Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccination may fail to sufficiently protect transplant recipients against coronavirus disease 2019 (COVID-19). We retrospectively evaluated COVID-19 in kidney transplant recipients (n = 226) after BNT162b2 mRNA vaccine administration. The control group consisted of unvaccinated patients (n = 194) during the previous pandemic wave. We measured anti-spike protein immunoglobulin G (IgG) levels and cellular responses, using enzyme-linked immunosorbent spot assay, in a prospective cohort after vaccination (n = 31) and recovery from COVID-19 (n = 19). COVID-19 was diagnosed in 37 (16%) vaccinated and 43 (22%) unvaccinated patients. COVID-19 severity was similar in both groups, with patients exhibiting a comparable need for hospitalization (41% vs. 40%, p = 1.000) and mortality (14% vs. 9%, p = .726). Short posttransplant periods were associated with COVID-19 after vaccination (p < .001). Only 5 (16%) patients achieved positive SARS-CoV-2 IgG after vaccination, and 17 (89%, p < .001) recovered from COVID-19 (median IgG levels, 0.6 vs. 52.5 AU/ml, p < .001). A cellular response following vaccination was present in the majority (n = 22, 71%), with an increase in interleukin 2 secreting T cells (p < .001). Despite detectable T cell immunity after mRNA vaccination, kidney transplant recipients remained at a high risk of severe COVID-19. Humoral responses induced by vaccination were significantly lower than that after COVID-19.
KEYWORDS: clinical research / practice, infectious disease, infection and infectious agents - viral, kidney transplantation / nephrology, SARS-CoV-2/COVID-19, vaccine
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; ELISpot, enzyme-linked immune absorbent spot assay; IFN-ɣ, interferon gamma; IL-2, interleukin 2; M, membrane protein; N, nucleoprotein; OR, odds ratio; ORF, open reading frame; PBMCs, peripheral blood mononuclear cells; PCR, polymerase chain reaction; RBD, receptor binding domain; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFC, spots forming cells
1. INTRODUCTION
Kidney transplant patients are among the most vulnerable to severe coronavirus disease 2019 (COVID-19) that is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). A high percentage of kidney recipients require hospitalization, and most studies indicate that mortality among them ranges between 10 and 30%.1, 2, 3, 4, 5, 6 In addition to chronic immunosuppression, adverse outcomes can be accounted for by the high proportion of kidney recipients who are elderly or have associated comorbidities.3, 4, 5, 6 Given the limited therapeutic options available, maximal efforts are directed toward the prevention of COVID-19.
Vaccination against SARS-CoV-2 is the preferred route to mitigate the impact of COVID-19. New mRNA vaccines show robust humoral and T cell immune responses and provide 95% protection against COVID-19 in the non-transplant population, including in elderly patients and those with comorbidities.7, 8, 9 The vaccine is highly efficacious even against SARS-CoV-2 variants of concern, such as B.1.1.7 (alpha) or B.1.617.2 (delta).10 , 11 Despite the fact that solid organ transplant recipients have not been included in COVID-19 vaccine trials, vaccination is recommended in this population.12 Kidney transplant recipients may exhibit long-term viral shedding; however, a significant majority develop anti-SARS-CoV-2 immunoglobulin G (IgG) antibodies and polyfunctional T cell immunity in levels that are comparable to non-immunosuppressed patients.13, 14, 15, 16, 17 Despite these promising data, a number of authors have reported poor humoral responses in transplant recipients after mRNA vaccination.18, 19, 20, 21, 22 Nevertheless, in contrast to the humoral response, cellular immunity was detected in a significantly high proportion of patients.22 , 23 From a clinical point of view, protection against COVID-19 in vaccinated patients after kidney transplantation remains unclear.
To determine the efficacy of vaccination, we retrospectively evaluated a cohort of kidney transplant recipients who received the BNT162b2 mRNA vaccine during January 2021, when the COVID-19 pandemic was at its peak in the Western Bohemia region. When the study began, the region experienced a 7-day incidence of 528 new cases per 100 000 population. The pandemic then peaked in March 2021, with values of 1050 new cases per 100 000 population (the “winter wave”), which corresponded to the highest incidence to date since the beginning of the pandemic. The course of COVID-19 was compared among a group of unvaccinated renal transplant recipients during the “autumn wave” of the pandemic, which began in early September 2020 with a 7-day incidence of 78 new cases per 100 000 population and peaked in late October 2020 at 817 cases per 100 000 population. In a follow-up prospective study, we measured the humoral and cellular immune responses after vaccination in another cohort who was vaccinated in February 2021 and compared them to the responses in patients who had recovered from COVID-19.
2. METHODS
2.1. Study population
The retrospective and prospective cohorts included adult kidney transplant recipients from a single transplant center (Charles University Teaching Hospital, Pilsen), all of whom were followed and treated at the center. All patients who received the BNT162b2 mRNA vaccine (Comirnaty, Pfizer/BioNTech, Mainz, Germany) in January 2021 (two doses at 4-week intervals) were retrospectively analyzed until the end of June 2021. Patients who contracted COVID-19 before vaccination were excluded. Two weeks after receiving the second dose, the patient was considered fully vaccinated. The control group consisted of unvaccinated patients with no previous diagnosis of COVID-19 who were retrospectively evaluated during the autumn wave from September 2020 to the end of December 2020. Patients with no history of COVID-19 who received BNT162b2 mRNA vaccination in February 2021 (two doses at 3-week intervals) were included in the prospective study. All patients were screened using polymerase chain reaction (PCR) for SARS-CoV-2 RNA from nasopharyngeal swabs, and SARS-CoV-2 IgG levels were determined at the time of the first dose. Individuals with positive PCR results and/or SARS-CoV-2 IgG were excluded. Both humoral (SARS-CoV-2 IgG) and cellular immune responses (enzyme-linked immunosorbent spot assay [ELISpot] technique) were evaluated 4 weeks after the second dose. The second group of the prospective study consisted of patients who had a previous COVID-19 infection 2–6 months prior to study participation, in whom humoral and cellular responses were assessed. The study was approved by the local ethics committee and conducted in compliance with the Declaration of Helsinki and Istanbul. Written informed consent was obtained from all the patients in the prospective cohort.
2.2. Assessment of SARS-CoV-2 humoral immunity
Peripheral venous blood samples were collected using the VACUETTE Blood Collection System (Greiner Bio-one Company, Kremsmünster, Austria). The serum was separated by a 10-min centrifugation at 1300× g and frozen at −80°C. Samples were thawed only once, just prior to the analysis. An automated chemiluminescent (CLIA) ACCESS SARS-CoV-2 IgG II assay (manufacturer’s catalog number C69057) was used to detect IgG antibodies against the receptor-binding domain (RBD) in the S1 subunit of the spike protein (IgG anti-S1-RBD) (Beckman Coulter, Brea, CA). Based on the manufacturer’s instructions, concentrations of antibodies >10 arbitrary units per milliliter (AU/mL) were considered to confirm the presence of antibodies against SARS-CoV-2.
2.3. Assessment of SARS-CoV-2 cellular immunity
The quantitation of interferon-gamma (IFN-ɣ) and interleukin-2 (IL-2)-producing cells was performed on isolated peripheral blood mononuclear cells (PBMCs) using a modified, dual-color, ELISpot analysis (Mabtech, Sweden), as described previously.24 PBMCs were obtained using density gradient centrifugation and stored in liquid nitrogen. Thawed PBMCs at a concentration of 2 × 105 cells per well were pipetted into ethanol-treated polyvinylidene difluoride 96-well microplates pre-coated with primary monoclonal antibodies specific for human IFN-γ and IL-2 (Mabtech, Sweden) and stimulated with antigens; an anti-CD3 monoclonal antibody (Mabtech, Sweden) and medium alone were used as positive and negative controls, respectively. Two sets of SARS-CoV-2 peptide pools were utilized for antigenic stimulation: a SARS-CoV-2 S1 scanning pool (Mabtech, Sweden) consisting of peptide 15-mers overlapping with 11 amino acids, covering the S1 domain of the spike protein (amino acids 13–685), and a SARS-CoV-2 SNMO-defined peptide pool (Mabtech, Sweden) containing 47 peptides from the human SARS-CoV-2 virus spike protein (S), nucleoprotein (N), membrane protein (M), and the open reading frame (ORF)-3a and ORF-7a proteins.25 All experiments were performed in duplicate wells. Cell cultures were cultivated in a CO2 incubator overnight, after which the plates were washed, dried, and incubated with detection antibodies and two fluorescent-labeled conjugates. Fluorescent spots were counted using an automated ELISpot reader equipped with filters for IFN-γ and IL-2 detection (AID, Germany). The mean spot count observed in the negative controls was subtracted from the count in the stimulated wells in each run. A result of ≥11–13 spot forming cells (SFC) per 2 × 105 cells for various stimulations was considered positive. This value corresponds to the 75th percentile in non-vaccinated patients with undetectable SARS-CoV-2 IgG, assuming a possible cross-reactivity due to previous infections with other common coronaviruses.26 , 27
2.4. Outcomes and follow-up
The primary endpoint in retrospective cohorts was the occurrence of COVID-19 in vaccinated and unvaccinated patients, diagnosed by a positive PCR test result for SARS-CoV-2 RNA from nasopharyngeal swabs or bronchoalveolar lavage; in rare cases, a positive rapid antigen test result was accepted. Secondary endpoints included COVID-19 severity and mortality, along with an analysis of potential factors that are predictive of COVID-19 development after vaccination. The course of COVID-19 was compared between the vaccinated and unvaccinated patients. The main parameters monitored in the prospective follow-up cohort were humoral and cellular immune responses 4 weeks after the second dose of vaccination. The humoral response of the cohort was compared with that of a group of patients who recovered from COVID-19. Other parameters monitored were the contraction of COVID-19 after vaccination and vaccination safety, as assessed by a questionnaire and laboratory monitoring of graft function 4 weeks after the completion of the vaccine course. The criteria for grading adverse events were as described above (Table S1).9 The follow-up period ended on June 30, 2021.
2.5. Statistical analysis
Quantitative parametric data were compared using Student’s t-test or Mann–Whitney U-test for non-parametric distribution. Qualitative data were analyzed using the χ2 or Fisher’s exact test. Due to the non-parametric distribution, the Wilcoxon signed-rank test was used to compare SARS-CoV-2 IgG levels and SFC counts after antigenic stimulation before and after vaccination. Recipient, donor, and transplant-related covariates (Table S2) were assessed as potential risk factors for COVID-19 after vaccination. First, logistic regression analyses were performed. Covariates with a p-value ≤ .2 were included in a multivariate forward stepwise logistic regression. Results are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Statistical calculations were performed using the SAS software (SAS Institute Inc., Cary, NC). Statistical significance was set at p < .05.
3. RESULTS
3.1. COVID-19 after vaccination
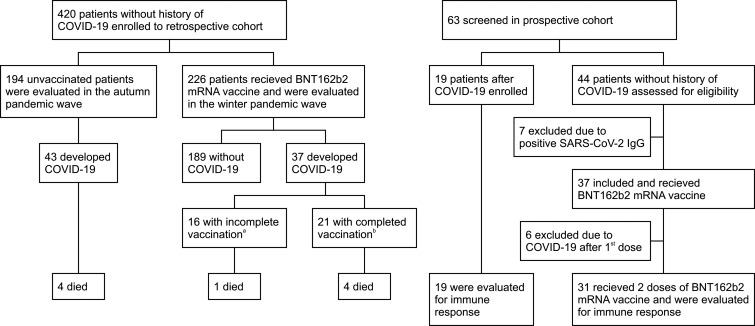
The retrospective cohort included 420 patients ( Figure 1). As of early January 2021, 226 patients received mRNA vaccines. Of these, 37 (16%) were later diagnosed with COVID-19. Sixteen of the patients who contracted COVID-19 had not been vaccinated completely; 21 patients were infected after completing the vaccination course. The median time from the first dose to the onset of COVID-19 was 45 days, with a maximum of 105 days, reflecting the decrease in cases in the region as a whole. During the autumn pandemic wave, 194 unvaccinated patients were evaluated, and 43 (22%) were diagnosed with COVID-19. Compared with the unvaccinated group, the mRNA vaccination patients had shorter posttransplant periods and were more likely to have diabetes and cardiovascular disease. The two groups were comparable in terms of other demographic characteristics and immunosuppressive therapy ( Table 1). The characteristics and treatment of COVID-19 are presented in Table 2 and S3. SARS-CoV-2 was confirmed using PCR in all but four patients. The B.1.1.7 variant was only detected in vaccinated patients during the winter pandemic wave (43% vs. 0%, p < .001). The course of COVID-19 was comparable between the vaccinated and unvaccinated groups, with both groups demonstrating a similar need for hospitalization (41% vs. 40%, p = 1.000). There was also no difference in mortality, with 5 (14%) deaths in the vaccinated group and 4 deaths (9%, p = .726) in the unvaccinated group. We did not observe a milder course of COVID-19 in patients with a complete course of vaccination compared to patients who were not vaccinated completely (Table S4).
FIGURE 1.
Flow of patients through the study. COVID-19, coronavirus disease 2019. aIncluded 5 patients with COVID-19 after 1st dose and 11 patients <14 days after 2nd dose. bMore than 14 days after 2nd dose
TABLE 1.
Characteristics of retrospective cohort according to vaccination status
| Unvaccinated (n = 194) | mRNA vaccination (n = 226) | p value | |
|---|---|---|---|
| Age (years) | 58 ± 13 | 58 ± 11 | .948 |
| Gender (male) | 130 (67) | 143 (63) | .485 |
| Time posttransplant (months) | 118 ± 85 | 88 ± 76 | <.001 |
| Previous transplantation | 21 (11) | 21 (9) | .720 |
| Cause of end stage renal disease | .054 | ||
| Chronic glomerulonephritis | 90 (46) | 111 (49) | |
| Diabetic nephropathy | 10 (5) | 14 (6) | |
| Polycystic kidney disease | 36 (19) | 40 (18) | |
| Hypertension/nephrosclerosis | 31 (16) | 21 (9) | |
| Other | 6 (16) | 34 (18) | |
| Duration of RRT (months) | 17 ± 17 | 19 ± 21 | .490 |
| Donor type (deceased) | 178 (92) | 210 (93) | .791 |
| Body mass index (kg/m2) | 29 ± 5 | 29 ± 5 | .087 |
| Diabetes | 44 (23) | 79 (35) | .008 |
| Hypertension | 192 (99) | 226 (100) | .413 |
| Cardiovascular disease | 55 (28) | 106 (47) | <.001 |
| Chronic pulmonary disease | 22 (11) | 26 (12) | .919 |
| Estimated GFRa (ml/min) | 49 ± 20 | 50 ± 20 | .993 |
| Immunosuppression at vaccination | |||
| Tacrolimus | 161 (83) | 195 (86) | .424 |
| Cyclosporine | 26 (13) | 25 (11) | .560 |
| Mycophenolate mofetil/sodium | 170 (88) | 200 (88) | .903 |
| Sirolimus | 5 (3) | 7 (3) | .980 |
| Depleting ALA within 6 months | 2 (1) | 2 (1) | .726 |
Note: Data are number of patients (percentage) or mean ± SD.
Abbreviations: ALA, antilymphocyte antibody; COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate; RRT, renal replacement therapy.
According to CKD-EPI formula.
TABLE 2.
Characteristics and treatment of COVID-19 in unvaccinated patients and after mRNA vaccination
| Unvaccinated (n = 43) | mRNA vaccination (n = 37) | p value | |
|---|---|---|---|
| Time from first dose of vaccine (days) | – | 52 ± 27 | |
| SARS-CoV−2 confirmation | |||
| PCR testa | 41 (95) | 35 (95) | 1.000 |
| Wild-type (WA1) | 41 (100) | 20 (57) | <.001 |
| B.1.1.7 variant | 0 (0) | 15 (43) | |
| Rapid Ag test | 2 (5) | 2 (5) | |
| Symptoms | |||
| Fever (≥38°C) | 24 (56) | 18 (49) | .820 |
| Cough | 32 (74) | 18 (49) | .057 |
| Fatigue/myalgia | 37 (86) | 35 (95) | .275 |
| Gastrointestinal | 14 (33) | 15 (41) | .492 |
| Acute kidney injury | 13 (30) | 12 (32) | 1.000 |
| Anosmia/ageusia | 8 (17) | 4 (11) | .367 |
| Confirmed pneumonia | 15 (35) | 14 (38) | .819 |
| Hospitalization | 17 (40) | 15 (41) | 1.000 |
| Treatment | |||
| Supplemental oxygenb | 14 (33) | 11 (30) | .814 |
| Dexamethasone | 13 (30) | 5 (14) | .107 |
| Remdesivir | 6 (14) | 10 (27) | .170 |
| SARS-CoV−2 mAbc | 0 (0) | 4 (11) | .042 |
| Antibiotics | 12 (28) | 10 (27) | 1.000 |
| Modification of immunosuppression | |||
| No changed | 3 (7) | 5 (14) | .461 |
| Mycophenolate mofetil withdrawale | 37 (86) | 30 (81) | .562 |
| Reduction of tacrolimusf | 17 (40) | 15 (41) | 1.000 |
| Deathg | 4 (9) | 5 (14) | .726 |
Note: Data are number of patients (percentage) or mean ± SD.
Abbreviations: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Nasopharyngeal swab or bronchoalveolar lavage.
Mechanical ventilation in 1 unvaccinated and 2 vaccinated patients, high-flow oxygen device in 3 unvaccinated and 1 vaccinated patient.
Casirivimab + imdevimab in 3, bamlanivimab in 1. SARS-CoV-2 mAb were not available during autumn pandemic wave. One patient progressed to severe COVID-19 and died in spite of casirivimab + imdevimab administration.
Including 1 vaccinated patient with fulminant course resulting in death within 12 h after admission.
4 unvaccinated patients and 5 after vaccination were not treated with mycophenolate mofetil at the time of diagnosis.
Targeting trough levels of 4–6 ng/ml in all hospitalized patients.
Including 1 vaccinated patient with sudden death after discharge >1 months after COVID-19 diagnosis.
When compared with the non-COVID-19 group patients, patients who contracted COVID-19 did so in a significantly reduced period of time following their transplant, had received renal replacement therapy for a shorter period of time, and were more likely to have received tacrolimus and antilymphocyte antibody depletion in the 6 months prior ( Table 3). However, the multivariate analysis showed that temporal proximity to transplantation was the only independent risk factor for contracting COVID-19 post-vaccination. The presence of cardiovascular disease was protective ( Table 4).
TABLE 3.
Characteristics of patients with and without COVID-19 after vaccination
| COVID-19 (n = 37) | Without COVID-19 (n = 189) | p value | |
|---|---|---|---|
| Age (years) | 55 ± 13 | 58 ± 11 | .192 |
| Gender (male) | 20 (54) | 123 (65) | .278 |
| Time posttransplant (months) | 34 ± 27 | 99 ± 78 | <.001 |
| Previous transplantation | 4 (11) | 17 (9) | .969 |
| Cause of end stage renal disease | .231 | ||
| Chronic glomerulonephritis | 22 (59) | 89 (47) | |
| Diabetic nephropathy | 2 (6) | 12 (6) | |
| Polycystic kidney disease | 4 (11) | 36 (19) | |
| Hypertension/nephrosclerosis | 3 (8) | 18 (10) | |
| Other | 6 (16) | 34 (18) | |
| Duration of RRT (months) | 12 ± 16 | 20 ± 21 | .010 |
| Donor type (deceased) | 36 (97) | 174 (92) | .433 |
| Body mass index (kg/m2) | 28 ± 5 | 30 ± 6 | .340 |
| Diabetes | 11 (30) | 68 (36) | .589 |
| Hypertension | 37 (100) | 189 (100) | – |
| Cardiovascular disease | 12 (32) | 94 (50) | .080 |
| Chronic pulmonary disease | 4 (11) | 22 (12) | .891 |
| Estimated GFRa (ml/min) | 46 ± 17 | 51 ± 20 | .149 |
| Immunosuppression at vaccination | |||
| Tacrolimusb | 36 (97) | 159 (84) | .062 |
| Cyclosporine | 0 (0) | 25 (13) | .039 |
| Mycophenolate mofetil/sodium | 32 (86) | 168 (89) | .891 |
| Sirolimus | 1 (3) | 6 (3) | .713 |
| Depleting ALA within 6 monthsc | 2 (5) | 0 (0) | .024 |
Note: Data are number of patients (percentage) or mean ± SD.
Abbreviations: ALA, antilymphocyte antibody; COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate; RRT, renal replacement therapy.
According to CKD-EPI formula.
Median tacrolimus trough levels were comparable in both groups (6.8 vs. 6.7 ng/ml, p = .767).
Thymoglobulin used for induction therapy in both patients.
TABLE 4.
Risk factors for COVID-19 after mRNA vaccination
| Odds ratio | 95% CI | p value | |
|---|---|---|---|
| Univariatea | |||
| Time posttransplant (per 1 month increase) | 0.971 | 0.946–0.996 | .026 |
| Age (per 1 year increase) | 0.977 | 0.947–1.008 | .144 |
| Chronic glomerulonephritis (ref. = other causes) | 1.757 | 0.858–3.594 | .123 |
| Cardiovascular disease (ref. = none) | 0.485 | 0.230–1.022 | .057 |
| Tacrolimus (ref. = cyclosporine or sirolimus)b | 6.791 | 0.897–51.4 | .064 |
| Estimated GFR (per 1 ml/min increase) | 0.986 | 0.968–1.005 | .150 |
| Multivariate | |||
| Time posttransplant (per 1 month increase) | 0.975 | 0.963–0.987 | <.001 |
| Cardiovascular disease (ref. = none) | 0.441 | 0.198–0.981 | .045 |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate.
Variables with p-value ≤ .2 are shown.
Both tacrolimus level (odds ratio, 1.036, 95% CI, 0.872–1.230 per 1 ng/ml increase, p = .688) and mycophenolate mofetil dose (odds ratio, 1.000, 95% CI, 1.000–1.001 per 1 mg/day increase, p = .361) did not reach significance.
3.2. SARS-CoV-2–specific immune response post-vaccination and after COVID-19: a prospective cohort
Of the 44 patients without a history of COVID-19, 7 were excluded due to the presence of SARS-CoV-2 IgG, and 37 patients received the mRNA vaccine. Six patients were diagnosed with COVID-19 after the first dose, one of whom died. These patients were excluded from the analysis (Figure 1). Thirty-one patients received both doses of the mRNA vaccine and subsequently had their humoral and cellular immune responses analyzed. The COVID-19 group consisted of 19 patients 2–6 months (median, 107 days) after diagnosis. All patients had mild or moderate disease (Table S5). Post-vaccination and post-COVID-19 patients did not differ in baseline demographics or immunosuppressive treatment ( Table 5).
TABLE 5.
Characteristics of patients after mRNA vaccination and patients recovered from COVID-19
| Vaccination (n = 37) | Post-COVID-19 (n = 19) | p value | |
|---|---|---|---|
| Age (years) | 51 ± 13 | 53 ± 9 | .555 |
| Gender (male) | 24 (65) | 15 (79) | .364 |
| Time posttransplant (months) | 100 ± 78 | 126 ± 87 | .350 |
| Previous transplantation | 5 (14) | 1 (5) | .652 |
| Cause of end stage renal disease | .243 | ||
| Chronic glomerulonephritis | 19 (51) | 8 (42) | |
| Diabetic nephropathy | 2 (5) | 0 (0) | |
| Polycystic kidney disease | 4 (11) | 4 (21) | |
| Hypertension/nephrosclerosis | 7 (19) | 1 (5) | |
| Other | 5 (14) | 4 (21) | |
| Duration of RRT (months) | 16 ± 13 | 25 ± 26 | .446 |
| Donor type (deceased) | 35 (95) | 16 (84) | .324 |
| Body mass index (m2/kg) | 29 ± 5 | 28 ± 4 | .340 |
| Diabetes | 8 (22) | 2 (11) | .467 |
| Hypertension | 36 (97) | 19 (100) | 1.000 |
| Cardiovascular disease | 11 (30) | 4 (21) | .543 |
| Chronic pulmonary disease | 3 (8) | 1 (5) | 1.000 |
| Estimated GFRa (ml/min) | 47 ± 19 | 53 ± 15 | .222 |
| Immunosuppression at vaccination/COVID−19 | |||
| Tacrolimus | 33 (89) | 16 (84) | .679 |
| Cyclosporine | 4 (11) | 3 (16) | .679 |
| Mycophenolate mofetil/sodium | 37 (100) | 19 (100) | 1.000 |
| Depleting ALA within 6 months | 1 (3) | 1 (5) | 1.000 |
Note: Data are number of patients (percentage) or mean ± SD.
Abbreviations: ALA, antilymphocyte antibody; COVID-19, coronavirus disease 2019; GFR, glomerular filtration rate; RRT, renal replacement therapy.
According to CKD-EPI formula.
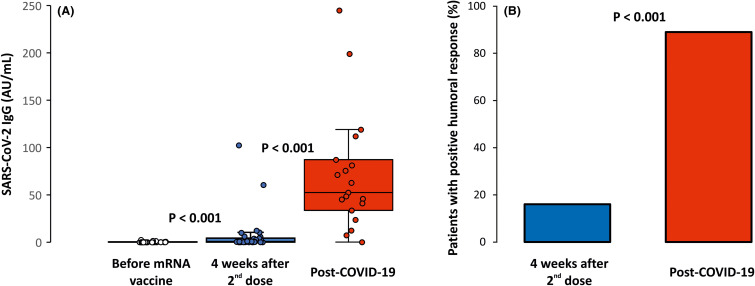
Although there was a significant, albeit very modest, increase in anti-RBD-S1 IgG levels after the completion of vaccination compared with pre-vaccination levels (0.4 ± 0.5 vs. 7.4 ± 20.8 AU/ml, p < .001), only 5 patients (16%) achieved a positive humoral response in contrast to a significant majority of 17 patients (89%, p < .001) who recovered from COVID-19. IgG levels were dramatically higher in the post-COVID-19 group than in the mRNA vaccine group (7.4 ± 20.8 vs. 71.8 ± 62.5 AU/ml, p < .001) ( Figure 2 and Table S6).
FIGURE 2.
Humoral response after mRNA vaccination and after COVID-19. IgG levels against S1 subunit of SARS-CoV-2 spike protein are shown (A) and the percentage of patients with positive response are defined by a value of ≥10 AU/ml (B). Minimum and maximum values excluding outliers represented by whiskers, median and interquartile range inside boxes. A Wilcoxon signed-rank test was used for comparison of IgG levels before and after vaccination, a Mann-Whitney U-test and χ2 test used for comparison of IgG levels and positive response after vaccination and after COVID-19. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
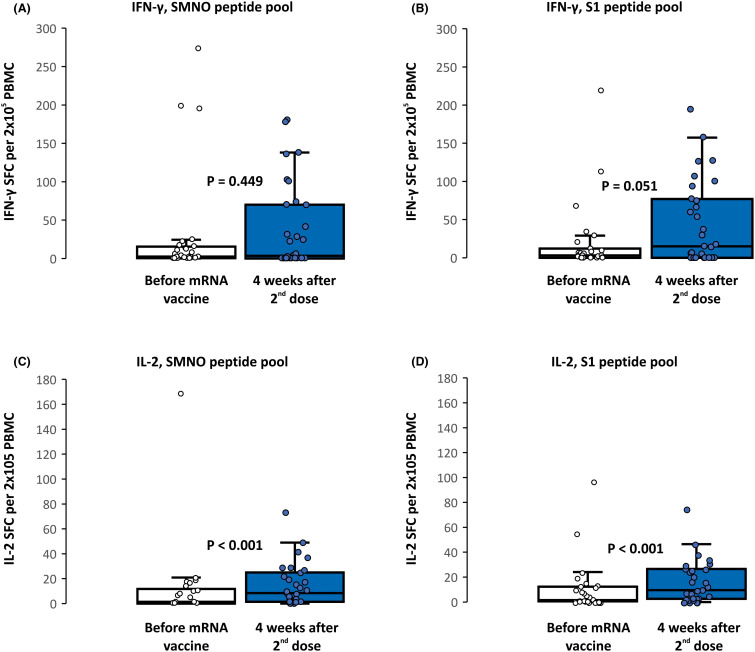
After vaccination, there was an increase in SARS-CoV-2–specific T cell counts assessed by ELISpot after stimulation with both the complex SNMO peptide pool and the S1 peptide pool ( Figure 3 and Table S7), although significant differences were observed only for IL-2–secreting cells (11 ± 30 vs. 15 ± 17 SFC per 2 × 105 PBMCs with SNMO peptide pool, and 10 ± 20 vs. 16 ± 17 SFC per 2 × 105 PBMCs with S1 peptide pool, p < .001). The percentage of patients with a positive cellular response increased from 26%–30% before vaccination to 45%–55% after vaccination (Table S4). A positive cellular response with at least one mode of stimulation (IFN-γ or IL-2 secreting cells) was achieved in 22 patients (71%) after vaccination, compared with 14 (47%, p = .071) before vaccination. All patients with a humoral response also showed a positive cellular response. An analysis of demographic factors and immunosuppressive treatment failed to show any factor that predicted response to the mRNA vaccine (Table S8). SARS-CoV-2-specific T cells could only be evaluated in 11 patients who recovered from COVID-19 with a comparable response to patients after vaccination (Table S7).
FIGURE 3.
Cellular response after mRNA vaccination by IFN-γ (A, B) or IL-2 (C, D) secreting T cells. Assessed by ELISpot after stimulation by SARS-CoV-2 SMNO or S1 antigen pools and expressed as SFC per 2 × 105 PBMC. Minimum and maximum values excluding outliers represented by whiskers, median and interquartile range inside boxes. Wilcoxon signed-rank test used for comparison. M, membrane protein; N, nucleoprotein; O, open reading frame proteins; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFC, spot forming cells; PBMC, peripheral blood mononuclear cells
3.3. The safety of the mRNA vaccine
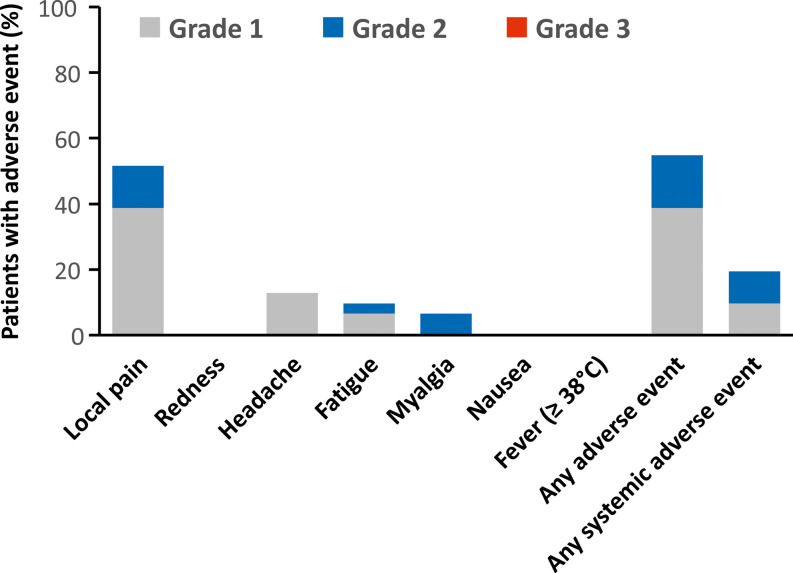
The first and second doses of the BNT162b2 mRNA vaccine were well tolerated. Local pain at the injection site was the most commonly reported adverse reaction (52%), with only mild to moderate reactions in all cases ( Figure 4). Systemic reactions occurred in 19% of patients. Renal function remained stable after vaccination, and none of the patients developed graft rejection.
FIGURE 4.
Local and systemic reactions reported after the BNT162b2 mRNA vaccination. Assessed in patients without COVID-19 after vaccination (n = 31). There were no grade 3 or 4 adverse reactions nor any hospitalizations related to vaccination. Serum creatinine remained stable 4 weeks after the second dose of vaccine (157 ± 97 μmol/L) compared to pre-vaccination level (162 ± 94 μmol/L)
4. DISCUSSION
Our study shows that despite a good safety profile, the lack of efficacy of the BNT162b2 mRNA vaccine is a major problem in the kidney transplant recipient population. As many as 80% of the vaccinated patients in our study failed to achieve a positive humoral response after two doses, in contrast to the 90% response rate in patients who had experienced a COVID-19 infection. In addition, anti-RBD-S1 IgG levels were dramatically higher after COVID-19 than after vaccination. Indicators of cellular response showed a significant increase after vaccination, and detectable SARS-CoV-2–specific immunity was demonstrable in 71% of patients, yet real protection from COVID-19 was minimal. In epidemically unfavorable conditions, 16% of the vaccinated patients became infected within 3 months, with a high proportion of severe cases and significant mortality. The course of COVID-19 was similar to that seen in unvaccinated renal transplant recipients during the previous autumn pandemic wave, with comparable mortality rates. Finally, patients with a completed course of vaccination did not have milder symptoms than those with incomplete vaccinations.
It should be emphasized that patient follow-up took place during the peak of the pandemic when SARS-CoV-2 variant B.1.1.7 (alpha), a strain with high transmissibility, was widespread among the population, which influenced the high prevalence of COVID-19 in our cohort.28 , 29 Despite recommendations to follow anti-epidemic measures even after vaccination, the incidence of COVID-19 was not lower in transplant recipients than in the general, largely unvaccinated population. In contrast, 37 infections in 226 vaccinated patients in 105 days, corresponding to a 7-day incidence of 1091 new cases per 100 000 population, exceeded the incidence in the general population over the same period (528 cases at the start of the study, with a peak of 1050 cases). Although we did not observe milder symptoms of COVID-19 in the vaccinated group in comparison with our autumn wave cohort, our data are not definitive, and it cannot be excluded that vaccination may attenuate the course of COVID-19. Our control group was not fully comparable to the vaccination cohort, as the patients had long posttransplant periods and were all infected with wild-type SARS-CoV-2. Adherence to anti-epidemic measures may be low in vaccinated patients. However, some effective COVID-19 treatment options, such as neutralizing monoclonal antibodies against the SARS-CoV-2 spike protein, were not available during the autumn wave. The outcomes of COVID-19 in our patients appear as adverse as in cohorts of renal transplant recipients before the vaccination era.2, 3, 4, 5, 6 Although the 14% mortality rate is near the lower end of previously published data, a number of studies have described outcomes predominantly in patients hospitalized for COVID-19.5 , 6 Our study covers all cases of COVID-19, including non-hospitalized patients, where mortality may be below 10%.3 Consistent with our observation, several recently published pilot studies have described the emergence of COVID-19 in renal transplant recipients after receiving the mRNA vaccines, with the need for hospitalization in up to 48% of these patients and mortality rates as high as 28%.30 , 31 To date, no factors associated with the development of COVID-19 after vaccination have been published. In our cohort, temporal proximity to transplantation was the strongest risk. This may be due not only to the high level of immunosuppression in patients shortly after transplantation but also to the need for frequent hospital visits with increased epidemiological risk. However, an analysis of immunosuppressive drugs did not show an association between individual immunosuppressive drugs and COVID-19. The reason for the protective effect of cardiovascular disease was not apparent. However, it can be speculated that patients without cardiovascular disease are mobile, have social contacts, and are therefore at great risk of being infected with SARS-CoV-2.
In a follow-up prospective study, only 16% of patients achieved positive anti-RBD-S1 IgG levels 4 weeks after the administration of two doses of the BNT162b2 mRNA vaccine. Inadequate humoral responses after vaccination have been repeatedly reported in solid organ transplant recipients.18, 19, 20, 21, 22, 23 Some authors describe a high proportion of patients able to generate anti-SARS-CoV-2 antibodies up to 54%.18 , 20 , 21 In contrast, many others have reported humoral response in a minimum (<10%) of vaccinated patients.19 , 22 The results may vary mainly due to the different patient mix and especially the intensity of immunosuppressive treatment. All of our patients were treated with mycophenolate mofetil (or sodium), which is associated with a low response to the vaccine.18 , 20 , 21 Although other risk factors have been described, the level of immunosuppression is crucial, which is indirectly confirmed by the significantly good humoral immunity after vaccination in the dialysis population despite the high prevalence of comorbidities.22 In contrast, the cellular immune response was present in almost three-quarters of patients after vaccination, which was comparable to patients who had recovered from COVID-19. Nevertheless, the very small number of patients examined after COVID-19 precludes a relevant comparison. After the second dose of vaccine, there was an increase in IFN-γ and IL-2 secreting cells both after stimulation with the SARS-CoV-2 complex peptide pool and with peptides covering the S1 domain of the spike protein. As expected, cellular immunity was already present before vaccination in a number of SARS-CoV-2 serologically negative patients without prior COVID-19, as described in previous studies using ELISpot assays.26 , 27 These cross-reactive SARS-CoV-2 specific T cells are likely induced by previous exposure to other circulating coronaviruses. The finding of a high number of IFN-γ-secreting cross-reactive cells before vaccination in several patients may explain the non-significant increase in these cells after vaccination. A significantly greater proportion of patients with a cellular response versus a humoral response has been recently published.22 , 23 Our clinical data and that of others suggest that it is questionable whether the level of cellular immunity induced by the mRNA vaccine confers a degree of protection against COVID-19 in patients after kidney transplantation. A detailed analysis of the SARS-CoV-2-specific T cell response showed not only a lower number of reactive cells but also significant functional abnormalities, such as impairment in effector cytokine production, memory differentiation, and cellular activation, in comparison with healthy controls or dialysis patients.22
The humoral response in patients who had recovered from COVID-19 was robust when compared to that in patients who had received the vaccination. A humoral response was present in virtually all patients, and they exhibited significantly high IgG levels. A similar finding has recently been reported in a cohort of lung transplant recipients.32 Both observations correlate well with the detailed analyses of post-COVID-19 immunity in transplant recipients, which document not only comparable SARS-CoV-2 IgG production, including neutralizing antibody titers but also functional T cell immunity with the ability to produce multiple pro-inflammatory cytokines that are comparable to the general population.14 , 16 , 17 Analogous to our results with COVID-19 is the stronger immune response to natural influenza infection, seen in transplant patients who had recovered from influenza, compared to transplant recipients who had received the influenza vaccine. The former group not only exhibited a great diversity of humoral response but also a multifold high CD4+ T cell immunity.33 , 34 The reason for the superior immune response to a natural infection in immunocompromised patients requires further study; however, one can speculate that it is the co-stimulation of innate immunity caused by a natural infection or an exposure to a broad spectrum of antigens than can be achieved with vaccination.33 , 35
Our study has limitations that need to be considered. The PCR test for SARS-CoV-2 was performed mainly in patients with suspected symptoms or in patients who had been in contact with COVID-19 positive individuals; only a minority of PCR tests were screening tests performed during hospitalizations for other reasons. Therefore, some asymptomatic COVID-19 cases may have been missed. However, the very high incidence of COVID-19 in our patients, exceeding the regional incidence, suggests that the number of undiagnosed COVID-19 cases was small. This is aided by the long-term organization of care, where even mild infections are managed primarily within the transplant center. Another limitation of our study is that the number of patients whose immune responses were measured after vaccination was limited. Given the relatively homogeneous immunosuppressive therapy used, it was not possible to determine the role of individual immunosuppressive drugs in response to vaccination. Finally, we used a standard two-dose vaccination schedule, and a third booster dose of mRNA vaccine is now recommended for transplant recipients, which may improve patient protection.
Our results have important clinical implications. Although vaccination efficacy is generally suboptimal in kidney transplant recipients, annual influenza vaccination is associated with a decrease in influenza-associated morbidity.36 , 37 Such evidence is lacking for COVID-19. The need for a change in our vaccination strategy is evident and may even become urgent with the spread of new SARS-CoV-2 variants, such as B.1.617.2 (delta), as these variants have a reduced sensitivity to antibody neutralization.38 Based on the success of the influenza vaccine, a high-dose vaccination or the administration of a third dose of the mRNA COVID-19 vaccine has been suggested.39 , 40 Pilot studies have already been conducted with partial success.41 , 42 Finally, even in patients who have completed their course of vaccination, it is important to insist on strict adherence to preventative measures in order to curtail the transmission of SARS-CoV-2. It is also important to consider treatment with neutralizing monoclonal antibodies against the SARS-CoV-2 spike protein if COVID-19 is detected.43
In conclusion, despite the administration of the BNT162b2 mRNA vaccine, kidney transplant recipients remain at a high risk of COVID-19 infection with a severe course and high mortality. The humoral response of vaccinated patients is significantly reduced compared to that of patients who have recovered from COVID-19. A cellular response is detectable in most patients; however, the level of protection against COVID-19 remains uncertain. Therefore, there is an urgent need to improve vaccination strategies for transplant recipients.
ACKNOWLEDGMENTS
This work was supported by the Charles University Research Fund (Progress Q39), and project “Fighting INfectious Diseases” (CZ.02.1.01/0.0/0.0/16_019/0000787) awarded by the Ministry of Education, Youth and Sports of the Czech Republic, financed from The European Regional Development Fund. The authors thank Mrs. Lenka Karlikova and Jana Havlova for their assistance in data collection.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information Ministry of Education, Youth and Sports of the Czech Republic, financed from The European Regional Development Fund, Grant/Award Number: FIND [CZ.02.1.0 1/0.0/0.0/16_019/0000787]; Lékarská Fakulta v Plzni, Univerzita Karlova, Grant/Award Number: Charles University Research Fund [Progress Q39]
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
Supplementary Material
REFERENCES
- 1.C4 article Implications of COVID-19 in transplantation. Am J Transplant. 2021;21(5):1801–1815. doi: 10.1111/ajt.16346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danziger-Isakov L, Blumberg EA, Manuel O, Sester M. Impact of COVID-19 in solid organ transplant recipients. Am J Transplant. 2021;21(3):925–937. doi: 10.1111/ajt.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Softeland JM, Friman G, von Zur-Muhlen B, et al. COVID-19 in solid organ transplant recipients: a national cohort study from Sweden. Am J Transplant. 2021;21(8):2762–2773. doi: 10.1111/ajt.16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villanego F, Mazuecos A, Perez-Flores IM, et al. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: analysis of the Spanish Registry. Am J Transplant. 2021;21(7):2573–2582. doi: 10.1111/ajt.16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: a multi-center cohort study. Clin Infect Dis. 2020:ciaa1097. doi: 10.1093/cid/ciaa1097. [DOI] [Google Scholar]
- 6.Chavarot N, Gueguen J, Bonnet G, et al. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am J Transplant. 2021;21(3):1285–1294. doi: 10.1111/ajt.16416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jalkanen P, Kolehmainen P, Hakkinen HK, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1):3991. doi: 10.1038/s41467-021-24285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glenn DA, Hegde A, Kotzen E, et al. Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. Kidney Int Rep. 2021;6(5):1407–1410. doi: 10.1016/j.ekir.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benotmane I, Gautier-Vargas G, Wendling MJ, et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am J Transplant. 2020;20(11):3162–3172. doi: 10.1111/ajt.16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thieme CJ, Anft M, Paniskaki K, et al. The magnitude and functionality of SARS-CoV-2 reactive cellular and humoral immunity in transplant population is similar to the general population despite immunosuppression. Transplantation. 2021;105(10):2156–2164. doi: 10.1097/TP.0000000000003755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Ruiz M, Olea B, Gimenez E, et al. SARS-CoV-2-specific cell-mediated immunity in kidney transplant recipients recovered from COVID-19. Transplantation. 2021;105(6):1372–1380. doi: 10.1097/TP.0000000000003672. [DOI] [PubMed] [Google Scholar]
- 16.Fava A, Donadeu L, Sabe N, et al. SARS-CoV-2-specific serological and functional T cell immune responses during acute and early COVID-19 convalescence in solid organ transplant patients. Am J Transplant. 2021;21(8):2749–2761. doi: 10.1111/ajt.16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magicova M, Fialova M, Zahradka I, et al. Humoral response to SARS-CoV-2 is well preserved and symptom dependent in kidney transplant recipients. Am J Transplant. 2021;21(12):3926–3935. doi: 10.1111/ajt.16746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozen-Zvi B, Yahav D, Agur T, et al. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: a prospective cohort study. Clin Microbiol Infect. 2021;27(8) doi: 10.1016/j.cmi.2021.04.028. 1173.e1-1173.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rincon-Arevalo H, Choi M, Stefanski AL, et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol. 2021;6(60):eabj1031. doi: 10.1126/sciimmunol.abj1031. [DOI] [PubMed] [Google Scholar]
- 20.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. doi: 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14):e150175. doi: 10.1172/JCI150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after MRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727–2739. doi: 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korber N, Behrends U, Hapfelmeier A, Protzer U, Bauer T. Validation of an IFNgamma/IL2 FluoroSpot assay for clinical trial monitoring. J Transl Med. 2016;14(1):175. doi: 10.1186/s12967-016-0932-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassaniti I, Percivalle E, Bergami F, et al. SARS-CoV-2 specific T-cell immunity in COVID-19 convalescent patients and unexposed controls measured by ex vivo ELISpot assay. Clin Microbiol Infect. 2021;27(7):1029–1034. doi: 10.1016/j.cmi.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong DSY, Fragkou PC, Schweitzer VA, Chemaly RF, Moschopoulos CD, Skevaki C. How to interpret and use COVID-19 serology and immunology tests. Clin Microbiol Infect. 2021;27(7):981–986. doi: 10.1016/j.cmi.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang P, Nair MS, Liu L, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 29.Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021;385(6):562–566. doi: 10.1056/NEJMsb2104756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caillard S, Chavarot N, Bertrand D, et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100(2):477–479. doi: 10.1016/j.kint.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tau N, Yahav D, Schneider S, Rozen-Zvi B, Abu Sneineh M, Rahamimov R. Severe consequences of COVID-19 infection among vaccinated kidney transplant recipients. Am J Transplant. 2021;21(8):2910–2912. doi: 10.1111/ajt.16700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havlin J, Svorcova M, Dvorackova E, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant. 2021;40(8):754–758. doi: 10.1016/j.healun.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirzel C, Chruscinski A, Ferreira VH, et al. Natural influenza infection produces a greater diversity of humoral responses than vaccination in immunosuppressed transplant recipients. Am J Transplant. 2021;21(8):2709–2718. doi: 10.1111/ajt.16503. [DOI] [PubMed] [Google Scholar]
- 34.L’Huillier AG, Ferreira VH, Hirzel C, et al. T-cell responses following natural influenza infection or vaccination in solid organ transplant recipients. Sci Rep. 2020;10(1):10104. doi: 10.1038/s41598-020-67172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gartner BC, Sester M. Diversity of antibody responses after influenza infection or vaccination-Needed or nice to have? Am J Transplant. 2021;21(8):2631–2632. doi: 10.1111/ajt.16554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Candon S, Thervet E, Lebon P, et al. Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am J Transplant. 2009;9(10):2346–2354. doi: 10.1111/j.1600-6143.2009.02787.x. [DOI] [PubMed] [Google Scholar]
- 37.Kumar D, Ferreira VH, Blumberg E, et al. A 5-year prospective multicenter evaluation of influenza infection in transplant recipients. Clin Infect Dis. 2018;67(9):1322–1329. doi: 10.1093/cid/ciy294. [DOI] [PubMed] [Google Scholar]
- 38.Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 39.Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis. 2018;66(11):1698–1704. doi: 10.1093/cid/cix1082. [DOI] [PubMed] [Google Scholar]
- 40.Haddadin Z, Krueger K, Thomas LD, Overton ET, Ison M, Halasa N. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipients. Am J Transplant. 2021;21(3):938–949. doi: 10.1111/ajt.16295. [DOI] [PubMed] [Google Scholar]
- 41.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.