfeeding decreased the risk of RSV hospitalisation beyond the age of 90 days. | S2 Medium (5) Direct |
| Blanken et al44 | The Netherlands | To update and validate a prediction rule for RSV hospitalisation in infants 33–35 weeks GA. | Prospective cohort | June 2008 and January 2011 | Estimated sample size 1000 | Risk and controls derivation cohort and validation cohorts | Breastfeeding <2 months contributed significantly and was identified as a risk factor for RSV hospitalisation. | S2 High (8) Direct |
| Vereen et al45 | USA | To assess association between breastfeeding and severity of acute RSV infection | Cross-sectional study | September to May 2004–2008 | 629 infants | BSS, length of hospital stay | Breast-fed infants did not differ in LOS compared with never breast-fed infants but trended towards lower BSS (no statistical significance). | S2 High (7) Direct |
| Carbonell- Estrany et al46 | Spain | To evaluate key risk factors for RSV hospitalisation in infants 32–35 weeks GA | Observational study: risk analysis | October 2005–April 2006 and October 2006–April 2007 | 190 RSV-hospitalised 4566 non- hospitalised | RSV hospitalisation predictive accuracy (ROC AUC) and NNT | Breastfeeding <2 months OR 3.26 (95% CI 1.96 to 5.42), p<0.001. Breastfeeding reduced the risk of RSV hospitalisation by 60%. ROC AUC: 0.776; NNT: 10.2 | S2 Medium (6) Direct |
| Korsten et al47 | The Netherlands | To improve published model for prediction of RSV-hospitalisation (<12 months) | Secondary analysis: two prospective cohorts | June 2008–February 2015 | 181 infants (127 in RISK-I, 54 RISK-II) | RSV hospitalisation, respiratory support | The updated model included breastfeeding ≤4 months (OR 1.6; 95% CI 1.2 to 2.2), p=0.003 hospitalised. | S2 High (7) Direct |
| Straňák et al48 | 23 countries | To identify a universal set of risk factors of development of severe RSV disease in preterm infants 33–35 weeks GA | Epidemiological study | 33 weeks and 35 weeks+6 days | 2390 infants | Incidence, severity, course, RSV-LRTI and non-RSV-LRTI | Not breastfeeding is associated with increased hospitalisation for RSV bronchiolitis p=0.0227, OR 1.84 (95% CI 1.09 to 3.10). |
P1 High (9) Direct |
| Maksic et al49 | Bosnia and Herzegovina | To investigate predictors for RSV hospitalisation, infants born <6 months before or during RSV season | Retrospective-prospective epidemiological study | 33–35 weeks GA 1 April 2013–28 February 2014 |
160 moderate-to-late preterm | Incidence, severity, outcomes of hospitalisation | Breastfeeding lowered the risk of RSV-LRTI hospitalisation (OR 0.34, 95% CI 0.08 to 1.62, p=0.340). Not breastfeeding increased the risk. | S2 High (7) Direct |
| Carbonell- Estrany et al59 | Spain | PICNIC and FLIP study (supplement article) | Secondary analysis PICNIC: prospective, cohort. FLIP: prospective case-control | PICNIC: November 2000–June 2001, and November 2001–June 2002 FLIP: October 2002–April 2003 |
PICNIC: 1760 infants FLIP: 186 cases, 371 controls | Hospital admission incidence, risk factors | Not receiving breast milk significantly associated with hospitalisation for RSV bronchiolitis. FLIP: significant association of RSV hospitalisation and breastfeeding duration. Breastfeeding >2 months is highly protective. | S2 Medium (5) Direct |
aOR, adjusted OR; AUC, area under the curve; BSS, bronchiolitis severity score; GA, gestational age; ICU, intensive care unit; LOS, length of stay; LRTI, lower respiratory tract infection; n/a, not available; NNT, number needed to treat; NSF, National Service Framework; PICNIC, Pediatric Investigators Collaborative Network on Infections in Canada; ROC, receiver operating characteristic curve; RR, risk ratio; RSV, respiratory syncytial virus.
Infants from eight middle-income countries were included; however, no eligible papers from low-income countries could be selected. Initial database search for articles conducted between May and September 2021 identified 3028 records, 1660 were removed due to being duplicates. One thousand three hundred sixty-eight studies were screened for title and abstract and 217 were eligible for full-text review. One hundred ninety-eight studies were excluded due to wrong intervention, design, outcomes, indication, age profile of the population, language or year of publication and RSV status. Nineteen articles describing RSV confirmed ALRI that collectively studied 16 787 infants were selected for data extraction. Ten of the included articles analysed data from prospective cohorts (two from secondary analysis of already existing cohorts using regression analysis and validation), three case-control studies, two cross-sectional studies, two predictive models, one epidemiological and one retrospective chart review.
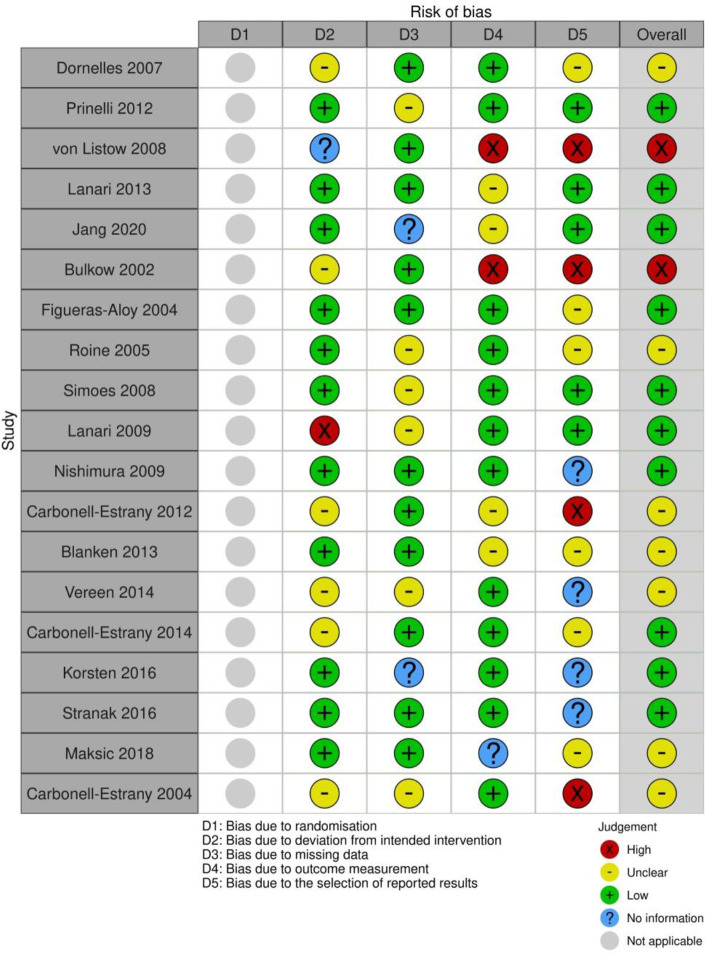
Risk-of-bias assessment of the studies, based on the five domains, yielded an overall low risk for 10/19 studies with 2/19 having a high risk and 7/19 categorised as unclear. An automated Robvis visualisation of the domain judgements is summarised in figure 2.
Figure 2.
Risk-of-bias assessment of the eligible studies by Robvis.
Based on the studies included, RSV-associated ALRI cases were either reported by a healthcare provider or parent or were retrospectively searched through hospital records and outcomes recorded. Majority of the RSV-associated ALRI (13/19) yielded high quality of evidence (7/10 or more).
While it is important to acknowledge a number of studies (including from LMICs) reporting on the association of breastfeeding with decreased rate and severity of non-RSV confirmed ALRI/bronchiolitis, they are not included in this systematic synthesis of RSV-specific evidence appraisal.25–29
Breastfeeding and the rate of RSV-associated ALRI
A study from Italy evaluated 1814 infants and concluded that crude risk ratio of never breast-fed infants was 7%, compared with 3.9% in ever breast-fed infants (never vs ever).30 31 Authors also suggested both exclusive and partial breastfeeding exhibiting a protective effect. Five out of the six studies that analysed incidence rates suggested that RSV bronchiolitis episodes among breast-fed infants was significantly lower compared with those with non-breast-feeding practices.30–34 A Brazilian cross-sectional study of 175 infants reported breastfeeding for <1 month increased rate of RSV bronchiolitis.30 A multicentre prospective cohort study from Italy observed that breastfeeding might confer protection from RSV bronchiolitis in both exclusively or partially breast-fed infants, even after weaning.33 A retrospective chart review of 411 infants stated that breastfeeding protects against frequency and severity of RSV bronchiolitis, and subsequent development of wheezing.34
Breastfeeding and hospitalisation rates
Four studies state that breastfeeding is significantly associated with decreased rates of RSV-associated ALRI admission to hospitals.30 33 35 36 An Italian multicentre study included 2154 newborns from 30 centres across the country, reported that infants <12 months faced a probability of hospitalisation for bronchiolitis of 4% in breast-fed and>8% among those never fed with BM.33 Fifteen out of 19 (83.3%) articles (table 2) concluded that breastfeeding is associated with lower risk of hospitalisation for PCR confirmed RSV bronchiolitis among infants.30 32 37–49 A prospective cohort study from Denmark evaluated 200 subjects and found exclusive breastfeeding even for the first 14 days of life offered some protection against hospitalisation (OR 0.21; 95% CI 0.06 to 0.79).47 The FLIP study, a prospective case-control study from Spain, included 186 cases and 371 age-matched controls and reported that breastfeeding for <2 months in preterm infants or no breastfeeding is an important risk factor for severe RSV infection requiring hospitalisation.38 A prediction model from The Netherlands suggested breastfeeding for <4 months as a significant predictor for RSV hospitalisation (OR 1.6; 95% CI 1.2 to 2.2, p=0.003).47
Supplemental oxygen use and ICU admission for mechanical ventilation
Three articles studied supplemental oxygen requirement in confirmed RSV bronchiolitis cases in infants <12 months.30 42 50 A prospective study of 175 infants demonstrated that longer duration of exclusive breastfeeding was associated with significantly lower duration of supplemental oxygen use and intensive care unit (ICU) admission.30 A Japanese multicentre prospective cohort study of 203 infants suggested that exclusive breastfeeding significantly reduced the requirement for oxygen therapy (OR 0.256; 95% CI 0.074 to 0.892, p=0.032).42 In addition, the incidence of supplemental oxygen use during follow-up was 5.7%, 12.7% and 16.3% in the exclusive breastfeeding, partial and non-breast-fed groups, respectively. A Korean study of 411 infants showed that the probability of oxygen administration was significantly higher in FF infants (13.5%) compared with exclusively breast-fed group (4.3%) (OR 3.807, 95% CI 1.22 to 11.90, p=0.021).34 ICU admission rate for mechanical ventilatory support was 1.1% for the fully breast-fed, 3.5% for partially breast-fed and 4.5% for never breast-fed.34-
Supportive evidence of relevance from studies not reporting decreased RSV-associated ALRI among the breast-fed
A Spanish prospective descriptive study of infants hospitalised with RSV observed a median LOS of 3.14 days among the breast-fed group and 2.82 days in the non-breast-fed group (p=0.004).51 However, breast-fed infants at admission had a lower bronchiolitis severity score (BSS), suggesting a relative protective role against severe bronchiolitis. A US cross-sectional study of 629 infants with RSV admissions performed a multivariate analysis.45 No significant association between breastfeeding and BSS (OR 0.97, 95% CI 0.69 to 1.39) was found. In their univariate analysis, infants ever breast-fed did not differ in LOS compared with never breast-fed (ever vs never), however trended towards a lower BSS distribution.45 These two studies did not find statistical significance of breastfeeding as a protective measure against the development of severe bronchiolitis.45 51
Discussion
Breastfeeding decreases RSV-associated ALRI episodes
In this systematic review, we appraised published evidence of protective association between breastfeeding and development of RSV-associated ALRI among infants. Several themes emerged in our analysis. Seven of the included studies documented breastfeeding decreasing the rate of RSV-associated ALRI and that the number and duration of episodes decreased as duration of breastfeeding increased.
The innate immune system is the primary defence for neonates, with neutrophils being the predominant cell type.52 53 Immune cells require time to produce an adequate amount of antibodies to effectively fight off infection, therefore infants are most susceptible to severe RSV outcomes in the first few months of life.54 While transplacental transfer of IgG antibodies in the last trimester of pregnancy is well described, it is proposed that mothers transfer antibodies postnatally via colostrum and BM to mucosal surfaces of their infants.4
Breastfeeding is safe and offers protection. Therefore, if optimal natural protection against RSV is to be achieved, there should be greater promotion of breastfeeding for both preterm and term infants. Moreover, breastfeeding offers the most environmentally friendly and cost-effective intervention in the absence of globally accessible and affordable immunoprophylaxis measures.54 55 There are, however, barriers to achieving the WHO breastfeeding standards as global practice. Primarily, inadequate knowledge and awareness about wider benefits of breastfeeding, especially among families of LMICs and, and in that context, missed educational opportunities.56 In addition, some may associate the act of breastfeeding as shameful, uncomfortable or inconvenient based on cultural beliefs; even in high-income regions of the world.57 A notable example being Ireland, with one of the lowest breastfeeding rates globally.57 58
Breastfeeding decreases RSV-associated ALRI severity
Breastfeeding during the first few months of life has a protective effect against severe RSV bronchiolitis outcomes. Nine of the included studies documented that lack of breastfeeding and breastfeeding for <2 months are significant risk factors for RSV-related hospital admissions.32 33 40 41 44 46 48 49 59 In addition, 18/19 articles suggests that breastfeeding significantly reduces the risk of hospitalisation, LOS and duration of supplemental oxygen use. Breastfeeding for 4–6 months and >6 months required less RSV-related hospital monitoring when compared with infants who never breast-fed. Furthermore, breastfeeding reduced bronchiolitis-associated healthcare utilisation, including emergency department (ED) presentation and ICU admission.28 29 34 39
Reflecting on available evidence, it is clear that without passive immunity delivered by MOM at birth, infants with underdeveloped immune systems are subjected to more severe disease outcomes. Hospitalisation with a confirmed RSV-associated ALRI in preterm infants was associated with more than twice the risk of acquiring long-term respiratory morbidity, such as asthma.60 More research needs to be conducted to determine the optimal duration of exclusive breastfeeding and if partial breastfeeding provides adequate protection against RSV-associated ALRI.
RSV-associated ALRI and seasonality
RSV has well-defined seasonal epidemics in both temperate and tropical regions, starting in late summer months in the tropics of each hemisphere, reaching peak numbers at temperate sites in winter months.61 During peak times, healthcare systems experience a demand surge, putting pressure on hospitals and staff. The median cost of infants treated in a paediatric ward is US$518.0 (217.0–768.9), intermediate care unit or paediatric high dependency unit (PHDU) is US$1305.2 (1051.4–1492.2) and in the paediatric intensive care unit (PICU) is US$2749.7 (1372.7–4159.9).62 A recent modelling study of infants born between 1 and 2 months before the peak of RSV season showed they had the highest risk of hospital admission due to RSV-associated ALRI, in comparison to infants born during other months and at the peak season.63 Infants born during that period have not had enough time to develop their immunity, resulting in increased RSV incidence during winter. This is also consistent with our observations, that breastfeeding <2 months or never is a significant factor for RSV hospitalisation.40 41 44 46 48 Further studies that focus on infants born and breast-fed during different months of the year and overall hospitalisation rates due to RSV should be performed to determine the true association of seasonality and disease severity.
Recent epidemiological trends in RSV-associated ALRI
Shortly after the lifting of physical distancing restrictions and COVID-19 lockdowns, with infants returning to crèche or daycare, there was an unprecedented and out-of-season influx of RSV cases in hospitals and burdened healthcare systems around the world.13 For example, in Australia, RSV cases began to increase during spring months and peaked in the summer, instead of the typical fall and winter months.12 Data from the USA demonstrated that admitted children were younger than previous years with a high proportion (81%) requiring ventilatory support (invasive/non-invasive).64 The median LOS increased from 3 to 4 days when compared with data from previous seasons. RSV infections that got competitively displaced or ‘elbowed out’ by SARS-CoV-2 during the initial phases of the COVID-19 pandemic are resurfacing with vigour.64
Emerging RSV preventative approaches
While this systematic review supports the role of breastfeeding in reducing the rate and severity of RSV-associated ALRI; as with other vaccine preventable infectious diseases, it is likely that the emerging RSV immunoprophylaxis choices during early infancy and new candidate RSV vaccines for maternal use during pregnancy could offer the crux of primary prevention against RSV-associated ALRI in the near future.
Currently available RSV-specific mAb, palivizumab is only indicated for high-risk populations, and therefore not available to most infants, who contribute to majority of RSV disease especially from LMICs.13–1513,14,15 However, long-acting RSV-specific mAb, nirsevimab, has been suggested as a single injection strategy and proposed for use among all infants through recent trials.18 65 However, this proposed prophylaxis will not be available until 2023 and possibly take years for wider recommendation, acceptability, affordability and uptake. Even then, the cost factor could preclude access to infants from LMICs. Maternal RSV vaccination during pregnancy, as part of a wider life-course immunisation approach, has been suggested with candidate vaccines in various phases of trials.17 55 66 67 However, based on the worldwide experience of currently offered maternal vaccinations, with some of them (eg, pertussis vaccine) having relatively low uptake in different parts of the world, it is plausible that the first RSV vaccine approved for use in pregnancy also would take years to gain confidence for universal recommendation and uptake.68–71
Strengths of the study
The strength of our systematic review is that it summarises available evidence of the benefits of breastfeeding as a cost-effective, universally available, adjunct primary prevention against RSV-associated ALRI. RSV prevention to date has largely been focused on mAb-based immunoprophylaxis against serious infections among the high-risk infants.13 14 Product costs and associated delivery expenses has prompted professional bodies and institutions in different countries to implement recommendations to rationalise and optimise its use.71 Breastfeeding offers passive immunity to a certain extent around the start of RSV season and may reduce hospital admissions and the critical care demand. Evidence synthesis provided by this review applies globally with most relevance to infants of LMICs, with highest RSV-related morbidity and mortality. Furthermore, extensive database searches included eligible articles published in English over the last two decades that addressed preset outcomes. By seeking reports from multiple databases, we minimise the risk of missing relevant studies and duplicate data were not included. Data from selected articles were meticulously extracted and all relevant information described in summary tables (Table 2). We conducted NSF scoring for quality assurance and grade of evidence. Our interpretations in support of breastfeeding are based on the majority of articles (13/19) included in evidence synthesis yielding a high grade of quality of evidence (7/10 or more). We have also subjected the eligible studies through a structured risk-of-bias assessment tool analysing five domains and 10/19 yielded a low risk-of-bias score with only two having an overall high score.
Since the completion of our systematic review, a prospective study of 969 infants from Spain published in late 2021 further strengthened our observations. While observing better outcomes for those exclusively breast-fed at 4 months, authors concluded that any breastfeeding was also associated with lower incidence of RSV bronchiolitis.72 Breastfeeding is currently the only low-cost, natural and universal primary prevention strategy that could be optimised and promoted as an adjunct to protect infants from severe RSV-associated ALRI. Overall, this systematic review that followed the PRISMA-P guidelines, Cochrane Handbook, SAGER guidelines and NSF evidence quality grading supports the impact of breastfeeding against severe RSV infections.22 23 73 74 It is important to demonstrate societal value of effective interventions such as breastfeeding to frontline clinicians who face challenges posed by RSV-associated ALRI during the winter peaks with overcrowding ED, bed shortages, in-hospital RSV outbreaks, lack of PHDU and PICU beds and often cancellation of scheduled life-saving surgeries for children.75–79 When there is no effective or standardised treatment for established RSV-associated ALRI, role of breastfeeding and its supportive preventative potential is demonstrated through this systematic review.
Limitations of the study
Following limitations are acknowledged: (1) this systematic review is limited to eligible studies from 2000 to 2021 published in English language; (2) articles describing RSV-associated ALRI and used interchangeably as RSV bronchiolitis or pneumonia were included at level 1 screening; (3) being the most frequently and severely affected RSV cohort, only infants <12 months of age were included; (4) studies describing preterm, term or mixed gestational cohorts were included; (5) studies that analysed secondary data of previously published primary studies could be open to publication bias; (6) while analysing hospitalisation for RSV-associated ALRI, a likely bias to the other factors could have contributed to admission or LOS (selection bias); (7) a review protocol was initially considered for PROSPERO international prospective register of systematic reviews. However, with PROSPERO giving priority approvals for COVID-19-related submissions, we had to complete the review considering the rapid worldwide increase in out-of-season RSV disease in 2021; (8) while initially designed to extract evidence from 1 January 2000 to 31 December 2021, reflecting on the rapid global resurgence of RSV disease after lifting the COVID-19 lockdowns, we limited the search to eligible publications until 21 September 2021; (9) a meta-analysis was not undertaken due to the widely heterogeneous nature of the articles included in this review. The published works demonstrated a mix of comparisons of different interventions (exclusive breastfeeding for 6 months, exclusive breastfeeding for 4 months, mixed or combined (breast and formula) of variable durations and MOM and DHM with different comparators). Inherent biases associated with the heterogeneous studies could also compound errors. Methods and outcomes demonstrated considerable diversity and therefore the authors concluded that they are not amenable to standard meta-analysis interrogations; however, merit a structured evidence synthesis; (10) potential contribution by environmental factors such as passive cigarette smoke exposure, crèche environment and birth order of infants were not analysed; (11) with no RCTs among the eligible studies (ethically challenging to evaluate the primary outcome by systematically excluding breastfeeding) and no eligible study from low-income countries specifically addressing breastfeeding and RSV-associated ALRI, our analysis of predominantly observational studies from middle-income and high-income countries with variable quality of evidence and inherent risk-of-bias, warrants interpretation with caution.
Conclusion
Breastfeeding has been shown to offer a universally available, easily accessible and low-cost adjunct primary prevention, conferring a degree of protection to infants against RSV-associated ALRI, based on observational studies of variable quality of evidence and an inherent risk-of-bias. This systematic review suggests that both exclusive and partial breastfeeding reduce the severity of RSV-associated ALRI, hospitalisation rate, LOS and supplemental oxygen requirement with or without ICU admission for ventilatory support. Therefore, mothers should be encouraged to initiate breastfeeding as soon as possible and continue as per WHO recommendations as a cost-effective intervention against severe RSV disease. Increased incidence, severity and out-of-season clustering of RSV infections in the post-COVID-19 environment should leverage a new impetus to highlight value of HM against RSV disease through global initiatives, in order to harness the bioactive and immunological value of one of the most environmentally friendly primary prevention— breastfeeding.
Acknowledgments
The authors wish to acknowledge the support offered by the patient representative of Limerick Neonatal Charity in this project to ensure patient and public involvement partnership. Support offered by the Library at University Hospital Limerick and University of Limerick is gracefully acknowledged.
Footnotes
Handling editor: Seye Abimbola
Twitter: @roykphilip
Contributors: RKP conceptualised and designed the study. GMM and RKP searched the literature and collected the data. GMM and RKP were responsible for accessing, reviewing and verifying eligible studies and for the integrity of datasets used. GMM and RKP prepared the draft manuscript. HP contributed to the methodology of systematic review and analysis of the results. CPD contributed to the intellectual, structured the manuscript and critically appraised the pooled observations. All authors participated in the interpretation of the data and writing of the manuscript. All authors confirm that they had full access to all the data used in the study and accept responsibility to submit for publication. The final submitted version of the manuscript was reviewed and approved by all authors. RKP acts as the guarantor responsible for the overall content of the published work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: RKP delivered a presentation, and participated in the speaker’s roundtable organised by Sanofi in 2021 on the topic of childhood respiratory infections. None of the presentation content is included in this manuscript. RKP received an honorarium for the presentation and roundtable participation. CPD delivered a global webinar for Abbott Diagnostics on the topic of rapid molecular tests for infectious diseases, including respiratory viruses. The presentation did not refer to RSV or the content of this paper. CPD received an honorarium for the webinar.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the 'Methods' section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Author note: Sex and Gender Equity in Research guidelines (SAGER): principles of sex and gender equity were applied to this systematic review. Please refer to methodology.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Formal approval from research ethics committee (REC) was not sought as the systematic review included only published literature and analysis of de-identified data.
References
- 1.Horta BL, Bahl R, Martines JC, et al. Evidence on the long-term effects of breastfeeding: systematic review and meta-analyses. WHO Library Cataloguing-in-Publication data, 2007. https://apps.who.int/iris/handle/10665/43623 [Google Scholar]
- 2.Victora CG, Bahl R, Barros AJD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016;387:475–90. 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- 3.Hanson LA. Human milk and host defence: immediate and long-term effects. Acta Paediatr Suppl 1999;88:42–6. 10.1111/j.1651-2227.1999.tb01299.x [DOI] [PubMed] [Google Scholar]
- 4.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013;60:49–74. 10.1016/j.pcl.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munblit D, Verhasselt V. Allergy prevention by breastfeeding: possible mechanisms and evidence from human cohorts. Curr Opin Allergy Clin Immunol 2016;16:427–33. 10.1097/ACI.0000000000000303 [DOI] [PubMed] [Google Scholar]
- 6.Bachrach VRG, Schwarz E, Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med 2003;157:237–43. 10.1001/archpedi.157.3.237 [DOI] [PubMed] [Google Scholar]
- 7.Glezen WP, Paredes A, Allison JE, et al. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981;98:708–15. 10.1016/S0022-3476(81)80829-3 [DOI] [PubMed] [Google Scholar]
- 8.Ramilo O, Mejias A. Measuring the burden of RSV in children to precisely assess the impact of preventive strategies. Pediatrics 2020;146:e20201727. 10.1542/peds.2020-1727 [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022;399:2047–64. 10.1016/S0140-6736(22)00478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010;375:1545–55. 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bont L, Checchia PA, Fauroux B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in Western countries. Infect Dis Ther 2016;5:271–98. 10.1007/s40121-016-0123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeoh DK, Foley DA, Minney-Smith CA, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis 2021;72:2199–202. 10.1093/cid/ciaa1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geskey JM, Thomas NJ, Brummel GL. Palivizumab: a review of its use in the protection of high risk infants against respiratory syncytial virus (RSV). Biologics 2007;1:33–43. [PMC free article] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134:415–20. 10.1542/peds.2014-1665 [DOI] [PubMed] [Google Scholar]
- 15.Linnane B, Kiernan MG, O'Connell NH, et al. Anti-RSV prophylaxis efficacy for infants and young children with cystic fibrosis in Ireland. Multidiscip Respir Med 2015;10:32. 10.1186/s40248-015-0029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019;7:e37–46. 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhi SA, Polack FP, Piedra PA, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 2020;383:426–39. 10.1056/NEJMoa1908380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin MP, Yuan Y, Takas T, et al. Single-dose Nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020;383:415–25. 10.1056/NEJMoa1913556 [DOI] [PubMed] [Google Scholar]
- 19.Leahy TR, McManus R, Doherty DG, et al. Interleukin-15 is associated with disease severity in viral bronchiolitis. Eur Respir J 2016;47:212–22. 10.1183/13993003.00642-2015 [DOI] [PubMed] [Google Scholar]
- 20.Dixon D-L, Griggs KM, Forsyth KD, et al. Lower interleukin-8 levels in airway aspirates from breastfed infants with acute bronchiolitis. Pediatr Allergy Immunol 2015;21:e691–6. 10.1111/j.1399-3038.2010.01011.x [DOI] [PubMed] [Google Scholar]
- 21.Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol 2009;2:222–31. [PMC free article] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner-Stokes L, Harding R, Sergeant J, et al. Generating the evidence base for the National service framework for long term conditions: a new research typology. Clin Med 2006;6:91–7. 10.7861/clinmedicine.6-1-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuinness LA, Higgins JPT. Risk-of-bias visualization (Robvis): an R package and shiny web APP for visualizing risk-of-bias assessments. Res Syn Meth 2020:1–7. 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 25.Koehoorn M, Karr CJ, Demers PA, et al. Descriptive epidemiological features of bronchiolitis in a population-based cohort. Pediatrics 2008;122:1196–203. 10.1542/peds.2007-2231 [DOI] [PubMed] [Google Scholar]
- 26.Lanari M, Prinelli F, Adorni F, et al. Risk factors for bronchiolitis hospitalization during the first year of life in a multicenter Italian birth cohort. Ital J Pediatr 2015;41:40. 10.1186/s13052-015-0149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JD, Dogaru C, Pescatore A, et al. Association of breastfeeding and respiratory infections in infants. Eur Respir J 2014;44:P3454. [Google Scholar]
- 28.Harvey SM, Murphy VE, Gibson PG, et al. The association between breastfeeding and respiratory health in infants born to women with asthma: a secondary analysis of two cohort studies. Eur Respir J 2019;54:PA5003. [Google Scholar]
- 29.Harvey SM, Murphy VE, Gibson PG, et al. Maternal asthma, breastfeeding, and respiratory outcomes in the first year of life. Pediatr Pulmonol 2020;55:1690–6. 10.1002/ppul.24756 [DOI] [PubMed] [Google Scholar]
- 30.Dornelles CTL, Piva JP, Marostica PJC. Nutritional status, breastfeeding, and evolution of infants with acute viral bronchiolitis. J Health Popul Nutr 2007;25:336–43. [PMC free article] [PubMed] [Google Scholar]
- 31.Prinelli F, Adorni F, Silvestri M, et al. Breastfeeding protects infants against lower respiratory tract infections caused by respiratory syncytial virus. results from the Italian cohort study Group on RSV. Selected abstracts / Early Human Development 2012;8852:S101–15. [Google Scholar]
- 32.von Linstow M-L, Høgh M, Nordbø SA, et al. A community study of clinical traits and risk factors for human metapneumovirus and respiratory syncytial virus infection during the first year of life. Eur J Pediatr 2008;167:1125–33. 10.1007/s00431-007-0643-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanari M, Prinelli F, Adorni F, et al. Maternal milk protects infants against bronchiolitis during the first year of life. results from an Italian cohort of newborns. Early Hum Dev 2013;89 Suppl 1:S51–7. 10.1016/S0378-3782(13)70016-1 [DOI] [PubMed] [Google Scholar]
- 34.Jang MJ, Kim YJ, Hong S, et al. Positive association of breastfeeding on respiratory syncytial virus infection in hospitalized infants: a multicenter retrospective study. Clin Exp Pediatr 2020;63:135–40. 10.3345/kjp.2019.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaur A, Singh K, Pannu MS, et al. The effect of exclusive breastfeeding on hospital stay and morbidity due to various diseases in infants under 6 months of age: a prospective observational study. Int J Pediatr 2016;2016:7647054 10.1155/2016/7647054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Yu H, Lin C, et al. The perinatal risk factors of acute bronchiolitis before one-year-old. Allergy 2019;74. [Google Scholar]
- 37.Bulkow LR, Singleton RJ, Karron RA, et al. Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics 2002;109:210–6. 10.1542/peds.109.2.210 [DOI] [PubMed] [Google Scholar]
- 38.Figueras-Aloy J, Carbonell-Estrany X, Quero J, et al. Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33-35 weeks in Spain. Pediatr Infect Dis J 2004;23:815–20. 10.1097/01.inf.0000136869.21397.6b [DOI] [PubMed] [Google Scholar]
- 39.Roine I, Fernandez JA, Vásquez A, et al. Breastfeeding reduces immune activation in primary respiratory syncytial virus infection. Eur Cytokine Netw 2005;16:206–10. [PubMed] [Google Scholar]
- 40.Simões EAF, Carbonell-Estrany X, Fullarton JR, et al. A predictive model for respiratory syncytial virus (RSV) hospitalisation of premature infants born at 33-35 weeks of gestational age, based on data from the Spanish FLIP study. Respir Res 2008;9:78. 10.1186/1465-9921-9-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lanari M, Silvestri M, Rossi GA. Respiratory syncytial virus risk factors in late preterm infants. J Matern Fetal Neonatal Med 2009;22 Suppl 3:102–7. 10.1080/14767050903194438 [DOI] [PubMed] [Google Scholar]
- 42.Nishimura T, Suzue J, Kaji H. Breastfeeding reduces the severity of respiratory syncytial virus infection among young infants: a multi-center prospective study. Pediatr Int 2009;51:812–6. 10.1111/j.1442-200X.2009.02877.x [DOI] [PubMed] [Google Scholar]
- 43.Carbonell X, Fullarton JR, Gooch KL, et al. The evolution of risk factors for respiratory syncytial virus-related hospitalisation in infants born at 32-35 weeks' gestational age: time-based analysis using data from the FLIP-2 study. J Perinat Med 2012;40:685–91. 10.1515/jpm-2011-0248 [DOI] [PubMed] [Google Scholar]
- 44.Blanken MO, Koffijberg H, Nibbelke EE, et al. Prospective validation of a prognostic model for respiratory syncytial virus bronchiolitis in late preterm infants: a multicenter birth cohort study. PLoS One 2013;8:e59161. 10.1371/journal.pone.0059161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vereen S, Gebretsadik T, Hartert TV, et al. Association between breast-feeding and severity of acute viral respiratory tract infection. Pediatr Infect Dis J 2014;33:986–8. 10.1097/INF.0000000000000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carbonell-Estrany X, Fullarton JR, Rodgers-Gray BS, et al. Can we improve the targeting of respiratory syncytial virus (RSV) prophylaxis in infants born 32-35 weeks' gestational age with more informed use of risk factors? J Matern Fetal Neonatal Med 2015;28:1133–41. 10.3109/14767058.2014.947573 [DOI] [PubMed] [Google Scholar]
- 47.Korsten K, Blanken MO, Nibbelke EE, et al. Prediction model of RSV-hospitalization in late preterm infants: an update and validation study. Early Hum Dev 2016;95:35–40. 10.1016/j.earlhumdev.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 48.Straňák Z, Saliba E, Kosma P, et al. Predictors of RSV LRTI hospitalization in infants born at 33 to 35 weeks gestational age: a large multinational study (PONI). PLoS One 2016;11:e0157446. 10.1371/journal.pone.0157446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maksić H, Heljić S, Skokić F, et al. Predictors and incidence of hospitalization due to respiratory syncytial virus (RSV)-associated lower respiratory tract infection (LRTI) in non-prophylaxed moderate-to-late preterm infants in Bosnia and Herzegovina. Bosn J Basic Med Sci 2018;18:279–88. 10.17305/bjbms.2018.2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JH, Kim CS, Chang YS, et al. Respiratory syncytial virus related readmission in preterm infants less than 34 weeks' gestation following discharge from a neonatal intensive care unit in Korea. J Korean Med Sci 2015;30 Suppl 1:S104–10. 10.3346/jkms.2015.30.S1.S104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flores-Gonzalez JC, Serrano-Moyano B, Lechuga-Sancho AM, et al. O-016 Breastfeeding as a protection factor for acute bronchiolitis. Arch Dis Child 2014;99:A28.1–A28. 10.1136/archdischild-2014-307384.85 [DOI] [Google Scholar]
- 52.Bozaykut A, Paketci A, Sezer RG, et al. Evaluation of risk factors for recurrent wheezing episodes. J Clin Med Res 2013;5:395–400. 10.4021/jocmr1543w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004;4:553–64. 10.1038/nri1394 [DOI] [PubMed] [Google Scholar]
- 54.Simister NE. Placental transport of immunoglobulin G. Vaccine 2003;21:3365–9. 10.1016/S0264-410X(03)00334-7 [DOI] [PubMed] [Google Scholar]
- 55.Ginsburg AS, Srikantiah P. Respiratory syncytial virus: promising progress against a leading cause of pneumonia. Lancet Glob Health 2021;9:e1644–5. 10.1016/S2214-109X(21)00455-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Surgeon G . The Surgeon General’s Call to Action to Support Breastfeeding: Barriers to Breastfeeding in the United States, 2011. Available: https://www.ncbi.nlm.nih.gov/books/NBK52682/ [Accessed 20 Jul 2021].
- 57.Purdy J, McAvoy H, Cotter N. Breastfeeding on the island of Ireland. Dublin: Institute of Public Health in Ireland; 2017. https://www.iphrepository.com/handle/123456789/166 [Accessed 20 Jul 2021]. [Google Scholar]
- 58.Gallagher L, Begley C, Clarke M. Determinants of breastfeeding initiation in Ireland. Ir J Med Sci 2016;185:663–8. 10.1007/s11845-015-1333-2 [DOI] [PubMed] [Google Scholar]
- 59.Carbonell-Estrany X, Figueras-Aloy J, Law BJ, et al. Identifying risk factors for severe respiratory syncytial virus among infants born after 33 through 35 completed weeks of gestation: different methodologies yield consistent findings. Pediatr Infect Dis J 2004;23:S193–201. 10.1097/01.inf.0000144664.31888.53 [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Garcia ML, Gonzalez-Carrasco E, Bracamonte T, et al. Impact of prematurity and severe viral bronchiolitis on asthma development at 6-9 years. J Asthma Allergy 2020;13:343–53. 10.2147/JAA.S258447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, Reeves RM, Wang X, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 2019;7:e1031–45. 10.1016/S2214-109X(19)30264-5 [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Martinez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Direct medical costs of RSV-related bronchiolitis hospitalizations in a middle-income tropical country. Allergol Immunopathol 2020;48:56–61. 10.1016/j.aller.2019.04.004 [DOI] [PubMed] [Google Scholar]
- 63.Li Y, Hodgson D, Wang X, et al. Respiratory syncytial virus seasonality and prevention strategy planning for passive immunisation of infants in low-income and middle-income countries: a modelling study. Lancet Infect Dis 2021;21:1303–12. 10.1016/S1473-3099(20)30703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agha R, Avner JR. Delayed seasonal RSV surge observed during the COVID-19 pandemic. Pediatrics 2021;148:e2021052089. 10.1542/peds.2021-052089 [DOI] [PubMed] [Google Scholar]
- 65.Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy Late-Preterm and term infants. N Engl J Med 2022;386:837–46. 10.1056/NEJMoa2110275 [DOI] [PubMed] [Google Scholar]
- 66.Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development - a global agenda. Vaccine 2016;34:2870–5. 10.1016/j.vaccine.2016.03.109 [DOI] [PubMed] [Google Scholar]
- 67.Schwarz TF, McPhee RA, Launay O, et al. Immunogenicity and safety of 3 formulations of a respiratory syncytial virus candidate vaccine in nonpregnant women: a phase 2, randomized trial. J Infect Dis 2019;220:1816–25. 10.1093/infdis/jiz395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manske JM. Efficacy and effectiveness of maternal influenza vaccination during pregnancy: a review of the evidence. Matern Child Health J 2014;18:1599–609. 10.1007/s10995-013-1399-2 [DOI] [PubMed] [Google Scholar]
- 69.Amirthalingam G, Andrews N, Campbell H, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet 2014;384:1521–8. 10.1016/S0140-6736(14)60686-3 [DOI] [PubMed] [Google Scholar]
- 70.Dunne CP, Spain E. Compulsory vaccination against COVID-19: a legal and ethical perspective on public good versus personal reticence. Ir J Med Sci 2022;25:1–6. 10.1007/s11845-022-02942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergin N, Murtagh J, Philip RK. Maternal vaccination as an essential component of life-course immunization and its contribution to preventive neonatology. Int J Environ Res Public Health 2018;15. 10.3390/ijerph15050847. [Epub ahead of print: 25 04 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gómez-Acebo I, Lechosa-Muñiz C, Paz-Zulueta M, et al. Feeding in the first six months of life is associated with the probability of having bronchiolitis: a cohort study in Spain. Int Breastfeed J 2021;16:82. 10.1186/s13006-021-00422-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higgins J, Green S. Cochrane Handbook for systematic reviews of interventions, 2008. Google Books. Available: https://training.cochrane.org/handbook/current/chapter-03 [Accessed 21 Jul 2021].
- 74.Information for Authors . Sex and gender equity research (SAGER) guidelines. BMJ Global Health. Available: https://gh.bmj.com/pages/authors [Accessed 03 Feb 2022].
- 75.Meenaghan S, Breatnach C, Smith H. Risk factors for respiratory syncytial virus bronchiolitis admissions. Ir Med J 2020;113:P9. [PubMed] [Google Scholar]
- 76.Pandolfi E, Gesualdo F, Rizzo C, et al. Breastfeeding and respiratory infections in the first 6 months of life: a case control study. Front Pediatr 2019;7:152. 10.3389/fped.2019.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Connor G, Tariq M, Greally P, et al. The changing epidemiology of the bronchiolitis epidemic in Tallaght Hospital. Ir Med J 2013;106:314-5 http://hdl.handle.net/10147/307047 [PubMed] [Google Scholar]
- 78.Vakrilova L, Nikolova SH, Slavov S, et al. An outbreak of RSV infections in a neonatology clinic during the RSV-season. BMC Pediatr 2021;21:567. 10.1186/s12887-021-03053-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mahase E. Winter pressure: RSV, flu, and covid-19 could push NHS to breaking point, report warns. BMJ 2021;374:n1802. 10.1136/bmj.n1802 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.