Abstract
Background
During acute respiratory distress syndrome, proinflammatory mediators inhibit natural anticoagulant factors, which alter the normal balance between coagulation and fibrinolysis leading to a procoagulant state. We hypothesize that pulmonary administration of anticoagulants might be beneficial to treat acute respiratory distress syndrome for their anticoagulant and antiinflammatory effects and reduce the risk of systemic bleeding.
Objectives
Our aim is to study the effects of nebulized antithrombin (AT) and combined AT and heparin in an animal model of acute lung injury.
Methods
Acute lung injury was induced in rats by the intratracheal administration of hydrochloric acid and lipopolysaccharide. AT alone (500 IU/kg body weight) or combined with heparin (1000 IU/kg body weight) were nebulized after the injury. Control groups received saline instead. Blood, lung tissue, bronchoalveolar lavage, and alveolar macrophages (AM) isolated from bronchoalveolar lavage were collected after 48 hours and analyzed.
Results
Nebulized anticoagulant treatments reduced protein concentration in the lungs and decreased injury‐mediated coagulation factors (tissue factor, plasminogen activator inhibitor‐1, plasminogen, and fibrinogen degradation product) and inflammation (tumor necrosis factor α and interleukin 1β) in the alveolar space without affecting systemic coagulation and no bleeding. AT alone reduced fibrin deposition and edema in the lungs. Heparin did not potentiate AT coagulant effect but promoted the reduction of macrophages infiltration into the alveolar compartment. Anticoagulants reduced nuclear factor‐kB downstream effectors in AM.
Conclusions
Nebulized AT and heparin attenuate lung injury through decreasing coagulation and inflammation without altering systemic coagulation and no bleeding. However, combined AT and heparin did not produce a synergistic effect.
Keywords: acute lung injury, antithrombin, heparin, inflammation, nebulization
Essentials
-
•
In acute respiratory distress syndrome inflammation and coagulation are disrupted.
-
•
We studied the effects of nebulized antithrombin and heparin in a rat model of acute lung injury.
-
•
Anticoagulants restored coagulation‐inflammation balance.
-
•
Local treatment with anticoagulants did not alter systemic coagulation.
Alt-text: Unlabelled Box
1. INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a condition characterized by a diffuse, intense inflammatory process and damage to both endothelial and epithelial cell barriers, resulting in marked extravasation of vascular fluid and entrance of neutrophils and monocytes in the alveolar space.1., 2. Moreover, endothelial and epithelial damage causes an increase of tissue factor (TF) expression, the first initiator of extrinsic coagulation cascade, and leads to a procoagulant state. This, together with an increase of plasminogen activator inhibitor (PAI‐1) and the reduction of fibrinolysis leads to the accumulation of fibrin deposition in the airspaces that potentiates inflammation and lung damage.3 An effective pharmacological therapy for ARDS is not yet available.2
In experimental acute lung injury (ALI) models and in ARDS patients, the beneficial effects of systemic anticoagulants were outweighed by systemic bleeding.4., 5. Preclinical and clinical studies found that local administration of nebulized anticoagulants improved pulmonary coagulopathy in ALI/ARDS, reducing the risk of systemic bleeding.6., 7., 8., 9.
Antithrombin (AT) has an important role in both anticoagulant and antiinflammatory pathways.10., 11., 12. Regarding its anticoagulant effects, AT neutralizes the activity of thrombin, factor Xa and other serine proteases by forming a complex between enzyme and inhibitor.13., 14. In the case of thrombin inhibition, the complex formed is known as thrombin‐antithrombin. This binding occurs at a relatively slow rate in the absence of its cofactor heparin, but is dramatically enhanced in its presence.13 In animal models of pulmonary infection and lung injury, nebulized AT was able to reduce pulmonary coagulation.15
Coagulation factors also promote the production of inflammatory cytokines such as interleukin 1β (IL‐1β), tumor necrosis factor α (TNF‐α) and IL‐6, by binding to protease‐activated receptors found in endothelial cells, mononuclear cells, fibroblasts, platelets, and smooth muscle cells.16., 17. Hence the inhibition of coagulation factors by AT results in a decrease of inflammatory cytokines secretion.12 In addition, AT has a coagulation‐independent antiinflammatory effect by binding to syndecan‐4 (a heparan sulfate proteoglycan, member of the syndecan family), which diminishes the release of reactive oxygen species or proteases by neutrophils.10 Moreover, the binding between AT and syndecan‐4 promotes the inhibition of nuclear factor‐kB (NF‐kB) in endothelial cells, which block the synthesis of proinflammatory cytokines.18., 19., 20., 21. Some studies showed that this independent effect is inhibited by heparin because heparin competes with syndecan‐4 to bind to AT because they share the same binding site.18., 22. However, recently it has been described that heparin also has antiinflammatory effects independent of its effect on AT.23
Additionally, it is well known that the deregulation of coagulation and inflammatory pathways lead to increased permeability and edema, another characteristic of ARDS.24., 25., 26. AT might enhance the alveolar epithelium restoration through its actions on the coagulant and the inflammatory cascades.
Based on previous observation that heparin enhances AT activity, we hypothesized that the local coadministration of both anticoagulants, AT and heparin, into the lung may perform a synergistic effect that will enhance the anticoagulant and antiinflammatory activities of both drugs and promote epithelial barrier restoration, improving ALI without causing systemic bleeding. The aim of this study is to analyze the effects of nebulized AT and the combination of AT and heparin in a rat model of ALI at 48 hours.
2. MATERIAL AND METHODS
2.1. Animals
Adult male Sprague‐Dawley rats (Charles River, Chatillon‐sur‐Chalaronne, France) initially weighing ~300 g were used as subjects. Animals were housed in pairs in clear filtered plastic cages deposited in ventilated racks. They were kept under controlled environmental conditions: temperature 23°C, 60% relative humidity, and reversed 12:12 hour light‐dark cycle. Food (A04 Scientific Animal Food & Engineering, Panlab) and tap water were available ad libitum. Enrichment material was placed inside the cages. The study was approved by the Animal Research Ethics Committee of Autonomous University of Barcelona and the Animal Experimentation Committee of Generalitat de Catalunya.
2.2. Experimental groups
Rats were randomly assigned into six experimental groups.
-
•
Control (n = 8): Saline intratracheal (IT) instillation at 0 and 2 hours followed by saline nebulization at 4, 12, and 28 hours.
-
•
Control + AT (n = 9): Saline IT instillation as in control group followed by an AT nebulization at 4 and 28 hours and a saline nebulization at 12 hours.
-
•
Control + AT+ heparin (n = 10): Saline IT instillation as in control group followed by a nebulization of AT combined with heparin at 4 and 28 hours and an extra dose of nebulized heparin at 12 hours.
-
•
HCl/LPS (n = 12): Hydrochloric acid (HCl) and lipopolysaccharide (LPS) IT instillation at 0 and 2 hours, respectively. Saline nebulization at 4, 12, and 28 hours after the first instillation.
-
•
HCl/LPS + AT (n = 12): HCl and LPS administration as in HCl/LPS group followed by an AT nebulization at 4 and 28 hours and a saline nebulization at 12 hours.
-
•
HCl/LPS + AT + heparin (n = 10): HCl and LPS administration as in HCl/LPS group and a nebulization of AT combined with heparin at 4 and 28 hours and an extra dose of nebulized heparin at 12 hours.
All animals were sacrificed at the same time, 48 hours after starting any treatment (Figure S1).
2.3. ALI induction
The current method of ALI induction was first established by our group.27 Briefly, ALI was induced in the corresponding experimental groups by the IT instillation of 300 μL of HCl (0.1 mol/L, pH = 1.3). According with the model of our group, 2 hours after HCl administration animals received an IT instillation of 500 μL of LPS from Escherichia coli 055:B5 (30 μg/g body weight) (Sigma Chemical) to prolong and enhance the damage.27 Control groups received saline solution (0.9% NaCl) instead. For this procedure, rats were slightly sedated with sevoflurane. Animals were weighed and supervised throughout all the experiment.
2.4. Nebulization
AT (Anbinex Grífols S.A.) and heparin (unfractionated heparin, Hospira Products Farmac) were used for this experiment. Both anticoagulants have been previously used in rat models with positive results, indicating that there is an interaction between them and the coagulation cascade of the animals.21., 28. For the nebulization of AT and heparin the AeronebPro nebulizer system (Aerogen Limited) was used. This system was connected to restraint tubes where animals were confined, allowing direct exposure of the nebulized agents to the rats. The oxygen flow used for the nebulization was constant at 2 L/min. Animals treated with AT alone received two doses of nebulized AT (500 IU/kg body weight) 4 and 28 hours after HCl or saline instillation. Animals treated simultaneously with AT and heparin received two doses of AT (500 IU/kg body weight) combined with heparin (1000 IU/kg body weight) 4 and 28 hours after the first instillation and an extra dose of heparin alone 12 hours after the initialization of the experiment because of the shorter half‐time life of this anticoagulant. Nontreated groups received nebulized saline instead. In all cases, the total volume of each nebulization was 700 μL/animal. Timing and doses were based on previous studies and bibliography.15., 28.
2.5. Tissue obtaining
Forty‐eight hours after the administration of HCl or saline, animals were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) and sacrificed via the exsanguination of the abdominal aorta artery. A small quantity of the obtained blood was used for gasometry (epoc Blood Analysis System, Alere Healthcare) and the rest was collected in citrate tubes (9NC coagulation sodium citrate 3.2% tube, Vacuette, Greiner‐Bio One) for plasma isolation and activated partial thromboplastin time (APTT) and prothrombin time (PT) measurements. APTT evaluates the integrity of the intrinsic coagulation pathway and PT determines the integrity of the extrinsic coagulation pathway. Both tests also assess factors of the common pathway of the coagulation cascade. APTT was determined using Pathromtin SL reagent (Siemens Healthcare Diagnosis Products GmbH) and PT was measured using Thromborel S reagent (Siemens Healthcare Diagnosis Products GmbH); both tests were performed by Echevarne Laboratories, Barcelona, Spain. Lungs were removed and weighed. The unilobular lung was either used for bronchoalveolar lavage (BAL) or histology and immunohistochemistry (IHC), whereas the multilobular lung was used for lung tissue analysis. The exact number of animals used for each analysis is indicated in the figure legends. Numbers were randomly assigned to each animal and the samples; hence, researchers were blinded while performing the analysis from the different experimental group at which each animal was assigned. Nevertheless, treatment administration was not blinded because the researcher prepared and nebulized the treatment to the animals.
2.6. Obtaining and processing BAL
BAL was obtained from the unilobular lung. The left bronchus was tied and a cut was performed under the ligation to remove the multilobular lung from the rest of the lung. To carry out the lavage, a syringe was connected to a cannula placed into the trachea and 5 mL of saline (0.9% NaCl) were gently flushed through the lung five times. Total cell numbers in BAL were counted using a hemocytometer (Neubauer, Marienfeld). To determine the proportion of each cellular type in lavage fluids, slides with 200 000 cells were prepared by cytocentrifugation (Shandon Cytospin 4, Thermo Electron Corporation) and Diff‐Quick staining performed (Panreac Quimica SAU). The rest of the BAL was centrifuged at 800× g for 10 minutes and the supernatant was stored at −80°C for further analysis. To isolate the alveolar macrophages (AM), the pellet was restored and cultivated in Petri dishes with RPMI 1640 medium (supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin [Gibco Big Cabin]) at 37°C for 1 hour to let the AM adhere. Once attached, AM were collected using TRIzol reagent (Thermo Fisher Scientific). Purity was checked by Diff‐Quick staining. AM were stored at −80°C for subsequent analysis.
2.7. Gene expression, cytokine and protein measurements
mRNA from lung tissue homogenate and AM was extracted using chloroform‐isopropanol isolation. Spectrophotometer ND‐1000 (Nanodrop, Thermo Fisher Scientific) was used to assess mRNA purity. mRNA was reverse‐transcribed into cDNA, and this was amplified using one‐step real‐time quantitative PCR (RT‐qPCR) system (7500 Real‐Time PCR System, Applied Biosystems, Thermo Fisher Scientific) using SYBR green (Kapa Biosystems, Merck Millipore) and the rat primers of the genes of interest (Table 1 ). The expression of all the following markers was analyzed in lung tissue using ΔΔCt method and GAPDH as housekeeping.
Table 1.
Rat primers used for real‐time polymerase chain reaction
| Gene | Functions | Forward Primer | Reverse Primer |
|---|---|---|---|
| GAPDH | Housekeeping | 5′CTGTGTCTTTCCGCTGTTTTC3′ | 5′TGTGCTGTGCTTATGGTCTCA3′ |
| IL1β | Pro‐inflammatory | 5′AAAAATGCCTCGTGCTGTCT3′ | 5′TCGTTGCTTGTCTCTCCTTG3′ |
| TNFα | Pro‐inflammatory | 5′AACTCCCAGAAAAGCAAGCA3′ | 5′CGAGCAGGAATGAGAAGAGG3′ |
| iNOS | Pro‐inflammatory | 5′CTTGGAGCGAGTTGTGGATT3′ | 5′GGTGGGAGGGGTAGTGATG3′ |
| Arg‐I | Anti‐inflammatory | 5′GGGAAGACACCAGAGGAGGT3′ | 5′TGATGCCCCAGATGACTTTT3′ |
| CCL2 | Macrophage recruitment | 5′GCTGCTACTCATTCACTGGC3′ | 5′GGTGCTGAAGTCCTTAGGGT3′ |
| CXCL1 | Neutrophil recruitment | 5′CCACACTCAAGAATGGTCGC3′ | 5′GTTGTCAGAAGCCAGCGTTC3′ |
| TF | Pro‐coagulation | 5′ACAATCTTGGAGTGGCAACC3′ | 5′TGGGACAGATAGGACCCTTG3′ |
| PAI‐1 | Pro‐coagulation | 5′AGGGGCAGCAGATAGACAGA3′ | 5′CACAGGGAGACCCAGGATAA3′ |
| Plasminogen | Anti‐coagulation | 5′AAACGAAAGGGACTCCAGGT3′ | 5′ TCTCGAAGCAAACCAGAGGT3′ |
Protein from the lung tissue homogenate was extracted using a protease inhibitor cocktail (Roche, Merck Millipore) mixed with orthovanadate sodic (1 mmol/L) and lysis buffer solution (25 mmol/L Tris‐HCl, pH 7.6, 150 mmol/L NaCl, 1% NP‐40, 1% sodium deoxycholate, 0.1% SDS). IL‐1β and TNF‐α were determined by multiplex assays following the manufacturer’s protocol (Luminex, Merck Millipore). Total protein concentration in BAL and lung homogenate was determined by the bicinchoninic acid method (Pierce, Thermo Scientific). TF (Cloud‐Clone), PAI‐1 (LifeSpan BioScience), and Fibrinogen Degradation Product (FDP; Cloud‐Clone) were measured in BAL using ELISAs.
2.8. Histopathology and IHC
Lungs used for histology and IHC examinations were slowly perfused with a fixative solution of 4% paraformaldehyde. Sections of 4 μm in thickness were stained with hematoxylin and eosin and evaluated under light microscopy using a Nikon Eclipse Ti microscope and ImageJ software (ImageJ 1.40 g; W. Rasband). They were analyzed by three independent investigators blinded to the treatment. Inflammation and damage were scored as follows: normal lung (0), hemorrhage (0‐1), peribronchial infiltration (0‐1), pulmonary edema (0‐2), alveolar hyperplasia (0‐3), and intra‐alveolar infiltration (0‐3). The total histopathology score was the sum of the scores of each variable, which were also separately evaluated. For IHC, a polyclonal rabbit anti‐human fibrinogen/fibrin antibody (1:6000; Abcam) followed by OmniMap anti‐Rabbit HRP (Roche, Merck Millipore) were used. Isotype and negative control were performed with Rabbit IgG polyclonal antibody (Abcam) and the omission of the primary antibody, respectively. Entire sections were digitized with NanoZoomer (Hamamatsu photonics) and fibrinogen/fibrin positive areas were quantified by ImageJ software (ImageJ 1.40g; W. Rasband) and expressed as a percentage of the total lung.
2.9. Statistical analysis
A power analysis using the Gpower computer program (Faul & Erdfelder, 1998) previously to start the experiment indicated that a total sample of 48 animals (eight animals/group) would be needed to detect large effects (0.5) with 80% power using an analysis of variance among factors with alpha at 0.05 at the end of the experiment. The sample size setting was also chosen based on similar studies by our group and other research groups.15., 29. Moreover, to obtain between 8 and 12 animals/group at the end of the experiment (48 hours), we used more animals because of the mortality of the injured groups (10%‐15%). In addition, different animals were used for histology studies and for BAL, macrophage activation, and cytokine lung measures. In the legend of each figure is the number of animals included in each analysis.
Gaussian distribution was tested applying by D’Agostino Pearson omnibus or Shapiro Wilk normality test. Possible outliers were determined by Tukey’s method (Q level of 5%). A two‐tailed analysis of variance was used to make comparisons among the experimental groups followed by a Bonferroni selected pair comparison post hoc test. Data were reported as mean ± standard error of the mean. Statistical analysis was performed with Prism 8.0 (GraphPad Software Inc.) and P ≤ 0.05 was considered statistically significant.
3. RESULTS
3.1. Acute lung injury model
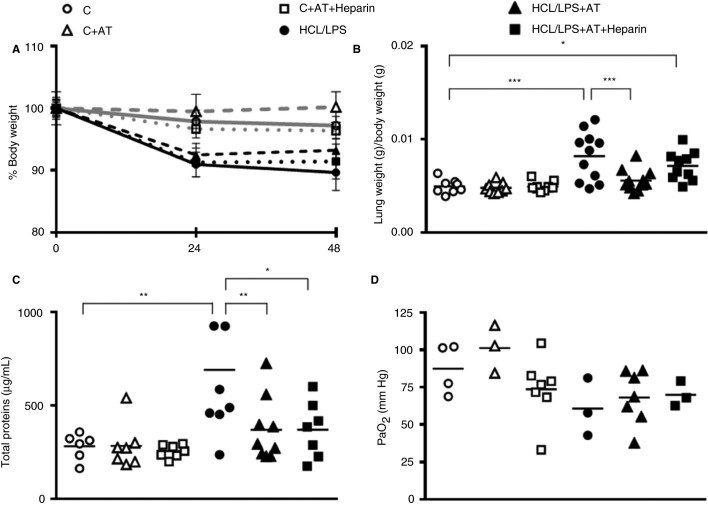
Total body weight of the different groups varied throughout the experiment. Although differences among groups did not reach statistical significance, HCl/LPS injured animals lost more body weight compared with control groups (Figure 1 A).
Figure 1.
Body weight analysis and alteration of the alveolar capillary barrier. (A) Comparison between groups of rat body weight at 24 and 48 hours. Values represent body weight percentage calculated in relation to baseline levels (n = 8‐12). (B) Lung/body weight ratio 48 hours after injury (n = 8‐11). (C) Protein concentration in bronchoalveolar lavage (BAL) at 48 hours (n = 6‐9). (D) Partial arterial oxygen pressure (PaO2) 48 hours after the injury (n = 3‐7). Data represent mean ± standard error of the mean (SEM). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001
In the HCl/LPS group, 92.3% of the animals survived (1 of 13 animals died). In the ALI group treated with AT alone, survival was also of 92.3% (1 of 13 animals died). However, in the ALI group treated with AT combined with heparin survival was lower, of 76.9% (3 of 13 animals died) (data not shown).
Nonsignificant differences were detected among groups in partial arterial oxygen pressure (Figure 1D).
3.2. Systemic coagulation alterations
The measure of aPTT and PT did not reveal systemic coagulation alterations in any treated group at 48 hours (Table S1). Moreover, no hemorrhage was found at a macroscopic level: neither at the abdominal cavity nor other organs, which indicates the absence of systemic bleeding.
3.3. Alveolar capillary barrier permeability
Regarding the lung weight, treatment with AT alone but not combined with heparin was able to significantly reduce the lung/body weight ratio, as a damage marker, which was increased in HCl/LPS group, returning it to control levels (Figure 1B). According to lung/body weight ratio, the protein concentration in the BAL increased in HCl/LPS group when compared with control group, and was decreased to control levels in ALI groups treated with AT alone and AT combined with heparin (Figure 1C).
3.4. Pulmonary coagulation and fibrinogen/fibrin deposition
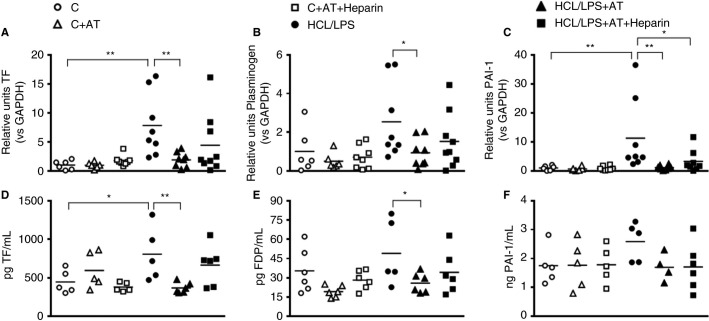
The effect of AT alone or combined with heparin on the expression of coagulation markers was assessed in lung tissue at 48 hours. The expression of TF significantly increased in HCl/LPS group. Nebulized treatment with AT alone but not with heparin was able to drop TF and plasminogen expression compared with the HCl/LPS group, and returned them to control group levels (Figures 2 A,B). In the case of PAI‐1, both treatments attenuated HCl/LPS effect and reduced PAI‐1 expression to control levels (Figure 2C).
Figure 2.
Coagulation factors levels of the experimental groups: RT‐qPCR analyses of (A) tissue factor (TF), (B) plasminogen ,and (C) plasminogen activator inhibitor (PAI‐1) in lung tissue homogenate at 48 hours after the induction of the injury. RNA levels shown in the graphs are expressed in relation to GAPDH expression (n = 6‐9). Protein levels in bronchoalveolar lavage (BAL) of (D) TF, (E) fibrinogen degradation product (FDP), and (F) PAI‐1 (n = 4‐6). Data represent mean ± SEM. *P ≤ 0.05, **P ≤ 0.01
Coagulation proteins concentrations were measured in BAL 48 hours after ALI induction. AT treatment significantly reduced TF and FDP levels compared with HCl/LPS group. Heparin did not potentiate the anticoagulant effect (Figures 2D,E). Levels of PAI‐1 did not significantly differ among groups although its concentration seemed to follow the same pattern as that found in mRNA expression in lung tissue (Figure 2F).
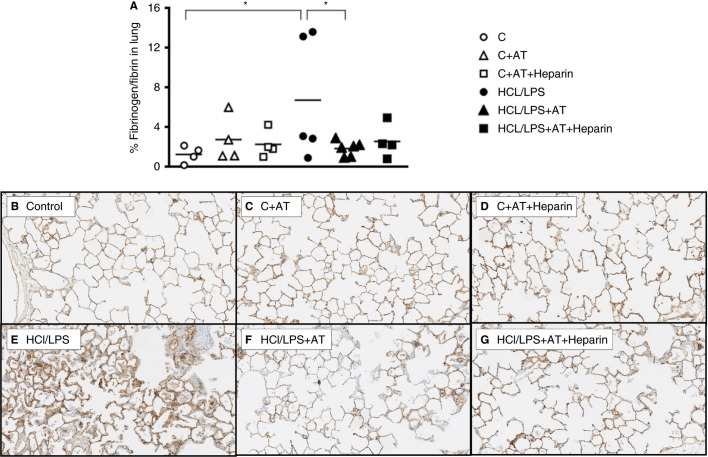
Fibrinogen/fibrin‐positive areas were found in the HCl/LPS group. AT treatment significantly decreased the presence of fibrinogen/fibrin, whereas the reduction of AT combined with heparin was nonsignificant (Figures 3 A‐G).
Figure 3.
Fibrinogen/fibrin deposition in the lung: immunohistochemistry of fibrinogen/fibrin‐stained sections in the lung. (A) Fibrinogen/fibrin positive areas 48 hours after induction of the injury (n = 4‐6). (B‐G) Representative images of fibrinogen/fibrin‐stained lung tissue sections, 100× amplification. Data represent mean ± SEM. *P ≤ 0.05
3.5. Effects on the infiltration of inflammatory cells in BAL and histology
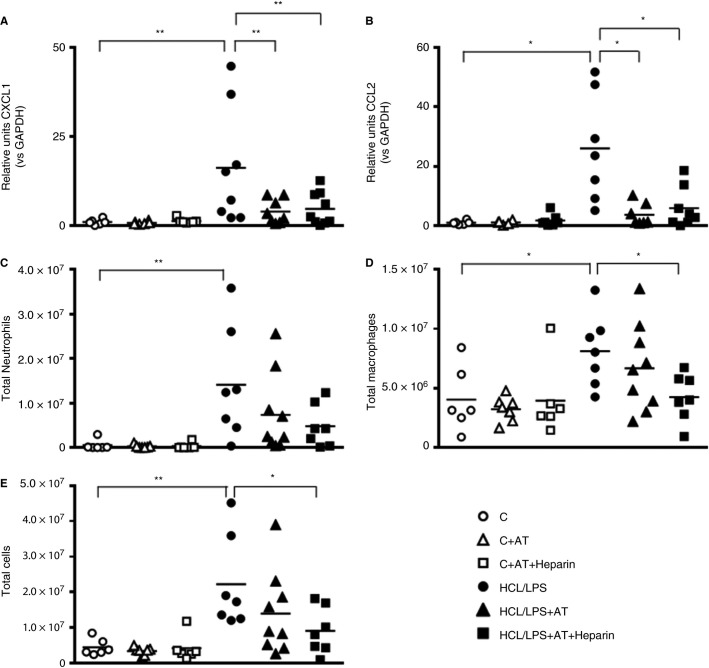
Forty‐eight hours after the induction of the injury, mRNA levels of chemoattractant cytokines of neutrophils (CXCL1) and monocytes (CCL2) in lung tissue were increased in the HCl/LPS group compared with the control group. Both AT and AT combined with heparin reversed these effects, returning CXCL1 and CCL2 mRNA expression to control levels (Figures 4 A,B). Regarding cellular numbers, there was an inflammatory cell infiltration in BAL; higher numbers of neutrophils and macrophages were found in the HCl/LPS group (Figures 4C‐E). AT combined with heparin treatment significantly reduced macrophage numbers and the total number of cells in BAL.
Figure 4.
Analyses of bronchoalveolar lavage (BAL) infiltration: RT‐qPCR analyses of (A) CXCL‐1 and (B) CCL2 chemoattractant chemokines in lung tissue 48 hours after damage (n = 6‐9). (C) Absolute neutrophil, (D) macrophages, and (E) total cell counts in the BAL of rats 48 hours after the induction of the injury (n = 6‐9). Data represent mean ± SEM. *P ≤ 0.05, **P ≤ 0.01
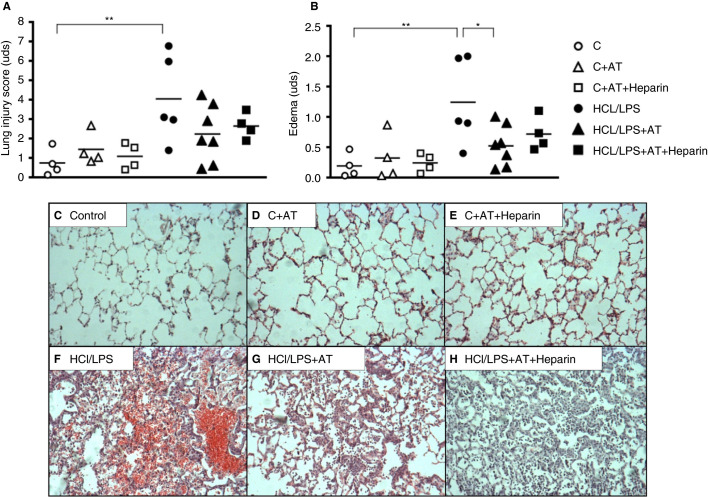
From a histological perspective, lung damage caused by the instillation of HCl and LPS was evidenced (with an increase in all the following lung injury markers: hemorrhage, pulmonary edema, alveolar pneumocyte hyperplasia, peribronchial and intraalveolar infiltration); each variable and the sum of the total histopathology score were increased (Figures 5 A,B). AT alone significantly reduced pulmonary edema of the histopathological score but not other variables (Figures 5B). However, no statistically differences were found when administering AT combined with heparin in any of the evaluated parameters. No bleeding was detected in the presence of anticoagulants in the control treated groups and nonadditional bleeding was found in injured treated groups. In fact, hemorrhage seemed to decrease in injured treated groups. Figure 5A‐H represents the lung histological damage 48 hours after inducing ALI through HCl/LPS instillation and the effect of anticoagulants treatment.
Figure 5.
Lung injury score and lung specimens. (A) Lung injury score 48 hours after induction of the injury (n = 4‐7). (B) Edema 48 hours after lung injury. (C‐H) Representative images of lung tissue sections stained with hematoxylin‐eosin, 100× amplification. Data represent mean ± SEM. *P ≤ 0.05, **P ≤ 0.01
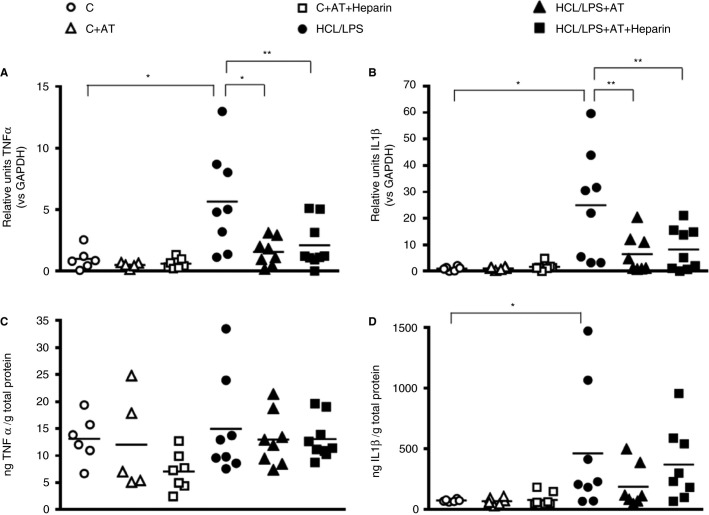
3.6. Pulmonary inflammatory response
In lung homogenates, both nebulized treatments, AT alone and AT combined with heparin, significantly decreased mRNA expression of TNF‐α and IL‐1β increased in the HCl/LPS group (Figures 6 A,B) at 48 hours. When looking at protein concentrations, no significant differences were observed in TNF‐α levels among groups (Figure 6C). Regarding IL‐1β, anticoagulants did not reduce its concentration, which was increased by HCl/LPS administration. Nevertheless, the results showed the same pattern than mRNA expression (Figure 6D).
Figure 6.
Inflammatory response. RT‐qPCR analyses of (A) TNF‐α and (B) IL‐1β in lung tissue homogenate (n = 5‐9). RNA levels shown in the graphs are expressed in relation to GAPDH expression and protein levels are corrected for the total protein concentration in lung tissue. Analyses were performed at 48 hours after the induction of the injury. Data represent mean ± SEM. *P ≤ 0.05, **P ≤ 0.01
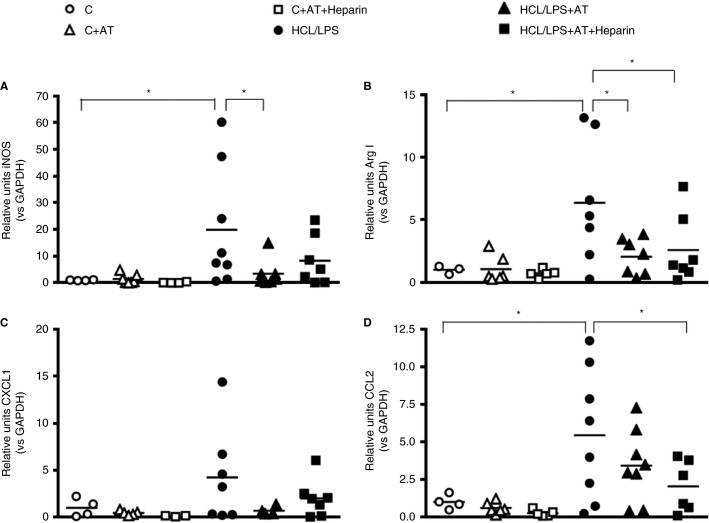
3.7. Alveolar macrophages response
Proinflammatory (inducible nitric oxide synthase [iNOS]) and anti‐inflammatory (Arginase‐I [Arg‐I]) expression were analyzed in macrophages obtained from the BAL of the animals 48 hours after the induction of ALI. iNOS levels were significantly reduced by AT treatment alone compared with HCl/LPS group (Figure 7 A), whereas both treatments were able to decrease Arg‐I levels, which were increased in HCl/LPS group (Figure 7B). CXCL1 and CCL2 expression were also analyzed in macrophages from BAL. Only when administered simultaneously AT and heparin the expression of CCL2 was significantly reduced (Figure 7D). However, a nonsignificant reduction was observed in CXCL1 when treated with AT alone and AT combined with heparin and in CCL2 when treated with AT alone (Figures 7C,D).
Figure 7.
Effect of anticoagulant treatments on alveolar macrophages: RT‐qPCR analyses of (A) iNOS, (B) Arg‐I, (C) CXCL1, and (D) CCL2 in alveolar macrophages of BAL 48 hours after the induction of the injury (n = 3‐7). RNA levels shown in the graphs are expressed in relation to GAPDH expression. Data represent mean ± SEM. *P ≤ 0.05
4. DISCUSSION
In this rat model of HCl/LPS‐induced ALI, we tried to elucidate the potential benefits of the local use of a combination of the two most common anticoagulants for ARDS treatment. Nebulized AT decreased lung injury by reducing lung permeability, edema, cellular infiltration, coagulation, and inflammation markers and fibrin deposition in the lungs. No synergic effect was observed when combining nebulized AT with heparin in coagulation or inflammation. Nevertheless, nebulization of both anticoagulants together potentiated the reduction of macrophages infiltration into the alveolar compartment.
The systemic administration of anticoagulants as a new treatment for ARDS has been previously evaluated in clinical studies and, although positive effects were observed, these were eclipsed by the presence of systemic bleeding.4., 5. Nevertheless, although systemic AT did not show increased bleeding in preclinical models, intravenous heparin did affect systemic coagulation.30., 31. The use of nebulized anticoagulants allows targeting the lungs reducing the major problem of systemic bleeding and allowing higher dosages.7 In a model of endotoxemia, nebulized heparin affected systemic coagulation without producing systemic bleeding,32 but in other studies neither nebulized heparin nor AT produced systemic effects.15., 33. Herein we focused on the activities of AT and whether they are increased by the combination of heparin. We did not perform a group with heparin alone because in a previous study our group analyzed the effects of nebulized heparin in a 24‐hour model of LPS‐induced ALI and found that heparin reduced pulmonary coagulation and inflammation without producing systemic bleeding.28 Our results suggest that the local administration of AT and AT combined with heparin by nebulization is safe and effective because the activities of both treatments were focused on the pulmonary compartment and no systemic alterations were observed.
One of the hallmarks of ARDS is the disruption of the alveolar capillary barrier, which increases the permeability in the lung, leading to protein‐rich edema and the presence of proinflammatory cells in the alveolar compartment.26 In our ALI model, although only AT alone was able to decrease lung weight and edema, nebulized AT alone as well as combined with heparin reduced protein concentration and cellular infiltration into the alveolar space. These results indicate a decrease of lung permeability that could be explained by the partial reestablishment of the alveolar capillary barrier being consistent with previous studies that showed the capacity of anticoagulants to reduce lung permeability.25., 28., 34., 35. Also, when we determined the histological damage analyzing the hallmarks of ALI, both treated groups with anticoagulants slightly improved the lesion although it was nonsignificant. Our ALI model was proved to last at least for 72 hours; 48 hours was most likely too short time to find a more pronounced difference among groups.27
The current study reveals that the local treatments with AT and AT combined with heparin have a beneficial effect attenuating pulmonary coagulopathy and fibrin deposition in the lungs. AT reduced the mRNA expression of key factors of the coagulation cascade such as TF, PAI‐1 and plasminogen, while AT combined with heparin only decreased PAI‐1. The same pattern was observed at protein level, with a significant reduction of TF and FDP when administering AT. A higher effect of anticoagulants at mRNA level may be due to the fact that mRNA goes through posttranscriptional modifications before being translated into protein, thus there is not a perfect correlation between mRNA expression and protein concentration.36 In addition, the sum of small alterations in the amount of a protein of the coagulation cascade may have a large impact on the final outcome as proteins are interconnected among them.37., 38. Also, fibrin deposition, the final outcome in the coagulation pathway, was significantly reduced by AT treatment in the lungs, proving an increased fibrinolysis.
Nevertheless, anticoagulant effect is not increased when treating with both anticoagulants, contrary to previous studies that showed AT anticoagulant effect is potentiated by the presence of heparin.13., 15., 39. This discrepancy may be due to the timing or the dosage. Here, for the first time, we nebulized both anticoagulants simultaneously in a 48‐hour ALI model with the addition of an extra dose of heparin alone as its half‐time life is shorter than AT’s. However, because we did not assess the actual concentration of AT and heparin in the lungs throughout the experiment, it is not possible to determine the actual AT and heparin interaction. Nonetheless, an excessive inhibition of procoagulant factors might also be deleterious, as was observed by Basterache et al,29 who suggested that the complete deficiency of TF in an ALI model lead to an increase of intra‐alveolar hemorrhage. In contrast, other studies indicate that high levels of TF result in higher depositions of fibrin, which is associated with a worst outcome.40., 41. In our results, nebulized anticoagulants reduced TF levels but not completely, attenuating lung coagulopathy and fibrin deposition in the lungs without producing intra‐alveolar hemorrhage.
In contrast to what other studies suggest, our results indicate that AT antiinflammatory effect is still maintained when combined with heparin. It is known that heparin competes with syndecan‐4 for binding AT and reduces the coagulant‐independent antiinflammatory effect of AT.
Nevertheless, heparin does not interfere in the antiinflammatory effects that AT produces through its action on the coagulation mediators. Moreover, our group recently proved that heparin has also antiinflammatory properties independent of its effect on AT.23 These would explain why a reduction in inflammation was observed in both treated groups, although the mechanisms through which AT and heparin act might be different. Furthermore, at a protein level, the antiinflammatory effect of both treatments was diluted, which could be explained by there not being a perfect correlation between mRNA expression and protein concentration. Moreover, no differences of TNF‐α concentrations but raised levels of mRNA expression at 48 hours could be attributed to a time effect because TNF‐α is released in the early stages of ARDS; a second peak is observed at later stages of this disease. Reduced inflammation and expression of some of the most abundant chemoattractant chemokines for neutrophils (CXCL1) and monocytes (CCL2) in lungs may also explain decreased numbers of proinflammatory cells in the alveolar space when administering anticoagulants.
There is a direct link between inflammation and coagulation, in which proinflammatory cytokines regulate TF expression.16 The competition of heparin with syndecan‐4 for the binding site of AT, a part of avoiding the reduction of proinflammatory cytokines might also explain why AT combined with heparin did not reduce coagulation at the same level than AT alone.
Moreover, our results confirmed previous studies that revealed the important role of AM in the development and the resolution of ARDS.28., 42., 43., 44. Treatment with AT and AT combined with heparin reduced AM expression of proinflammatory (iNOS) and antiinflammatory (Arg‐I) mediators and decreased the expression of mediators of recruitment (CXCL1 and CCL2), indicating an interaction between both anticoagulants and macrophages. In a previous study, we found that nebulized heparin was able to decrease the effectors of the NF‐kB pathway through AM.28 In this study, AT and AT combined with heparin also reduced the expression of proinflammatory cytokines (iNOS, CXCL1, and CCL2) regulated by the NF‐kB pathway in AM. These results suggest that anticoagulants could bind to macrophages blocking the NF‐kB pathway and reducing, in turn, the inflammatory response.
In our results, both AT alone or combined with heparin demonstrated beneficial effects attenuating pulmonary coagulation and inflammation but not completely, limiting their amplification but allowing their protective host response against injury. Systemic administration of AT or AT alpha‐1 protease inhibitor in a model of intranasal LPS or intravenous LPS with subsequent injurious ventilation did not attenuate lung injury.45 Although in both studies AT was administered as a treatment in the early phase of ALI, favorable results found in our model might be ascribed to the time because we observed differences at 48 hours and Juschten et al did not find ameliorations at 6 hours; in addition, nebulized anticoagulants might allow higher dosages and increased local efficacy.
It is important to remark on the limitations of our study. Although animal models are helpful to elucidate the mechanisms involved in a disease and to develop new treatments, they cannot be completely equated with human diseases. ARDS heterogeneity and complexity are difficult to reflect in a simplified model such as the HCl/LPS model. In addition, AT dose was chosen based on a pilot study and heparin dosage was taken from previous experiments. However, because it was the first time that both anticoagulants were administered together and nebulized and because of the different lifespan of the two anticoagulants, it is difficult to assess whether the concentration of both anticoagulants was constant throughout the experiment. Nevertheless, the high concentrations of anticoagulants and the nebulizer system used guarantee that a high proportion of AT and heparin reached the alveolar space, although for future studies it would be interesting to monitor AT and heparin levels throughout the experiment to have a clearer outlook of the actual concentration and their interaction.
In conclusion, nebulized AT alone or combined with heparin attenuated lung injury in our rat model of ALI without affecting systemic coagulation and no bleeding. Nevertheless, the combination of AT and heparin did not produce a synergistic effect. It would be interesting to extend the treatment for as long as our ALI model lasts (72 hours) to check whether differences are found in the outcome. If these positive results are confirmed, preclinical data should be transferred to clinical studies to assess the potential benefits of nebulized AT and AT combined with heparin in ARDS patients.
4.1. Addendum
M. Camprubí‐Rimblas participated in the study design, performed the experiments, took part in the analysis and interpretation of data, and cowrote the paper. N. Tantinyà performed the experiments, participated in the analysis and interpretation of data, and cowrote the paper. R. Guillamat‐Prats participated in the study design, the interpretation of data, and collaborated in the drafting of the manuscript and its final approval. J. Bringué participated in the performance of the experiments and the critical revision of the manuscript. F. Puig designed the ALI in vivo model and participated in the critical revision of the manuscript and final approval of the manuscript. M. Nieves Gómez participated in the performance of the experiments and the final approval of the manuscript. L. Blanch participated in the critical revision of the manuscript and the final approval of the manuscript. A. Artigas participated in the study design, the interpretation of data, the critical revision of the manuscript, and the final approval of the manuscript.
This work was supported by Grifols; Dr. Artigas received a research grant from Grifols and was awarded with the Grifols Antithrombin Research Awards (GATRA). This work was also supported by Institut d’Investigació i Innovació Parc Taulí, CIBERES, and the Spanish Society of Intensive Care Medicine SEMICYUC.
CONFLICT OF INTEREST
The authors state that they do not have any conflict of interest.
ACKNOWLEDGEMENTS
We thank Dr. Francisco M. Mota (senior manager medical affairs, EU/ROW Intensive Care Grifols, S.A.) for his support and advice.
Grifols
Footnotes
Marta Camprubí‐Rimblas and NeusTantinyà contributed equally to this paper.
Manuscript handled by: Marcel Levi
Final decision: Marcel Levi, 18 November 2019
Supporting Information
REFERENCES
- 1.Thompson B.T., Chambers R.C., Liu K.D. Acute respiratory distress syndrome. N Engl J Med. 2017;377(19):1904–1905. doi: 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 2.Villar J., Blanco J., Kacmarek R.M. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care. 2016;22(1):1–6. doi: 10.1097/MCC.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 3.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31(Suppl):S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 4.Warren B.L., Eid A., Singer P., et al. KyberSept Trial Study Group. Caring for the critically ill patient. High‐dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286(15):1869–1878. doi: 10.1001/jama.286.15.1869. [DOI] [PubMed] [Google Scholar]
- 5.Eid A., Wiedermann C.J., Kinasewitz G.T. Early administration of high‐dose antithrombin in severe sepsis: single center results from the KyberSept‐trial. Anesth Analg. 2008;107(5):1633–1638. doi: 10.1213/ane.0b013e318184621d. [DOI] [PubMed] [Google Scholar]
- 6.Murakami K., McGuire R., Cox R.A., et al. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock. 2002;18(3):236–241. doi: 10.1097/00024382-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Juschten J., Tuinman P.R., Juffermans N.P., Dixon B., Levi M., Schultz M.J. Nebulized anticoagulants in lung injury in critically ill patients – an updated systematic review of preclinical and clinical studies. Ann Transl Med. 2017;5(22):444. doi: 10.21037/atm.2017.08.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enkhbaatar P., Cox R.A., Traber L.D., et al. Aerosolized anticoagulants ameliorate acute lung injury in sheep after exposure to burn and smoke inhalation. Crit Care Med. 2007;35(12):2805–2810. doi: 10.1097/01.ccm.0000291647.18329.83. [DOI] [PubMed] [Google Scholar]
- 9.Schultz M.J., Haitsma J.J., Zhang H., Slutsky A.S. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia – a review. Crit Care Med. 2006;34(3):871–877. [PubMed] [Google Scholar]
- 10.Okajima K., Uchiba M. The anti‐inflammatory properties of antithrombin III: new therapeutic implications. Semin Thromb Hemost. 1998;24(1):27–32. doi: 10.1055/s-2007-995820. [DOI] [PubMed] [Google Scholar]
- 11.Levi M., van der Poll T., Büller H.R. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 12.Esmon C.T. The interactions between inflammation and coagulation. Br J Haematol. 2005;131(4):417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 13.Marcum J.A., McKenney J.B., Rosenberg R.D. Acceleration of thrombin‐antithrombin complex formation in rat hindquarters via heparinlike molecules bound to the endothelium. J Clin Invest. 1984;74(2):341–350. doi: 10.1172/JCI111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damus P.S., Rosenberg R.D. Antithrombin‐heparin cofactor. Meth Enzymol. 1976;45:653–669. doi: 10.1016/s0076-6879(76)45056-5. [DOI] [PubMed] [Google Scholar]
- 15.Hofstra J.J., Cornet A.D., de Rooy B.F., et al. Nebulized antithrombin limits bacterial outgrowth and lung injury in Streptococcus pneumoniae pneumonia in rats. Crit Care. 2009;13(5):R145. doi: 10.1186/cc8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93(3):212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 17.Coughlin S.R. Thrombin signalling and protease‐activated receptors. Nature. 2000;407(6801):258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 18.Wiedermann C.J. Clinical review: molecular mechanisms underlying the role of antithrombin in sepsis. Crit Care. 2006;10(1):209. doi: 10.1186/cc4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagiwara S., Iwasaka H., Matsumoto S., Noguchi T. High dose antithrombin III inhibits HMGB1 and improves endotoxin‐induced acute lung injury in rats. Intensive Care Med. 2008;34(2):361–367. doi: 10.1007/s00134-007-0887-5. [DOI] [PubMed] [Google Scholar]
- 20.Vuong T.T., Reine T.M., Sudworth A., Jenssen T.G., Kolset S.O. Syndecan‐4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing. J Histochem Cytochem. 2015;63(4):280–292. doi: 10.1369/0022155415568995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okajima K. Regulation of inflammatory responses by natural anticoagulants. Immunol Rev. 2001;184:258–274. doi: 10.1034/j.1600-065x.2001.1840123.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaneider N.C., Egger P., Dunzendorfer S., Wiedermann C.J. Syndecan‐4 as antithrombin receptor of human neutrophils. Biochem Biophys Res Commun. 2001;287(1):42–46. doi: 10.1006/bbrc.2001.5534. [DOI] [PubMed] [Google Scholar]
- 23.Camprubí‐Rimblas M., Guillamat‐Prats R., Lebouvier T., et al. Role of heparin in pulmonary cell populations in an in‐vitro model of acute lung injury. Respir Res. 2017;18(1):89. doi: 10.1186/s12931-017-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capaldo C.T., Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788(4):864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puig F., Fuster G., Adda M., et al. Barrier‐protective effects of activated protein C in human alveolar epithelial cells. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0056965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware L.B. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 27.Puig F., Herrero R., Guillamat‐Prats R., et al. A new experimental model of acid‐ and endotoxin‐induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2016;311(2):L229–L237. doi: 10.1152/ajplung.00390.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chimenti L., Camprubí‐Rimblas M., Guillamat‐Prats R., et al. Nebulized heparin attenuates pulmonary coagulopathy and inflammation through alveolar macrophages in a rat model of acute lung injury. Thromb Haemost. 2017;117(11):2125–2134. doi: 10.1160/TH17-05-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastarache J.A., Sebag S.C., Clune J.K., et al. Low levels of tissue factor lead to alveolar haemorrhage, potentiating murine acute lung injury and oxidative stress. Thorax. 2012;67(12):1032–1039. doi: 10.1136/thoraxjnl-2012-201781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi G., Hofstra J.J., Roelofs J.J., et al. Antithrombin inhibits bronchoalveolar activation of coagulation and limits lung injury during Streptococcus pneumoniae pneumonia in rats. Crit Care Med. 2008;36(1):204–210. doi: 10.1097/01.CCM.0000292012.87482.F4. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Sun J.F., Cui X., et al. The effect of heparin administration in animal models of sepsis: a prospective study in Escherichia coli‐challenged mice and a systematic review and metaregression analysis of published studies. Crit Care Med. 2011;39(5):1104–1112. doi: 10.1097/CCM.0b013e31820eb718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofstra J.J., Vlaar A.P., Cornet A.D., et al. Nebulized anticoagulants limit pulmonary coagulopathy, but not inflammation, in a model of experimental lung injury. J Aerosol Med Pulm Drug Deliv. 2010;23(2):105–111. doi: 10.1089/jamp.2009.0779. [DOI] [PubMed] [Google Scholar]
- 33.Cornet A.D., Hofstra J.J., Vlaar A.P., et al. Nebulized anticoagulants limit coagulopathy but not inflammation in pseudomonas aeruginosa‐induced pneumonia in rats. Shock. 2011;36(4):417–423. doi: 10.1097/SHK.0b013e31822bcef0. [DOI] [PubMed] [Google Scholar]
- 34.Rehberg S., Yamamoto Y., Sousse L.E., et al. Antithrombin attenuates vascular leakage via inhibiting neutrophil activation in acute lung injury. Crit Care Med. 2013;41(12):e439–e446. doi: 10.1097/CCM.0b013e318298ad3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ning F., Wang X., Shang L., et al. Low molecular weight heparin may prevent acute lung injury induced by sepsis in rats. Gene. 2015;557(1):88–91. doi: 10.1016/j.gene.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Beyer A., Aebersold R. On the dependency of cellular protein levels on mRNA abundance. Cell. 2016;165(3):535–550. doi: 10.1016/j.cell.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Palta S., Saroa R., Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515–523. doi: 10.4103/0019-5049.144643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith S.A., Travers R.J., Morrissey J.H. How it all starts: initiation of the clotting cascade. Crit Rev Biochem Mol Biol. 2015;50(4):326–336. doi: 10.3109/10409238.2015.1050550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rehberg S., Yamamoto Y., Sousse L.E., et al. Advantages and pitfalls of combining intravenous antithrombin with nebulized heparin and tissue plasminogen activator in acute respiratory distress syndrome. J Trauma Acute Care Surg. 2014;76(1):126–133. doi: 10.1097/TA.0b013e3182ab0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gando S. Tissue factor in trauma and organ dysfunction. Semin Thromb Hemost. 2006;32(1):48–53. doi: 10.1055/s-2006-933340. [DOI] [PubMed] [Google Scholar]
- 41.Ozolina A., Sarkele M., Sabelnikovs O., et al. Activation of coagulation and fibrinolysis in acute respiratory distress syndrome: a prospective pilot study. Front Med. 2016;3:64. doi: 10.3389/fmed.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnston L.K., Rims C.R., Gill S.E., McGuire J.K., Manicone A.M. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47(4):417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aggarwal N.R., King L.S., D’Alessio F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306(8):L709–L725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herold S., Mayer K., Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juschten J., Ingelse S.A., Maas M.A.W., et al. Antithrombin plus alpha‐1 protease inhibitor does not affect coagulation and inflammation in two murine models of acute lung injury. Intensive Care Med Exp. 2019;7(Suppl 1):36. doi: 10.1186/s40635-019-0240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.