Abstract
Although strategies to mitigate barriers to colorectal cancer (CRC) screening have proven successful in some parts of the US, few of these strategies have been studied in rural, American Indian communities that may exhibit unique culturally driven attitudes toward and knowledge of colorectal cancer and experience increased barriers to healthcare access. In this study, we describe the results of a survey among CRC screen-eligible members of Zuni Pueblo (N = 218) on an array of questions regarding CRC screening behaviors, knowledge, satisfaction with and access to healthcare services, social support for CRC screening, perceptions toward FOBT, and preference for evidence-based interventions or strategies for improving CRC screening rates. Results from the multivariable model suggest age, having a regular healthcare provider, and harboring fewer negative perceptions toward FOBT are key drivers of ever completing CRC screening. Respondents reported strong support for Community Guide-recommended interventions and strategies for increasing CRC screening for nearly all proposed interventions. Results confirm the need for multilevel, multicomponent interventions, with a particular focus on improving Zuni Pueblo community members’ access to a regular source of care, improving knowledge of CRC risk factor, and addressing negative perceptions toward CRC screening. These results provide critical, community-specific insight into better understanding the drivers of low guideline-adherent screening rates and inform local healthcare providers and community leaders of context-specific strategies to improve CRC screening in Zuni Pueblo.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10900-023-01196-7.
Keywords: Colorectal cancer screening, American Indian/Alaska Native health, Fecal occult blood test, Colonoscopy
Introduction
Colorectal cancer (CRC) has the third highest mortality rate of all cancers in the United States (US) [1]. This high mortality rates exists despite a decades-long trend of improvements in CRC screening rates and survivability [2]. These improvements have been largely attributed to the reduction of system- and patient-level barriers to CRC screening, which is a highly effective strategy for reducing CRC incidence through the identification and removal of pre-cancerous polyps and reducing CRC mortality through the early detection and treatment of CRC. Strategies to mitigate screening barriers have been studied extensively and many have been found to be effective, particularly when combined into multilevel, multicomponent interventions [3, 4]. These strategies include patient-level interventions such as mailing fecal occult blood tests (FOBTs) to patients [5, 6], patient navigators guiding patients through the CRC screening process [7, 8], patient education [9, 10], patient reminders [11, 12], and providing financial incentives for CRC screening completion [13, 14]. Effective system-level interventions include employing patient navigators, academic detailing for primary care providers (i.e., face-to-face education for providers on CRC screening best practices) [15, 16] and electronic notification alerts via a provider’s electronic health record (EHR) to flag patients who are due for CRC screening [17, 18].
However, despite the improvements in CRC screening and survivability at the national level, significant disparities exist in CRC screening, incidence, and mortality among different races and ethnicities and across regions. In 2020 at the national level, non-Hispanic American Indian/Alaska Native (AIAN) and non-Hispanic Black (NHB) are consistently found to have greater proportions of distant-stage CRC diagnoses and lower CRC survival [19]. Within some regions and states, these disparities are even larger. In New Mexico in 2017 (the most recent year these data are available) for example, a state with already below average CRC guideline-concordant screening rates (US: 67.7%, NM: 60.8%), CRC screening disparities are the largest between non-Hispanic Whites (NHW) (69.2%) and AIAN (40.9%) [20]. These same disparities carry over into CRC incidence (NHW: 32.3 new CRC cases per 100k, AIAN: 43.0 new CRC cases per 100k) and CRC mortality (NHW: 12.6 CRC deaths per 100k, AIAN: 17.4 CRC deaths per 100k) [20]. These disparities require further attention from cancer prevention researchers and healthcare providers. When the patient- and system-level interventions discussed earlier have doubled CRC screening rates from approximately 40% to over 80% among both NHW and NHB in other regions of the US from 2000 to 2019 [21], it begs numerous questions about the generalizability of those same interventions within the context of other subpopulations and communities that continue to significantly lag in CRC screening rates and outcome measures. Perhaps these interventions have simply not been consistently implemented in some communities. Alternatively, perhaps these interventions have not been as effective due to idiosyncratic structural, cultural, and/or socioeconomic matters not encountered elsewhere. For example, AIAN communities such as the Pueblo of Zuni in western New Mexico exhibit low CRC screening rates and an array of healthcare delivery challenges. These challenges include but are not limited to: the Pueblo’s rural location (approximately 150 miles from the nearest gastroenterology clinic); nearly 85% of residents speak Zuni language at home; a substantial proportion (27%) are in poverty; nearly 1 in 5 residents are unemployed; the community experiences high rates of chronic disease [22], and the Pueblo’s Indian Health Service-managed Health Center, which is classified as a Rural Health Professional Shortage Area, must often prioritize acute care services over preventive health [22].
In this study, we describe the results of a survey conducted in 2020 and 2021 among CRC screen-eligible members of Zuni Pueblo in rural, western New Mexico. The intention of the survey was to collect data on an array of questions regarding CRC screening behaviors, CRC and general cancer knowledge, satisfaction with and access to healthcare services, social support for CRC and general cancer screening, perceptions of CRC screening modalities, and preferred methods for improving CRC screening rates. The results from the survey will provide critical, community-specific insight into better understanding the drivers of the low guideline-adherent screening rates and inform local healthcare providers and community leaders of culturally appropriate, context-specific strategies to improve CRC screening, incidence, and mortality rates in Zuni Pueblo.
Methods
After receiving approval from the Zuni Pueblo Tribal Council, the Southwest Tribal Institutional Review Board (IRB) (SWT-2018-004), and the University of New Mexico Health Sciences Center IRB (HRRC # 18–264), we conducted an observational, cross-sectional study using a population-based survey. The survey was administered on Zuni Pueblo in October 2020 through April 2021. Zuni is a Pueblo Tribe located approximately 150 miles west of Albuquerque, New Mexico, USA. With a population of approximately 11,000, Zuni is a rural community with a median age of 32.8 years and a median household income of $39,706. The Zuni community is served by the Zuni Comprehensive Health Center (ZCHC)—an Indian Health Service-managed, 45-bed center offering emergency, inpatient, outpatient, and obstetric services. ZCHC does not offer gastroenterology services. The closest healthcare facility offering colonoscopy is 70 miles away in Fort Defiance, Arizona or in in Albuquerque. Zuni Pueblo is one of 27 Tribal Nations served by the Indian Health Service (IHS) Albuquerque Area. In 2019, the Area reported a 31.2% guideline-adherent CRC screening rate [23].
Study Sample and Survey Design
The survey sample was created using a complete list of all streets within Zuni Pueblo. Streets were then selected in random order and all houses on the selected streets received recruitment flyers issued by Zuni Health Initiative (ZHI) staff. ZHI staff also handed out recruitment flyers at high-traffic community locations and issued public service announcements on the community radio station. As a precaution for COVID-19, surveys were administered over the phone. To qualify, participants had to self-identify as American Indian, a member of Zuni tribe, or be married to a Zuni tribal member, and had to be 50–75 years old. All qualified participants received a merchandise card for their participation on the survey. The final completed sample consisted of 218 participants. The survey, which was administered by ZHI staff in English, took approximately 25 min to complete.
Survey development was guided by the Health Behavior Framework (HBF), which contends health intentions and behaviors are jointly determined by individual and healthcare system factors, as well as social, environmental, and personal barriers [24]. The HBF has been used to study drivers of health behavior in diverse cancer control study settings and populations [25–28]. The survey included 61 questions on basic demographic information, health history, healthcare utilization (see Table 1), and preferences for various evidence-based strategies designed to increase CRC screening derived from the Community Preventive Services Task Force’s Community Guide [4] (see Table 2). Four composite scores, which were adapted from other HBF-guided studies [25, 26], were generated to measure knowledge of CRC risk factors (15 items), satisfaction with their healthcare providers (3 items), social support for screening (4 items), and negative perceptions toward FOBT (4 items) (see Table 3). Cronbach’s α was calculated for the satisfaction with healthcare composite score as it was derived as a mean Likert score (α = 0.660). The remaining composite scores (knowledge, social support for screening, and perceptions toward FOBT) were scored using a summed approach. The dependent variable was a self-report of ever having completed a colonoscopy or FOBT. Due to the preliminary nature of the study and disruption of preventive services due to COVID-19, this analysis focuses on ever being screened for CRC rather than being up to date with CRC screening. The survey instrument was refined through discussions with the project’s Tribal Advisory Panel, which comprises representatives from Zuni tribal leadership, and Zuni stakeholders and local organizations (i.e., Health and Wellness program directors, Community Health Representatives, and cancer survivors). Given the nature of the sensitive subject matter, the Tribal Advisory Panel also provided feedback on structuring questions in a culturally appropriate manner and removing others altogether.
Table 1.
Characteristics of Survey Respondents (N = 218)
| Variable | Ever Completed FOBT or Colonoscopy? | P | |||
|---|---|---|---|---|---|
| No | Yes | Total | |||
| What is your age? | 56.42 (SE 0.61) | 61.3 (SE 0.64) | 59.24 (SE 0.48) | 0.000 | |
| Sex | Male | 52 (56.5%) | 57 (45.2%) | 109 (50.0%) | 0.100 |
| Female | 40 (43.5%) | 69 (54.8%) | 109 (50.0%) | ||
| Which of the following best describes the annual household income from all sources? | Less than $10,000 per year | 48 (52.2%) | 45 (35.7%) | 93 (42.7%) | 0.050 |
| $10,000 - $19,999 per year | 22 (23.9%) | 38 (30.2%) | 60 (27.5%) | ||
| More than $20,000 per year | 22 (23.9%) | 43 (34.1%) | 65 (29.8%) | ||
| What is the highest level of education that you completed? | No High School or GED | 13 (14.1%) | 20 (15.9%) | 33 (15.1%) | 0.330 |
| HS Diploma or GED | 42 (45.7%) | 45 (35.7%) | 87 (39.9%) | ||
| Some College or College Graduate | 37 (40.2%) | 61 (48.4%) | 98 (45.0%) | ||
| Currently employed in any capacity? | Not Currently Employed | 51 (55.4%) | 57 (45.2%) | 108 (49.6%) | 0.137 |
| Currently Employed | 41 (44.6%) | 69 (54.8%) | 110 (50.5%) | ||
| In general, would you say your physical health is: | Fair or Poor | 20 (21.7%) | 45 (35.7%) | 65 (29.8%) | 0.026 |
| Good, Very Good, or Excellent | 72 (78.3%) | 81 (64.3%) | 153 (70.2%) | ||
| Has your doctor ever told you that you have cancer? | No | 89 (96.7%) | 105 (84.0%) | 194 (89.4%) | 0.003 |
| Yes | 3 (3.3%) | 20 (16.0%) | 23 (10.6%) | ||
| Does (or Did) anyone in your family have a cancer? | No | 46 (50.0%) | 47 (37.9%) | 93 (43.1%) | 0.076 |
| Yes | 46 (50.0%) | 77 (62.1%) | 123 (56.9%) | ||
| Do you have a health care provider that you go to regularly? | No | 55 (59.8%) | 31 (24.6%) | 86 (39.5%) | 0.000 |
| Yes | 37 (40.2%) | 95 (75.4%) | 132 (60.6%) | ||
| How many times have you been to an Indian Health Service health clinic in the past three years? | Never | 14 (15.2%) | 8 (6.4%) | 22 (10.1%) | 0.000 |
| One time | 15 (16.3%) | 9 (7.2%) | 24 (11.1%) | ||
| Two times | 19 (20.7%) | 13 (10.4%) | 32 (14.8%) | ||
| Three times or more | 44 (47.8%) | 95 (76.0%) | 139 (64.1%) | ||
Table 2.
Preferences for Community Guide-recommended interventions and strategies designed to increase CRC screening (ranked most to least preferred)
| We would like to implement programs that can help increase screening for cervical cancer. What types of programs would you like us to implement? | Ever Completed FOBT or Colonoscopy? | P | |||
|---|---|---|---|---|---|
| No | Yes | Total | |||
| Reminders such as letters, postcards, emails, or phone messages | No | 6 (6.5%) | 12 (9.5%) | 18 (8.3%) | 0.426 |
| Yes | 86 (93.5%) | 114 (90.5%) | 200 (91.7%) | ||
| One-on-one education | No | 9 (9.8%) | 10 (7.9%) | 19 (8.7%) | 0.633 |
| Yes | 83 (90.2%) | 116 (92.1%) | 199 (91.3%) | ||
| Printed materials such as letters, brochures, and newsletters | No | 10 (10.9%) | 11 (8.7%) | 21 (9.6%) | 0.597 |
| Yes | 82 (89.1%) | 115 (91.3%) | 197 (90.4%) | ||
| Having community health representatives (CHRs) or patient navigators help obtain screening | No | 13 (14.1%) | 17 (13.5%) | 30 (13.8%) | 0.893 |
| Yes | 79 (85.9%) | 109 (86.5%) | 188 (86.2%) | ||
| Having flexible clinic hours | No | 15 (16.3%) | 18 (14.3%) | 33 (15.1%) | 0.681 |
| Yes | 77 (83.7%) | 108 (85.7%) | 185 (84.9%) | ||
| Offering transportation to the clinic | No | 16 (17.4%) | 18 (14.3%) | 34 (15.6%) | 0.533 |
| Yes | 76 (82.6%) | 108 (85.7%) | 184 (84.4%) | ||
| Public service announcements (PSAs) on the radio | No | 12 (13.0%) | 24 (19.1%) | 36 (16.5%) | 0.238 |
| Yes | 80 (87.0%) | 102 (81.0%) | 182 (83.5%) | ||
| Videos in the clinic waiting room | No | 17 (18.5%) | 21 (16.7%) | 38 (17.4%) | 0.728 |
| Yes | 75 (81.5%) | 105 (83.3%) | 180 (82.6%) | ||
| Offering translation or interpretation services at the clinic | No | 18 (19.6%) | 21 (16.7%) | 39 (17.9%) | 0.581 |
| Yes | 74 (80.4%) | 105 (83.3%) | 179 (82.1%) | ||
| Reduce co-payments for testing | No | 18 (19.6%) | 24 (19.1%) | 42 (19.3%) | 0.924 |
| Yes | 74 (80.4%) | 102 (81.0%) | 176 (80.7%) | ||
| Offering screening through non-clinical settings | No | 18 (19.6%) | 25 (19.8%) | 43 (19.7%) | 0.960 |
| Yes | 74 (80.4%) | 101 (80.2%) | 175 (80.3%) | ||
| Home visits for education | No | 18 (19.6%) | 32 (25.4%) | 50 (22.9%) | 0.312 |
| Yes | 74 (80.4%) | 94 (74.6%) | 168 (77.1%) | ||
| Group education | No | 25 (27.2%) | 29 (23.0%) | 54 (24.8%) | 0.482 |
| Yes | 67 (72.8%) | 97 (77.0%) | 164 (75.2%) | ||
| Offering childcare services | No | 26 (28.3%) | 36 (28.6%) | 62 (28.4%) | 0.960 |
| Yes | 66 (71.7%) | 90 (71.4%) | 156 (71.6%) | ||
| Using social media, such as Facebook, YouTube, Twitter | No | 35 (38.0%) | 57 (45.2%) | 92 (42.2%) | 0.288 |
| Yes | 57 (62.0%) | 69 (54.8%) | 126 (57.8%) | ||
Table 3.
Bivariate Results of Composite Scores and Scale Composition
| Construct | Scale | Ever Completed FOBT or Colonoscopy? | P | ||
|---|---|---|---|---|---|
| No | Yes | Total | |||
| Cancer Knowledge Composite Score | Sum | 8.16 (SD 1.92) | 8.83 (SD 1.75) | 8.55 (SD 1.85) | 0.008 |
| Which of the following increase a person’s chance of developing CRC? |
Yes = 1 No = 0 |
||||
| Having relatives with colorectal cancer | 66 (71.7%) | 102 (81.0%) | 168 (77.1%) | 0.142 | |
| Having non-cancerous growths in the colon or rectum | 58 (63.0%) | 99 (78.6%) | 157 (72.0%) | 0.014 | |
| A diet low in fruit and vegetables | 57 (62.0%) | 92 (73.0%) | 149 (68.4%) | 0.105 | |
| A diet low in fiber and high in fat | 46 (50.0%) | 80 (63.5%) | 126 (57.8%) | 0.053 | |
| A diet high in processed meat | 54 (58.7%) | 81 (64.3%) | 135 (61.9%) | 0.480 | |
| Not being physically active | 64 (69.6%) | 97 (77.0%) | 161 (73.9%) | 0.275 | |
| Drinking excess alcohol | 61 (66.3%) | 91 (72.2%) | 152 (70.0%) | 0.373 | |
| Tobacco use | 67 (72.8%) | 92 (73.0%) | 159 (72.9%) | 0.548 | |
| Having diabetes | 27 (29.4%) | 34 (27.0%) | 61 (28.0%) | 0.761 | |
| Being overweight | 61 (66.3%) | 95 (75.4%) | 156 (71.6%) | 0.171 | |
| Having had another type of cancer |
No = 1 Yes = 0 |
28 (30.4%) | 40 (31.8%) | 68 (31.2%) | 0.883 |
| Too much stress in your life | 46 (50.0%) | 56 (44.4%) | 102 (46.8%) | 0.492 | |
| Sitting on hot or cold surfaces | 52 (56.5%) | 77 (61.1%) | 129 (59.2%) | 0.577 | |
| Using preservatives in food | 32 (34.8%) | 39 (31.0%) | 71 (32.6%) | 0.562 | |
| Being exposed to medical x-rays | 32 (34.8%) | 38 (30.2%) | 70 (32.1%) | 0.557 | |
| Satisfaction with Healthcare Composite Score | Mean | 3.89 (SD 0.58) | 4.01 (SD 0.66) | 3.96 (SD 0.63) | 0.173 |
| The hospital or clinic I usually go to provide me with good healthcare overall. | Strongly Agree = 5 Strongly Disagree = 1 | 3.78 (SD 0.84) | 3.78 (SD 0.92) | 3.78 (SD 0.88) | 0.968 |
| The health care providers I usually see treat me with dignity and respect. | 3.87 (SD 0.78) | 4.04 (SD 0.84) | 3.98 (SD 0.82) | 0.126 | |
| I feel comfortable talking to healthcare providers when I have a health problem. | 4.01 (SD 0.65) | 4.20 (SD 0.80) | 4.12 (SD 0.75) | 0.067 | |
| Social Support for Screening Composite Score | Sum | 2.01 (SD 0.85) | 2.31 (SD 1.09) | 2.18 (SD 1.00) | 0.030 |
| My family and friends would support me in doing a stool blood test. | Yes = 1 No = 0 | 84 (91.3%) | 110 (87.3%) | 194 (89.0%) | 0.389 |
| With regards to FOBT, I want to do what my family & friends think I should do. | 67 (72.8%) | 95 (75.4%) | 162 (74.3%) | 0.754 | |
| Have any of your family and friends ever suggested you do a stool blood test? | 17 (18.5%) | 39 (31.0%) | 56 (25.7%) | 0.042 | |
| Ever discussed your personal risk for getting CRC with friends or relatives? | 17 (18.5%) | 47 (37.3%) | 64 (29.4%) | 0.003 | |
| Negative Perceptions Toward FOBT Composite Score | Sum | 1.70 (SD 1.16) | 1.06 (SD 1.26) | 1.33 (SD 1.26) | 0.000 |
| It is inconvenient to do the test yourself? | Yes = 1 No = 0 | 42 (45.7%) | 39 (31.0%) | 81 (37.2%) | 0.033 |
| It is too unpleasant to handle stool? | 40 (43.5%) | 39 (31.0%) | 79 (36.2%) | 0.065 | |
| You have no time to do the test? | 28 (30.4%) | 27 (21.4%) | 55 (25.2%) | 0.156 | |
| You do not know how to do the test? | 46 (50.0%) | 29 (23.0%) | 75 (34.4%) | 0.000 | |
Statistical Analyses
We compared those who had never completed FOBT or colonoscopy with those who had across each of our independent variables, composite scores, and preferences for CRC screening programs using χ² tests and t-tests where appropriate. We used logistic regression to assess the associations between respondent characteristics and receipt of FOBT or colonoscopy. The initial logistic regression model included all variables from the survey, save for the 15 variables on screening awareness programs as these questions were future oriented (i.e., “What types of programs would respondents like us to implement?”) and our dependent variable and model was past oriented (i.e., receipt of any FOBT or colonoscopy). After fitting the initial logistic regression model, we simplified it in an effort to find the best balance of fit and parsimony while still including the major components of the HBF. The variables that were removed sequentially included: exercise frequency, availability of transportation, annual physicals, marital status, height, and weight. Odds ratios, standard errors, and 95% confidence intervals are reported from the simplified model. Model-adjusted predicted probabilities for having ever completed FOBT or colonoscopy were estimated for those with and without regular providers from ages 50–75. All analyses were conducted using STATA 17 (College Station, TX).
Results
The 218 respondents were split equally between males and females and had a mean age of 59. Nearly 45% of respondents reported earning less than $10,000 annually and a similar percentage reported having some college or having graduated college. Over 60% of respondents reported having a regular source of care and nearly two-thirds reported visiting an IHS clinic three or more times over the past three years. For our primary variable of interest, 92 out of 218 respondents (42%) reported having never completed an FOBT or colonoscopy. See Table 1 for more respondent characteristics.
Regarding preferences for evidence-based interventions or strategies to increase CRC screening awareness, all fifteen interventions or strategies received at least 58% support among respondents. Patient reminders, one-on-one education, use of printed education materials, having patient navigators help obtain screening, and having flexible clinic hours, garnered the most support (92%, 91%, 90%, 86%, and 85%, respectively). Conversely, home visits for education, group education, offering childcare services, and social media campaigns all garnered the least support (77%, 75%, 72%, and 58%, respectively). No significant differences for preference were observed between those who had and those who had never completed FOBT or colonoscopy for any of the 15 interventions or strategies screening awareness. See Table 2 for more information on respondents’ preferences.
The cancer knowledge composite score, which was a summed score across 15 single point-scored questions yielded a mean of 8.55 (SD 1.85), a minimum of 3, and a maximum of 15. This score suggests that, on average, respondents correctly answered approximately 60% (9 out of 15) of the questions related to knowledge of CRC risk. The satisfaction with healthcare composite score, which was a mean score of three Likert score-based questions, yielded a mean of 3.96 (SD 0.63), a minimum of 1.67, and a maximum of 5, suggesting respondents, on average, “agreed” with all three component questions. The social support for screening composite score, which was a summed score across four single point-scored questions yielded a mean of 2.18 (SD 1.00), a minimum of 0, and a maximum of 4, suggesting moderate levels of social support. Finally, the composite score for negative perceptions toward FOBT, which was a summed score across four single point-scored questions yielded a mean of 1.33 (SD 1.26), a minimum of 0, and a maximum of 4, suggesting respondents, on average, had only mildly negative perceptions toward FOBT. See Table 3 for more composite score characteristics.
In bivariate analyses, significant differences were observed between those who had and those who had never completed FOBT or colonoscopy across an array of independent variables. Respondents who had completed FOBT or colonoscopy were significantly: older; more likely to earn more; less likely to rate their health as good, very good, or excellent; more likely to have already been diagnosed with any cancer; more likely to have a regular source of care; and more likely to have visited an IHS clinic three or more times in the past three years (see Table 1). For the four composite scores, respondents who had completed FOBT or colonoscopy had significantly greater cancer knowledge, more social support for screening, and fewer negative perceptions toward FOBT (see Table 3).
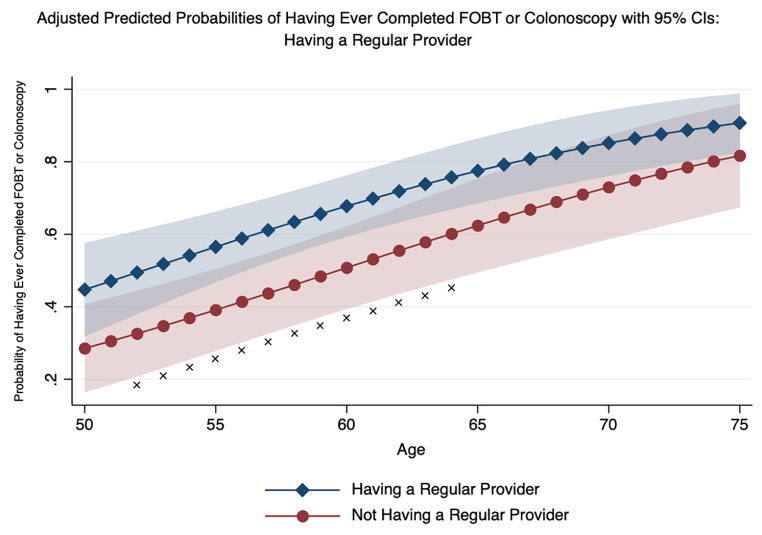
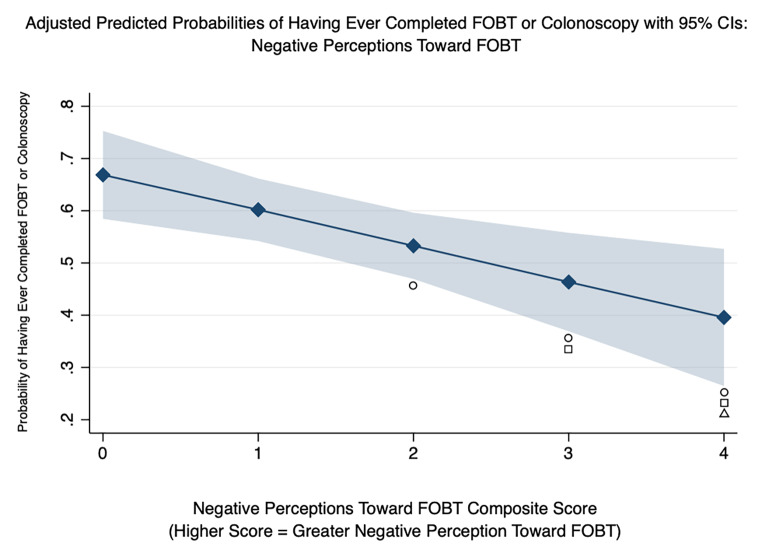
Table 4 presents the odds ratios for ever having received FOBT or colonoscopy from the logistic regression model. The odds of FOBT or colonoscopy receipt were 13% higher for each subsequent year of age (OR: 1.13, 95% CI: 1.06–1.19) and 2.4 times higher for respondents who reported having a regular healthcare provider (OR: 2.40, 95% CI: 1.16–4.95). Out of the four composite scores, only the negative perceptions toward FOBT composite score was significantly associated with ever having received FOBT or colonoscopy, suggesting a one-point increase in negative perceptions was associated with a 33% decreased odds of FOBT or colonoscopy receipt (OR: 0.67, 95% CI: 0.50–0.89). Using the model’s adjusted estimates, two plots were created to illustrate the marginal impact of having a regular source of care across categories of age (see Fig. 1) and having a higher composite score for negative perceptions toward FOBT (see Fig. 2). Figure 1 highlights the significant association of having a regular source of care and CRC screening—particularly from ages 52 to 64 when patients are in their earliest years of screening eligibility. Figure 2 highlights the significant association between greater negative perceptions and lower probability of CRC screening. Specifically, an individual who did not indicate inconvenience, unpleasantness, lack of time, or lack of knowledge about completing an FOBT (composite score = 0) was significantly more likely to have completed an FOBT or colonoscopy than an individual who did indicate two, three, or all four of the same indicators.
Table 4.
Logistic Regression Results: Odds Ratios for Ever Having Been Screened for Colorectal Cancer
| Odds Ratio | Std. err. | P > z | 95% Conf. Interval | ||
|---|---|---|---|---|---|
| Age | 1.125 | 0.033 | 0.000 | 1.062 | 1.192 |
| Sex | |||||
| Male | REF | REF | REF | REF | REF |
| Female | 1.006 | 0.368 | 0.987 | 0.491 | 2.059 |
| Income | |||||
| Less than $10k | REF | REF | REF | REF | REF |
| $10k-20k | 1.287 | 0.547 | 0.553 | 0.560 | 2.960 |
| More than $20k | 1.501 | 0.669 | 0.362 | 0.627 | 3.594 |
| Education | |||||
| High School or Less | REF | REF | REF | REF | REF |
| Some College or College Graduate | 0.727 | 0.282 | 0.412 | 0.340 | 1.556 |
| Employment | |||||
| Not Currently Employed | REF | REF | REF | REF | REF |
| Currently Employed | 0.699 | 0.261 | 0.338 | 0.336 | 1.455 |
| Self-Rated Health | |||||
| Fair or Poor | REF | REF | REF | REF | REF |
| Good, Very Good, or Excellent | 0.692 | 0.287 | 0.374 | 0.307 | 1.558 |
| Previous Cancer Diagnosis (Self) | |||||
| No | REF | REF | REF | REF | REF |
| Yes | 3.945 | 2.997 | 0.071 | 0.890 | 17.483 |
| Previous Cancer Diagnosis (Family) | |||||
| No | REF | REF | REF | REF | REF |
| Yes | 1.056 | 0.391 | 0.882 | 0.511 | 2.183 |
| Have regular health care provider? | |||||
| No | REF | REF | REF | REF | REF |
| Yes | 2.398 | 0.888 | 0.018 | 1.161 | 4.954 |
| No. of Visits to IHS in past 3 years? | |||||
| Zero | REF | REF | REF | REF | REF |
| One time | 0.783 | 0.584 | 0.743 | 0.181 | 3.379 |
| Two times | 0.763 | 0.527 | 0.695 | 0.197 | 2.954 |
| Three times or more | 2.713 | 1.657 | 0.102 | 0.819 | 8.981 |
| Cancer Knowledge Composite Score | 1.182 | 0.110 | 0.072 | 0.985 | 1.418 |
| Social Support Composite Score | 1.366 | 0.248 | 0.085 | 0.957 | 1.948 |
| Negative Perceptions Toward FIT Composite Score | 0.667 | 0.097 | 0.005 | 0.503 | 0.886 |
| Satisfaction with Healthcare Composite Score | 0.812 | 0.245 | 0.491 | 0.450 | 1.468 |
| Constant | 0.000 | 0.001 | 0.000 | 0.000 | 0.026 |
Fig. 1.
Adjusted Predictions of Having Ever Completed FIT or Colonoscopy by Category of Having a Regular Provider and Age with 95% Confidence Intervals
X = P < 0.05
Fig. 2.
Adjusted Predictions of Having Ever Completed FIT or Colonoscopy by Composite Score for Negative Perceptions Toward FOBT.
○ Statistically different (P < 0.05) from Composite Score 0.
□ Statistically different (P < 0.05) from Composite Score 1.
Δ Statistically different (P < 0.05) from Composite Score 2.
Discussion
Although strategies to mitigate barriers to CRC screening have proven successful in other parts of the US [3, 21], few of these strategies have been studied in rural, American Indian communities that may exhibit unique culturally driven attitudes toward and knowledge of cancer [29, 30] and also experience increased barriers to healthcare access [31]. Zuni Pueblo is one such community located in an IHS Area with low guideline-adherent CRC screening rates and increased barriers to healthcare access [22]. In this study, we described the results of a survey among CRC screen-eligible members of Zuni Pueblo on an array of questions regarding CRC screening behaviors, knowledge, satisfaction with and access to healthcare services, social support for CRC screening, perceptions toward FOBT, and preference for evidence-based interventions or strategies for improving CRC screening rates. These results provide critical, community-specific insight into better understanding the drivers of the low guideline-adherent screening rate and inform local healthcare providers and community leaders of community-specific strategies to improve CRC screening.
Results from the multivariable model suggest age, having a regular healthcare provider, and harboring fewer negative perceptions toward FOBT are key drivers of ever completing CRC screening. Our finding of a positive association between age and receipt of CRC screening has been found by others in studies of American Indian communities [32–36]. Similarly, not having a regular provider and thus, having fewer chances to be recommended for CRC screening has also been found in other AI CRC studies [29, 33, 34, 36]. As highlighted in Fig. 1, younger individuals without a regular provider are among the least likely to have ever completed colonoscopy or FOBT. Additionally, since administering the survey, the US Preventive Services Task Force (USPSTF) has updated the recommended starting age for CRC screening from 50 to 45 [37]. Our results suggests that this new, younger age group may prove difficult to be screened. However, Fig. 1 also suggests that having a regular provider significantly improves the probability of CRC screening. Therefore, measures must be taken to engage Zuni Pueblo members, particularly those who are younger and healthier and are not as likely to utilize routine care or annual checkups. Fortunately, our summaries of respondents’ preferences for Community Guide-recommended interventions or strategies can help inform Zuni health program development to target these very individuals. For example, having flexible clinic hours, offering childcare services, creating home visits for education, and having community health representatives (CHRs) help obtain screening, were all highly preferred evidence-based interventions or strategies that may be particularly well-suited for younger individuals with family caretaking and employment commitments that otherwise restrict access to traditional healthcare settings.
Additionally, our finding of a negative association between negative perceptions toward FOBT and receipt of CRC screening have also been found in other studies, though none of these were specific to AI communities [38–40]. Fig. 2 highlights the importance of reducing negative perceptions toward FOBT as those with no reported negative perceptions toward FOBT were significantly more likely to have received colonoscopy or FOBT than those with two or more reported negative perceptions. Therefore, Zuni community and healthcare leaders should design and employ interventions and strategies aimed at reducing these perceptions. Yet again, our summaries of results of respondents’ preferences for Community Guide-recommended interventions can help inform Zuni health program development. For example, negative perceptions held by screening-hesitant patients may need to be addressed in private, confidential settings. This could be achieved through one-on-one education, home visits for education, and possibly offering screening through nonclinical settings—all three of which were highly preferred by survey respondents. Additionally, improving access to a regular provider would also logically increase the ability for screening-hesitant patients to discuss their concerns in a private setting with a trusted, knowledgeable provider. Strategies to improve this type of access was described in the preceding paragraph and were also highly preferred by survey respondents.
Respondents reported strong support for Community Guide-recommended interventions or strategies for increasing CRC screening for nearly all proposed interventions. Apart from using social media, all interventions garnered at least 72% support. The top four most-preferred interventions all involved knowledge generation, with two of the four calling on CHRs or healthcare providers to provide personalized education on CRC and how to obtain screening. Importantly, no significant differences were observed for any of the 15 interventions or strategies between those who had and those who had never received CRC screening. This finding suggests that these evidence-based interventions or strategies are important for not just those who have never been screened, but also for those who have and will need to be screened again, which is particularly important for communities such as Zuni Pueblo given the likely high reliance on annual FOBT versus colonoscopy every ten years. Although this study only focused on ever having received FOBT or colonoscopy, factors related to repeat CRC screening can be similar but also present unique challenges. For example, in a study on repeat FOBT completion, out of 69 patients with zero clinic visits in the year after completing an FOBT, zero of those 69 participants completed their second FOBT within a year [41]. Therefore, if the Zuni community were to implement an array of multilevel interventions as discussed, these interventions would need to be equally effective for both first-time and repeat screening patients. Fortunately, this study’s results suggest the Community Guide-recommended interventions or strategies are equally preferable to both patient categories.
All of the aforementioned factors, and strategies to address these factors, confirm the need for multilevel, multicomponent interventions [3]. Patients who lack a regular source of care are unlikely to receive preventive and routine care where CRC screening recommendations are most often made [29, 34] and where negative perceptions can be addressed by providers. Therefore, provider education or EHR alerts alone are not likely to improve FOBT completion for patients who are sporadically or never seen. Similarly, rarely seen patients are likely not empaneled to an IHS provider, likely preventing them from ever being flagged by a clinic to receive mailed FOBTs or reminders via mail, phone, or text message. Additionally, these impersonal methods of communication likely do not possess the valence necessary to overcome strong negative perceptions toward FOBT, particularly for those who have never been screened before. Other research has shown that negative perceptions toward FOBT are the highest for those who have never before completed the test [39, 42]. Therefore, these system-level interventions are necessary but not sufficient for improving CRC screening. Additional measures must also be taken to engage Zuni Pueblo members, particularly those who are younger and healthier and are not as likely to utilize routine care or annual checkups. This will likely require that more awareness of CRC screening be generated outside of healthcare facilities, likely by community health representatives (CHRs) and use of printed and digital media. An array of individual-level interventions, combined with system-level interventions, should be employed by healthcare and community organizations.
Several limitations to this study should be noted. First, the cross-sectional nature of the survey and the quasi-convenience sampling strategy used limit the generalizability of the findings. Additionally, selection bias may have occurred where individuals who had already completed CRC screening were more inclined to respond to recruitment efforts. That 58% of our respondents had completed FOBT or colonoscopy is possibly indicative of this bias, given the 31.2% guideline-adherent screening rates for the IHS Albuquerque Area [23]. However, certainly not all 58% of our respondents who had ever been screened were up to date. The survey also included self-reported CRC screening utilization and knowledge, which may over- or under-estimate true behaviors and knowledge. However, the major strength of this study is its focus on Zuni adults and their CRC screening knowledge, perceptions, and behaviors.
Conclusion
This study highlighted the relationship between CRC screening behaviors, individual- and healthcare-level factors, and social and personal barriers to healthcare within a culturally unique American Indian population. These results provide critical, community-specific insight into better understanding the drivers of the low guideline-adherent screening rates and inform local healthcare providers and community leaders of context-specific strategies to improve CRC screening in Zuni Pueblo. We believe this is the first study of its kind for this Tribe and expect these results can help lead to improve health outcomes.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge the Tribal stakeholders, including the Zuni Tribal Governor and Tribal Council members and the Tribal Advisory Panel members who contributed to the study activities. The authors are also grateful to the Zuni people for welcoming us into their lives, and sincerely thank the men and women from the Zuni Pueblo who participated in the survey. The authors also acknowledge the contributions of the University of New Mexico Health Sciences Center staff (Donica Ghahate and Keith Kelly) and the UNMCCC’s Behavioral Measurement and Population Science Shared Resource staff (Joseph Rodman).
Author Contributions
Conceptualization: NE, SIM; Methodology: NE, VSP, MK, DK; Formal analysis and investigation: NE, KC, JS, VSP, MK, DK, SIM; Writing – original draft preparation: NE; Writing – review and editing: KC, JS, VSP, MK, DK, SL, SIM; Funding acquisition: SIM; Resources: JS, SL, SIM; Supervision: SIM.
Funding Statement
This research was supported by the UNM Comprehensive Cancer Center (UNMCCC) Support Grant NIH/NCI P30CA118100 (Tomkinson, PI), UNMCCC institutional pilot awards (PP-U1418-RS, PP-U1402-CaC, Mishra, PI), the UNMCCC Behavioral Measurement and Population Science and the Biostatistics Shared Resources, and the Institutional Development Award (IDeA) from the NIH/NIGMS P20GM103451 under the New Mexico IDeA Networks of Biomedical Research (NM-INBRE) Developmental Research Project Program (Mishra, PI of the Developmental Research Project).
Declarations
Competing Interests
None of the authors have any commercial associations that pose, or have the appearance of posing, a conflict of interest in connection with the submitted article, including but not limited to: employment, consultancies, stock ownership or other equity interests, patent-licensing arrangements, honoraria, paid expert testimony, or personal relationships.
Ethics Approval
This study received approval from the Zuni Pueblo Tribal Council, the Southwest Tribal Institutional Review Board (IRB) (SWT-2018-004), and the University of New Mexico Health Sciences Center IRB (HRRC # 18–264).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel, R. L., Miller, K. D., Fuchs, H. E. (2021). Cancer Statistics, CA: A Cancer Journal for Clinicians 2021; 71: 7–33. 10.3322/caac.21654 [DOI] [PubMed]
- 2.Centers for Disease Control and Prevention, & Cancer, C. D. C. (2021). An Update on Cancer Deaths in the United States. In:Prevention CfDCaed;
- 3.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA internal medicine. 2018;178:1645–1658. doi: 10.1001/jamainternmed.2018.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Community Preventive Services Task Force (CPSTF). Cancer Screening: Multicomponent Interventions—Colorectal Cancer. The Community Guide 2016.
- 5.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: a randomized controlled trial in a safety-net health system. Cancer. 2016;122:456–463. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman SN, Liss DT, Brown T, et al. Comparative effectiveness of multifaceted outreach to initiate colorectal cancer screening in community health centers: a randomized controlled trial. Journal of general internal medicine. 2015;30:1178–1184. doi: 10.1007/s11606-015-3234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietrich AJ, Tobin JN, Robinson CM, et al. Telephone outreach to increase colon cancer screening in medicaid managed care organizations: a randomized controlled trial. The Annals of Family Medicine. 2013;11:335–343. doi: 10.1370/afm.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braschi CD, Sly JR, Singh S, et al. Increasing colonoscopy screening for latino Americans through a patient navigation model: a randomized clinical trial. Journal of Immigrant and Minority Health. 2014;16:934–940. doi: 10.1007/s10903-013-9848-y. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman AS, Lowenstein LM, Kamath GR, et al. An entertainment-education colorectal cancer screening decision aid for african american patients: a randomized controlled trial. Cancer. 2017;123:1401–1408. doi: 10.1002/cncr.30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vernon SW, Bartholomew LK, McQueen A, et al. A randomized controlled trial of a tailored interactive computer-delivered intervention to promote colorectal cancer screening: sometimes more is just the same. Annals of Behavioral Medicine. 2011;41:284–299. doi: 10.1007/s12160-010-9258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sequist TD, Zaslavsky AM, Colditz GA, et al. Electronic patient messages to promote colorectal cancer screening: a randomized controlled trial. Archives of internal medicine. 2011;171:636–641. doi: 10.1001/archinternmed.2010.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller CJ, Robinson RF, Smith JJ, et al. Text message reminders increased colorectal cancer screening in a randomized trial with Alaska native and american indian people. Cancer. 2017;123:1382–1389. doi: 10.1002/cncr.30499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Miller S, Koch M, et al. Financial incentives for promoting colorectal cancer screening: a randomized, comparative effectiveness trial. Official journal of the American College of Gastroenterology| ACG. 2016;111:1630–1636. doi: 10.1038/ajg.2016.286. [DOI] [PubMed] [Google Scholar]
- 14.Kullgren JT, Dicks TN, Fu X, et al. Financial incentives for completion of fecal occult blood tests among veterans: a 2-stage, pragmatic, cluster, randomized, controlled trial. Annals of internal medicine. 2014;161:S35–S43. doi: 10.7326/M13-3015. [DOI] [PubMed] [Google Scholar]
- 15.Ornstein S, Nemeth LS, Jenkins RG, et al. Colorectal cancer screening in primary care: translating research into practice. Medical care. 2010;48:900. doi: 10.1097/MLR.0b013e3181ec5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basch CE, Zybert P, Wolf RL, et al. A randomized trial to compare alternative educational interventions to increase colorectal cancer screening in a hard-to-reach urban minority population with health insurance. Journal of Community Health. 2015;40:975–983. doi: 10.1007/s10900-015-0021-5. [DOI] [PubMed] [Google Scholar]
- 17.Fortuna RJ, Idris A, Winters P, et al. Get screened: a randomized trial of the incremental benefits of reminders, recall, and outreach on cancer screening. Journal of general internal medicine. 2014;29:90–97. doi: 10.1007/s11606-013-2586-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendren S, Winters P, Humiston S, et al. Randomized, controlled trial of a multimodal intervention to improve cancer screening rates in a safety-net primary care practice. Journal of general internal medicine. 2014;29:41–49. doi: 10.1007/s11606-013-2506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pankratz VS, Kosich M, Edwardson N, et al. American Indian/Alaska native and black colon cancer patients have poorer cause-specific survival based on disease stage and anatomic site of diagnosis. Cancer Epidemiology. 2022;80:102229. doi: 10.1016/j.canep.2022.102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.New Mexico Indicator Based Information System (NM-IBIS). New Mexico Health Indicator Data & Statistics: Colorectal Cancer. In:Health NMDoed;2013–2017 https://ibis.doh.nm.gov/indicator/summary/CancerScrColoRec.html
- 21.Doubeni CA, Corley DA, Zhao W, et al. Association between Improved Colorectal screening and racial disparities. New England Journal of Medicine. 2022;386:796–798. doi: 10.1056/NEJMc2112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narva AS, Romancito G, Faber T, et al. Managing CKD by Telemedicine: the Zuni Telenephrology Clinic. Advances In Chronic Kidney Disease. 2017;24:6–11. doi: 10.1053/j.ackd.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Indian Health Services (2019). FY 2019 GPRA/GPRAMA Integrated Data Collection System (IDCS) National Results. In:Services DoHaHed;
- 24.Bastani R, Glenn BA, Taylor VM, et al. Integrating theory into community interventions to reduce liver cancer disparities: the Health Behavior Framework. Preventive Medicine. 2010;50:63–67. doi: 10.1016/j.ypmed.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra SI, Bastani R, Crespi CM, et al. Results of a randomized trial to increase mammogram usage among samoan women. Cancer Epidemiology Biomarkers & Prevention. 2007;16:2594–2604. doi: 10.1158/1055-9965.EPI-07-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guadagnolo BA, Cina K, Helbig P, et al. Medical mistrust and less satisfaction with health care among native Americans presenting for cancer treatment. Journal Of Health Care For The Poor And Underserved. 2009;20:210–226. doi: 10.1353/hpu.0.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu SP, Taylor V, Yasui Y, et al. Promoting culturally appropriate colorectal cancer screening through a health educator: a randomized controlled trial. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2006;107:959–966. doi: 10.1002/cncr.22091. [DOI] [PubMed] [Google Scholar]
- 28.Bastani R, Marcus AC, Maxwell AE, et al. Evaluation of an intervention to increase mammography screening in Los Angeles. Preventive medicine. 1994;23:83–90. doi: 10.1006/pmed.1994.1012. [DOI] [PubMed] [Google Scholar]
- 29.Batai K, Sanderson PR, Hsu CH, et al. Factors Associated with Cancer Screening among Hopi Men. Journal of Cancer Education. 2022;37:915–923. doi: 10.1007/s13187-020-01900-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cartwright, K., Leekity, S., Sheche, J. (2022). Health Literacy, Health Numeracy, and Cancer Screening Patterns in the Zuni Pueblo: Insights from and Limitations of “Standard” Questions. Journal of Cancer Education [DOI] [PMC free article] [PubMed]
- 31.Itty TL, Hodge FS, Martinez F. Shared and unshared barriers to Cancer Symptom Management among Urban and Rural American Indians. The Journal of Rural Health. 2014;30:206–213. doi: 10.1111/jrh.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muus KJ, Baker-Demaray T, McDonald LR, et al. Body mass index and cancer screening in older american indian and Alaska native men. The Journal of Rural Health. 2009;25:104–108. doi: 10.1111/j.1748-0361.2009.00206.x. [DOI] [PubMed] [Google Scholar]
- 33.Maly AG, Steel TL, Fu R, et al. Colorectal cancer screening among american Indians in a Pacific Northwest tribe: Cowlitz Tribal BRFSS Project, 2009–2010. Public Health Reports. 2014;129:280–288. doi: 10.1177/003335491412900310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roh S, Burnette CE, Lee KH, et al. Correlates of receipt of colorectal cancer screening among american Indians in the Northern Plains. Social Work Research. 2016;40:95–104. doi: 10.1093/swr/svw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumacher MC, Slattery ML, Lanier AP, et al. Prevalence and predictors of cancer screening among american indian and Alaska native people: the EARTH study. Cancer Causes & Control. 2008;19:725–737. doi: 10.1007/s10552-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SR, Joshweseoma L, Saboda K, et al. Cancer screening on the Hopi reservation: a model for success in a native american community. Journal of community health. 2015;40:1165–1172. doi: 10.1007/s10900-015-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, J. S., Perdue, L. A., Henrikson, N. B. (2021). Screening for colorectal cancer: an evidence update for the US preventive services task force. [PubMed]
- 38.Bujang NNA, Lee YJ, Mohd-Zain SAS, et al. Factors associated with colorectal cancer screening via immunochemical fecal occult blood test in an average-risk population from a multiethnic, middle-income setting. JCO global oncology. 2021;7:333–341. doi: 10.1200/GO.20.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers, J., O’Carroll, R., Brownlee, L. (Colorectal cancer screening and perceived disgust: The importance of the “Ick” factor in faecal occult blood test uptake. Colorectal Cancer: Open Access 2016). ; 2
- 40.Jones RM, Woolf SH, Cunningham TD, et al. The relative importance of patient-reported barriers to Colorectal Cancer Screening. American Journal of Preventive Medicine. 2010;38:499–507. doi: 10.1016/j.amepre.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liss DT, Petit-Homme A, Feinglass J, et al. Adherence to repeat fecal occult blood testing in an Urban Community Health Center Network. Journal of Community Health. 2013;38:829–833. doi: 10.1007/s10900-013-9685-x. [DOI] [PubMed] [Google Scholar]
- 42.von Wagner C, Knight K, Halligan S, et al. Patient experiences of colonoscopy, barium enema and CT colonography: a qualitative study. The British journal of radiology. 2009;82:13–19. doi: 10.1259/bjr/61732956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.