Abstract
Poor responses to mRNA COVID-19 vaccine have been reported after 2 vaccine injections in kidney transplant recipients (KTRs) treated with belatacept. We analyzed the humoral response in belatacept-treated KTRs without a history of SARS-CoV-2 infection who received three injections of BNT162b2-mRNA COVID-19 vaccine. We also investigated vaccine immunogenicity in belatacept-treated KTRs with prior COVID-19 and characterized symptomatic COVID-19 infections after the vaccine in belatacept-treated KTRs. Among the 62 belatacept-treated KTRs (36 [58%] males), the median age (63.5 years IQR [51–72]), without COVID-19 history, only four patients (6.4%) developed anti-SARS-CoV-2 IgG with low antibody titers (median 209, IQR [20–409] AU/ml). 71% were treated with mycophenolic acid and 100% with steroids in association with belatacept. In contrast, in all the 5 KTRs with prior COVID-19 history, mRNA vaccine induced a strong antibody response with high antibody titers (median 10 769 AU/ml, IQR [6410–20 069]) after two injections. Seroprevalence after three-vaccine doses in 35 non-belatacept-treated KTRs was 37.1%. Twelve KTRs developed symptomatic COVID-19 after vaccination, including severe forms (50% of mortality). Breakthrough COVID-19 occurred in 5% of fully vaccinated patients. Administration of a third dose of BNT162b2 mRNA COVID-19 vaccine did not improve immunogenicity in KTRs treated with belatacept without prior COVID-19. Other strategies aiming to improve patient protection are needed.
KEYWORDS: belatacept, COVID-19 mRNA vaccine, kidney transplant recipients, three doses
Abbreviations: ATG, antithymocyte globulin; AU, arbitrary units; CNI, calcineurin inhibitors; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KT, kidney transplantation; KTR, kidney transplant recipient; MPA, mycophenolic acid; mTOR, mammalian target of rapamycin; RT-PCR, reverse transcriptase-polymerase chain reaction; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCr, serum creatinine
1. INTRODUCTION
In contrast to immunocompetent individuals, poor immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccines have been observed in kidney transplant recipients (KTRs) after two vaccine injections. Several studies demonstrated seroconversion rates between 30% and 54% in patients treated with calcineurin inhibitors (CNI)-based immunosuppressive therapy.1, 2, 3, 4, 5 However, immune responses were particularly low in KTRs treated with belatacept, with a seroconversion rate of 0 to 5.7% in three studies.6, 7, 8
These findings, associated with reports of symptomatic COVID-19 infections following mRNA vaccine in immunosuppressed patients9, 10, 11, 12 lead the French National Authority for Health to recommend, on April 2021, the use of the third injection in this population.13 The first report of post-vaccinal humoral responses after three injections14 demonstrated a significant improvement of vaccine immunogenicity in solid organ transplant patients, mostly treated with CNI. The prevalence of anti-SARS-CoV-2 antibodies was, respectively, 40% and 68% after the second and the third dose.
Moreover, immunocompetent individuals with prior SARS-CoV-2 infection exhibited stronger immune responses after mRNA vaccine,15 which prompted the recommendation of a single vaccine dose in these patients. However, no previous study reported immune responses to mRNA SARS-CoV-2 vaccine in belatacept-treated KTRs with prior COVID-19 infection.
We aimed in this study to report the humoral response in belatacept-treated KTRs without a history of SARS-CoV-2 infection who received three injections of BNT162b2 mRNA COVID-19 vaccine. We also investigated vaccine immunogenicity in belatacept-treated KTRs with prior COVID-19 infection and characterized symptomatic COVID-19 infections after mRNA vaccine in KTRs treated with belatacept.
2. METHODS
2.1. Patients
We retrospectively included all KTRs followed at Necker’s Hospital, Paris, France and treated with belatacept, who received three injections of BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech) and had their anti-SARS-CoV-2 spike (S) protein antibodies assessed.
The main indications for belatacept conversion in our center include: a low eGFR associated with renal chronic vascular lesions; CNI nephrotoxicity on a graft biopsy or clinically suspected nephrotoxicity and prolonged delayed graft function. Patients were excluded if they were treated with eculizumab in association with belatacept (to avoid underestimating humoral response under belatacept, as eculizumab therapy has been associated with weak vaccinal responses16).
Epidemiological, clinical data, and post-vaccinal humoral responses of non-belatacept-treated KTRs with available anti-SARS-CoV-2 serology were also retrospectively analyzed.
KTRs who presented COVID-19 infection (defined as the presence of a positive SARS-CoV-2 on reverse transcriptase-polymerase chain reaction (RT-PCR) testing performed on nasopharyngeal swab or positive anti-SARS-CoV-2 IgG antibodies directed against the SARS-CoV-2 nucleocapsid protein) before vaccination were identified and their data were analyzed separately. Additionally, patients who developed a symptomatic COVID-19 infection after vaccination (at least 1 dose) were identified and COVID-19 infections (severity and outcome) were characterized.
The following clinical and epidemiological data were retrospectively collected using hospital databases and clinical records: age, gender, transplant date, transplant range, induction immunosuppressive therapy, acute rejection treatment in 24 months before vaccination, maintenance immunosuppression associated with belatacept (type and doses) at vaccination time and serum creatinine (estimated glomerular filtration rate [eGFR]) at first vaccine injection.
Written informed consent was obtained to collect clinical data for the prospective transplant database Données Informatiques Validées en Transplantation (DIVAT). The study was approved by the Institutional Review Board of Necker Hospital. In addition, all patients provided informed consent for blood collection and serum samples were collected and stored as part of the Necker biobank (DC-2009–95).
2.2. mRNA vaccination modalities
The two first injections of 30-μg doses of BNT162b2 mRNA COVID-19 vaccine (Pfizer-BioNTech), were administered intramuscularly 28 days apart to all patients. The third injection was performed between 26 and 95 days after the second injection.
Anti-SARS-CoV-2 post-vaccinal humoral response against S protein was assessed using the SARS-CoV-2 IgG II Quant antibody test (Abbott, USA) according to the manufacturer’s instructions (titers >50 arbitrary units per mL [AU/ml] were considered as positive). This test had been demonstrated to display a high specificity (>99%) and sensitivity (90%).17
2.3. Statistical analysis
Categorical data were expressed as numbers and percentages, while continuous variables were expressed as medians (interquartile ranges [IQR]) or as mean (standard deviation, SD), as appropriate. Continuous and categorical variables were both compared using t tests or Mann–Whitney tests, and chi-squared tests or Fisher’s exact tests, as appropriate, after verification of the skewness of the distribution. Values of p < .05 in two-tailed tests were considered statistically significant. All analyses and figures were, respectively, conducted with Stata v15.0 (StataCorp, College Station, TX, USA) and GraphPad Prism 6.
3. RESULTS
3.1. Poor post-vaccinal humoral response in KTRs converted to belatacept without prior COVID-19 infection
Sixty-two KTRs without prior COVID-19 infection received three injections of BNT162b2 mRNA COVID-19 vaccine between January 12 and May 21, 2021 ( Table 1). Of them, 36 (58%) were male and the median age was 63.5 (51–72) years. Immunosuppressive induction consisted in antithymocyte globulin (ATG) in 20 patients (32%). Belatacept conversion was performed at a median of 11.5 (3–26) months after transplantation. At vaccination, 44 patients (71%) were treated with mycophenolic acid (MPA) (median dose: 1000 [750–1500] mg/day), 8 (13%) with mammalian target of rapamycin (mTOR) inhibitors (median serum concentration: 4.9 [6.1–6.6] ng/ml), 3 (5%) with azathioprine and 62 (100%) with steroids (median dose: 7.5 [5–10] mg/day) in adjunction to belatacept. The median basal serum creatinine was 137 (105.1–180) μmol/L and median eGFR 44.4 (33.2–59.7) ml/min/1.73 m2. None of the patients was treated for rejection in the 24 months preceding vaccination.
TABLE 1.
Baseline characteristics and post-vaccinal humoral responses of the 62 KTRs converted to belatacept, who received three injections of BNT162b2 mRNA COVID-19 vaccine
| Variables | Whole cohort, N = 62 | Non responders, N = 58 (94%) | Responders, N = 4 (6%) |
|---|---|---|---|
| Age, median (IQR) | 63.5 (51–72) | 64 (51.5–72) | 51.5 (42–58) |
| Sex (males), N (%) | 36 (58) | 33 (57) | 3 (75) |
| KT >1, N (%) | 6 (10) | 5 (8.6) | 1 (25) |
| Induction immunosuppressive therapy | |||
| Antithymocyte globulin, N (%) | 20 (32) | 20 (34) | 0 |
| Basiliximab, N (%) | 42 (68) | 38 (66) | 4 (100) |
| Rituximab at induction, N (%) | 10 (16) | 10 (17) | 0 |
| Maintenance immunosuppressive therapy | |||
| Calcineurin inhibitors, N (%) | 2 (3) | 2 (3) | 0 |
| Azathioprine, N (%) | 3 (5) | 1 (2) | 2 (50) |
| Mycophenolic acid, N (%) | 44 (71) | 42 (72) | 2 (50) |
| Dose, median (IQR) | 1000 (750–1500) | 1000 (750–1500) | 1000 (1000–1000) |
| mTOR-i (everolimus), N (%) | 8 (13) | 8 (14) | 0 |
| Dose, median (IQR) | 4.9 (6.1–6.6) | 4.9 (6.1–6.6) | 0 |
| Steroids, N (%) | 62 (100) | 48 (100) | 4 (100) |
| Dose, median (IQR) | 7.5 (5–10) | 7.5 (5–10) | 5 (5–6.25) |
| Belatacept, N (%) | 62 (100) | 48 (100) | 4 (100) |
| Time between KT and belatacept conversion, months, median (IQR) | 11.5 (3–26) | 12 (3–30) | 3.5 (2.5–8.5) |
| At first vaccine injection | |||
| SCr at first vaccine injection, median (IQR) | 137 (105.1–180) | 136.9 (105.1–178.2) | 151 (105.8–193) |
| eGFR at first vaccine injection, median (IQR) | 44.4 (33.2–59.7) | 44.4 (33.2–59.7) | 42.0 (34.5–61.5) |
| Time between KT and first injection, months, median (IQR) | 47.5 (25.3–79) | 52 (25–82) | 41.5 (33–46) |
| Time between belatacept conversion and first injection, months, median (IQR) | 28.8 (16.5–51.7) | 28 (16.5–55) | 36 (26.5–40) |
| SARS-CoV–2 anti-Spike IgG titer at first injection, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Outcomes after first vaccine injection | |||
| SARS-CoV–2 anti-Spike IgG titer at first injection, median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Positive SARS-CoV–2 serology 28 days after 1 injection, N (%) | 0 | 0 | 0 |
| SARS-CoV–2 anti-Spike IgG titer 28 days after first injection, AU/ml, median (IQR) | 1.5 (0–3) | 1 (0–3) | 2 (2–2) |
| Outcomes after second vaccine injection | |||
| Positive SARS-CoV–2 serology after 2 injections, N (%) | 0 | 0 | 0 |
| SARS-CoV–2 anti-Spike IgG titer after second injection, median (IQR) | 0 (0–0) | 0 (0–0) | 3 (1.5–4.5) |
| Outcomes after third vaccine injection | |||
| Delay between second and third injections, days, median (IQR) | 69.5 (40–84) | 71 (40–84) | 44.5 (32–63) |
| Delay between third injection and serology, days, median (IQR) | 28 (28–33) | 28 (28–33) | 34.5 (29–41) |
| Positive SARS-CoV–2 serology after 3 injections, N (%) | 4 (6.4) | 0 | 4 (100) |
| SARS-CoV–2 anti-Spike IgG titer after third injection, AU/ml, median (IQR) | 0 (0–1) | 0 (0–0) | 298 (209–409) |
Abbreviations: AU, arbitrary units; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KT, kidney transplantation; SCr, serum creatinine.
The first dose of vaccine was initiated in a median time of 47.5 (25.3–79) months after transplantation and 28.8 (16.5–51.7) months after conversion to belatacept. The first two doses were given to all patients 28 days apart, and the third dose was administered in a median time of 69.5 (40–84) days after the second dose. The median time between the third injection and anti-SARS-CoV-2 antibodies assessment was 28 (28–33) days. We observed a favorable safety profile: no patient presented severe systemic events.
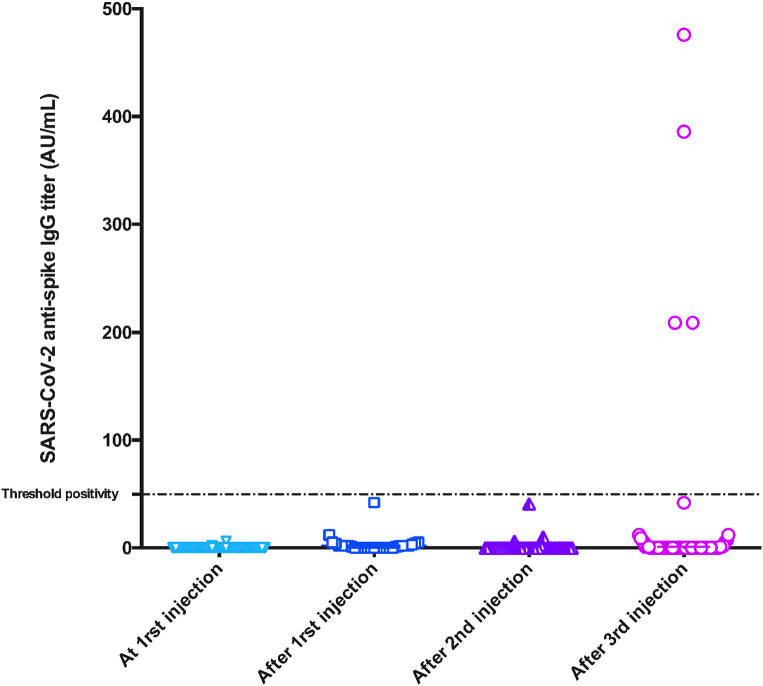
Only four patients (6.4%) developed anti-SARS-COV-2 antibodies after the third injection ( Figure 1). All four patients had negative anti-S serology at first vaccine injection. None of them had seroconverted after the first nor the second injection. The median antibodies titer in these patients was 298 (209–409) AU/ml. The four patients (median age of 51.5 [42–58] years) were vaccinated in a median time of 43 (32.6–51.2) months after transplantation and 36.2 (26.5–39.7) months after conversion to belatacept. Two of them were treated with MPA (1000mg/day) while the two other patients were treated with azathioprine. All four were treated with steroids (median dose 5 [5–6.25] mg/day) in association with belatacept.
FIGURE 1.
Humoral response following anti-SARS-CoV2 mRNA vaccine in 62 KTRs treated with belatacept without prior COVID-19 infection. Anti-SARS-CoV-2 IgG at first injection and after first, second, and third injection. 0% of patients had a positive IgG response after first and second injections. After the third injection, only four (6.4%) patients exhibited positive IgG response with low IgG titers (median IgG titers: 298 AU/ml, IQR [209–409]). AU, arbitrary unit [Color figure can be viewed at wileyonlinelibrary.com]
In the 58 remaining patients, the median antibodies titer was dramatically low (0 AU/ml [0–0]).
Thirty-five consecutive non-belatacept-treated KTRs without prior COVID-19 were included Epidemiological and transplant characteristics of these patients were comparable to belatacept patients except for age (KTRs treated with belatacept were significantly older) (Table S1). In contrast to belatacept patients, 13/35 (37.1%) patients developed humoral response after the third vaccine dose.
3.2. Effective post-vaccinal humoral response in KTRs converted to belatacept with prior COVID-19 infection
Five KTRs (1 [20%] males and median age of 66 [46–67] years) who developed COVID-19 infection before vaccination were identified ( Table 2). Of them, two (40%) received ATG at induction. Belatacept conversion was performed at a median time of 2 (0–3) months after transplantation and the median time between belatacept conversion and vaccination was 18 (9–34) months. 80% of them received MPA (median dose: 1000 [1000–1000] mg/day) and steroids (median dose: 10 [8–10] mg/day) in association with belatacept. One patient received mTOR-I (i.e., everolimus). None of the patients was treated for rejection in the 24 months preceding vaccination.
TABLE 2.
Characteristics and post-vaccinal humoral responses to BNT162b2 mRNA COVID-19 vaccine of KTRs converted to belatacept with prior COVID-19 infections
| Variables | KTRs with prior COVID–19 infection, N = 5 |
|---|---|
| Age, median (IQR) | 66 (46–67) |
| Sex (males), N (%) | 1 (20) |
| KT >1, N (%) | 0 |
| Induction immunosuppressive therapy | |
| Antithymocyte globulin, N (%) | 2 (40) |
| Basiliximab, N (%) | 3 (60) |
| Rituximab at induction, N (%) | 0 |
| Maintenance immunosuppressive therapy | |
| Calcineurin inhibitors, N (%) | 0 |
| Azathioprine, N (%) | 0 |
| Mycophenolic acid, N (%) | 4 (80) |
| Dose, median (IQR) | 1000 (1000–1000) |
| mTOR-i (everolimus), N (%) | 1 (20) |
| Dose, median (IQR) | 2.4 (2.4–2.4) |
| Steroids, N (%) | 5 (100) |
| Dose, median (IQR) | 10 (10–10) |
| Belatacept, N (%) | 5 (100) |
| Time between KT and belatacept conversion, months, median (IQR) | 2 (0–3) |
| At first vaccine injection | |
| SCr at first vaccine injection, median (IQR) | 134 (83–145) |
| eGFR at first vaccine injection, median (IQR) | 39 (32.4–63.4) |
| Time between COVID–19 infection and first injection, days, median (IQR) | 344 (276–347) |
| Time between KT and first injection, months, median (IQR) | 19 (12–36) |
| Time between belatacept conversion and first injection, months, median (IQR) | 18 (9–34) |
| SARS-CoV–2 anti-Spike IgG titer at first injection, median (IQR) | |
| Positive SARS-CoV–2 serology at first injection, N (%) (N = 5) | 3 (60) |
| SARS-CoV–2 anti-Spike IgG titer at first injection, median (IQR) (N = 5) | 76 (39–290) |
| Outcomes after first injection | |
| Positive serology 28 days after first injection, N (%) (N = 2) | 2 (100) |
| SARS-CoV–2 anti-Spike IgG titer 28 days after first injection, median (IQR) (N = 2) | 720 (695–745) |
| Outcomes after second injection | |
| Patients who received 2 injections, N (%) | 5 (100) |
| Positive serology after 2 injections, N (%) (N = 4) | 4 (100) |
| SARS-CoV–2 anti-Spike IgG titer after second injection, median (IQR) (N = 4) | 10 769 (6410–20 069) |
| Outcomes after third injection | |
| Patients who received 3 injections, N (%) | 2 (40) |
| Time between second and third injection, days, median (IQR) | 40 (36–69) |
| Positive serology after 3 injections, N (%) | 2 (100) |
| SARS-CoV–2 anti-Spike IgG titer after third injection, median (IQR) | 2175 (1665–2685) |
Abbreviations: AU, arbitrary units; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KT, kidney transplantation; SCr, serum creatinine.
Diagnosis of COVID-19 infection was established on a positive anti-SARS-CoV-2 PCR in patients with typical symptoms of COVID-19 in all cases (100%).
The five patients received two vaccine injections including two patients who received three injections. The median time between COVID-19 infection and first vaccine injection was 344 (276–347) days and median IgG titer was 76 (39–290) AU/ml at the time of the first injection. The second dose was performed 28 days after the first injection and the time between the second and third injection was 40 (36–69) days.
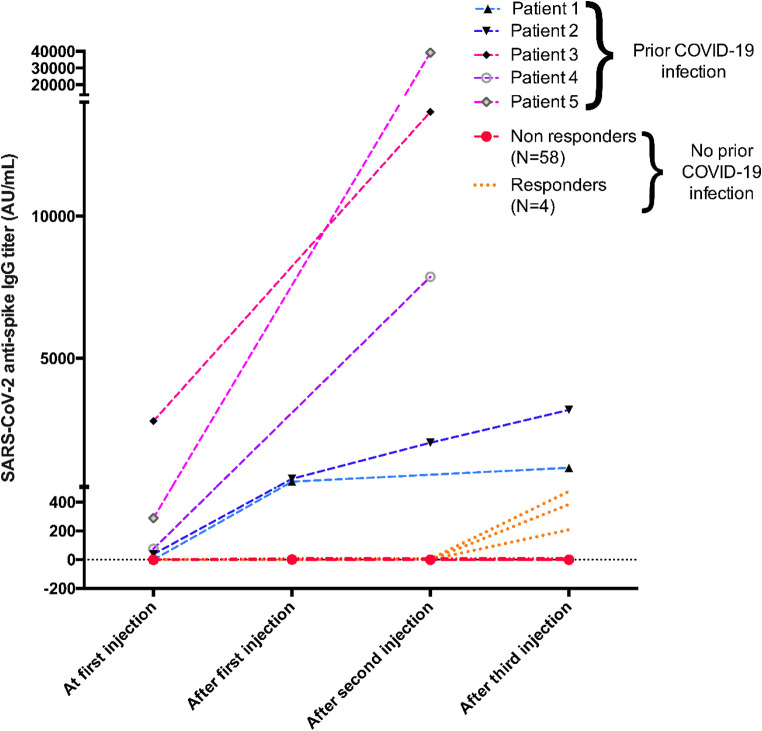
In contrast to KTRs without prior COVID-19 infection before vaccination, all patients developed a strong antibody response after vaccination. Antibodies titers increased in all patients after one injection with a median IgG titer of 720 (695–745) AU/ml, and humoral response was boosted after the second and third injections ( Figure 2). In the five patients with two injections, titers increased to 10 769 AU/ml (6410–20069) and in the two patients with three injections, titers increased to 2175 AU/ml (1665–2685).
FIGURE 2.
Humoral response following anti-SARS-CoV2 mRNA vaccine in five KTRs treated with belatacept with prior COVID-19 infection and in 62 KTRs without prior history of COVID-19. Patients with prior COVID-19 (Patients 1, 2, 3, 4, and 5) exhibited strong antibody responses after vaccine injections. In contrast, only 4/62 KTRs without prior COVID-19 seroconverted after three injections of vaccine with low IgG titers (AU, arbitrary unit)
No patient presented severe systemic side effect.
3.3. Symptomatic COVID-19 infection after BNT162b2 mRNA vaccine in KTRs converted to belatacept
One hundred and eighty-one KTRs treated with belatacept in our center received ≥2 COVID-19 BNT162b2 vaccines. Among them, 12 patients (6.6%) developed a symptomatic COVID-19 in a median time of 18 (8–30) days after their last vaccine infusion, including 9 (5.0%) ≥14 days after the second dose (breakthrough COVID-19) ( Table 3).
TABLE 3.
Characteristics of KTRs converted to belatacept with symptomatic COVID-19 infection after BNT162b2 mRNA vaccine
| Variables | Whole cohort, N = 12 |
|---|---|
| Age, median (IQR) | 69.5 (54.5–75) |
| Sex (males), N (%) | 7 (58) |
| KT >1, N (%) | 1 (8) |
| Comorbidities | |
| BMI >30 kg/m2, N (%) | 3 (25) |
| Diabetes mellitus, N (%) | 8 (67) |
| Hypertension, N (%) | 12 (100) |
| Active cancer, N (%) | 1 (8) |
| History of MCVE, N (%) | 8 (67) |
| Coronary heart disease, N (%) | 7 (58) |
| Peripheral artery disease, N (%) | 1 (8) |
| History of stroke, N (%) | 4 (33) |
| Chronic respiratory disease, N (%) | 7 (58) |
| Induction immunosuppressive therapy | |
| Antithymocyte globulin, N (%) | 5 (42) |
| Basiliximab, N (%) | 7 (58) |
| Rituximab at induction, N (%) | 0 |
| Maintenance immunosuppressive therapy | |
| Calcineurin inhibitors, N (%) | 0 |
| Azathioprine, N (%) | 1 (8) |
| Mycophenolic acid, N (%) | 9 (75) |
| Dose, median (IQR) | 1000 (1000–2000) |
| mTOR-i (everolimus), N (%) | 0 |
| Steroids, N (%) | 12 (100) |
| Dose, median (IQR) | 10 (7–10) |
| Belatacept, N (%) | 12 (100) |
| Time between KT and belatacept conversion, months, median (IQR) | 10.5 (2–69) |
| SCr at first injection, median (IQR) | 152 (119–179) |
| eGFR at first injection, median (IQR) | 40.4 (32.5–46.3) |
| Outcomes after first injection | |
| Time between KT and SARS-CoV–2 infection, months, median (IQR) | 65 (50–100) |
| Time between last belatacept infusion and SARS-CoV–2 infection, days, median (IQR) | 18 (8–30) |
| Time between last vaccine injection and SARS-CoV–2 infection, days, median (IQR) | 31.5 (15–58) |
| Patients with at least 2 injections before SARS-CoV–2 infection, N (%) | 8 (67) |
| Infection requiring hospitalization, N (%) | 8 (67) |
| Length of stay in hospital, days, median (IQR) | 18 (11–21) |
| Hospitalization in ICU, N (%) | 3 (25) |
| Mechanical ventilation, N (%) | 2 (67) |
| Treatment | |
| High dose steroids | 6 (50) |
| Tocilizumab | 4 (33) |
| Monoclonal antibodies | 1 (8) |
| Convalescent plasma | 1 (8) |
| Death, N (%) | 6 (50) |
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; IQR, interquartile range; KT, kidney transplantation; MCVE, major cardio-vascular event (coronary heart disease, peripheral artery disease, and history of stroke); SCr, serum creatinine.
All 12 patients presented ≥2 comorbidities (hypertension in all, diabetes in 8 patients, history of major cardio-vascular event [coronary heart disease, peripheral artery disease, and history of stroke] in 8 and chronic respiratory disease in 7). None of the patients was treated for rejection in the 24 months preceding vaccination.
COVID-19 occurred after the first injection in three patients, and after the second injection in eight patients, in a median time of 31.5 (15–58) days after injection. One patient also developed COVID-19 6 days after the third dose. The whole population follow-up was, however, short (median follow-up of 44 days [40–49] after the third injection). No patients had developed COVID-19 infection prior to vaccine. Among the six patients with available data on anti-SARS-CoV-2 antibodies between the second vaccine dose and COVID-19 infection, all had negative serology (0 [0–0] AU/ml).
Eight (67%) patients required hospitalization, including three in Intensive Care Unit. Six received high doses of steroids, four were treated with tocilizumab, one was administered monoclonal antibodies (not available in our center during the first and second outbreaks) and one received convalescent plasma. Four over eight patients presented secondary complications during hospitalization including septic shock in one patient, stroke in one, norovirus infection in one, and femoral neck fracture in 1. 6 (50%) patients died. Causes of death were: acute respiratory failure in four patients, myocardial infarction in one, and septic shock in one.
Thus, vaccination did not confer protection against severe disease in this high-risk population.
4. DISCUSSION
We report in this study the humoral response after three injections of BNT162b2 mRNA COVID-19 vaccine in KTRs treated with belatacept. We found that seroconversion rate remained dramatically weak (6.4%) after three injections in patients without prior COVID-19 infection. In contrast, belatacept-treated KTRs with a history of COVID-19 before the vaccine exhibited a strong humoral response and anti-S antibodies titers increased after the vaccine in all patients. Twelve patients developed symptomatic COVID-19 after at least one vaccine injection including 75% of patients requiring hospitalization and 50% of mortality.
We and others6, 7, 8 reported a poor post-vaccinal response after two injections of BNT162b2 mRNA vaccine in KTRs treated with belatacept. Seroconversion rate after the third dose was close to that observed after two injections (6.3% vs. 0%–5.7%) and antibodies titers remained drastically low, suggesting that additional injections would not be beneficial in these patients. Interestingly, Kamar et al.14 reported post-vaccinal humoral responses of 68% after three injections. Among these patients, 12 were treated with belatacept. The proportion of patients given belatacept was higher among non-responders. However, seroconversion rates were higher (seroconversion occurred in 5/12 patients). A possible explanation could be a maintenance immunosuppressive therapy associated with belatacept or the use of belatacept without MPA or steroids. In fact, the intensity of immunosuppression (particularly MPA therapy) has been associated with a lower likelihood of developing post-vaccinal humoral response in previous studies.1 , 5 Of our patients, 71% were treated with MPA and all had steroids in association with belatacept.
This poor humoral response could be explained by several underlying mechanisms: belatacept had been shown to play a direct and active role at several steps of the humoral response by reducing plasmablast differentiation, Ig production, and the expression of the major transcription factor involved in plasma cell function, Blimp-1, in a T cell–independent manner.18 Moreover, belatacept modulates B cell-Tfh crosstalk, leading to an impaired germinal center formation and an improper antibody response in KTRs treated with belatacept.18 , 19 Interestingly, a recent study reporting immune responses to mRNA vaccines, showed that most spike-specific Th cells expressed the coactivating molecule CD28; and could therefore be theoretically be inhibited by belatacept.20
COVID-19 infection has been demonstrated to be more severe in KTRs with increased morbidity and mortality.21, 22, 23 Many studies reported COVID-19 infections following COVID-19 vaccination in KTRs.9, 10, 11, 12 In our cohort, 12 patients developed COVID-19 following at least one vaccine injection and nine patients after ≥2 injections, including severe forms (50% of mortality). Breakthrough infection, defined by Centers for disease Control and Prevention (CDC) as COVID-19 infection ≥14 days postvaccine series completion, then occurred in 5% of belatacept-treated KTRs in our center. This rate was higher compared to breakthrough infections in general population (0.01%) and in solid organ transplants (0.23 to 2.52%).24 , 25 In a cohort of 459 solid organ transplant, Malinis et al.24 reported three patients (0.65%) who developed breakthrough infections. Interestingly, two of them were treated with belatacept. The breakthrough infection rate in our study was comparable to previously reported de novo COVID-19 rates in unvaccinated solid organ transplant patients (5%)26and in COVID-19 rate reported in our center during the first outbreak.27 Additionally, postvaccinal mortality rate was 50% in patients (25% in patients with breakthrough infection). In contrast with previous data that showed a lower mortality rate (9.3%) in breakthrough COVID-19 in comparison with unvaccinated patients,25 vaccination in our belatacept-treated KTRs did not provide protection against severe forms of the disease.
The follow-up was too short after the third dose for analyzing COVID-19 infection rate in KTRs treated with belatacept. Given the persistent weak humoral response after three injections, COVID-19 infection risk is likely to be similar to after two injections. Thus, patients should maintain barrier measures.
In addition to the different strategies aiming to increase vaccinal response to COVID-19 vaccine in KTRs (as the use of a third dose or increasing vaccine dose), a complementary reflection about the degree and type of immunosuppressive therapy during COVID-19 vaccination is needed. Interestingly, mTOR inhibition may enhance vaccine responses, particularly in old patients. A recent study demonstrated that activation of mTORC1 at late endosomes misdirects T cell fate decision in older individuals but not in young individuals.28 mTORC1 inhibition by low dose of rapamycin produced an enhanced primary and memory recall CD8+ T cell response in mice and promoted follicular helper cell over T helper cell 1 generation.28, 29, 30
In our study, patients treated with mTORi in association with belatacept did not exhibit post-vaccinal humoral responses. Thus, in some selected KTRs, a strategy evaluating the effectiveness on vaccinal responses of a planned temporary belatacept interruption during the vaccination period with a replacement by mTORi-based therapy, could be investigated. The benefit-risk balance of this strategy should be assessed for each patient and a close follow-up is warranted during this period. Dose of steroids and MPA could also be reduced during this period for some low immunological risk patients.
In addition to vaccination, other preventive strategies aiming to improve patient’s protection should be evaluated. Monoclonal antibodies might provide an alternative tool for the prevention of COVID-19 in KTRs. Their use as a preventive strategy in patients who failed to develop post-vaccinal responses needs to be assessed.
Conversely, the humoral response was strongly boosted in KTRs treated with belatacept with prior COVID-19 infection suggesting that vaccination strategy should be different in this particular category of belatacept-treated KTRs and two vaccine doses are probably sufficient to boost their immunity. Interestingly, immunosuppression was reduced during COVID-19 infection (including belatacept withdrawal), which may lead to the development of a stronger natural immunity to SARS-CoV-2 infection.
The main limitations of our study are the lack of data about the cellular response after the vaccine. The weak immune response did not allow us to analyze independent risk factors of non-response in these patients. As we performed an unmatched analysis between belatacept and non-belatacept-treated patients, we cannot totally rule out that KTRs treated with belatacept may present intrinsic differences that could impact the vaccinal response. Also, there is a small number of patients in the group of KTRs with prior COVID-19 infection. However, all patients had a similar post-vaccinal response with increasing antibodies titers after vaccination. The specificity and sensitivity of the Abbott serological assay refer to the diagnosis of past COVID-19 infection, not vaccine response. Finally, the short follow-up after the third dose did not allow us to assess the occurrence of symptomatic COVID-19 in these patients.
5. CONCLUSION
In conclusion, the administration of the third dose of BNT162b2 mRNA COVID-19 vaccine did not improve immunogenicity in KTRs treated with belatacept without prior COVID-19 infection. Other strategies aiming to improve patient’s protection are still needed. Barrier measures and indirect protection as vaccination of household members and caregivers of these patients are crucial. In case of prior COVID-19 infection, humoral response to the vaccine is effective. COVID-19 vaccination is then strongly encouraged in this population to enhance natural immunity to SARS-CoV-2.
ACKNOWLEDGMENTS
We thank Guillaume Monge and Laurene Cachera for their technical assistance.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Drs Chavarot and Morel contributed equally to this article.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27(8) doi: 10.1016/j.cmi.2021.04.028. 1173.e1-1173.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498–1500. doi: 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavarot N, Ouedrani A, Marion O, et al. Poor Anti-SARS-CoV-2 humoral and T-cell responses after 2 injections of mRNA vaccine in kidney transplant recipients treated with belatacept. Transplantation. 2021;105(9):e94–e95. doi: 10.1097/TP.0000000000003784. [DOI] [PubMed] [Google Scholar]
- 7.Ou MT, Boyarsky BJ, Motter JD, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021. 10.1097/TP.0000000000003780 [DOI] [PMC free article] [PubMed]
- 8.Bertrand D, Hamzaoui M, Lemée V, et al. Antibody and T cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J Am Soc Nephrol. 2021. 10.1681/ASN.2021040480 [DOI] [PMC free article] [PubMed]
- 9.Caillard S, Chavarot N, Bertrand D, et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100(2):477–479. doi: 10.1016/j.kint.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali NM, Alnazari N, Mehta SA, et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation. 2021;105(9):e104–e106. doi: 10.1097/TP.0000000000003836. [DOI] [PubMed] [Google Scholar]
- 11.Tsalouchos A, Rossolini GM, Maggi L, Mazzoni A, Annunziato F, Dattolo PC. COVID-19 in a kidney transplant recipient after mRNA-based SARS-CoV-2 vaccination. Transpl Infect Dis. 2021. 10.1111/tid.13649 [DOI] [PMC free article] [PubMed]
- 12.Tsapepas D, Paget K, Mohan S, Cohen DJ, Husain SA. Clinically significant COVID-19 following SARS-CoV-2 vaccination in kidney transplant recipients. Am J Kidney Dis. 2021;78(2):314–317. doi: 10.1053/j.ajkd.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DGS-Urgent. Vaccins contre la Covid-19: modalites d’administration des rappels. https://www.mesvaccins.net/textes/dgs_urgent_n43_vaccination_modalites_d_administration_des_rappels.pdf. Published 2021. Accessed November 4, 2021.
- 14.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27(6):981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gäckler A, Kaulfuß M, Rohn H, et al. Failure of first meningococcal vaccination in patients with atypical haemolytic uraemic syndrome treated with eculizumab. Nephrol Dial Transplant. 2020;35(2):298–303. doi: 10.1093/ndt/gfy225. [DOI] [PubMed] [Google Scholar]
- 17.Harritshøj LH, Gybel-Brask M, Afzal S, et al. Comparison of sixteen serological SARS-CoV-2 immunoassays in sixteen clinical laboratories. J Clin Microbiol. 2021. 10.1128/JCM.02596-20 [DOI] [PMC free article] [PubMed]
- 18.Leibler C, Thiolat A, Hénique C, et al. Control of humoral response in renal transplantation by belatacept depends on a direct effect on B cells and impaired T follicular helper-B cell crosstalk. J Am Soc Nephrol. 2018;29(3):1049–1062. doi: 10.1681/ASN.2017060679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Yin H, Xu J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig: endogenous alloreactive B cell responses. Am J Transplant. 2013;13(9):2280–2292. doi: 10.1111/ajt.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sattler A, Angermair S, Stockmann H, et al. SARS–CoV-2–specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest. 2020;130(12):6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21(3):1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavarot N, Gueguen J, Bonnet G, et al. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2021;21(3):1285–1294. doi: 10.1111/ajt.16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toapanta N, Torres IB, Sellarés J, Chamoun B, Serón D, Moreso F. Kidney transplantation and COVID-19 renal and patient prognosis. Clin Kidney J. 2021;14(Suppl 1):i21–i29. doi: 10.1093/ckj/sfab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malinis M, Cohen E, Azar MM. Effectiveness of SARS-CoV-2 vaccination in fully vaccinated solid organ transplant recipients. Am J Transplant. 2021;21(8):2916–2918. doi: 10.1111/ajt.16713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin CX, Moore LW, Anjan S, et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021. 10.1097/TP.0000000000003907 [DOI] [PMC free article] [PubMed]
- 26.Elias M, Pievani D, Randoux C, et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. J Am Soc Nephrol. 2020;31(10):2413–2423. doi: 10.1681/ASN.2020050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavarot N, Fournier C, Burger C, Amrouche L, Legendre C. COVID-19: a one-center experience in Paris. Transplant Direct. 2021;7(1):e647. doi: 10.1097/TXD.0000000000001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin J, Kim C, Xia Q, et al. Activation of mTORC1 at late endosomes misdirects T cell fate decision in older individuals. Sci Immunol. 2021;6(60):eabg0791. doi: 10.1126/sciimmunol.abg0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araki K, Turner AP, Shaffer VO, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray JP, Staron MM, Shyer JA, et al. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T Helper 1 and follicular B helper T cells. Immunity. 2015;43(4):690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.