Summary
Brain edema is a fatal pathological state in which brain volume increases as a result of abnormal accumulation of fluid within the brain parenchyma. A key attribute of experimentally induced brain edema – increased brain water content (BWC) – needs to be verified. Various methods are used for this purpose: specific gravimetric technique, electron microscopic examination, magnetic resonance imaging (MRI) and dry/wet weight measurement. In this study, the cohort of 40 rats was divided into one control group (CG) and four experimental groups with 8 rats in each group. The procedure for determining BWC using dry/wet weight measurement was initiated 24 h after the completion of edema induction by the water intoxication method (WI group); after the intraperitoneal administration of Methylprednisolone (MP) together with distilled water during edema induction (WI+MP group); 30 min after osmotic blood brain barrier disruption (BBBd group); after injection of MP via the internal carotid artery immediately after BBBd (BBBd+MP group). While induction of brain edema (WI, BBBd) resulted in significantly higher BWC, there was no increase in BWC in the MP groups (WI+MP, BBBd+MP), suggesting a neuroprotective effect of MP in the development of brain edema.
Keywords: Brain water content, Dry/wet weight measurement, Cytotoxic edema, Vasogenic edema
Introduction
Brain edema is a fatal pathological state in which brain volume increases as a result of abnormal accumulation of fluid within the brain parenchyma. The abnormal accumulation of fluid causes an increase of brain volume, defined as brain water content (BWC), along with elevation of intracranial pressure (ICP) because of an enclosed rigid skull [1–2].
The current classification [3] of brain edema includes cytotoxic (cellular), ionic (extracellular), and vasogenic (extracellular) edema. In cytotoxic (cellular) edema, the nature of which is the osmotically controlled redistribution of water between the cellular and extracellular compartments, there is no increase in BWC. In extracellular edema, water flows into the brain from the capillaries across the blood-brain barrier (BBB) and this leads to an increase in BWC. Extracellular edemas differ in several aspects. In ionic edema the BBB is intact, the fluid does not contain proteins, and penetration of ions and water into the extracellular space is mediated by ion channels and transporters exclusively due to the osmotic gradient. The basic condition of vasogenic edema is the disruption of BBB, the fluid contains proteins, and the driving force for the penetration of solutes and water into the extracellular space is a hydrostatic gradient. Cytotoxic edema (cellular) manifests several minutes after the brain insult (trauma, ischemia, water intoxication), ionic edema (extracellular) is formed immediately after the onset of cytotoxic edema, and vasogenic edema (extracellular) with protein influx due to BBB disruption occurs within hours after the initial brain insult. This temporal sequence leads to a logical interpretation of ionic edema as an extracellular component of cytotoxic edema, since it actually increases BWC shortly after the initial brain insult [4].
In general, any of the following methods can be used in animal models to determine BWC: dry/wet weight measurements [5–18], specific gravimetric technique [19–25], electron microscopic examination [26–28], and magnetic resonance imaging (MRI) [27,29–32]. It turns out that the most historically used method – measuring dry and wet weight - still has its place in determining BWC, although current highly sophisticated MRI methods are able to accurately determine brain water distribution in addition to BWC [32].
In our previous work, we compared BWC, degree of myelin damage, and locomotor activity at different levels of hyperhydration used for water intoxication (WI) [33].
In this study we used the determination of BWC by dry/wet weight measurement to demonstrate the neuroprotective effect of Methylprednisolone (MP) on the development of brain edema. To induce cytotoxic edema, we used the water intoxication method [34–35] and to model vasogenic edema we used the method of osmotic disruption of the BBB by intracarotid injection of 20 % Mannitol [34,36]. Methylprednisolone (Solu-Medrol®, Pfizer) was injected intraperitoneally during induction of cytotoxic edema or via the internal carotid artery after induction of vasogenic edema by osmotic BBBd [34].
In the discussion, we compared the results of this study with MRI findings and ICP values.
Methods
All experiments were approved by the Ethical Committee of the First Faculty of Medicine (Charles University in Prague) and were in agreement with the Guidelines of the Animal Protection Law of the Czech Republic and Guidelines for the treatment of laboratory animals EU Guidelines 86/609/EEC. For experiments, male rats of the Wistar strain weighing 400 to 410 g of our own breed were used.
The cohort consisted of a total of 40 rats divided into one control group and four experimental groups with 8 rats in each group. Intact animals formed the control group (CG group). The experimental groups were (1) WI group (the edema induction by the water intoxication method); (2) WI+MP group (the intraperitoneal administration of MP together with distilled water during the edema induction; (3) BBBd group (the edema induction by osmotic blood brain barrier disruption); (4) BBBd+MP group (the injection of MP via the internal carotid artery 10 min after osmotic BBBd).
Induction of edema by water intoxication (WI) and osmotic disruption of BBB (BBBd)
WI consists of fractional administration of distilled water + vasopressin. The modified method of WI is based on intraperitoneal (i.p.) administration of distilled water (DW) in the total amount corresponding to 20 % of body weight in three consecutive doses over 24 h (after eight hours) with a simultaneous administration of desmopressin. Each sub-dose represented one-third of the total dose of 0.032 mg/kg of desmopressin (1-desamino-8-D-arginine vasopressin) (OCTOSTIM®, Ferring). Desmopressin is an antidiuretic hormone, which potentiates the effect of hyperhydration by inducing hyponatremia.
Osmotic BBBd consists of injection of 20 % Mannitol into the internal carotid artery. Animals were put into the state of general anesthesia using intraperitoneal application of thiopental in the dose of 4 mg/100 g of inhalation anesthesia by isoflurane (Florante ®, AbbVie Ltd.) and allowed to ventilate spontaneously throughout the procedure. Starting from a skin incision along the midline between the upper end of the sternum and the mandible, the common carotid artery (CCA) was exposed with a standard microsurgical technique and, before its bifurcation, also the proximal portions of the external carotid artery (ECA), which was ligated close beyond the bifurcation. An intraluminal catheter was introduced into the ICA trunk through the arteriotomy for injection of Mannitol 20 % (200 g in 1000 ml of water for injection, 1098 mosmol/l) in a dose of 5 ml/kg at a rate of 0.12 ml/sec. With the application over and the catheter removed, the ICA was ligated. The surgery concluded with a single-layer suture.
Administration of MP
Methylprednisolone (Solu-Medrol®, Pfizer) was applied either intraperitoneally (the total amount of MP 100 mg/kg b.w. was divided into three subdoses and administered during edema induction with each dose of the distilled water and vasopressin) or via the internal carotid artery (total dose 50 mg/kg b.w.) 10 min after the osmotic BBBd.
Brain water content measurement
The procedure for determining BWC consisted of the following steps – decapitation, immediate removal of the brain, weighing the brain and determining the wet weight, placing the brain in a thermostat at 85 °C for six days, weighing the brain again and determining the dry weight, and determining BWC as a percentage according to equation: BWC (%) = (wet weight – dry weight)/wet weight × 100. According to the experimental protocol, the procedure was initiated (1) 24 h after the completion of edema induction by the WI method (WI group); (2) 24 h after the intraperitoneal administration of MP together with distilled water during edema induction (WI+MP group); (3) 30 min after osmotic BBBd (BBBd group); (4) 30 min after injection of MP via the internal carotid artery 10 min after BBBd (BBBd+MP group).
Results
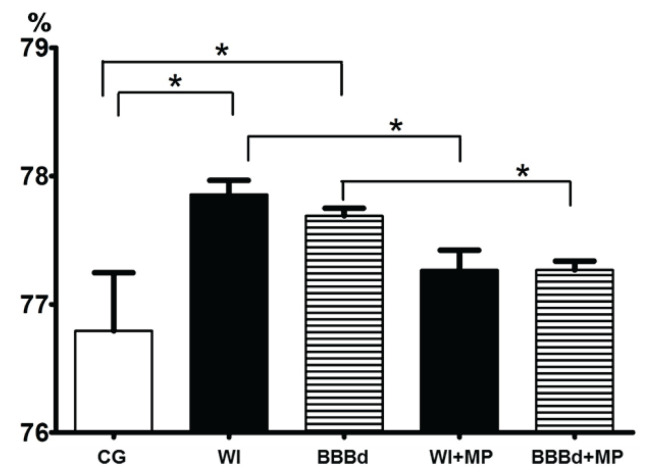
The results of the BWC measurements are shown in Figure 1. All data were statistically processed using parametric ANOVA and non-parametric Kruskal-Wallis tests for a statistically significant difference value of p<0.05.
Fig. 1.
Brain water content – BWC (%). x-axis: BWC (%), y-axis: columns with average value ± SD, CG=control group (intact animals); WI (WI group – the edema induction by the water intoxication method); BBBd (BBBd group – the edema induction by osmotic blood brain barrier disruption); WI+MP (WI+MP group – the intraperitoneal administration of MP together with distilled water during the edema induction); BBBd+MP (BBBd+MP group – the injection of MP via the internal carotid artery immediately after osmotic BBBd). (*) denotes statistically significant difference (p<0.05).
A statistically significant increase in BWC was found between intact animals (CG) and those in which edema was induced (WI, BBBd); a statistically significant decrease in BWC was found between animals with edema induced by the WI method (WI) and animals with MP applied intraperitoneally (WI+MP), and between BBBd-induced edema animals (BBBd) and animals with MP applied to the carotid artery (BBBd+MP); no statistically significant difference was found between intact animals (CG) and MP-treated animals (WI+MP, BBBd+MP). While induction of brain edema (groups WI and BBBd) resulted in significantly higher BWC, there was no increase in BWC in the MP groups (group WI+MP and BBBd+MP), suggesting a neuroprotective effect of MP in the development of brain edema. The mechanisms by which the neuroprotective effect of MP is achieved, and the relevant conditions required for the execution of these mechanisms, are described in detail in our previous work [34]. Briefly, the neuroprotective effect of MP is based on its antioxidative ability, which allows it to influence the pathogenic process in nerve cells at different levels. Due to the high molecular weight, MP must be administered when the BBB is open, i.e. during edema induction by the WI method or after osmotic BBBd.
Discussion
Determining BWC in experimental models of brain edema is of paramount importance; in addition to water accumulation, a key attribute of experimentally induced brain edema is the increase in BWC [1–2]. And this must be demonstrated. Each laboratory dealing with the studying or research of brain edema uses the method of determining BWC in experimental animals according to its habits and technical possibilities.
So far, four different methods have been described for the determination of BWC, as mentioned in the introduction. Nowadays MRI is able not only to pinpoint the BWC, but also to determine the predominant localization of water in the cellular or extracellular compartment, depending on the type of edema, and thus some methods of determining BWC (electron microscopy and specific gravimetric technique) have partially lost their original validity. On the other hand, despite the leading role of MRI in identifying the BWC and water distribution, the determination of BWC using dry and wet weight measurement retains its significant validity even today. Electron microscopy has been used to demonstrate the presence of water in brain tissue since 1960, when Luse and Harris [26] described changes associated with brain edema in rabbits, and gradually, with the development of knowledge about different types of edema, their typical features have also crystallized into electron microscopy findings [27]. In the MRI era, the role of electron microscopy as a stand-alone method to determine brain water content has diminished significantly, but it still serves as a complementary examination [28]. Use of specific gravity in the measurement of cerebral edema was for the first time documented by Nelson et al. in 1971 [19] and later upgraded by Marmarou et al [20–21]. The pros and cons of measuring water content gravimetrically compared to the dry/wet weight method were defined by Schigeno et al. [25]. The historically important gravimetric method for determining the BWC [22,24] is still part of the armamentarium of some laboratories, studying experimental brain edema [23].
Although in the 1980s it was already possible to detect brain edema using MRI [27,29] only in this millennium it has been possible to reliably determine the distribution of water in the brain. Quantification of the apparent diffusion coefficient (ADC) or T2 relaxation time has proven to be a useful tool for investigating experimental brain edema [2]. Cellular and extracellular edema after brain injury can be differentiated by a combination of ADC and T2 imaging. ADC is an indicator of the magnitude of the diffusion of water molecules within the tissue, and diffusion imaging provides information about the cellular architecture such as cellular size, membranes and volume fraction. ADC increases with higher extracellular volume and amount of fluids, and it is reduced when cell swelling is observed due to narrowing of the extracellular space within the cerebral parenchyma. T2 (transverse relaxation time of excited protons) is related to water content and vascular permeability. In general, reduced ADC values correlate with a cellular edema (and vice versa increased ADC values correlate with an experimental extracellular edema) whereas increased T2 values reflect the development of extracellular edema [15,30–32].
Historically, the relationship between wet and dry weight of the brain in a laboratory animal was probably first defined by Elliot and Jasper in 1949: „If W and D are respectively the fresh and dry weight of the brain of a normal animal and P is the percentage dry weight, then W = D × 100/P” [5]. Since the 1960s, brain edema in animal models has been quantified by comparing the water content of the affected tissue with normal brain. The water content has been expressed as % brain water, determined from the difference in wet and dry weights (i.e. the water weight) divided by the wet weight (BWC % = [(wet weight -dry weight)/wet weight] × 100 %) [6–7]. Keep et al. in 2012 warned, based on a very detailed analysis, that this change in % brain water content can be misleading, as ‘small’ changes in % brain water content actually reflect much bigger changes in brain swelling. They concluded that using either water content, expressed as g/g dry weight, or a measure of brain swelling, better reflect the impact of edema after stroke and brain injury. Their precise work indicates that the importance of BWC% lies in the demonstration of the existence of edema, not in the determination of its severity [8]. Briefly, this method consists of several steps: weighing the whole brain or parts of it (= tissue) after removal from the skull (wet weight); placing the tissue in a thermostat at a defined temperature for a defined time; weighing the tissue a second time (dry weight); determining the BWC according to the aforementioned equation [9]. Although the description of the procedure looks simple and clear, there are many variations in its realization. In its original version [6] the tissue was placed in an incubator at 56 °C, and weighed periodically thereafter for a total of 144 h. In each instance, the weight decreased with time, 95 to 99 % of the loss occurring in the first 24 h, and a constant level being reached at 120 h. In our laboratory we use a modified version: temperature 85 °C, drying time 6 days [33]. The most commonly used version is a temperature of 100 °C and a drying time of 24 h, which reflects the fact that 99 % of the weight is lost in this time interval [10–14]. Further modifications consist of a temperature of 105 °C and a drying time of 24 and/or 48 h, or a temperature of 50 and/or 60 °C and a drying time to achieve a constant weight [15–18].
As mentioned above, cytotoxic/cellular edema does not increase BWC, as it represents only a redistribution of fluid between the cellular and extracellular compartments in favor of the cellular, but the nature of extracellular edema – ionic and vasogenic – is the inflow of water into the brain from capillaries and thus an increase in BWC. From the time course after a brain insult, it follows that ionic edema occurs immediately after cytotoxic edema and is succeeded in a few hours by vasogenic edema. MRI with ADC accurately defines the distribution of water in the cells or in the extracellular compartment, but does not differentiate ionic edema from vasogenic edema.
In our previous study [38], we induced cellular edema by the WI method, and MRI performed after 24 h demonstrated the presence of extracellular edema, which corresponds to the aforementioned time course of edema development. This study demonstrated BWC increase after the induction of vasogenic – extracellular edema by the osmotic BBBd method, but also after the induction of cytotoxic – primarily cellular edema by the WI method. The procedure for determining BWC by the method of dry/wet weight measurement was started 24 h after the completion of WI, i.e. at a time when the present edema was already extracellular, without being able to determine whether it was ionic or vasogenic. In this regard, the dry/wet weight method is comparable in its informative value to the current possibilities of MRI.
In the same MRI study [38], there was a significant decrease in ADC values in MP-treated animals, which could be determined very accurately. Mean ADC values dropped from 684±77×10−6 mm2/s to 612±34×10−6 mm2/s in the cortex, and from 793±44×10−6 mm2/s to 759±50×10−6 mm2/s in the hippocampus after intraperitoneal MP application; mean ADC values dropped from 684±77×10−6 mm2/s to 599±25×10−6 mm2/s in the cortex, and from 793±44×10−6 mm2/s to 730±48×10−6 mm2/s in the hippocampus after MP application into carotid artery. This decrease in ADC values documented the neuroprotective effect of MP.
The current study showed a statistically significant decrease in BWC in MP-treated animals, but this decrease cannot be mathematically defined very precisely. Therefore, determination of BWC by measurement of dry/wet weights should be used to demonstrate the existence of edema, not to determine its severity as stated above [8].
The results of another previous study [37] confirmed, that brain edema induced by WI and osmotic BBBd methods leads to an increase in intracranial pressure (ICP). The ICP monitoring procedure began immediately after completion of WI or osmotic BBBd and continued for 72 h. In the experimental model of extracellular edema (BBBd) the elevation of ICP started very early but was of short duration whereas in the experimental model of cellular edema (WI) elevation of ICP was present during the whole period of monitoring. Peak ICP elevation was within 24–36 h after completion of WI and within 12–24 h after completion of osmotic BBBd. The findings of increased ICP are consistent with a statistically significantly increased BWC as determined by dry/wet weight measurements beginning 24 h after completion of edema induction by the WI method and 30 min after osmotic BBBd in the current study.
We can state that the results of this study clearly demonstrated the ability of dry/wet weight measurement to demonstrate not only an increase in BWC after inducing both types of edema (cellular and/or extracellular), but also to confirm the neuroprotective effect of MP on the development of brain edema. Although MRI must be the preferred choice for determining BWC today, the dry/wet weight method is still a useful tool in these experiments.
Acknowledgements
Supported by grant Progress Q35/LF1
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Klatzo I. Presidential Address. Neuropathological aspects of brain edema. J Neuropathol Exp Physiol. 1967;26:1–14. doi: 10.1097/00005072-196701000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Michinaga S, Koyama Y. Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci. 2015;16:9949–9975. doi: 10.3390/ijms16059949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stokum JA, Gerzanich V, Simard JM. Molecular pathophysiology of cerebral edema. J Cereb Blood Flow Metab. 2016;36:513–538. doi: 10.1177/0271678X15617172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szczygielski J, Kopańska M, Wysocka A, Oertel J. Cerebral microcirculation, perivascular unit, and glymphatic system: role of aquaporin-4 as the gatekeeper for water homeostasis. Front Neurol. 2021;12:767470. doi: 10.3389/fneur.2021.767470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliot KAC, Jasper H. Measurement of experimentally induced brain swelling and shrinkage. Am J Physiol. 1949;157:122–129. doi: 10.1152/ajplegacy.1949.157.1.122. [DOI] [PubMed] [Google Scholar]
- 6.Adachi M, Feigin I. Cerebral oedema and the water content of normal white matter. J Neurol Neurosurg Psychiat. 1966;29:446–450. doi: 10.1136/jnnp.29.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faas FH, Ommaya AK. Brain tissue electrolytes and water content in experimental concussion in the monkey. J Neurosurg. 1968;28:137–144. doi: 10.3171/jns.1968.28.2.0137. [DOI] [PubMed] [Google Scholar]
- 8.Keep RF, Hua Y, Xi G. Brain water content. A misunderstood measurement? Transl Stroke Res. 2012;3:263–265. doi: 10.1007/s12975-012-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, Batchelor TT, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;20:2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Wu X, Yu S, Fauzee NJ, Wu J, Li L, Zhao J, Zhao Y. Neuroprotective capabilities of Tanshinone IIA against cerebral ischemia/reperfusion injury via anti-apoptotic pathway in rats. Biol Pharm Bull. 2012;35:164–170. doi: 10.1248/bpb.35.164. [DOI] [PubMed] [Google Scholar]
- 11.Hu CY, Guo YQ, Hao YH, Zheng LN, Qi YH. Research on mechanism of sevoflurane in alleviating cerebral ischemia-reperfusion injury in rats through JNK signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:3907–3914. doi: 10.26355/eurrev_202004_20857. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Xu X, Wu YG, Lyu L, Zhou ZW, Zhang JN. Dexmedetomidine attenuates traumatic brain injury: action pathway and mechanisms. Neural Regen Res. 2018;13:819–826. doi: 10.4103/1673-5374.232529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaya M, Gulturk S, Elmas I, Kalayci R, Arican N, Kocyildiz ZC, Kucuk M, Yorulmaz H, Sivas A. The effects of magnesium sulfate on blood-brain barrier disruption caused by intracarotid injection of hyperosmolar mannitol in rats. Life Sci. 2004;76:201–212. doi: 10.1016/j.lfs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Marchi N, Betto G, Fazio V, Fan Q, Ghosh C, Machado A, Janigro D. Blood-brain barrier damage and brain penetration of antiepileptic drugs: role of serum proteins and brain edema. Epilepsia. 2009;50:664–677. doi: 10.1111/j.1528-1167.2008.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerriets T, Stolz E, Walberer M, Müller C, Kluge A, Bachmann A, Fisher M, Kaps M, Bachmann G. Noninvasive quantification of brain edema and the space-occupying effect in rat stroke models using magnetic resonance imaging. Stroke. 2004;35:566–571. doi: 10.1161/01.STR.0000113692.38574.57. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Deng S, Lei Q, He Q, Ren Y, Zhang Y, Nie J, Lu W. miR-7-5p affects brain edema after intracerebral hemorrhage and its possible mechanism. Front Cell Dev Biol. 2020;8:598020. doi: 10.3389/fcell.2020.598020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He HY, Ren L, Guo T, Deng YH. Neuronal autophagy aggravates microglial inflammatory injury by downregulating CX3CL1/fractalkine after ischemic stroke. Neural Regen Res. 2019;14:280–288. doi: 10.4103/1673-5374.244793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwab M, Bauer R, Zwiener U. The distribution of normal brain water content in Wistar rats and its increase due to ischemia. Brain Res. 1997;749:82–87. doi: 10.1016/S0006-8993(96)01165-1. [DOI] [PubMed] [Google Scholar]
- 19.Nelson SR, Mantz ML, Maxwell JA. Use of specific gravity in the measurement of cerebral edema. J Appl Physiol. 1971;30:268–271. doi: 10.1152/jappl.1971.30.2.268. [DOI] [PubMed] [Google Scholar]
- 20.Marmarou A, Poll W, Shulman K, Bhagavan H. A simple gravimetric technique for measurement of cerebral edema. J Neurosurg. 1978;49:530–537. doi: 10.3171/jns.1978.49.4.0530. [DOI] [PubMed] [Google Scholar]
- 21.Marmarou A, Tanaka K, Shulman K. An improved gravimetric measure of cerebral edema. J Neurosurg. 1982;56:246–253. doi: 10.3171/jns.1982.56.2.0246. [DOI] [PubMed] [Google Scholar]
- 22.Hayasaki K, Marmarou A, Barzó P, Fatouros P, Corwin F. Detection of brain atrophy following traumatic brain injury using gravimetric techniques. Acta Neurochir Suppl. 1997;70:75–77. doi: 10.1007/978-3-7091-6837-0_23. [DOI] [PubMed] [Google Scholar]
- 23.Chavarria L, Oria M, Romero-Giménez J, Alonso J, Lope-Piedrafita S, Cordoba J. Brain magnetic resonance in experimental acute-on-chronic liver failure. Liver Int. 2013;33:294–300. doi: 10.1111/liv.12032. [DOI] [PubMed] [Google Scholar]
- 24.Kuroiwa T, Nagaoka T, Ueki M, Yamada I, Miyasaka N, Akimoto H. Different apparent diffusion coefficient: water content correlations of gray and white matter during early ischemia. Stroke. 1998;29:859–865. doi: 10.1161/01.STR.29.4.859. [DOI] [PubMed] [Google Scholar]
- 25.Shigeno T, Brock M, Shigeno S, Fritschka E, Cervós-Navarro J. The determination of brain water content: microgravimetry versus drying-weighing method. J Neurosurg. 1982;57:99–107. doi: 10.3171/jns.1982.57.1.0099. [DOI] [PubMed] [Google Scholar]
- 26.Luse SA, Harris B. Electron microscopy of the brain in experimental edema. J Neurosurg. 1960;17:439–446. doi: 10.3171/jns.1960.17.3.0439. [DOI] [PubMed] [Google Scholar]
- 27.Barnes D, McDonald WI, Johnson G, Tofts PS, Landon DN. Quantitative nuclear magnetic resonance imaging: characterisation of experimental cerebral oedema. J Neurol Neurosurg Psychiatry. 1987;50:125–133. doi: 10.1136/jnnp.50.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Shi X, Chen Z, Geng J, Wang Y, Feng H, Zhu G, Chen Q. Edaravone reduces iron-mediated hydrocephalus and behavioral disorder in rat by activating the Nrf2/HO-1 pathway. J Stroke Cerebrovasc Dis. 2018;27:3511–3520. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Hilal SK, Maudsley AA, Simon HE, Perman WH, Bonn J, Mawad ME, Silver AJ, Ganti SR, Sane P, Chien IC. In vivo NMR imaging of tissue sodium in the intact cat before and after acute cerebral stroke. AJNR Am J Neuroradiol. 1983;4:245–249. [PMC free article] [PubMed] [Google Scholar]
- 30.Schuhmann MU, Stiller D, Skardelly M, Mokktarzadeh M, Thomas S, Brinker T, Samii M. Determination of contusion and oedema volume by MRI corresponds to changes of brain water content following controlled cortical impact injury. Acta Neurochir Suppl. 2002;81:213–215. doi: 10.1007/978-3-7091-6738-0_55. [DOI] [PubMed] [Google Scholar]
- 31.Badaut J, Ashwal S, Tone B, Regli L, Tian HR, Obenaus A. Temporal and regional evolution of aquaporin-4 expression and magnetic resonance imaging in a rat pup model of neonatal stroke. Pediatr Res. 2007;62:248–254. doi: 10.1203/PDR.0b013e3180db291b. [DOI] [PubMed] [Google Scholar]
- 32.Naessens DMP, Coolen BF, de Vos J, VanBavel E, Strijkers GJ, Bakker ENTP. Altered brain fluid management in a rat model of arterial hypertension. Fluids Barriers CNS. 2020;17:41. doi: 10.1186/s12987-020-00203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozler P, Riljak V, Pokorný J. Both water intoxication and osmotic BBB disruption increase brain water content in rats. Physiol Res. 2013;62(Suppl 1):S75–S80. doi: 10.33549/physiolres.932566. [DOI] [PubMed] [Google Scholar]
- 34.Kozler P, Marešová D, Pokorný J. Effect of methylprednisolone on experimental brain edema in rats - own experience reviewed. Physiol Res. 2021;70(Suppl 3):S289–S300. doi: 10.33549/physiolres.934818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 36.Kroll RA, Neuwelt EA. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1099. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- 37.Kozler P, Maresova D, Pokorny J. Cytotoxic brain edema induced by water intoxication and vasogenic brain edema induced by osmotic BBB disruption lead to distinct pattern of ICP elevation during telemetric monitoring in freely moving rats. Neuro Endocrinol Lett. 2019;40:249–256. [PubMed] [Google Scholar]
- 38.Kozler P, Herynek V, Marešová D, Perez PD, Šefc L, Pokorný J. Effect of methylprednisolone on experimental brain edema in magnetic resonance imaging. Physiol Res. 2020;69:919–926. doi: 10.33549/physiolres.934460. [DOI] [PMC free article] [PubMed] [Google Scholar]