Abstract
The neutralizing monoclonal antibody combination of tixagevimab/cilgavimab has been shown to reduce the risk of SARS-CoV-2 infection in unvaccinated individuals during the Alpha (B.1.1.7) and Delta (B.1.617.2) waves. However, data on the efficacy and safety of tixagevimab/cilgavimab in vaccinated solid organ transplant recipients during the Omicron wave is limited. To address this, we conducted a retrospective cohort study comparing 222 solid organ transplant recipients (SOTRs) who received tixagevimab/cilgavimab for pre-exposure prophylaxis and 222 vaccine-matched solid organ transplant recipients who did not receive tixagevimab/cilgavimab. Breakthrough SARS-CoV-2 infections occurred in 11 (5%) of SOTRs who received tixagevimab/cilgavimab and in 32 (14%) of SOTRs in the control group (p < .001). In the tixagevimab/cilgavimab group, SOTRs who received the 150–150 mg dose had a higher incidence of breakthrough infections compared to those who received the 300–300 mg dose (p = .025). Adverse events were uncommon, occurring in 4% of our cohort and most were mild. There was no significant change in serum creatinine or liver chemistries in kidney and liver transplant recipients, respectively. In conclusion, we found that tixagevimab/cilgavimab use is safe and associated with a lower risk of breakthrough SARS-CoV-2 infection in vaccinated solid organ transplant recipients during the Omicron wave.
KEYWORDS: COVID-19, kidney transplant, prophylaxis, SARS-CoV-2
Abbreviations: IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOTR, solid organ transplant recipient
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in solid organ transplant recipients (SOTRs) is associated with higher mortality compared to immunocompetent individuals.1 Since antiviral responses to SARS-CoV-2 vaccines in SOTRs are attenuated,2 additional strategies such as monoclonal antibody pre-exposure prophylaxis have been developed.3 Tixagevimab and cilgavimab are neutralizing monoclonal antibodies directed against different epitopes of the receptor-binding domain of SARS-CoV-2 spike protein that have been associated with a lower risk of SARS-CoV-2 infection when used for pre-exposure prophylaxis in unvaccinated individuals.4 Based on that, tixagevimab/cilgavimab received emergency use authorization from the US Food and Drug Administration (FDA) for pre-exposure prophylaxis against SARS-CoV-2 infection in high-risk populations.5 However, post hoc analysis of the PROVENT trial showed a slightly higher incidence of serious cardiovascular events in the tixagevimab/cilgavimab group.5 Furthermore, data on the safety and efficacy of tixagevimab/cilgavimab in SOTRs during the Omicron wave are limited as only a small number of SOTRs were included in the trial, all were unvaccinated, and the trial was performed during the period when the Alpha (B.1.1.7) and Delta (B.1.617.2) variants were most prevalent.4
2. MATERIALS AND METHODS
2.1. Study design
To evaluate the safety and efficacy of tixagevimab/cilgavimab in SOTRs in a real-world setting during the Omicron period, we conducted a retrospective multicenter cohort study of kidney, liver, lung, and multiorgan transplant recipients at our institutions (Massachusetts General Hospital and Brigham and Women’s Hospital) who received tixagevimab/cilgavimab for SARS-CoV-2 pre-exposure prophylaxis. Tixagevimab/cilgavimab was prioritized to be given to SOTRs deemed to be at high risk for breakthrough and severe SARS-CoV-2 infection by their transplant physicians (allocation scheme in supplementary methods). Since the number of vaccines received is an important risk factor for the development of Omicron breakthrough infections,6 , 7 a control group of SOTRs matched for number of vaccines (categorized into three groups: 0–2, 3, or 4–5 vaccines) at our centers who did not receive tixagevimab/cilgavimab was used for comparison. The Mass General Brigham Research Patient Data Registry was used to identify both the tixagevimab/cilgavimab and the matched control groups, and then the electronic health record was reviewed manually for each patient. The primary outcome was the development of any breakthrough SARS-CoV-2 infection, defined as a newly positive polymerase chain reaction or antigen test, whether performed for symptoms or for another indication. Secondary outcomes included hospitalization or death from SARS-CoV-2 infection, changes in allograft function and occurrence of adverse events after receiving tixagevimab/cilgavimab. Posttransplant laboratory monitoring of allograft function was done per the institutions’ monitoring standard without additional specific monitoring after tixagevimab/cilgavimab. Adverse event monitoring was performed by manual review of the electronic health record. The study was approved by the Mass General Brigham institutional review board (protocol numbers: 2021P001235, 2019P002526, and 2017P000336). Data are reported in compliance with the STROBE reporting guidelines.
2.2. Statistical analysis
Continuous variables are listed as mean ± standard deviation or median (interquartile range, IQR) depending on distribution. Categorical variables are listed as counts or percentages. Differences between continuous variables of two unpaired samples were assessed using an unpaired t-test or Mann–Whitney U-test depending on distribution. Differences between continuous variables of two-paired samples were assessed using a paired t-test or Wilcoxon matched-pairs signed-rank test depending on distribution. Differences between proportions of categorical variables were assessed using Pearson’s Chi-squared test. Kaplan–Meier curves were used to estimate the incidence of SARS-CoV-2 infection in the tixagevimab/cilgavimab and control groups with differences assessed using the log-rank test. Follow-up for each patient in the control group was started on the same day that their vaccine-matched counterpart in the tixagevimab/cilgavimab group received tixagevimab/cilgavimab to control for variation in disease incidence. Patients were censored at occurrence of the outcome (i.e., SARS-CoV-2 infection), death or their last follow-up. A two-sided α level of 0.05 was considered statistically significant. SPSS v24 (Chicago, IL) and GraphPad Prism v9.1.2 (San Diego, CA) were used for statistical analysis and creation of figures, respectively.
3. RESULTS
3.1. Patient characteristics
Two hundred and twenty-two solid organ transplant recipients were included in the study ( Table 1). The median age was 65 years (IQR 55–72), 39% were female and the median time from transplantation to tixagevimab/cilgavimab administration was 3.8 years (IQR 1.9–8.2). 7% had a history of prior SARS-CoV-2 infection and > 99% had received at least one dose of SARS-CoV-2 vaccine. All tixagevimab/cilgavimab doses were given between December 28, 2021 and April 13, 2022 (Figure S1). 90 (40.5%) SOTRs received the 150–150 mg dose, 131 (59.0%) received the 300–300 mg dose and one (0.5%) received the 450–450 mg dose. The control group had similar characteristics to the tixagevimab/cilgavimab group, with the exception of a higher proportion of prior SARS-CoV-2 infection in the control group (19% vs. 7%, p < .001).
TABLE 1.
Baseline characteristics of tixagevimab/cilgavimab and control groups
| Characteristic | Tixagevimab/cilgavimab group (n = 222) | Control group (n = 222) | p value |
|---|---|---|---|
| Age (years), median (IQR) | 65 (55–72) | 64 (54–70) | .094a |
| Female sex, n (%) | 86 (39) | 92 (41) | .561b |
| Type of transplant, n (%) | .235b | ||
| Kidney | 114 (51.4) | 114 (51.4) | |
| Lung | 77 (34.7) | 70 (31.5) | |
| Liver | 17 (7.7) | 27 (12.2) | |
| Kidney/heart | 7 (3.2) | 1 (0.5) | |
| Kidney/liver | 4 (1.8) | 7 (3.2) | |
| Lung/heart | 2 (0.9) | 1 (0.5) | |
| Lung/kidney | 1 (0.5) | 1 (0.5) | |
| Pancreas/kidney | 0 (0) | 1 (0.5) | |
| Years from transplantation, median (IQR) | 3.8 (1.9–8.2) | 4.3 (2.7–7.0) | .076a |
| Number of vaccines received, n (%) | .323b | ||
| None | 2 (0.9) | 0 (0.0) | |
| One | 3 (1.4) | 0 (0.0) | |
| Two | 15 (6.8) | 20 (9.0) | |
| Three | 91 (41.0) | 91 (41.0) | |
| Four | 106 (47.7) | 107 (48.2) | |
| Five | 5 (2.3) | 4 (1.8) | |
| Months from last vaccine, median (IQR) | 2.3 (1.1–5.2) | 2.5 (0.7–5.5) | .178a |
| History of SARS-CoV-2 infection, n (%) | 15 (7) | 42 (19) | <.001b |
| History of CAD, n (%) | 55 (25) | 58 (26) | .744b |
| History of heart failure, n (%) | 50 (23) | 43 (19) | .414b |
| Tixagevimab-cilgavimab dose, n (%) | |||
| 150–150 mg | 90 (40.5) | N/A | N/A |
| 300–300 mg | 131 (59.0) | N/A | |
| 450–450 mg | 1 (0.5) | N/A |
Abbreviations: CAD, coronary artery disease; IQR, interquartile range; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
Statistics by Mann–Whitney U-test.
Statistics by Pearson’s Chi-squared test.
3.2. Breakthrough infection
At a mean follow-up of 87 ± 30 days after tixagevimab/cilgavimab administration, 11 SOTRs (5%) developed breakthrough SARS-CoV-2 infections, of whom one required hospitalization and none died ( Table 2). Infections occurred at a median of 81 days (IQR 15–97) after tixagevimab/cilgavimab administration and at a median of 6.1 months (IQR 2.8–8.3) since last vaccination. In the control group, 32 (14%) SOTRs developed SARS-CoV-2 infections, of whom six were hospitalized and three died at a mean follow-up of 82 ± 28 days (Table S1). Infections occurred at a median of 4.9 months (IQR 2.9–6.5) since last vaccination.
TABLE 2.
Breakthrough infections in the tixagevimab/cilgavimab group
| # | Date of infection | Most common variant at the timea | SOT | Number of vaccines | Months since last vaccine | T/C dose (mg) | Days since T/C | Hosp. | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jan. 19th 2022 | B.1.1.529 | Kidney | 4 | 3.9 | 150–150 | 7 | No | None | Recovered |
| 2 | Feb. 5th 2022 | B.1.1.529 | Lung | 3 | 0.9 | 150–150 | 8 | Yes | Remdesivir | Recovered |
| 3 | March 2nd 2022 | B.1.1.529 | Lung | 4 | 1.1 | 150–150 | 19 | No | Bamlanivimab | Recovered |
| 4 | March 29th 2022 | BA.2 | Liver | 3 | 7.4 | 150–150 | 46 | No | Bebtelovimab | Recovered |
| 5 | April 13th 2022 | BA.2 | Lung | 2 | 6.1 | 150–150 | 91 | No | Bebtelovimab | Recovered |
| 6 | April 21st 2022 | BA.2 | Lung | 3 | 8.1 | 150–150 | 99 | No | Bebtelovimab | Recovered |
| 7 | April 24th 2022 | BA.2 | Kidney | 4 | 2.2 | 300–300 | 11 | No | Bebtelovimab | Recovered |
| 8 | May 6th 2022 | BA.2 | Lung | 3 | 8.5 | 150–150 | 114 | No | MAB at outside institution | Recovered |
| 9 | May 15th 2022 | BA.2 | Lung | 4 | 9.0 | 150–150 | 95 | No | MAB at outside institution | Recovered |
| 10 | May 15th 2022 | BA.2 | Kidney | 4 | 3.4 | 150–150 | 81 | No | Bebtelovimab | Recovered |
| 11 | May 30th 2022 | BA.2.12.1 | Kidney | 3 | 9.5 | 150–150 | 102 | No | Bebtelovimab | Recovered |
Abbreviations: MAB, monoclonal antibody; SOT, solid organ transplant; T/C, Tixagevimab/cilgavimab.
Based on the most prevalent SARS-CoV-2 variant in region 1 (including Massachusetts) from the Center for Disease Control and Prevention COVID data tracker: https://covid.cdc.gov/covid-data-tracker/#variant-proportions.
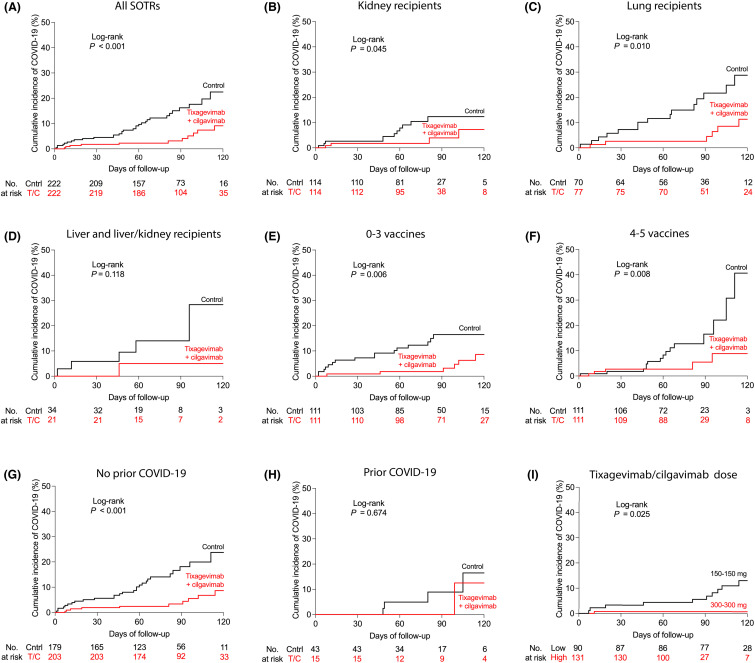
Survival analysis showed a lower incidence of SARS-CoV-2 infection in the tixagevimab/cilgavimab group compared to the control group ( Figure 1A, log-rank p < .001). Stratified analysis by organ type showed a significantly lower incidence of SARS-CoV-2 infection in kidney and lung transplant recipients who received tixagevimab/cilgavimab compared to those who did not, but the difference was not statistically significant in liver and liver/kidney transplant recipients (Figure 1B–D, log-rank p = .045, .010, and .118, respectively). Stratified analysis by number of vaccines showed a lower incidence of SARS-CoV-2 infection in SOTRs who received tixagevimab/cilgavimab whether they had received 0–3 or 4–5 vaccine doses (Figure 1E,F, log-rank p = .006 and .008, respectively). Given the difference in the proportions of SOTRs with a history of prior SARS-CoV-2 infection between the tixagevimab/cilgavimab and control groups, we also performed stratified analysis by prior SARS-CoV-2 infection status. In SOTRs without a history of prior SARS-CoV-2 infection, we found a significantly lower incidence of SARS-CoV-2 infection in SOTRs who received tixagevimab/cilgavimab compared to those who did not (Figure 1G, log-rank p < .001). However, there was no significant difference in the incidence of SARS-CoV-2 infection between the tixagevimab/cilgavimab and control groups in SOTRs with a prior history of SARS-CoV-2 infection (Figure 1H, log-rank p = .674). In the tixagevimab/cilgavimab group, the incidence rate of breakthrough SARS-CoV-2 infection was higher in those who received the lower (150–150 mg) dose of tixagevimab/cilgavimab compared to those who received the higher dose of 300–300 mg (Figure 1I, log-rank p = .025).
FIGURE 1.
Efficacy of tixagevimab/cilgavimab for pre-exposure prophylaxis in solid organ transplant recipients (SOTRs). (A) Kaplan–Meier estimates of the cumulative incidence of breakthrough coronarivus disease 19 (COVID-19) in SOTRs who received tixagevimab/cilgavimab (n = 222) and vaccine-matched SOTRs controls (n = 222) stratified by (B–D) solid organ type, (E, F) number of SARS-CoV-2 vaccines, (G, H) history of prior COVID-19, and (I) tixagevimab/cilgavimab dose. (A–I) Statistic by log-rank test
3.3. Safety and adverse events
Adverse events were uncommon in the tixagevimab/cilgavimab group, occurring only in nine SOTRs (4%) at a median of 15 days (IQR 5–22) after tixagevimab/cilgavimab administration. The most common adverse events were nausea, vomiting, or diarrhea (n = 4, 1.8%), headache (n = 3, 1.4%), and abdominal pain (n = 2, 0.9%). Two patients (0.9%) developed new lung infiltrates with negative infectious evaluation, thought to be pneumonitis. In terms of cardiovascular adverse events, one patient (0.5%) developed a mild heart failure exacerbation, and one (0.5%) developed new atrial fibrillation requiring cardioversion.
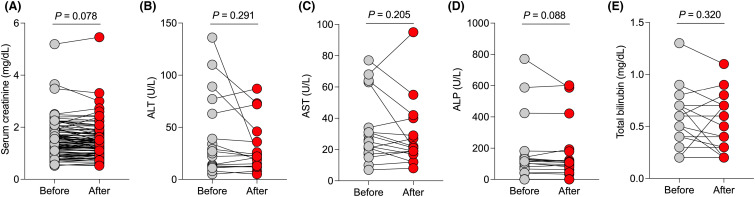
In kidney transplant recipients who had serum creatinine measured after tixagevimab/cilgavimab administration (n = 92), there was no significant change in serum creatinine levels measured at a median of 30 days (IQR 20–46) after tixagevimab/cilgavimab compared to baseline ( Figure 2A, p = .078). In liver transplant recipients who had liver chemistries measured after tixagevimab/cilgavimab administration (n = 16), there was no significant change in alanine aminotransferase (Figure 2B, p = .291), aspartate aminotransferase (Figure 2C, p = .205), alkaline phosphatase (Figure 2D, p = .088) or total bilirubin levels (Figure 2E, p = .320) measured at a median of 22 days (IQR 12–43) after tixagevimab/cilgavimab administration.
FIGURE 2.
Safety of tixagevimab/cilgavimab for pre-exposure prophylaxis in solid organ transplant recipients (SOTRs). (A) Serum creatinine levels in kidney transplant recipients before and after tixagevimab/cilgavimab administration (n = 92). (B) Alanine aminotransferase (ALT), (C) aspartate aminotransferase (AST), (D) alkaline phosphatase (ALP), and (E) total bilirubin levels in liver transplant recipients after tixagevimab/cilgavimab administration (n = 16). (A) Statistic by paired t-test. (B–E) Statistic by Wilcoxon matched-pairs signed rank test
4. DISCUSSION
In this study, we report our early experience with using tixagevimab/cilgavimab for pre-exposure prophylaxis in SOTRs during the Omicron wave. We found that tixagevimab/cilgavimab use was associated with a significantly lower risk of SARS-CoV-2 breakthrough infection in SOTRs, compared to a control group of vaccine-matched SOTRs at our centers. Hospitalizations and deaths due to SARS-CoV-2 infection were also numerically lower in the tixagevimab/cilgavimab group but given the small number of events our study was underpowered to find differences in these outcomes.
Since SOTRs who were deemed by their physicians to be at higher risk for breakthrough and severe SARS-CoV-2 infection were preferentially referred to receive tixagevimab/cilgavimab, this could have biased our findings toward a smaller difference in the incidence of breakthrough infections between the two groups if the tixagevimab/cilgavimab group had been compared to an unmatched control group consisting of all SOTRs who did not receive tixagevimab/cilgavimab at our centers at the time. Instead, to reduce the effect of confounding variables on the outcome of breakthrough infection, we used a control group of SOTRs from our centers who were vaccine-matched, had similar ages and organ types, and stratified analysis was performed. To reduce the effect of the changing SARS-CoV-2 incidence in the population on the outcome of breakthrough infection, follow-up for each control subject was started on the same date that their matched counterpart in the tixagevimab/cilgavimab group received tixagevimab/cilgavimab. While most characteristics were balanced between the two groups, we found that a history of prior SARS-CoV-2 infection was less common in SOTRs in the tixagevimab/cilgavimab group. To address its potential impact on the outcome of SARS-CoV-2 breakthrough infection, we stratified our analysis by prior SARS-CoV-2 infection history. Since infection-naïve individuals are at higher risk of SARS-CoV-2 infections compared to those with prior SARS-CoV-2 infection,8, 9, 10, 11, 12 the presence of more infection-naïve SOTRs in the tixagevimab/cilgavimab group would likely bias our study findings toward a higher incidence of breakthrough infection in the tixagevimab/cilgavimab group, and therefore result in an underestimate of the difference between the two groups. Interestingly, we also found no significant difference in the incidence of breakthrough infection between the tixagevimab/cilgavimab and control groups in the subgroup of SOTRs who had prior SARS-CoV-2 infection. This finding in combination with recent data showing no significant difference in the risk of symptomatic breakthrough Omicron infection in kidney transplant recipients who received tixagevimab/cilgavimab compared to those who had high-titer anti-spike antibody responses to vaccination but did not receive tixagevimab/cilgavimab13 suggest that SOTRs with no prior SARS-CoV-2 infection or those with low anti-spike antibody responses to vaccination may benefit most from tixagevimab/cilgavimab pre-exposure prophylaxis.
We also found that tixagevimab/cilgavimab use was associated with a lower incidence of breakthrough infection regardless of whether SOTRs had received 0–3 or 4–5 vaccines doses. Separate analysis of SOTRs who received 0–2 or 5 vaccine doses was not performed due to the small number of SOTRs in these groups. In addition, we found that tixagevimab/cilgavimab use was associated with a lower risk of breakthrough infection in kidney and lung transplant recipients. There was no statistically significant difference in the risk of breakthrough infection between liver or liver/kidney transplant recipients who did and did not receive tixagevimab/cilgavimab. However, given the small number of liver and liver/kidney transplant recipients in our cohort, our study was underpowered to find differences in this subgroup of SOTRs. Importantly, we also found a higher incidence of breakthrough infections in the low-dose (150–150 mg) compared to the high-dose (300–300 mg) tixagevimab/cilgavimab cohort, suggesting a lower risk of breakthrough infections in the high-dose group, which supports the FDA’s revised recommendation to use the higher dose for pre-exposure prophylaxis in SOTRs.14 A randomized controlled trial comparing the two doses would provide further evidence supporting the difference in breakthrough infection between the two doses in SOTRs.
We also found that tixagevimab/cilgavimab was safe with a low rate of adverse events and no evidence of allograft injury in kidney or liver transplant recipients. It is important to note, however, that since monitoring for adverse events was done retrospectively through review of the electronic health record, adverse events that were not recorded in the electronic health record could have been missed. Furthermore, post-tixagevimab/cilgavimab laboratory assessment of allograft function was only available in 73% and 76% of kidney and liver transplant recipients, respectively. Prospective systematic monitoring for adverse events and allograft function would provide stronger evidence of the safety of tixagevimab/cilgavimab in SOTRs.
Our study adds to the literature by including vaccinated SOTRs, which were not included in the original trial,4 evaluating breakthrough infections during the Omicron period, as opposed to the Alpha and Delta period during which the original trial was conducted, and by including patients who received the higher tixagevimab/cilgavimab dose (300–300 mg), as opposed to the lower dose (150–150 mg) that was evaluated in the trial.4 The limitations of our study include its observational nature, retrospective design, and differences between the tixagevimab/cilgavimab and control groups that could have influenced the rate of breakthrough infection. Given the small number of events in the tixagevimab/cilgavimab group, multivariable Cox regression to adjust for potential confounding variables was not performed and instead we used matching and a stratified analysis approach. Furthermore, our study was conducted during a period when the B.1.1.529, BA.2, and BA.2.12.1 Omicron lineages were most prevalent in Massachusetts,15 therefore the protective effects of tixagevimab/cilgavimab in vaccinated SOTRs cannot be generalized to BA.4 and BA.5 lineages. Despite these limitations, our study adds evidence of the efficacy and safety of tixagevimab/cilgavimab for pre-exposure prophylaxis in vaccinated SOTRs during the Omicron wave.
ACKNOWLEDGMENT
None.
FUNDING INFORMATION
The study was supported in part by the Harold and Ellen Danser Endowed/Distinguished Chair in Transplantation at Massachusetts General Hospital (Boston, MA, USA).
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data to support the findings in the study are available from the corresponding author upon request.
Funding information Massachusetts General Hospital, Grant/Award Number: Harold and Ellen Danser Endowed/Distinguished Chai
Footnotes
Ayman Al Jurdi and Leela Morena are considered co-first authors.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
Figure S1 Dates of tixagevimab/cilgavimab administration.
Table S1. Breakthrough infections in the control group.
REFERENCES
- 1.Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21(3):1295–1303. doi: 10.1111/ajt.16424. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Jurdi A, Gassen RB, Borges TJ, et al. Non-invasive monitoring for rejection in kidney transplant recipients after SARS-CoV-2 mRNA vaccination. Front Immunol. 2022;13:838985. doi: 10.3389/fimmu.2022.838985. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loo Y-M, McTamney PM, Arends RH, et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med. 2022;14(635):eabl8124. doi: 10.1126/scitranslmed.abl8124. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N Engl J Med. 2022;386:2188–2200. doi: 10.1056/NEJMoa2116620. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tixagevimab and Cilgavimab (Evusheld) for pre-exposure prophylaxis of COVID-19. Jama. 2022;327(4):384–385. doi: 10.1001/jama.2021.24931. doi: [DOI] [PubMed] [Google Scholar]
- 6.Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a Nationwide setting. N Engl J Med. 2022;386(17):1603–1614. doi: 10.1056/NEJMoa2201688. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386(13):1288–1290. doi: 10.1056/NEJMc2200133. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid immunity to SARS-CoV-2. N Engl J Med. 2022;25:2201–2212. doi: 10.1056/NEJMoa2118946. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall VJ, Foulkes S, Charlett A, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet (London, England). 2021;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet (London, England). 2021;397(10280):1204–1212. doi: 10.1016/S0140-6736(21)00575-4. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheehan MM, Reddy AJ, Rothberg MB. Reinfection rates among patients who previously tested positive for coronavirus disease 2019: a retrospective cohort study. Clin Infect Dis. 2021;73(10):1882–1886. doi: 10.1093/cid/ciab234. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertrand D, Laurent C, Lemée V, et al. Efficacy of anti SARS-CoV-2 monoclonal antibodies prophylaxis and vaccination on omicron COVID-19 in kidney transplant recipients. Kidney Int. 2022 doi: 10.1016/j.kint.2022.05.007. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA. FDA authorizes revisions to Evusheld dosing. 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-revisions-evusheld-dosing
- 15.CDC: Variant proportions. 2022. Accessed May 1, 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Dates of tixagevimab/cilgavimab administration.
Table S1. Breakthrough infections in the control group.
Data Availability Statement
Data to support the findings in the study are available from the corresponding author upon request.