Abstract
Background
A relatively high mortality of severe coronavirus disease 2019 (COVID‐19) is worrying, and the application of heparin in COVID‐19 has been recommended by some expert consensus because of the risk of disseminated intravascular coagulation and venous thromboembolism. However, its efficacy remains to be validated.
Methods
Coagulation results, medications, and outcomes of consecutive patients being classified as having severe COVID‐19 in Tongji hospital were retrospectively analyzed. The 28‐day mortality between heparin users and nonusers were compared, as was a different risk of coagulopathy, which was stratified by the sepsis‐induced coagulopathy (SIC) score or D‐dimer result.
Results
There were 449 patients with severe COVID‐19 enrolled into the study, 99 of them received heparin (mainly with low molecular weight heparin) for 7 days or longer. D‐dimer, prothrombin time, and age were positively, and platelet count was negatively, correlated with 28‐day mortality in multivariate analysis. No difference in 28‐day mortality was found between heparin users and nonusers (30.3% vs 29.7%, P = .910). But the 28‐day mortality of heparin users was lower than nonusers in patients with SIC score ≥4 (40.0% vs 64.2%, P = .029), or D‐dimer >6‐fold of upper limit of normal (32.8% vs 52.4%, P = .017).
Conclusions
Anticoagulant therapy mainly with low molecular weight heparin appears to be associated with better prognosis in severe COVID‐19 patients meeting SIC criteria or with markedly elevated D‐dimer.
Keywords: coagulopathy, coronavirus disease 2019, D‐dimer, low molecular weight heparin, sepsis
Essentials
-
•
Heparin treatment has been recommended for COVID‐19, however, its efficacy remains to be validated.
-
•
The 28‐day mortality between heparin users and nonusers were compared in stratified patients.
-
•
The 28‐day mortality of heparin users were lower than nonusers In patients with SIC score ≥ 4 or D‐dimer > 3.0 μg/mL.
-
•
Heparin treatment appears to be associated with better prognosis in severe COVID‐19 patients with coagulopathy.
Alt-text: Unlabelled Box
1. INTRODUCTION
As recent studies described,1., 2., 3. severe coronavirus disease 2019 (COVID‐19) is commonly complicated with coagulopathy, and disseminated intravascular coagulation (DIC) may exist in the majority of deaths. Because of the evidence of virus infection and respiratory dysfunction, many patients with severe COVID‐19 meet the Third International Consensus Definitions for Sepsis (Sepsis‐3).4 In addition, long‐term bed rest and likely receiving hormone treatment also increase the risk of venous thromboembolism (VTE) in severe COVID‐19. For these reasons, the active application of anticoagulants (such as heparin) for patients with severe COVID‐19 has been recommended by some expert consensus in China5; however, its efficacy remains to be validated.
The International Society of Thrombosis and Haemostasis (ISTH) has proposed a new category identifying an earlier phase of sepsis‐associated DIC called “sepsis‐induced coagulopathy” (SIC),6 that patients who meet the diagnostic criteria of SIC benefit from anticoagulant therapy have been confirmed.7 Our study aimed to validate the usefulness of SIC score and other coagulation parameters, in screening out patients who can benefit from anticoagulant through retrospective analysis.
2. METHODS
Consecutive patients with severe COVID‐19 admitted to Tongji Hospital of Huazhong University of Science and Technology in Wuhan from January 1 to February 13, 2020, were retrospectively enrolled. Exclusion criteria were a bleeding diathesis, hospital stay < 7 days, lack of information about coagulation parameters and medications, and age < 18 years. A retrospective review of the characteristics of these patients was performed through the electronic medical record system of our hospital, the medications and outcomes (28‐day mortality) were monitored up to March 13, 2020. This study was approved by the Ethics Committee of Tongji Hospital (Wuhan, China).
The diagnosis of COVID‐19 was according to World Health Organization interim guidance8 and confirmed by RNA detection of the SARS‐CoV‐2 in a clinical laboratory of the Tongji hospital. Severe COVID‐19 was defined as meeting any one of following items, according to the Diagnosis and Treatment Plan of COVID‐19 suggested by National Health Commission of China9: Respiratory rate ≥30 breaths/min; arterial oxygen saturation ≤93% at rest; PaO2/FiO2 ≤ 300 mm Hg.
The SIC score system including prothrombin time (PT), platelet count, and sequential organ failure assessment (SOFA) was described in Table 1 ,6 in which the SOFA score contained four items and was originally developed by an international group of experts to describe the time course of muitiple organ dysfunctions using a limited number of routinely measured variables.10 Meanwhile, in our previous study,3 higher D‐dimer and PT on admission were associated with poor prognosis in patients with COVID‐19. Hence these three parameters were included in this study and the results were recorded at the time the patient meeting the definition of severe COVID‐19. Anticoagulant treatment group was defined as receiving unfractionated heparin or low molecular weight heparin (LMWH) for 7 days or longer,11 which was the most commonly used anticoagulant therapy for COVID‐19 in our hospital.
Table 1.
ISTH SIC scoring system
| Item | Score | Range |
|---|---|---|
| Platelet count (×109/L) | 1 | 100‐150 |
| 2 | <100 | |
| PT‐INR | 1 | 1.2‐1.4 |
| 2 | >1.4 | |
| SOFA score | 1 | 1 |
| 2 | ≥2 | |
| Total score for SIC | ≥4 |
Abbreviations: INR, International Normalized Ratio; SOFA, sequential organ failure assessment.
The coagulation tests, including PT and D‐dimer, were detected using a STA‐R MAX coagulation analyzer and original reagents (Diagnostica Stago). The platelet counts were analyzed by a Sysmex XE‐2100 hematology analyzer (Sysmex).
Normally and abnormally distributed quantitative variables were compared using the Student's t‐test and the Mann‐Whitney U test, respectively. Categorical variables were compared using the chi‐squared test. The results were given as the mean ± standard deviation, median (interquartile range), or number (percentage), wherever appropriate. Categorical and consecutive variables were evaluated by logistic regression analysis for their ability to predict 28‐day mortality. A P value of < .05 was considered statistically significant. Data were analyzed using SPSS 21.0 for Windows (SPSS Inc).
3. RESULTS
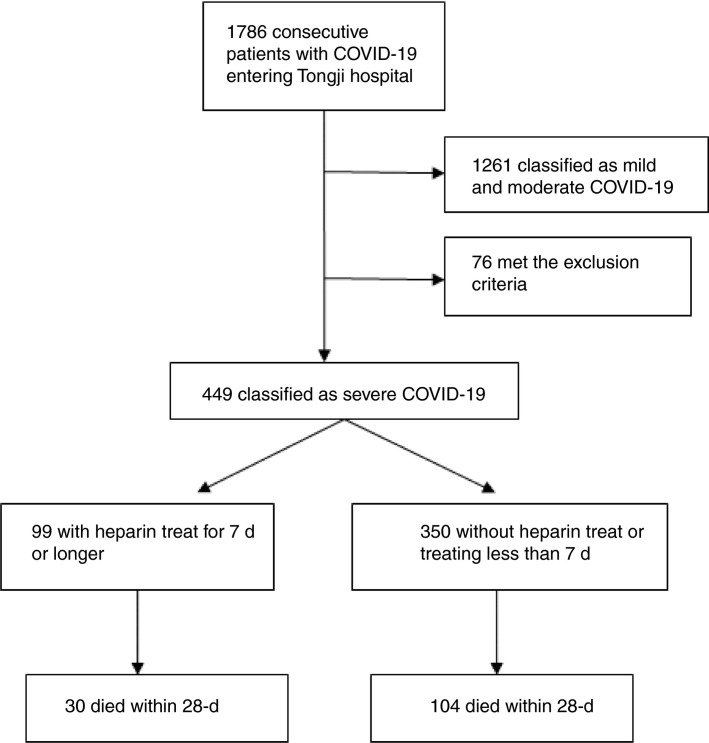
A total of 449 patients (181 females and 268 males) classified as severe COVID‐19 were enrolled into the study from consecutive 1786 confirmed cases. The mean age at disease onset was 65.1 ± 12.0 years. Two hundred and seventy‐two (60.6%) patients had one or more chronic underlying diseases, mainly including hypertension (n = 177, 39.4%), diabetes (n = 93, 20.7%), and heart diseases (n = 41, 9.1%). Ninety‐nine (22.0%) patients received heparin treatment for at least 7 days, in which 94 received LMWH (40‐60 mg enoxaparin/d) and five received unfractionated heparin (10 000‐15 000 U/d), no anticoagulants other than heparin had been used for 7 days or longer in our patients. All patients received antiviral and appropriate supportive therapies after admission. Ninety‐seven (21.6%) patients met the SIC criteria (total score ≥ 4) when they were classified as severe cases. By the end of March 13, 315 (70.2%) patients were still survived and 134 (29.8%) patients had died (Figure 1 ). No difference on the 28‐day mortality was found between heparin users and nonusers (30.3% vs 29.7%, P = .910).
Figure 1.
The enrollment of patients with severe COVID‐19
The parameters of coagulation tests and clinical characteristics between survivors and nonsurvivors were compared (Table 2 ). Then these parameters were examined in a multivariate logistic regression model to identify independent correlative factor of 28‐day mortality of severe COVID‐19 (Table 3 ). The D‐dimer, PT, and age were positively, and platelet count was negatively, correlated with 28‐day mortality.
Table 2.
Clinical and coagulation characteristics of patients being classified as severe COVID‐19
| Parameters | Normal range | Total (n = 449) | Survivors (n = 315) | Nonsurvivors (n = 134) | P values |
|---|---|---|---|---|---|
| Age (years) | 65.1 ± 12.0 | 63.7 ± 12.2 | 68.7 ± 11.4 | <.001 | |
| Sex ratio (male/female) | 268/181 | 178/137 | 90/44 | .036 | |
| With underlying diseases | 272 (60.6%) | 181 (57.5%) | 91 (67.9%) | .136 | |
| Receiving heparin | 99 (22.0%) | 69 (21.9%) | 30 (22.4%) | .910 | |
| Meeting SIC criteria | 97 (21.6%) | 42 (13.3%) | 55 (41.0%) | <.001 | |
| Coagulation parameters | |||||
| PT (s) | 11.5‐14.5 | 15.2 ± 5.0 | 14.6 ± 2.1 | 16.5 ± 8.4 | <.001 |
| Platelet count (×109/L) | 125‐350 | 215 ± 100 | 231 ± 99 | 178 ± 92 | <.001 |
| D‐dimer (μg/mL) | <0.5 | 1.94 (0.90‐9.44) | 1.47 (0.78‐4.16) | 4.70 (1.42‐21.00) | <.001 |
Table 3.
Multivariate correlative factors of 28‐day mortality in severe COVID‐19
| Multivariate analysis |
||
|---|---|---|
| Odds ratio (95% CI) | P value | |
| Age | 1.033 (1.013‐1.055) | .002 |
| Sex ratio | 0.677 (0.425‐1.078) | .100 |
| With underlying diseases | 0.861 (0.538‐1.379) | .534 |
| Treating with heparin | 1.647 (0.929‐2.921) | .088 |
| Prothrombin time | 1.107 (1.008‐1.215) | .033 |
| Platelet count | 0.996 (0.993‐0.998) | .001 |
| D‐dimer | 1.058 (1.028‐1.090) | <.001 |
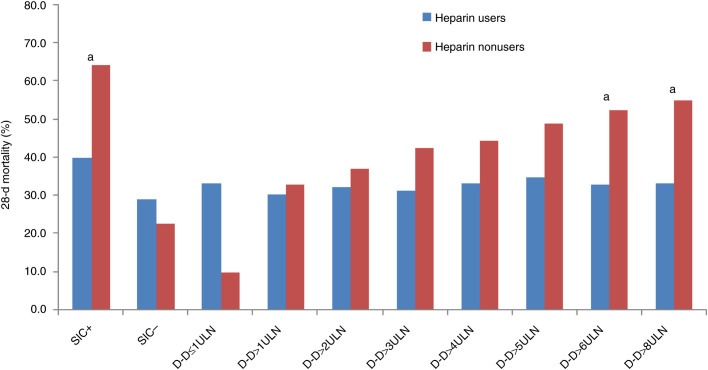
The association between heparin treatment and outcome in stratified patients according to SIC score or D‐dimer result were evaluated (Table 4 and Figure 2 ). The heparin treat was associated with lower mortality in patients with SIC score ≥ 4 (40.0% vs 64.2%, P = .029), but not in those with SIC score < 4 (29.0% vs 22.6%, P = .419). Because patients were stratified by D‐dimer result, the mortality in heparin users basically maintained at same level, but in nonusers, the mortality rose with the rising D‐dimer. When D‐dimer exceeding 3.0 μg/mL (6‐fold of upper limit of normal, six ULN), approximate 20% reduction in mortality with heparin treat was found (32.8% vs 52.4%, P = .017).
Table 4.
The association between heparin treatment and outcomes in stratified patients
| Patients with | 28‐day mortality |
Univariate analysis |
||
|---|---|---|---|---|
| Treating with heparin, % | Nontreating with heparin, % | Odds ratio (95% CI) | P value | |
| SIC score ≥ 4 (n = 97) | 40.0 | 64.2 | 0.372 (0.154‐0.901) | .029 |
| SIC score ≤ 4 (n = 352) | 29.0 | 22.6 | 1.284 (0.700‐2.358) | .419 |
| D‐dimer ≤ 1 ULN (n = 34) | 33.3 | 9.7 | 4.667 (0.320‐68.03) | .260 |
| D‐dimer > 1 ULN (n = 415) | 30.2 | 32.7 | 0.934 (0.569‐1.533) | .788 |
| D‐dimer > 2 ULN (n = 317) | 32.1 | 36.9 | 0.810 (0.477‐1.375) | .435 |
| D‐dimer > 3 ULN (n = 253) | 31.1 | 42.5 | 0.611 (0.344‐1.086) | .093 |
| D‐dimer > 4 ULN (n = 224) | 33.3 | 44.5 | 0.623 (0.345‐1.127) | .118 |
| D‐dimer > 5 ULN (n = 190) | 34.9 | 48.8 | 0.563 (0.301‐1.050) | .071 |
| D‐dimer > 6 ULN (n = 161) | 32.8 | 52.4 | 0.442 (0.226‐0.865) | .017 |
| D‐dimer > 8 ULN (n = 150) | 33.3 | 54.8 | 0.412 (0.207‐0.817) | .011 |
Abbreviation: ULN, upper limit of normal (0.5 μg/mL for D‐dimer).
Figure 2.
A paired bar chart showing the mortality between heparin users and nonusers in stratified patients. D‐D, D‐dimer; SIC+, SIC score ≥ 4; SIC−, SIC score < 4; ULN, upper limit of normal (0.5 μg/mL); a, P < .05 between heparin users and nonusers
4. DISCUSSION
The dysfunction of endothelial cells induced by infection results in excess thrombin generation and fibrinolysis shutdown, which indicated a hypercoagulable state in patient with infection,12., 13. such as COVID‐19. In addition, the hypoxia found in severe COVID‐19 can stimulate thrombosis through not only increasing blood viscosity, but also a hypoxia‐inducible transcription factor‐dependent signaling pathway.14 As evidence, occlusion and microthrombosis formation in pulmonary small vessels of critical patient with COVID‐19 has been reported from a recent lung organ dissection.15 Hence, early application of anticoagulant therapy in severe COVID‐19 was suggested in China for improving outcome5; however, no specific inclusion or exclusion criteria has been pointed out so far. As anticoagulant was seldom used in early stage because of a lack of understanding of this disease, and increasingly used later during this outbreak of COVID‐19, we could retrospectively include enough cases to analyze the difference on outcomes between patients with and without receiving anticoagulant.
LMWH was the most commonly used anticoagulant in our hospital for preventing DIC and VTE in patients, also because of its anti‐inflammatory effect.16 Another reason is that other anticoagulants, such as recombinant soluble thrombomodulin or antithrombin, is unavailable in China. The prophylactic dose of LMWH was used in most of our heparin users, bleeding complications were unusual and commonly mild, and it is not known if higher doses would have been better. Because the evidence suggests that the prevalence and genetic risk factors of VTE vary significantly among ethnic populations, and the incidence of VTE in Asian populations (21‐29 cases per 100 000 individuals per year) is low,17., 18. a higher dose of LMWH could be considered in non‐Asian patients with severe COVID‐19. However, the effectiveness of anticoagulant therapy for sepsis‐associated DIC is still controversial19., 20.; even the Japanese guideline for management of sepsis is against the use of heparin or heparin analogs as a standard treatment in sepsis‐associated DIC,21 and some studies suggested that septic patients might just benefit from early recognition and specific treatment.22., 23. As platelet counts decline and PT prolongation is correlated with increased mortality, and hypofibrinogenemia is not common in sepsis, the ISTH developed the SIC criteria to guide anticoagulant therapy. The usefulness of this simple score has been validated previously.7
Because organ dysfunction is mainly limited in lung and virus is the main pathogen, the coagulation feature of severe COVID‐19 might not be identical with sepsis in general. Perhaps because of the reactively increased thrombopoietin following pulmonary inflammation,24 platelet count may not be a sensitive marker for coagulopathy of COVID‐19, in current study, only 21.6% of patients with severe COVID‐19 met the SIC criteria, which suggested limited patients needing anticoagulant treat. However, as an indirect marker of coagulation activation, markedly elevated D‐dimer (>6 ULN) also suggested benefit from heparin treat, in a larger group of severe patients (161 of 449, 35.9%).
Because the activation of coagulation also contributes to compartmentalization of pathogens and reduces their invasion,25 anticoagulant treatment in patients without significant coagulopathy has potential risk. This may explain the relatively higher mortality of heparin users compared with nonusers in patients with D‐dimer ≤ 1 ULN, although the difference was not statistically significant (P = .260).
There were several limitations in the current study. First, potential selection bias exists in this retrospective study; for instance, LMWH might tend to be used in patients with targeted symptom or medical history, which we have not controlled. Second, because insufficient medical resources at the early stage of the COVID‐19 outbreak in Wuhan, China, the severity and mortality of the included patients might not be representative. Third, the influence of other therapies on these patients has not been evaluated; in addition, as we enrolled patients over a 6‐week period, It is possible that some nonpharmacological change has taken place in the management of patients as the doctors learned more about this disease over the period. Nonetheless, this study included a large critical patient population, and due to lack of specific drugs against the infection of SARS‐CoV‐2 up to now,26 the majority of patients with severe COVID‐19 should have received similar supportive treatment after admission. Hence, we believe that the results of current study still have certain clinical significance.
In conclusion, a relatively high mortality of severe COVID‐19 is worrying; our study suggests that anticoagulants may not benefit the unselected patients, instead, only the patients meeting SIC criteria or with markedly elevated D‐dimer may benefit from anticoagulant therapy mainly with LMWH. Further prospective studies are needed to confirm this result.
AUTHOR CONTRIBUTIONS
N. Tang drafted the manuscript. N. Tang, H. Bai, X. Chen, and J. Gong collected and analyzed the data. D. Li interpreted the data. N. Tang and Z. Sun designed the study.
Funding information
National Mega Project on Major infectious Disease Prevention of China. (No.2017ZX10103005‐007).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
Acknowledgments
National Mega Project on Major Infectious Disease Prevention of China 2017ZX10103005‐007
Footnotes
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 24 Mar 2020
REFERENCES
- 1.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanghai Clinical Treatment Expert Group for. COVID‐19 Comprehensive treatment and management of coronavirus disease 2019: expert consensus statement from Shanghai (in Chinese) Chin J Infect Dis. 2020;38 doi: 10.3760/cma.j.issn.1000-6680.2020.0016. [DOI] [Google Scholar]
- 6.Iba T., Levy J.H., Warkentin T.E., et al. Diagnosis and management of sepsis‐induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. 2019;17(11):1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 7.Iba T., Nisio M.D., Levy J.H., Kitamura N., Thachil J. New criteria for sepsis‐induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance. Published January 28, 2020. https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐whennovel‐coronavirus‐(ncov)‐infection‐is‐suspected. Accessed January 31, 2020
- 9.National Health Commission of China. The diagnosis and treatment plan for the novel coronavirus disease (the seventh edition). 2020.
- 10.Vincent J.L., Moreno R., Takala J., et al. The SOFA (Sepsis‐related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis‐Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 11.Venclauskas L., Llau J.V., Jenny J.Y., Kjaersgaard‐Andersen P., Jans Ø., ESA VTE Guidelines Task Force European guidelines on perioperative venous thromboembolism prophylaxis: day surgery and fast‐track surgery. Eur J Anaesthesiol. 2018;35(2):134–138. doi: 10.1097/EJA.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 12.Levi M., van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt F.C.F., Manolov V., Morgenstern J., et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9(1):19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta N., Zhao Y.Y., Evans C.E. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Luo W, Yu H, Gou J, et al.Clinical pathology of critical patient with novel coronavirus pneumonia (COVID‐19). Preprints. 2020, 2020020407.
- 16.Poterucha T.J., Libby P., Goldhaber S.Z. More than an anticoagulant: do heparins have direct anti‐inflammatory effects? Thromb Haemost. 2017;117(3):437–444. doi: 10.1160/TH16-08-0620. [DOI] [PubMed] [Google Scholar]
- 17.Stein P.D., Kayali F., Olson R.E., Milford C.E. Pulmonary thromboembolism in Asians/Pacific Islanders in the United States: analysis of data from the National Hospital Discharge Survey and the United States Bureau of the Census. Am J Med. 2004;116(7):435–442. doi: 10.1016/j.amjmed.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Zakai N.A., McClure L.A. Racial differences in venous thromboembolism. J Thromb Haemost. 2011;9(10):1877–1882. doi: 10.1111/j.1538-7836.2011.04443.x. [DOI] [PubMed] [Google Scholar]
- 19.Aikawa N., Shimazaki S., Yamamoto Y., et al. Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock. 2011;35(4):349–354. doi: 10.1097/SHK.0b013e318204c019. [DOI] [PubMed] [Google Scholar]
- 20.Liu X.L., Wang X.Z., Liu X.X., et al. Low‐dose heparin as treatment for early disseminated intravascular coagulation during sepsis: a prospective clinical study. Exp Ther Med. 2014;7(3):604–608. doi: 10.3892/etm.2013.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida O., Ogura H., Egi M., et al. The Japanese clinical practice guidelines for management of sepsis and septic shock 2016 (J‐SSCG 2016) Acute Med Surg. 2018;5(1):3–89. doi: 10.1002/ams2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umemura Y., Yamakawa K., Ogura H., Yuhara H., Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta‐analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518–530. doi: 10.1111/jth.13230. [DOI] [PubMed] [Google Scholar]
- 23.Iba T., Gando S., Thachil J. Anticoagulant therapy for sepsis‐associated disseminated intravascular coagulation: the view from Japan. J Thromb Haemost. 2014;12(7):1010–1019. doi: 10.1111/jth.12596. [DOI] [PubMed] [Google Scholar]
- 24.Menter D.G., Kopetz S., Hawk E., et al. Platelet "first responders" in wound response, cancer, and metastasis. Cancer Metastasis Rev. 2017;36(2):199–213. doi: 10.1007/s10555-017-9682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun H., Wang X., Degen J.L., Ginsburg D. Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood. 2009;113(6):1358–1364. doi: 10.1182/blood-2008-07-170506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID‐19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]