Abstract
Objective
Acute respiratory distress syndrome (ARDS) frequently complicates critical illness. We hypothesized that an infusion of recombinant human activated protein C (rh-APC), a natural anticoagulant, would attenuate pulmonary coagulopathy and injury.
Methods
In this sub study of a multicenter open-label randomized controlled trial of patients with ARDS, we compared an intravenous (i.v.) infusion of rh-APC (24 mcg kg−1 h−1 for 96 h) with placebo. Patients with sepsis or septic shock were excluded.
Results
In 27 patients serial non-directed bronchoalveolar lavage fluid (NBLF) samples were obtained: 16 patients were treated with rh-APC and 11 patients with placebo. The rh-APC infusion was associated with higher APC levels in plasma during the infusion period of 4 days (P = 0.001), as well as higher APC levels in NBLF up to day 5 after the start of the infusion (P = 0.028). An infusion of rh-APC was associated with lower levels of thrombin–antithrombin complexes (P = 0.009) and soluble tissue factor (P = 0.011) in NBLF, compared with treatment with placebo. An infusion of rh-APC affected fibrinolysis, as plasminogen activator activity levels in NBLF were higher in the patients treated with rh-APC (P = 0.01), presumably as a result of lower NBLF levels of plasminogen activator inhibitor 1, (P = 0.01). The rh-APC infusion decreased the lung injury score (P = 0.005) and simplified the acute physiology score (P = 0.013) on day 5, when compared with baseline. The rh-APC infusion was not associated with bleeding complications.
Conclusion
An infusion of rh-APC in patients with ARDS attenuates pulmonary coagulopathy and injury.
Keywords: ARDS, coagulopathy, critically ill, rh-APC
Introduction
Acute respiratory distress syndrome (ARDS) and its milder form, previously known as acute lung injury (ALI) 1, occur at rates between 30 and 80 per 100 000 person-years and are a common cause of respiratory insufficiency and admission to the intensive care unit (ICU) 2., 3.. ARDS is associated with high morbidity and mortality 2. In spite of efforts to find a specific treatment for ALI/ARDS 4, treatment nowadays is merely supportive and directed at avoiding additional harm (by mechanical ventilation).
Pulmonary coagulopathy, which results from activation of coagulation, defective anticoagulant pathways and inhibition of fibrinolysis, is invariably present in ARDS and is likely to contribute to its pathogenesis 5., 6., 7., 8.. In patients with ARDS, pulmonary activated protein C (APC) levels are depressed 8., 9., 10., 11.. Infusion of recombinant human (rh) APC was found to reduce mortality of patients with severe sepsis and a high disease severity 12, although this was offset by other trial evidence and a recently published study of patients with septic shock, which even led to market withdrawal of commercial rh-APC (Xigris®)13. Of note, in the phase III trial that initially resulted in licensing of Xigris®, administration of rh-APC was particularly effective in patients who presented with severe community-acquired pneumonia as the source of sepsis 14. Therefore, the beneficial effect of infusion of rh-APC in patients with severe sepsis as found in some studies could, at least in part, be attributed to the effects of rh-APC on coagulopathy in the lung 14. This is supported by results of pre-clinical studies in rats evaluating the effect of an infusion of rh-APC in models of ARDS showing anticoagulant effects in the lungs 15., 16.. Also, one previous study in healthy volunteers demonstrated an infusion of rh-APC to affect pulmonary coagulation after intrapulmonary challenge with endotoxin 9.
To date, no data exist on the effects of an infusion of rh-APC on the pulmonary coagulopathy present in patients with ARDS. We hypothesized that an infusion of rh-APC would attenuate this pulmonary coagulopathy, which could be beneficial to patients with ARDS. Therefore, we performed an open-label randomized controlled trial of patients with ARDS comparing an intravenous (i.v.) infusion of rh-APC with placebo, with respect to the pulmonary and systemic pro- and anticoagulant balance and organ functions.
Patients and methods
Study design
This is a sub study of INFectious and INFlammatory Acute Lung Injury/Acute Respiratory Distress Syndrome (INFALI), a multicenter open-label randomized controlled trial of patients with ALI/ARDS (trial registration number ISRCTN 52566874). The Ethics Committee of VU University Medical Center, Amsterdam, the Netherlands, approved the study protocol. Written informed consent was obtained from all patients or their next of kin before inclusion into the trial.
Inclusion and exclusion criteria
Patients, over 18 years of age and admitted to the mixed medical–surgical ICUs of one of two participating university hospitals, were to be enrolled within 24 h after ARDS was diagnosed irrespective of the need for (invasive) ventilatory support. ARDS was diagnosed using the North American European Consensus Conference (NAECC) definition 17. Patients were excluded if rh-APC treatment was indicated based on current national guidelines at the time of the study (i.e. severe sepsis or septic shock). Additional exclusion criteria were: platelet count < 30 × 109 L−1, any major surgery within 12 h before inclusion, acute bleeding, severe head trauma, intracranial surgery or stroke within 3 months before inclusion, known intracranial abnormalities (e.g. malignancies or other tumors, arteriovenous malformation), known hypercoagulability (e.g. protein C resistance, hereditary deficiency of protein C, protein S or antithrombin, or anticardiolipin or antiphospholipid antibodies), congenital hemorrhagic diathesis, pregnancy or breast feeding, liver cirrhosis with portal hypertension and/or esophageal varices, the presence of an epidural catheter; severely immune-compromised status (e.g. HIV-infected patients with CD4 count < 50 mL, and patients treated with immunosuppressive medication after bone marrow or solid organ transplantation). The following concomitant medications were reasons for exclusion: heparin in a therapeutic dose (within 8 h of study entry), coumarin derivatives at any dose (within 7 days of study entry), acetylsalicylic acid at a dose > 650 mg day−1 (within previous 3 days of study entry), thrombolytic therapy at any dose (within previous 3 days of study entry), glycoprotein IIb/IIIa inhibitors at any dose (within 7 days of study entry), antithrombin at any dose (within 3 days of study entry) and previous treatment with rh-APC (at any time within study entry). A prophylactic dose of low-molecular-weight heparin was acceptable.
Treatment protocol
All patients were treated according to international guidelines, by the discretion of the supervising intensivists. If needed, mechanical ventilation was performed after endotracheal intubation, in a pressure-controlled mode, aiming at a maximum airway pressures < 35 cmH2O, and tidal volumes ≤ 6 mL kg−1 predicted ideal body weight, with or without proning. Antibiotic therapy was guided by Gram-stains and cultures, according to local guidelines for antimicrobial therapy. Fluid therapy consisted of crystalloids, with or without gelatins and/or hydroxyethyl starches, in order to maintain an arterial blood pressure (MAP > 70 mmHg) and diuresis (> 30 mL h−1).
Treatment assignment
Patients were randomly assigned to an infusion of rh-APC or placebo (normal saline). Prior to the start of the trial, sealed opaque envelopes, containing the treatment assignment for each patient, were numbered through block randomization, with six blocks of patients.
Study protocol
Rh-APC (Eli Lilly, Indianapolis, IN, USA), at a dose of 24 mcg kg−1h−1, or placebo was infused at a constant rate for a total of 96 h 12. Infusion of rh-APC was interrupted 1 h before any invasive percutaneous procedure or major surgery. When no bleeding complications occurred, infusion of rh-APC was resumed 1 h after a percutaneous procedure, and 12 h after major surgery, in line with international guidelines.
Data collection
Upon enrollment data on baseline demographics, co-morbidity and reasons of admission to the intensive care unit (ICU), as well as hemodynamic and respiratory parameters were collected. The acute physiology, age and chronic health evaluation II score (APACHE II) 18, the simplified acute physiology score (SAPS II) 19, the sequential organ failure assessment score (SOFA) 20 and the lung injury score (LIS) 21 were calculated daily until day 5 and every second day thereafter. The number of ventilator-free days was assessed, defined as the number of days from randomization to day 28 after achieving unassisted breathing for at least two consecutive calendar days. If a patient who achieved unassisted breathing, subsequently required additional assisted breathing, and once again achieved unassisted breathing, only the ventilator-free days after beginning the final period of unassisted breathing were counted. Patients who died before day 28 were assigned zero ventilator-free days. Both 28-day, ICU and in-hospital mortality were assessed. Follow-up was discontinued after 1 year.
Non-directed bronchoalveolar lavage and blood sampling
Non-directed bronchoalveolar lavage (NBL) was performed by an experienced intensivist by instilling 20 mL of sterile 0.9% saline via a 50-cm 14-gauge tracheal suction catheter as described previously 22. In short, the distal end of the suction catheter was inserted through the endotracheal tube until resistance was encountered. The 20 mL of sterile 0.9% saline was instilled over 10–15 s, and (in part) aspirated before withdrawal of the catheter. The volume of aspirated NBL-fluid (F) was at least 10 mL. Before NBL was performed, blood was collected, preferably from an indwelling arterial catheter or central venous catheter. Sometimes peripheral blood sampling was necessary.
Assays
NBLF and blood samples were centrifuged at 1500 × g for 15 min and the supernatant was stored at −80 °C until assays were performed. Levels of APC were determined using an enzyme capture assay using monoclonal antibody HAPC 1555 and chromogenic substrate Spectrozyme Pca (American Diagnostics, Greenwich, CT). Thrombin–antithrombin complexes (TATc) and soluble tissue factor (sTF) were measured using ELISA (TATc; Behring, Marburg, Germany; sTF: American Diagnostics, Greenwich, CT, USA). Antithrombin (AT), plasminogen activator activity (PAA) and plasminogen activator inhibitor (PAI)-1 were measured by automated amidolytic assays 23.
Statistical analysis
Data were checked for distribution with help of the Kolmogorov–Smirnov test. Data were expressed as medians (interquartile ranges), or absolute numbers where appropriate. Non-parametric data were analyzed using the Mann–Whitney U, Wilcoxon signed-ranks and Fisher's exact test. A P-value of < 0.05 was considered statistically significant. Exact P-values are given unless < 0.001. Statistical analysis was performed using SPSS 19.0 (SPSS, Chicago, IL, USA) and Prism 5.0 (GraphPad Software, San Diego, CA, USA).
Results
Between 1 January 2007 and 1 May 2011 9484 patients were assessed for eligibility. Of these patients 1274 were diagnosed with ARDS, of which 1203 were excluded or refused participation in the trial, leaving 71 patients for study inclusion. The most common reasons for the large number of patients excluded from participation was that many patients with ARDS had concurrent organ failure, were concomitantly treated with anticoagulant medication or had an increased risk of bleeding. A consort diagram is shown in Fig. 1 . In 44 patients it was not considered safe (prone positioning, high levels of PEEP) or appropriate (not fully sedated) to perform NBL (by the treating intensivist). Patient characteristics, hemodynamic and respiratory baseline values, as well as disease severity scores are shown in Table 1 . Groups were comparable with respect to disease severity and pulmonary condition. The most frequent cause of ARDS was pneumonia. In both groups, no bleeding complications or other serious adverse events were observed.
Figure 1.
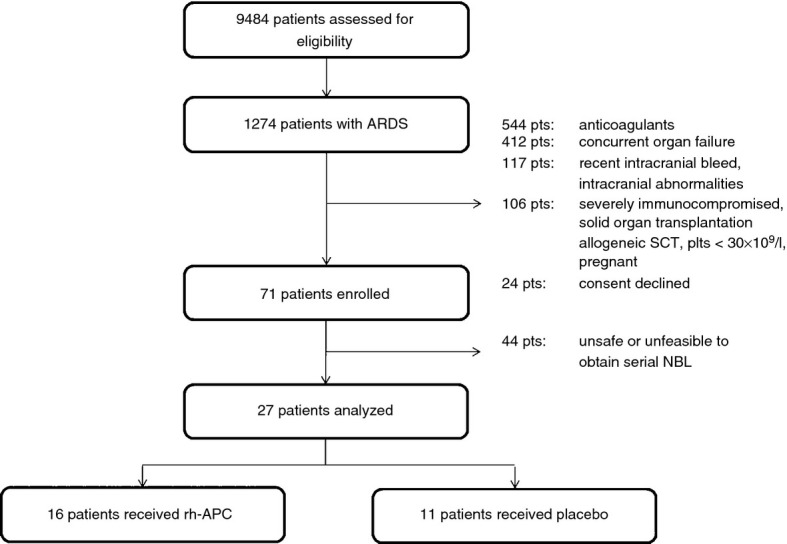
CONSORT diagram. ARDS, acute respiratory distress syndrome; rh-APC, recombinant human activated protein C; SCT, stem cell transplantation; plts, platetelets; NBL, non-directed bronchoalveolar lavage
Table 1.
Baseline characteristics
| Rh-APC n= 16 | Placebo n = 11 | P-value | |
|---|---|---|---|
| General | |||
| Age | 64 (23) | 61.5 (28.5) | 0.47 |
| Gender (male) | 7 | 7 | 0.44 |
| Reason of admission | |||
| Pneumonia | 7 | 8 | – |
| Abdominal sepsis | 4 | 0 | 0.22 |
| Sepsis | 5 | 2 | – |
| Near-drowning | 0 | 1 | – |
| APACHE 2 | 14 (8.25) | 15 (4) | 0.46 |
| SAPS 2 | 37 (19.5) | 34.5 (5.25) | 0.31 |
| SOFA | 6 (2.5) | 5.5 (6) | 0.44 |
| Temperature (°C) | 36.8 (2.25) | 36.9 (2.35) | 0.90 |
| Heart rate (/min) | 113 (55) | 111 (33.25) | 0.86 |
| MAP (mmHg) | 75.5 (10.75) | 73.5 (22.75) | 0.79 |
| Vasopressor | 11 | 7 | 0.16 |
| Creatinine (μmoL/L) | 79 (78.75) | 86 (111) | 0.82 |
| Respiratory | |||
| Respiratory rate (/min) | 25 (9.75) | 21.5 (13.25) | 0.41 |
| PaO2/FiO2-ratio | 150 (70) | 190 (88.25) | 0.15 |
| PEEP (cm H2O) | 11 (4.75) | 9.5 (2.75) | 0.12 |
| LIS | 2.5 (0.75) | 2.1 (1) | 0.16 |
Data are expressed as medians (interquartile ranges), or absolute numbers where appropriate.
Rh-APC, Recombinant human activated protein C; MAP, mean arterial pressure; PaO2, partial arterial oxygen tension; FiO2, fraction of inspired oxygen; PEEP, positive end expiratory pressure; APACHE, Acute Physiology and Chronic Health Evaluation; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; LIS, Lung Injury Score.
Systemic anticoagulant effects of infusion of rh-APC
An Infusion of rh-APC was associated with higher APC levels in plasma during treatment (P=0.001). After discontinuation of an infusion of rh-APC, APC levels in plasma became comparable to those in patients receiving placebo (Fig. 2 ). An infusion of rh-APC was associated with lower TATc levels in plasma during and after treatment with rh-APC, and levels remained lower until day 7 (P=0.014) (Fig. 2). An infusion of rh-APC was associated with higher PAA levels in plasma during and after treatment (until day 7) with rh-APC (P=0.014) (Fig. 2), and lower PAI-1 levels in plasma (P=0.014) (Fig. 2).
Figure 2.
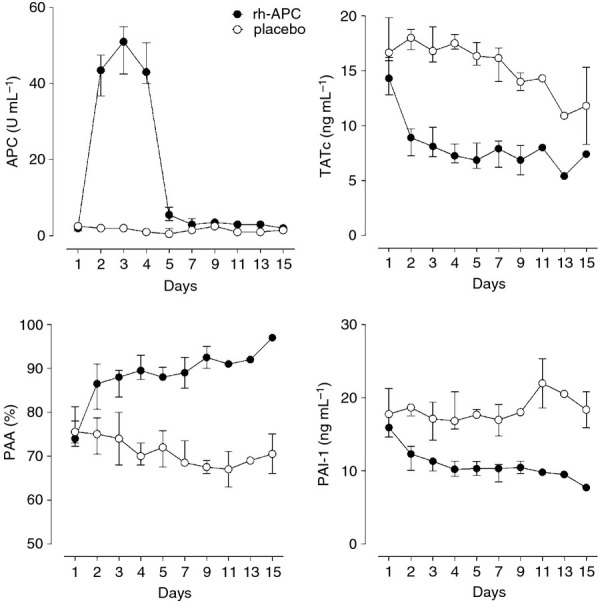
Effect of recombinant human activated protein (rh-APC) administration on systemic levels of APC, thrombin–antithrombin complexes (TATc), plasminogen activator activity (PAA) and plasminogen activator inhibitor-1 (PAI-1). Differences in APC were significant on days 2–5 (P < 0.01). Differences in TATc, PAA and PAI-1 were significant on days 2–5 (P < 0.01) and day 7 (P < 0.05).
Pulmonary anticoagulant effects of infusion of rh-APC
An Infusion of rh-APC was associated with increased APC levels in NBLF (P = 0.028), which remained higher than in patients treated with placebo up to the end of the observation period (Fig. 3 ). An infusion of rh-APC was associated with a reduction in the thrombin generation marker TATc in NBLF, during and after an infusion of rh-APC (P=0.009) (Fig. 3). However, AT levels in NBLF were similar in both study groups (Fig. 3). PAA levels in NBLF were higher in patients treated with rh-APC (P=0.01) (Fig. 3). Similar as in plasma, levels of PAI-1 were lower in NBLF (P=0.01) (Fig. 3). Levels of sTF in NBLF showed a marked decline in patients treated with rh-APC (P=0.011) (Fig. 3).
Figure 3.
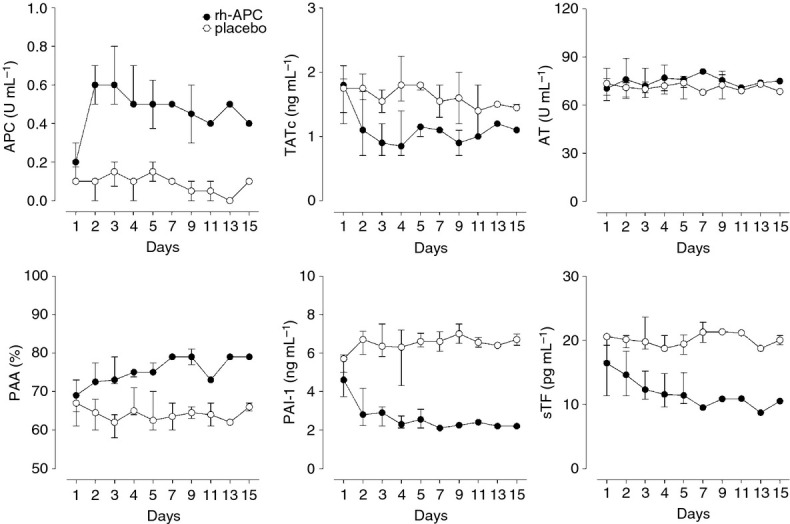
Effect of recombinant human activated protein (rh-APC) administration on levels of APC, thrombin–antithrombin complexes (TATc), antithrombin (AT), plasminogen activator activity (PAA), plasminogen activator inhibitor-1 (PAI-1) and soluble tissue factor (sTF) in non-directed bronchoalveolar lavage fluid. Differences in APC, PAA, PAI-1 and sTF were significant on days 2–4 (P < 0.01) and day 5 (P < 0.05). Differences in TATc were significant on days 2–5 (P < 0.01). There were no significant differences in AT.
Clinical parameters
In patients treated with rh-APC the baseline LIS (P = 0.005) and SAPS (0.013) had decreased on day 5, with a reduced SOFA score following on day 15 (P = 0.046). In patients treated with placebo, there was a decrease in the SOFA score on day 5 (P = 0.047), which did not endure until day 15. The number of ventilator-free days appeared to be less in patients treated with Rh-APC, but statistical significance was not reached. Mortality, both ICU and in-hospital, was similar in both groups (Table 2 ).
Table 2.
Clinical parameters
| Rh-APC n = 16 | Placebo n = 11 | P-value | |
|---|---|---|---|
| SOFA | |||
| Day 1 | 8 (3) | 9 (2) | 0.67 |
| Day 5 | 8 (2.75) | 8 (2) | 0.70 |
| Day 15 | 4.5 (4.75) | 8 (5) | 0.12 |
| SAPS | |||
| Day 1 | 47 (28.5) | 36 (25) | 0.32 |
| Day 5 | 38.5 (14.25) | 34 (18) | 0.43 |
| Day 15 | 31.5 (8) | 35 (5) | 0.24 |
| LIS | |||
| Day 1 | 2.75 (1.5) | 2.5 (0.5) | 0.12 |
| Day 5 | 2.1 (1.2) | 2.75 (1.7) | 0.58 |
| Day 15 | 1.3 (1.4) | 2.75 (3.25) | 0.92 |
| Days on ventilator | 8.5 (14) | 8 (24) | 0.86 |
| Ventilator-free days | 11 (22) | 11 (24) | 0.75 |
| 28-day mortality | 3 | 2 | 1 |
| ICU mortality | 4 | 5 | 0.41 |
| In-hospital mortality | 5 | 5 | 0.69 |
Data are expressed as medians (interquartile ranges), or absolute numbers where appropriate.
Rh-APC, Recombinant human activated protein C; SOFA, Sequential Organ Failure Assessment; LIS, Lung Injury Score; SAPS, Simplified Acute Physiology Score; ICU, intensive care unit.
Discussion
We have analyzed the effect of systemically administered rh-APC on pulmonary coagulopathy in patients with ARDS. The results are summarized as follows: (i) an infusion of rh-APC increased APC levels in the pulmonary compartment in patients with ARDS, (ii) an infusion of rh-APC attenuated systemic coagulopathy, (iii) and pulmonary coagulopathy in patients with ARDS, (iv) an infusion of rh-APC resulted in a faster resolution of pulmonary dysfunction, and (v) an infusion of rh-APC was not associated with bleeding complications.
Reduced levels of (activated) protein C are associated with non-pulmonary organ system dysfunction and increased mortality in ARDS 11. Equally, high levels of PAI-1 prognosticate a poor outcome 11., 24.. Although it has been suggested that APC may have beneficial effects in ARDS 5., 25., as demonstrated in numerous animal models of ARDS, and in a human model with intrapulmonary delivered endotoxin (LPS) 9, this is the first study to investigate this hypothesis in clinical patients. Even although rh-APC is no longer licensed for the treatment of severe sepsis, it still may have a role in the treatment of ARDS.
APC is a natural anticoagulant that inactivates coagulation factors (F)Va and VIIIa. Furthermore, it decreases the synthesis and expression of sTF on leukocytes 26., 27.. Systemic administration of rh-APC expectedly resulted in increased plasma levels of APC during the infusion period of 96 h after which plasma levels of APC returned to levels comparable to the placebo group. In line with this finding, as has been demonstrated previously, systemic levels of TATc and PAI-1 decreased whereas PAA increased 28.
Levels of TATc and sTF have previously been used as markers for local coagulation activation 9. At baseline, concentrations of TATc and sTF were comparable in the rh-APC and placebo group, indicating a comparable amount of coagulation activation. The plasma levels of both TATc and sTF in NBLF were markedly reduced after the infusion of rh-APC. In addition, APC was detectable in NBLF of these patients and still increased as compared with baseline on day 5, when the rh-APC infusion had already been stopped. This is an interesting finding, as APC was no longer detectable in the blood within 2 h after the infusion was stopped in the majority of patients with sepsis 29. This suggests that APC can enter the alveolar space after i.v. administration and that its pulmonary clearance is relatively slow.
During sepsis, systemic fibrinolysis is inhibited mainly as a result of elevated levels of PAI-130. In the alveolar compartment of patients with ARDS, fibrinolysis is suppressed as well 31. APC may augment fibrinolysis through inhibition of PAI-1. Our results show that APC markedly reduced PAI-1 levels in NBLF. However, PAA was enhanced systemically compared with baseline, but this enhancement did not reach statistical significance in NBLF. A possible explanation may be that tissue-type plasminogen activator (tPA) and urokinase-plasminogen activator (uPA), together responsible for the largest part of PAA, are bound to their inhibitor PAI-1. That way, the net PAA may have been decreased because of heightened formation of PAI-1. Comparable observations were made in the alveolar compartment of patients with pneumonia, and in healthy volunteers challenged with i.v. or intrapulmonary LPS 9., 32., 33..
The present study on the effects of rh-APC on pulmonary coagulopathy in patients with ARDS, is in line with previous findings in sepsis and a human model of lung injury 9., 34.. Systemic administration of rh-APC results in reduced activation of coagulation both systemically and in the alveolar compartment. These anticoagulant effects are exerted through inactivation of FVa and FVIIIa, and as a consequence inhibition of thrombin formation. This may in part be enhanced by inhibition of TF, which may have prevented protein C consumption 27. A profibrinolytic effect of APC could not be demonstrated in the alveolar compartment, as opposed to the systemic effects. Our findings are in conflict with two earlier studies investigating the effect of rh-APC, administered in a similar fashion as in our study, in human volunteers intravenously challenged with LPS, which did not observe an effect of the treatment on coagulation activation 35., 36.. In these studies, however, only systemic markers of coagulation were measured. Furthermore, systemic coagulopathy caused by endotoxin in healthy volunteers may differ from systemic coagulopathy in critically ill patients.
The aforementioned effects, may well have resulted in the faster resolution of pulmonary dysfunction in the patients treated with rh-APC, as expressed by the more rapid decrease in LIS, as compared with patients treated with placebo. The LIS consists of a PaO2/FiO2 ratio, level of PEEP, compliance and pulmonary radiography. These single components improved over time, although the difference between both treatment groups did not become statistically significant. Yet, this did not translate into a reduced number of ventilator-free days, compared with the placebo group, which may be attributable to the relatively small sample size.
Our study has some important limitations. We chose to analyze the effects of this trial on systemic and pulmonary coagulation only in intubated and mechanically ventilated patients in which it was deemed safe to perform serial NBL, thereby inducing selection bias. We may have missed out on the more severe cases of ARDS, which factor on the other hand may be balanced by the patients that were not sedated enough to undergo NBL. Also, the relatively small study populations may have resulted in a limited statistical power to detect clinical differences between study groups. Furthermore, the study population of our trial differed significantly from that of most clinical trials of ARDS because of exclusion of those patients with severe sepsis or septic shock who had an indication for rh-APC treatment in line with international guidelines at the time of the study, and with the exclusion of patients with increased risk of bleeding. Indeed, we had to exclude 94% of patients with ALI. This is, however, in line with a clinical trial comparing rh-APC with placebo with similar in- and exclusion criteria 37.
Finally, this trial was initiated when rh-APC was still in use. Nowadays, commercial rh-APC (Xigris®) has been taken off the market, and its role in the treatment of severe sepsis appears to have subsided, although controversies remain 1. However, our trial may contribute to identifying new areas in which rh-APC can possibly be of value in the near future.
Conclusion
This study demonstrates for the first time in selected mechanically ventilated patients with ARDS that systemically administered rh-APC has a powerful pulmonary anticoagulant effect. In patients treated with rh-APC, this resulted in a faster resolution of pulmonary dysfunction as expressed by an improvement of LIS, and organ dysfunction in general as expressed by an improved SAPS. Together with previous (animal) studies, the present study underlines the potential of APC as a therapy for ARDS.
Disclosure of Conflict of Interests
This study was partly funded by Eli Lilly, Indianapolis, IN, USA. Eli Lilly provided the study medication and financed the execution of this study.
Addendum
Study concept and design: A. Cornet, J-J. Hofstra, M. Schultz, A. Groeneveld, A. Beishuizen. Acquisition of data: A. Cornet, J-J. Hofstra, A. Vlaar. Analysis and interpretation of data: A. Cornet, M. Schultz, A. Groeneveld, A. Beishuizen. Drafting of the manuscript: A. Cornet, J-J. Hofstra, M. Schultz, A. Groeneveld, A. Beishuizen. Critical revision of the manuscript for important intellectual content: A. Vlaar, P. Tuinman, M. Levi, A. Girbes, M. Schultz, A. Groeneveld, A. Beishuizen. Administrative, technical,and material support: A. Cornet, J-J. Hofstra, M. Levi, A. Girbes. Study supervision: M. Schultz, A. Groeneveld, A. Beishuizen.
References
- 1.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute Respiratory Distress Syndrome: the Berlin Definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Wind J., Versteegt J., Twisk J., van Bindels A.J., Spijkstra J.J., Girbes A.R., Groeneveld A.B. Epidemiology of acute lung injury and acute respiratory distress syndrome in The Netherlands: A survey. Respir Med. 2007;101:2091–8. doi: 10.1016/j.rmed.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Cepkova M., Matthay M.A. Pharmacotherapy of acute lung injury and the acute respiratory distress syndrome. J Intensive Care Med. 2006;21:119–43. doi: 10.1177/0885066606287045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz M.J., Haitsma J.J., Zhang H., Slutsky A.S. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia–a review. Crit Care Med. 2006;34:871–7. [PubMed] [Google Scholar]
- 6.Dahlem P., Bos A.P., Haitsma J.J., Schultz M.J., Meijers J.C., Lachmann B. Alveolar fibrinolytic capacity suppressed by injurious mechanical ventilation. Intensive Care Med. 2005;31:724–32. doi: 10.1007/s00134-005-2588-2. [DOI] [PubMed] [Google Scholar]
- 7.Dahlem P., Bos A.P., Haitsma J.J., Schultz M.J., Wolthuis E.K., Meijers J.C., Lachmann B. Mechanical ventilation affects alveolar fibrinolysis in LPS-induced lung injury. Eur Respir J. 2006;28:992–8. doi: 10.1183/09031936.06.00133104. [DOI] [PubMed] [Google Scholar]
- 8.Glas G.J., van der Sluijs K.F., Schultz M.J., Hofstra J.J., van der Poll T., Levi M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J Thromb Haemost. 2013;11:17–25. doi: 10.1111/jth.12047. [DOI] [PubMed] [Google Scholar]
- 9.van der Poll T., Levi M., Nick J.A., Abraham E. Activated protein C inhibits local coagulation after intrapulmonary delivery of endotoxin in humans. Am J Respir Crit Care Med. 2005;171:1125–8. doi: 10.1164/rccm.200411-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware L.B., Fang X., Matthay M.A. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L514–21. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
- 11.Ware L.B., Matthay M.A., Parsons P.E., Thompson B.T., Januzzi J.L., Eisner M.D. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–8. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard G.R., Vincent J.L., Laterre P.F., La Rosa S.P., Dhainaut J.F., Lopez-Rodriguez A., Steingrub J.S., Garber G.E., Helterbrand J.D., Ely E.W., Fisher C.J., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 13.Ranieri V.M., Thompson B.T., Barie P.S., Dhainaut J.F., Douglas I.S., Finfer S., Gardlund B., Marshall J.C., Rhodes A., Artigas A., Payen D., Tenhunen J., Al-Khalidi H.R., Thompson V., Janes J., Macias W.L., Vangerow B., Williams M.D. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 14.Laterre P.F., Garber G., Levy H., Wunderink R., Kinasewitz G.T., Sollet J.P., Maki D.G., Bates B., Yan S.C., Dhainaut J.F. Severe community-acquired pneumonia as a cause of severe sepsis: data from the PROWESS study. Crit Care Med. 2005;33:952–61. doi: 10.1097/01.ccm.0000162381.24074.d7. [DOI] [PubMed] [Google Scholar]
- 15.Sebag S.C., Bastarache J.A., Ware L.B. Therapeutic modulation of coagulation and fibrinolysis in acute lung injury and the acute respiratory distress syndrome. Curr Pharm Biotechnol. 2011;12:1481–96. doi: 10.2174/138920111798281171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware L.B., Camerer E., Welty-Wolf K., Schultz M.J., Matthay M.A. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L307–11. doi: 10.1152/ajplung.00157.2006. [DOI] [PubMed] [Google Scholar]
- 17.Bernard G.R., Artigas A., Brigham K.L., Carlet J., Falke K., Hudson L., Lamy M., Legall J.R., Morris A., Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 18.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 19.Le Gall J.R., Lemeshow S., Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 20.Vincent J.L., Moreno R., Takala J., Willatts S., de Mendonca M.A., Bruining H., Reinhart C.K., Suter P.M., Thijs L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 21.Murray J.F., Matthay M.A., Luce J.M., Flick M.R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–3. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 22.Tuinman P.R., Vlaar A.P., Cornet A.D., Hofstra J.J., Levi M., Meijers J.C., Beishuizen A., Schultz M.J., Groeneveld A.J., Juffermans N.P. Blood transfusion during cardiac surgery is associated with inflammation and coagulation in the lung: a case control study. Crit Care. 2011;15:R59. doi: 10.1186/cc10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi M., de Boer J.P., Roem D., ten Cate J.W., Hack C.E. Plasminogen activation in vivo upon intravenous infusion of DDAVP. Quantitative assessment of plasmin-alpha 2-antiplasmin complex with a novel monoclonal antibody based radioimmunoassay. Thromb Haemost. 1992;67:111–16. [PubMed] [Google Scholar]
- 24.Gunther A., Mosavi P., Heinemann S., Ruppert C., Muth H., Markart P., Grimminger F., Walmrath D., Temmesfeld-Wollbrück B., Seeger W. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000;161:454–62. doi: 10.1164/ajrccm.161.2.9712038. [DOI] [PubMed] [Google Scholar]
- 25.Cornet A.D., van Nieuw Amerongen G.P., Beishuizen A., Schultz M.J., Girbes A.R., Groeneveld A.B. Activated protein C in the treatment of acute lung injury and acute respiratory distress syndrome. Expert Opin Drug Discov. 2009;4:219–27. doi: 10.1517/17460440902721204. [DOI] [PubMed] [Google Scholar]
- 26.Shua F., Kobayashia H., Fukudomeb K., Tsuneyoshib N., Kimotob M., Teraoa T. Activated protein C suppresses tissue factor expression on U937 cells in the endothelial protein C receptor-dependent manner. FEBS Lett. 2000;477:208–12. doi: 10.1016/s0014-5793(00)01740-3. [DOI] [PubMed] [Google Scholar]
- 27.Esmon C.T. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 28.Mosnier L.O., Zlokovic B.V., Griffin J.H. The cytoprotective protein C pathway. Blood. 2007;109:3161–72. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 29.Macias W.L., Dhainaut J.F., Yan S.C., Helterbrand J.D., Seger M., Johnson G., III, Small D.S. Pharmacokinetic-pharmacodynamic analysis of drotrecogin alfa (activated) in patients with severe sepsis. Clin Pharmacol Ther. 2002;72:391–402. doi: 10.1067/mcp.2002.128148. [DOI] [PubMed] [Google Scholar]
- 30.Raaphorst J., Johan Groeneveld A.B., Bossink A.W., Erik H.C. Early inhibition of activated fibrinolysis predicts microbial infection, shock and mortality in febrile medical patients. Thromb Haemost. 2001;86:543–9. [PubMed] [Google Scholar]
- 31.Suffredini A.F., Harpel P.C., Parrillo J.E. Promotion and subsequent inhibition of plasminogen activation after administration of intravenous endotoxin to normal subjects. N Engl J Med. 1989;320:1165–72. doi: 10.1056/NEJM198905043201802. [DOI] [PubMed] [Google Scholar]
- 32.Choi G., Schultz M.J., van Till J.W., Bresser P., van der Zee J.S., Boermeester M.A., Levi M., van der Poll T. Disturbed alveolar fibrin turnover during pneumonia is restricted to the site of infection. Eur Respir J. 2004;24:786–9. doi: 10.1183/09031936.04.00140703. [DOI] [PubMed] [Google Scholar]
- 33.Schultz M.J., Millo J., Levi M., Hack C.E., Weverling G.J., Garrard C.S., van der Poll T. Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax. 2004;59:130–5. doi: 10.1136/thorax.2003.013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhainaut J.F., Yan S.B., Margolis B.D., Lorente J.A., Russel J.A., Freebairn J.A., Spapen H.D., Riess H., Basson B., Johnson G., III, Kinasewitz G.T. Drotrecogin alfa (activated)(recombinant human activated protein C) reduces host coagulopathy response in patients with severe sepsis. Thromb Haemost. 2003;90:642–53. doi: 10.1160/TH02-11-0270. [DOI] [PubMed] [Google Scholar]
- 35.Derhaschnig U., Reiter R., Knobl P., Baumgartner M., Keen P., Jilma B. Recombinant human activated protein C (rhAPC; drotrecogin alfa [activated]) has minimal effect on markers of coagulation, fibrinolysis, and inflammation in acute human endotoxemia. Blood. 2003;102:2093–8. doi: 10.1182/blood-2003-02-0416. [DOI] [PubMed] [Google Scholar]
- 36.Kalil A.C., Coyle S.M., Um J.Y., La Rosa S.P., Turlo M.A., Calvano S.E., Sundin D.P., Nelson D.R., Lowry S.F. Effects of drotrecogin alfa (activated) in human endotoxemia. Shock. 2004;21:222–9. doi: 10.1097/01.shk.0000116778.27924.79. [DOI] [PubMed] [Google Scholar]
- 37.Liu K.D., Levitt J., Zhuo H., Kallet R.H., Brady S., Steingrub J., Tidswell M., Siegel M.D., Soto G., Peterson M.W., Chessnut M.S., Philips C., Weinacker A., Thompson B.T., Eisner M.D., Matthay M.A. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178:618–23. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalil A.C., LaRosa S.P. Effectiveness and safety of drotrecogin alfa (activated) for severe sepsis: a meta-analysis and metaregression. Lancet Infect Dis. 2012;12:678–86. doi: 10.1016/S1473-3099(12)70157-3. [DOI] [PubMed] [Google Scholar]


