Key Points
-
•
COVID-19 infection risk is similar for long-term BMT survivors and non-BMT participants.
-
•
Among BMT survivors, COVID-19 infection risk is higher among those who are unemployed and not masking.
Visual Abstract
Abstract
There is limited information regarding COVID-19 in long-term blood or marrow transplant (BMT) survivors. We leveraged the BMT Survivor Study (BMTSS) to address this gap. BMTSS included patients who underwent BMT at 1 of 3 sites in the United States between 1974 and 2014 and survived ≥2 years after BMT. A sibling cohort serves as a non-BMT comparison group. Participants (2430 BMT survivors; 780 non-BMT participants) completed the BMTSS survey between October 2020 and November 2021 about COVID-19 testing, risk mitigation behaviors, morbidity, and health care use. Median age at BMT was 46 years (range, 0-78 years) and median follow-up since BMT was 14 years (6-46 years); 76% were non-Hispanic White, 54% had received allogeneic BMT. The risk of COVID-19 infection was comparable for BMT survivors vs non-BMT participants (15-month cumulative incidence, 6.5% vs 8.1%; adjusted odd ratio [aOR] = 0.93; 95% confidence interval [CI], 0.65-1.33; P = .68). Among survivors, being unemployed (aOR 1.90; 95% CI, 1.12-3.23; P = .02; reference: retired) increased the odds of infection; always wearing a mask in public was protective (aOR = 0.49; 95% CI, 0.31-0.77; P = .002; reference: not always masking). When compared with COVID-positive non-BMT participants, COVID-positive BMT survivors had higher odds of hospitalization (aOR = 2.23; 95% CI, 0.99-5.05; P = .05); however, the odds of emergency department visits were comparable (aOR = 1.60; 95% CI = 0.71-3.58; P = .25). COVID-19 infection status did not increase the odds of hospitalization among BMT survivors (aOR = 1.32; 95% CI = 0.89-1.95; P = .17) but did increase the odds of emergency department visits (aOR = 2.63; 95% CI, 1.74-3.98; P <.0001). These findings inform health care providers about the management of care for long-term BMT survivors during the ongoing pandemic.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues strong after almost 3 years; almost 100 million cases and over a million deaths have occurred in the United States.1 In March 2022, the US Centers for Disease Control and Prevention (CDC) changed their guidelines to liberalize precautions in the setting of effective vaccines, while recommending that high-risk individuals take additional precautions recommended by their health care providers.2 However, the definition of specific high-risk populations continues to evolve. Therefore, it is critical to identify vulnerable populations to facilitate appropriate decisions about risk mitigation at the individual level.
There is ample evidence that people undergoing active cancer treatment with conventional therapy or blood or marrow transplantation (BMT) are at an elevated risk for severe COVID-19 infection with higher rates of hospitalization, intensive care unit (ICU) admissions, and death.3, 4, 5, 6, 7, 8 Conventionally-treated cancer survivors are at lower risk of severe COVID-19 infection than those on active treatment.9,10 However, little is known about the risk and severity of COVID-19 in long-term survivors of BMT (> 5 years [y] post-BMT), as much of the literature around COVID-19 infection among BMT survivors either limits analysis to the first 2 years after BMT.8,11,12 Both the prevalence and severity of COVID-19 infection may continue to remain elevated among BMT survivors because of the high intensity of therapeutic exposures, the prolonged periods of immune suppression, and the substantial burden of multimorbidity, which increases with time after BMT.13, 14, 15, 16, 17, 18 It is critical to understand the risk of COVID-19 in the growing population of long-term BMT survivors19 and identify subpopulations at the highest risk to allow both survivors and health care providers to make appropriate decisions in the face on the ongoing pandemic. We addressed this gap by leveraging the BMT survivor study (BMTSS).
Methods
Study design
BMTSS is a cohort study with the overarching goal of understanding the long-term outcomes in BMT recipients. The cohort includes patients who received allogeneic or autologous BMT at City of Hope (COH), University of Minnesota (UMN), or University of Alabama at Birmingham (UAB) between 1 January 1974 and 31 December 2014, and survived ≥2 years after BMT. Siblings of BMT recipients serve as a non-BMT comparison group.
Participation in BMTSS involves periodic completion of the BMTSS survey by BMT survivors and the non-BMT comparison group. The baseline BMTSS survey included the following content: specific chronic health conditions as diagnosed by a health care provider (along with age at diagnosis), relapse of primary cancer, development of subsequent neoplasms, and history of chronic graft-versus-host disease (cGvHD). The survey also asked for sociodemographic characteristics (race/ethnicity, education, employment, annual household income, and health insurance). The reliability and validity of the BMTSS survey shows that BMT survivors are able to report their outcomes with a high degree of accuracy.20 Details of conditioning (conditioning intensity [myeloablative (MAC) or nonmyeloablative/reduced intensity (NMA/RIC)]), stem cell source (bone marrow, peripheral blood stem cells [PBSCs], or cord blood), and donor type (autologous or allogeneic) were captured from the institutional transplant databases and/or medical records. The institutional review board (IRB) at UAB serves as the single IRB of record for the participating sites (UMN and COH); participants have provided informed consent according to the Declaration of Helsinki.
We invited BMT survivors and siblings who had completed the baseline BMTSS survey before 1 March 2020 at age ≥18 years, to complete the BMTSS_2020 survey (see supplemental materials for COVID-19 related questions). The BMTSS_2020 survey asked respondents if they were tested for COVID-19 since 1 March 2020. If they answered yes, we asked them to provide the testing date and results for each time they were tested. They were asked if anyone in their home, social circle, or work environment was diagnosed with COVID-19 (defined as COVID-positive contact). Participants were asked about details pertaining to their risk mitigation behaviors (masking in public, social distancing from nonhousehold contact, and frequent handwashing), with possible responses including, yes-always, yes-sometimes, and never. The survey asked participants about employment status and health care use since 1 March 2020 (including emergency department [ED] visits and hospitalization). Respondents did not specify if health care use was related to COVID-19. The survey obtained updated information regarding key morbidities (kidney problems, heart disease, blood clot, stroke, problems with learning/memory, subsequent neoplasms, and recurrence of primary cancer), with an age at onset of these conditions. At the time of survey initiation (October 2020), COVID-19 vaccines were not available, precluding us from gathering information on vaccination status. Overall, 2430 BMT survivors (80.3% response rate) and 780 non-BMT comparison group (80.6% response rate) completed the BMTSS_2020 survey between October 2020 and November 2021 (supplemental Figure 1).
Statistical analysis
Risk of COVID-19 infection: BMT survivors vs non-BMT cohort
Kaplan-Meier techniques were used to describe the cumulative incidence of COVID-19 infection; the person-months at risk were computed from 1 March 2020 until survey completion. We conducted a Cox regression analyses to examine the hazards of developing COVID-19 infection in the BMT survivor cohort vs non-BMT cohort. We adjusted the multivariable model for demographic characteristics (age at survey [<65 years, ≥65 years], race/ethnicity [non-Hispanic White, African American, Hispanic, other], sex, annual household income [<$50 000, $50 000-74 999, ≥$75 000], education [<high school, high school to some college, ≥college], insurance [yes/no], employment [employed, not employed, retired]), severe/life-threatening chronic health conditions (yes/no), COVID-positive contact (yes/no), and risk mitigation behaviors (always masking, always maintaining social distancing, and always frequent hand washing).
Risk of COVID-19 infection: analysis restricted to BMT survivors
We conducted Cox regression analyses to identify factors (demographic variables, clinical characteristics [BMT type, history of cGvHD, length of follow-up from BMT, primary cancer type, risk of relapse at BMT, and conditioning intensity], severe/life-threatening chronic health conditions, COVID-positive contact, and risk mitigation behaviors) associated with the odds of developing COVID-19 infection. Backward selection was used to retain variables with P value < .1 in the multivariable logistic regression model.
Hospitalizations or ED visits among COVID-19 positive individuals: BMT survivor cohort vs non-BMT cohort
Treating the non-BMT cohort as the reference group, we used logistic regression to examine the odds of hospitalization among COVID-19 positive individuals. The model was adjusted for demographic characteristics, chronic health conditions, COVID-positive contacts, and risk mitigation behaviors. Similar models were constructed to examine the odds of ED visits among COVID-19 positive individuals.
Risk of hospitalization or ED visits: analysis restricted to BMT survivors
Using logistic regression analyses, we examined the odds of hospitalization. The primary exposure was COVID-19 infection. Other variables examined included BMT type, cGvHD, length of follow-up from BMT, primary cancer type, risk of relapse at BMT, conditioning intensity, demographics, COVID-positive contact, and risk mitigation behaviors. Similar models were constructed to examine the odds of ED visits by COVID-19 status among BMT survivors.
Given the lack of details regarding vaccination status, we repeated all the analyses for patients who completed the survey during the prevaccine era as well as those who participated in the postvaccine era (based on US Food and Drug Administration [FDA] approval of the first COVID-19 vaccine on 11 December 2020). We used SAS v9.4 (SAS Institute Inc., Cary, NC) for statistical analyses. All reported P values are 2-sided and considered significant at an α-level of .05.
Results
Study population
As shown in Table 1, 2430 BMT survivors and 780 non-BMT participants responded between October 2020 and March 2022 (supplemental Figure 2). Among BMT survivors, the median age at BMT was 46 years (range, 0-78 years) and median age at survey completion was 63 years (20-91 years); 76.3% were non-Hispanic White (1855/2430), and 52.8% were male. The most common indications for BMT included leukemia (41.4%), lymphoma (32.7%), and plasma cell dyscrasias (18.4%); 54% had received allogeneic BMT and 66.3% had received MAC. Fifty-four percent of the allogeneic BMT recipients carried a history of cGvHD. The median length of follow-up was 14 years (6-46 years) from BMT.
Table 1.
Characteristics of the study participants
| Characteristics | BMT survivors (n = 2430), n (%) | Non-BMT comparison group (n = 780), n (%) | P value |
|---|---|---|---|
| Age at study participation | |||
| Median (range) | 63 (20-91) | 62 (21-88) | .17 |
| <65 y | 1317 (54.2) | 448 (57.4) | .11 |
| ≥65 y | 1113 (45.8) | 332 (42.6) | |
| Sex | |||
| Female | 1147 (47.2) | 502 (64.4) | <.0001 |
| Race/ ethnicity | |||
| African American | 131 (5.4) | 20 (2.6) | <.0001 |
| Hispanic | 257 (10.6) | 44 (5.6) | |
| Non-Hispanic White | 1855 (76.3) | 686 (88.0) | |
| Other | 187 (7.7) | 30 (3.8) | |
| Annual household income | |||
| <$50 000 | 649 (26.7) | 142 (18.2.0) | <.0001 |
| $50 000-74 999 | 371 (15.3) | 146 (18.7) | |
| ≥$75 000 | 1069 (44.0) | 423 (54.2) | |
| Missing | 341 (14.0) | 69 (8.9) | |
| Education | |||
| <High school | 377 (15.5) | 80 (10.3) | <.0001 |
| High school to college | 883 (36.4) | 262 (33.8) | |
| ≥College | 1169 (48.1) | 433 (55.9) | |
| Have insurance | |||
| Yes | 2381 (98.0) | 757 (97.7) | .55 |
| Contact with COVID-19 test positive individuals (family members/friends/at work) | |||
| Yes | 1081 (44.5) | 442 (56.7) | <.0001 |
| Always wears mask | |||
| Yes | 2146 (88.3) | 662 (84.9) | .01 |
| Always stays 6 feet away | |||
| Yes | 1728 (71.1) | 464 (59.5) | <.0001 |
| Always washes hands | |||
| Yes | 1208 (49.7) | 322 (41.3) | <.0001 |
| Chronic health conditions (severe/life-threatening) | |||
| No | 190 (7.8) | 159 (20.4) | <.01 |
| Grade 1 | 156 (6.4) | 86 (11.0) | |
| Grade 2 | 736 (30.3) | 298 (38.3) | |
| Grade 3 | 925 (38.1) | 182 (23.4) | |
| Grade 4 | 423 (17.4) | 54 (6.9) | |
| COVID-19 test positive | |||
| Yes | 121 (5.0) | 47 (6.0) | .25 |
| Employment | |||
| Employed | 1038 (42.9) | 456 (58.7) | <.0001 |
| Not employed | 401 (16.6) | 52 (6.7) | |
| Retired | 981 (40.5) | 269 (34.6) | |
| Age at BMT (y) | |||
| Median (range) | 46 (0-78) | ||
| <45 y | 1136 (46.7) | ||
| 45-64 y | 1086 (44.7) | ||
| ≥65 y | 208 (8.6) | ||
| BMT type | |||
| Autologous | 1117 (46.0) | ||
| Allogeneic without chronic GvHD | 610 (25.1) | ||
| Allogeneic with chronic GvHD | 703 (28.9) | ||
| BMT survivors follow-up (y) | |||
| Median (range) | 14 (6-46) | ||
| <10 y | 571 (23.5) | ||
| 10-19 y | 1122 (46.2) | ||
| ≥20 y | 737 (30.3) | ||
| BMT survivors primary cancer | |||
| Leukemia | 1006 (41.4) | ||
| Lymphoma | 794 (32.7) | ||
| Plasma cell dyscrasias | 448 (18.4) | ||
| Other | 182 (7.5) | ||
| BMT survivors conditioning intensity | |||
| Myeloablative conditioning | 1610 (66.3) | ||
| Nonmyeloablative/ reduced intensity conditioning | 820 (33.7) | ||
| Disease status at BMT | |||
| High-risk | 1061 (43.7) | ||
Characteristics of the 780 non-BMT participants are in Table 1. Compared with the non-BMT cohort, the BMT survivors were more likely to be female (64.4% vs 47.2%; P < .001), non-Hispanic white (88.0% vs 76.3%; P < .001), and had higher income (≥$75 000: 54.2% vs 44%; P < .001) and education (≥college: 55.9% vs 48.1%; P < .001), and were more likely to be employed (58.7% vs 42.9%; P < .001). The BMT participants were more likely to have severe/ life-threatening chronic health conditions (92.2% vs 79.6%; P < .001) (conditions delineated in supplemental Table 1). Characteristics of the subcohorts in the pre- and postvaccine era are in supplemental Table 2.
Risk mitigation behaviors and COVID-19 infection: BMT survivors vs non-BMT participants
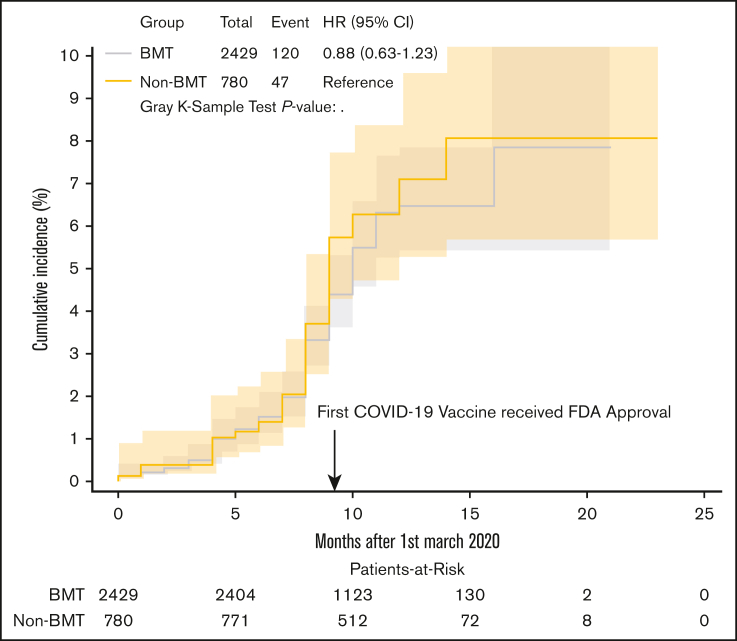
As detailed in Table 1, BMT survivors were more likely to report always wearing masks in public (88.3% vs 84.9%; P = .01), always maintaining social distancing (71.1% vs 59.5%; P < .001), and always washing hands (49.7% vs 41.3%; P < .001). BMT survivors were less likely to report COVID-19 positive contacts (44.5% vs 56.7%; P < .001). The proportion of participants who underwent COVID-19 testing over the entire study period did not differ between the 2 cohorts (48.3% vs 48.0%; P = 1.0). The cumulative incidence of COVID-19 infection at 15 months from 1 March 2020 was 6.5% (95% confidence interval [CI], 5.3-7.8) in BMT survivors vs 8.1% (95% CI, 5.5-11.2) in the non-BMT cohort (P = .45) (Figure 1). Multivariable analyses adjusted for sociodemographics, risk mitigation behaviors, and chronic health conditions showed that the hazard ratio (HR) of COVID-19 infections in BMT survivors were comparable with that of the non-BMT cohort (adjusted hazard ratio [aHR] = 0.93; 95% CI, 0.65-1.33; P = .68) (Table 2). The majority of COVID-19 infections (86% among survivors; 94% among siblings) occurred in the postvaccine era. The adjusted odd ratio (aOR) of COVID-19 infections in BMT survivors were comparable with that of the non-BMT comparison group in both the prevaccine (aOR = 1.58; 95% CI, 0.40-6.15; P = .51) and postvaccine era (aOR = 0.92; 95% CI, 0.62-1.36; P = .66), supplemental Table 2.
Figure 1.
Cumulative incidence of COVID-19 in BMT survivors vs non-BMT comparison.
Table 2.
Risk of COVID-19 infection in BMT survivors vs non-BMT comparison group
| Risk factors |
Univariate analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| HR | HR (95% CI) | P value | HR | aHR 95% CI | P value | |
| BMT survivors vsnon-BMTcomparison group | ||||||
| Non-BMT comparison group | REF | REF | ||||
| BMT survivors | 0.88 | 0.63-1.23 | .45 | 0.93 | 0.65-1.33 | .68 |
| Age at study participation | ||||||
| <65 y | REF | |||||
| ≥65 y | 0.58 | 0.42-0.80 | .001 | |||
| Race/ethnicity | ||||||
| Non-Hispanic White | REF | .049∗ | REF | .047∗ | ||
| African American | 1.41 | 0.78- 2.55 | .25 | 1.68 | 0.92- 3.08 | .09 |
| Hispanic | 1.34 | 0.85- 2.11 | .20 | 1.30 | 0.81- 2.09 | .27 |
| Other | 0.34 | 0.12-0.91 | .03 | 0.38 | 0.14-1.02 | .06 |
| Sex | ||||||
| Female | REF | |||||
| Male | 1.02 | 0.76-1.39 | .88 | |||
| Annual household income | ||||||
| ≥$75 000 | REF | REF | ||||
| <$75 000 | 1.32 | 0.96- 1.80 | .09 | 1.38 | 1.00-1.91 | .05 |
| Education | ||||||
| ≥College | REF | .08∗ | ||||
| High school to some college | 1.29 | 0.92-1.81 | .14 | |||
| <High school | 1.58 | 1.04-2.40 | .03 | |||
| Have insurance | ||||||
| No | REF | |||||
| Yes | 1.00 | 0.37-2.71 | .99 | |||
| Chronic health conditions | ||||||
| Grade 0-2 | REF | REF | ||||
| Grade 3-4 | 1.21 | 0.89-1.64 | .22 | 1.43 | 1.03-1.98 | .03 |
| Contact with COVID–19 test positive individuals (family members/ friends/at work) | ||||||
| No | REF | REF | ||||
| Yes | 5.7 | 3.75-8.66 | <.001 | 5.32 | 3.47-8.16 | <.001 |
| Always wear mask | ||||||
| No | REF | REF | ||||
| Yes | 0.48 | 0.34-0.69 | <.001 | 0.55 | 0.38-0.80 | .002 |
| Always stay 6 feet away | ||||||
| No | REF | |||||
| Yes | 0.73 | 0.54-1.00 | .05 | |||
| Always wash hands | ||||||
| No | REF | |||||
| Yes | 1.02 | 0.76-1.39 | .88 | |||
| Employment | ||||||
| Retired | REF | .002∗ | REF | .09∗ | ||
| Employed | 1.83 | 1.27-2.63 | .001 | 1.35 | 0.92- 1.98 | .13 |
| Not employed | 2.03 | 1.28-3.23 | .003 | 1.69 | 1.05-2.71 | .03 |
REF, reference group.
P value for whole group.
COVID-19 infection among BMT survivors
As shown in Table 3, factors associated with increased hazards of COVID-19 infection included being unemployed (aHR = 1.90; 95% CI, 1.12-3.23; P = .02; reference: retired), and COVID-positive contact (aHR = 4.48; 95% CI, 2.82-7.12; P < .001; reference: no contact). Among risk mitigation behaviors, always wearing a mask in public was the only protective factor (aHR = 0.49; 95% CI, 0.31-0.77; P = .002; reference: not always masking). As shown in supplemental Table 4, African American race and COVID-positive contact were associated with risk of infection in the prevaccine era. In the postvaccine era, Hispanic ethnicity, COVID-positive contact, and high-risk disease increased the risk of infection, whereas always wearing a mask was protective.
Table 3.
Factors associated with COVID-19 infection among BMT survivors
| Risk factors |
Univariate analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| HR ratio | 95% CI | P value | aHR ratio | 95% CI | P value | |
| Age at study participation | ||||||
| <65 y | REF | |||||
| ≥65 y | 0.54 | 0.37-0.79 | .002 | |||
| Race/ethnicity | ||||||
| Non-Hispanic White | REF | .03c | REF | .08∗ | ||
| African American | 1.49 | 0.77-2.87 | .23 | 1.50 | 0.75-3.02 | .25 |
| Hispanic | 1.67 | 1.03-2.69 | .04 | 1.56 | 0.95-2.57 | .08 |
| Other | 0.43 | 0.16-1.18 | .10 | 0.47 | 0.17-1.29 | .14 |
| Sex | ||||||
| Female | REF | |||||
| Male | 0.87 | 0.60-1.24 | .43 | |||
| Primary cancer | ||||||
| Leukemia + lymphoma | REF | .44 | REF | .10 | ||
| PCD | 1.31 | 0.86-2.00 | .21 | 1.51 | 0.96-2.39 | .08 |
| Other | 0.96 | 0.46-1.98 | .90 | 0.66 | 0.30-1.45 | .30 |
| Contact with COVID–19 test positive individuals (family members/friends/at work) | ||||||
| No | REF | REF | ||||
| Yes | 5.00 | 3.17-7.87 | <.001 | 4.48 | 2.82-7.12 | <.0001 |
| Always wears mask | ||||||
| No | REF | REF | ||||
| Yes | 0.47 | 0.31-0.73 | .001 | 0.49 | 0.31-0.77 | .002 |
| Always stays 6 feet away | ||||||
| No | REF | |||||
| Yes | 0.63 | 0.44-0.91 | .01 | |||
| Always washes hand | ||||||
| No | REF | |||||
| Yes | 1.06 | 0.74-1.52 | .74 | |||
| Annual household income | ||||||
| ≥$75 000 | REF | |||||
| <$75 000 | 1.34 | 0.92-1.95 | .13 | |||
| Education | ||||||
| ≥College | REF | .42∗ | ||||
| <High school | 1.38 | 0.85-2.24 | .19 | |||
| High school/college | 1.10 | 0.73-1.64 | .65 | |||
| Have insurance | ||||||
| No | REF | |||||
| Yes | 0.91 | 0.29-2.86 | .87 | |||
| Chronic health conditions | ||||||
| Grade 0-2 | REF | |||||
| Grade 3-4 | 1.17 | 0.81-1.68 | .40 | |||
| Employment | ||||||
| Retired | REF | .01∗ | REF | .057∗ | ||
| Employed | 1.84 | 1.19-2.84 | .006 | 1.45 | 0.91-2.30 | .12 |
| Not employed | 2.14 | 1.28-3.59 | .004 | 1.90 | 1.12-3.23 | .02 |
| BMT type | ||||||
| Autologous | REF | .72∗ | ||||
| Allogeneic without chronic GvHD | 0.84 | 0.51-1.38 | .49 | |||
| Allogeneic with chronic GvHD | 0.87 | 0.56-1.35 | .54 | |||
| BMT survivorsfollow-upyears | ||||||
| ≥20 y | REF | .58∗ | ||||
| <10 y | 1.03 | 0.64-1.65 | .92 | |||
| 10-19 y | 0.83 | 0.55-1.27 | .40 | |||
| BMT survivors conditioning intensity | ||||||
| Nonmyeloablative/reduced intensity | REF | |||||
| Myeloablative conditioning | 0.98 | 0.67-1.44 | .91 | |||
| Disease status at BMT | ||||||
| Standard risk | REF | |||||
| High-risk | 1.31 | 0.92-1.88 | .13 | |||
P value for whole group. Bold values indicate significant associations.
Health care utilization
Hospitalizations
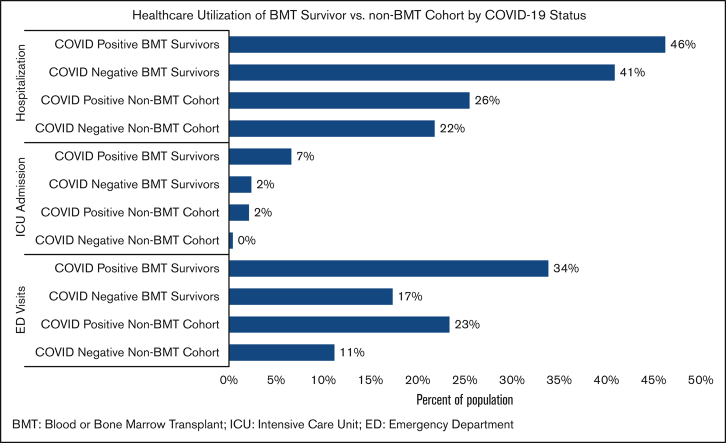
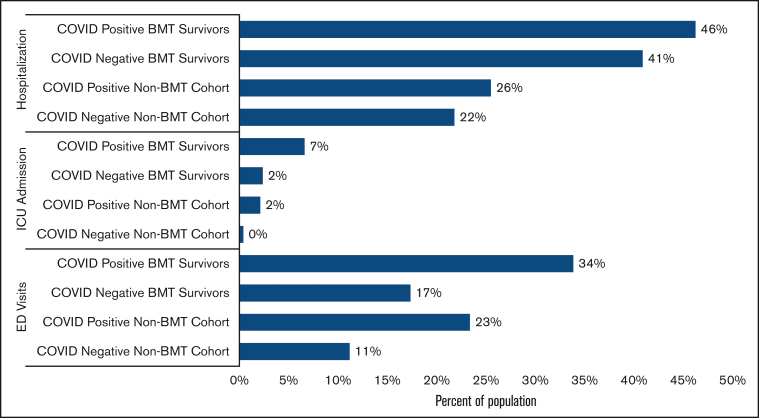
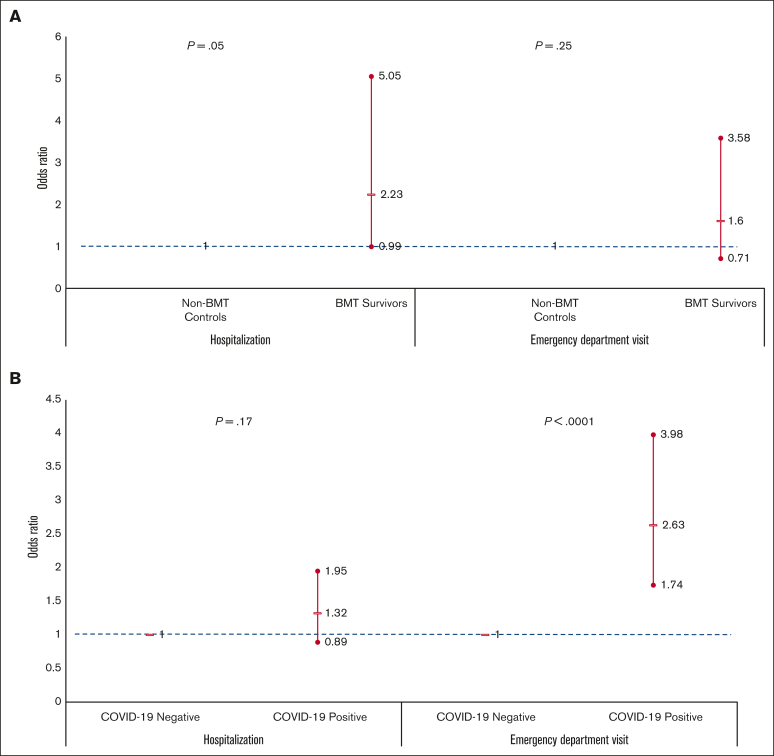
Forty-three percent of the BMT survivors (COVID-19 positive survivors: 46%; COVID-19 negative survivors: 41%; attributable difference: 5%) and 22% of the non-BMT controls (COVID-positive controls: 26%; COVID-19 negative controls: 22%; attributable difference: 4%) were admitted to the hospital between 1 March 2020 and 31 March 2022 (Figure 2). Multivariable regression analysis revealed 2.2-fold higher odds of hospitalization for COVID-19 positive survivors (aOR = 2.23; 95% CI, 0.99-5.05; P = .053; reference: non-BMT COVID-19 positive participants) (Figure 3A; supplemental Table 5). Among BMT survivors, the adjusted odds of hospitalization were not increased for COVID-19 positive BMT survivors compared with that of COVID-19 negative survivors (aOR = 1.32; 95% CI, 0.89-1.95; P = .17) (Figure 3B; supplemental Table 6). Overall, 4% of the BMT survivors (COVID-19 positive survivors: 7%; COVID negative survivors: 2%; attributable difference: 5%) and 0.5% of siblings (COVID-19 positive siblings: 2%; COVID-19 negative siblings: 0%; attributable difference: 2%) were admitted to the ICU (Figure 2). The risk factors for hospitalization among BMT survivors during the pandemic included severe/life-threatening chronic health conditions (aOR = 1.89; 95% CI, 1.58-2.25; P = <0.001; reference: grade 0-2 conditions).
Figure 2.
Health care use of BMT survivor vs non-BMT cohort by COVID-19 status. BMT, blood or bone marrow transplant; ICU, intensive care unit; ED, emergency department.
Figure 3.
Odds of hospitalization and emergency department visits. (A) Among COVID-19 positive individuals: BMT survivors vs non-BMT comparison group (controlling for clinical and sociodemographic factors). (B) Among BMT survivors: COVID-19 positive vs COVID-19 negative survivors (controlling for clinical and sociodemographic factors). Showing odds ratio and 95%CI.
ED visits
During the pandemic, 19% of the BMT survivors (COVID-19 positive survivors: 34%; COVID-19 negative survivors: 17%; attributable difference: 17%) and 12% of non-BMT controls (COVID-19 positive controls: 23%; COVID-19 negative controls: 11%; attributable difference: 12%) reported ED visits (Figure 2). In multivariable regression, there was no difference in the odds of ED visits for COVID-19 positive BMT survivors (aOR = 1.60; 95% CI, 0.71-3.58; reference: non-BMT controls) (Figure 3A; supplemental Table 7). Among BMT survivors, the adjusted odds of ED visits were increased for COVID-19 positive BMT survivors compared with that of the COVID-19 negative survivors (aOR = 2.63; 95% CI, 1.74-3.98) (Figure 3B; supplemental Table 8). The risk factors for ED among BMT survivors during the pandemic also included severe/life-threatening chronic health conditions (aOR = 1.50; 95% CI, 1.20-1.87; P = .0004; reference: grade 0-2 conditions).
Discussion
In this study, we found that long-term BMT survivors were not at an increased risk for COVID-19 infection when compared with a non-BMT comparison group. Risk of COVID-19 infection in BMT survivors was driven by similar factors as in the general population, such as lack of masking and vulnerable sociodemographic characteristics. Additionally, BMT survivors with COVID-19 infection were more likely to be hospitalized than the non-BMT comparison group with COVD-19 infections, but the odds of ED visits were comparable. Among BMT survivors, having COVID-19 infections did not increase the odds of hospitalizations, but did increase the odds of ED visits. Severe/life-threatening chronic health conditions increased the odds of hospitalizations for BMT survivors, independent of COVID-19 infection. This information is critical for BMT survivors and clinicians to make decisions about risk mitigation during the ongoing pandemic. The study highlights the importance of ongoing survivorship care during the pandemic.
BMT survivors did not have increased risk of COVID-19 infection compared with that of the non-BMT comparison group. This contrasts with information about people undergoing active cancer treatment, who are at an increased risk of COVID-19 infection compared with noncancer cohorts.11,18,21 People receiving active cancer treatment may be at increased risk of COVID-19 infections because of both abnormal immune systems and frequent contact with health care facilities, thus increasing the risk of nosocomial transmission. Although both risk factors would be present at the time of BMT, they would wane over time. This is illustrated by the rates of COVID-19 infection among BMT survivors in the present study (6%) compared with that of Veteran’s Affairs (VA) patients undergoing active cancer treatment (8%) over a slightly shorter duration.22 However, unmeasured differences in risk mitigation could also counterbalance any increased risk in COVID-19 infection because of biologic or health care related reasons. In our study, BMT survivors were more vigilant about risk mitigation behaviors (masking and social distancing) than the non-BMT cohort, which may represent a more cautious approach to COVID-19. Although this study can reassure BMT survivors about their risk of acquiring COVID-19, risk mitigation should continue.
Among BMT survivors, lack of masking as well as vulnerable sociodemographic characteristics such as being unemployed were risk factors for COVID-19 infection, mirroring what has been found in the general population.23, 24, 25, 26 Sociodemographic factors are associated with COVID-19 infection among children (Hispanic race and public insurance)3 and adults (Black race) undergoing active treatment for cancer.18,19,21 Thus, clinicians can provide similar counsel to long-term BMT survivors about their risk of contracting COVID-19 as provided to healthy individuals.
Although BMT survivors had higher rates of hospitalizations than the non-BMT comparison group, it appears that the high prevalence was primarily driven by post-BMT complications, rather than COVID-19 infection. In fact, the difference in hospitalization rates between COVID-19 positive and negative survivors was only 5% (46% vs 41%), much smaller than the COVID-19 attributable hospitalization rate among VA patients with cancer and with and without COVID-19 (12%; 44% vs 32%).22 Similarly, the COVID-19 attributable ICU admission rate was 5% among BMT survivors (7% vs 2%), compared with 12% in the VA cancer population (20% vs 8%).22 However, emergency department visits were associated with COVID-19 status, potentially indicating that BMT survivors with COVID-19 were sick enough or worried enough to visit the ED but not sick enough to require hospitalization. Even during the ongoing pandemic, management of BMT-related sequelae is essential and may potentially mitigate morbidity and mortality. Missed or delayed care during the pandemic has been associated with increased mortality27,28 and a previous study indicates that there was an overall increase in inpatient mortality for non–COVID-19 admissions early in the pandemic.29 How the pandemic affected survivorship care for BMT survivors remains unclear. There are reports of fears of in-person appointments leading to missed care for cancer survivors30 as well as reports that the shift to telemedicine may have actually improved survivorship care access for some.31,32 It will be important to examine the long-term effects of the pandemic on morbidity and non–COVID-19-related mortality in the BMTSS population as well as how shifts to telehealth impact care for survivors.
Overall, this study provides valuable information to help BMT survivors and their clinicians navigate the pandemic. However, additional information is required to help with more nuanced decision-making and recommendations. First, this survey began before COVID-19 vaccines were available so it cannot address efficacy of vaccination in BMT survivors. There is evidence that COVID-19 vaccination helps prevent COVID-19 in patients with cancer but not as effectively as in patients without cancer33; however, data in BMT survivors are lacking. Second, there are data that BMT recipients have increased risk of severe COVID-19 infection shortly after their transplants,8 which was not observed in the long-term BMT survivors. There is most likely a slow decrease in COVID-19 risk as people get further out from BMT; a more nuanced understanding of how risk changes over time and what aspects of the immune system need to be reconstituted to prevent severe COVID-19 would facilitate clinician-patient conversations.
Although this study provides critical information for BMT survivors and their clinicians, it has several limitations. First, given that this study did not routinely monitor for COVID-19 but instead relied on health care system or self-administered tests for clinical or personal reasons. Testing for COVID-19 was often required for individuals having contact with the health care system during the pandemic, a potential concern was that such increased testing could have resulted in higher rates of COVID-19 in survivors. However, the proportion of survivors vs non-BMT participants who underwent COVID-19 testing did not differ significantly. Given that individuals were not required to routinely screen for COVID-19 for the study, this study most likely missed some asymptomatic infections. Similarly, individuals reported the use of health care facilities (eg, hospitalization) during the time frame of interest, but not the underlying reason for that health care contact. Based on the different rates of hospitalization, ICU admission, and ED visits between COVID-19 positive and COVID-19 negative individuals, we can make informed estimates about the percent of each that were because of COVID-19; but it is only an estimate, particularly because there were clinical and sociodemographic differences between survivors with and without COVID-19 that could influence hospitalization rates (higher rates of grade 3-4 chronic health conditions among COVID-19 positive survivors than COVID-19 negative survivors). Information about COVID-19 positive contacts and risk mitigation behaviors was gathered via self-report. Although self-reports have shown over-reporting of risk mitigation behaviors,34,35 both groups would have been affected similarly. Although we ask about contact with COVID-19 positive individuals and other key aspects of risk mitigation, there are other aspects that we do not have data about (eg, working remotely, number of individuals in the home). We also lack information about the vaccination status. However, examination of the study cohort participating in the prevaccine era vs in the postvaccine era yielded similar results. Similarly, we lack information about whether BMT survivors were on immunosuppressive therapy, but do have information about whether they had active cGvHD. Finally, this study covered a period between October 2020 and November 2021 in this evolving pandemic; we need to be mindful that with changes in vaccine availability, dominant variants, and risk mitigation behaviors, these results could shift. However, most cases occurred in the postvaccine era in our study.
These issues notwithstanding, this large study of long-term BMT survivors finds that the BMT survivors are not at increased risk of COVID-19 infection compared with that of non-BMT controls. The risk factors for COVID-19 infection mirror the risk factors in the general population, such as lack of masking and vulnerable sociodemographic subpopulations and presence of comorbidities. These findings will help survivors and clinicians make appropriate decisions to manage the care for long-term BMT survivors during the ongoing pandemic.
Conflict-of-interest disclosure: D.J.W. reports research support from Incyte and FATE, outside the submitted work. The remaining authors declare no competing financial interests.
Acknowledgments
This work was supported in part by the National Cancer Institute (R01 CA078938 [S.B.]; U01 CA213140 [S.B.]), and the Leukemia and Lymphoma Society (R6502-16 [S.B.]).
Authorship
Contribution: S.B. had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis; E.E.J., S.B., D.J.W., and S.H.A. contributed to study conception and design; S.B., L.H., J.W., E.R., S.L., N.B., A.B., H.S.T., L.F., and S.H.A. collected and assembled the data; S.B., Q.M., W.L., L.H., and F.L.W. analyzed and interpreted the data; E.E.J. and S.B. drafted the manuscript;. S.B., L.H., W.L., E.R., L.F., and N.B. provided administrative, technical, or material support; S.B., D.J.W., and S.H.A. supervised the study; and all authors critically reviewed the manuscript for important intellectual content and approved the final manuscript.
Footnotes
Data are available on request from the corresponding author, Smita Bhatia (smitabhatia@uabmc.edu).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Johns Hopkins University & Medicine Coronavirus Resource Center. COVID-19 United States cases by county. 2023. https://coronavirus.jhu.edu/us-map
- 2.Centers for Disease Control and Prevention Science Brief: indicators for monitoring COVID-19 community levels and making public health recommendations. 2022. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/indicators-monitoring-community-levels.html [PubMed]
- 3.Johnston EE, Martinez I, Davis ES, et al. SARS-CoV-2 in childhood cancer in 2020: a disease of disparities. J Clin Oncol. 2021;39(34):3778–3788. doi: 10.1200/JCO.21.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukkada S, Bhakta N, Chantada GL, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol. 2021;22(10):1416–1426. doi: 10.1016/S1470-2045(21)00454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavez-MacGregor M, Lei X, Zhao H, Scheet P, Giordano SH. Evaluation of COVID-19 mortality and adverse outcomes in US Patients with or without cancer. JAMA Oncol. 2022;8(1):69–78. doi: 10.1001/jamaoncol.2021.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute. COVID-19: what people with cancer should know. 2020. https://www.cancer.gov/about-cancer/coronavirus/coronavirus-cancer-patient-information
- 8.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185–e193. doi: 10.1016/S2352-3026(20)30429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jimenez-Kurlander L, Antal Z, DeRosa A, et al. COVID-19 in pediatric survivors of childhood cancer and hematopoietic cell transplantation from a single center in New York city. Pediatr Blood Cancer. 2021;68(3):e28857. doi: 10.1002/pbc.28857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Sutradhar R, Alexander S, et al. Risk of COVID-19 infections and of severe complications among survivors of childhood, adolescent, and young adult cancer: a population-based study in Ontario, Canada. J Clin Oncol. 2022;40(12):1281–1290. doi: 10.1200/JCO.21.02592. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt NS, Sharma A, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in pediatric and early adolescent and young adult hematopoietic stem cell transplant recipients: a cohort study. Transplant Cell Ther. 2022;28(10):696.e1–696.e7. doi: 10.1016/j.jtct.2022.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shahzad M, Chaudhary SG, Zafar MU, et al. Impact of COVID-19 in hematopoietic stem cell transplant recipients: A systematic review and meta-analysis. Transpl Infect Dis. 2022;24(2):e13792. doi: 10.1111/tid.13792. [DOI] [PubMed] [Google Scholar]
- 13.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2021;21(5):277–291. doi: 10.1038/s41577-020-00457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Champlin R. 6th edition. Holland-Frei Cancer Medicine; 2003. Selection of autologous or allogeneic transplantation.http://www.ncbi.nlm.nih.gov/books/NBK12844/ [Google Scholar]
- 16.Yew PY, Alachkar H, Yamaguchi R, et al. Quantitative characterization of T-cell repertoire in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2015;50(9):1227–1234. doi: 10.1038/bmt.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun CL, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant. 2013;19(7):1073–1080. doi: 10.1016/j.bbmt.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Blood. 2010;116(17):3129–3139. doi: 10.1182/blood-2009-06-229369. quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majhail NS, Tao L, Bredeson C, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transplant. 2013;19(10):1498–1501. doi: 10.1016/j.bbmt.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25(11):1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fillmore NR, La J, Szalat RE, et al. Prevalence and outcome of COVID-19 infection in cancer patients: a national veterans affairs study. J Natl Cancer Inst. 2020;113(6):691–698. doi: 10.1093/jnci/djaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175(2):176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths. Ann Intern Med. 2021;174(3):362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vahidy FS, Nicolas JC, Meeks JR, et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10(8):e039849. doi: 10.1136/bmjopen-2020-039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czeisler MÉ. Delay or avoidance of medical care because of COVID-19–related concerns — United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250–1257. doi: 10.15585/mmwr.mm6936a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith M, Vaughan Sarrazin M, Wang X, et al. Risk from delayed or missed care and non-COVID-19 outcomes for older patients with chronic conditions during the pandemic. J Am Geriatr Soc. 2022;70(5):1314–1324. doi: 10.1111/jgs.17722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dang A, Thakker R, Li S, Hommel E, Mehta HB, Goodwin JS. Hospitalizations and mortality from non-SARS-CoV-2 causes among medicare beneficiaries at US hospitals during the SARS-CoV-2 pandemic. JAMA Netw Open. 2022;5(3):e221754. doi: 10.1001/jamanetworkopen.2022.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leach CR, Kirkland EG, Masters M, et al. Cancer survivor worries about treatment disruption and detrimental health outcomes due to the COVID-19 pandemic. J Psychosoc Oncol. 2021;39(3):347–365. doi: 10.1080/07347332.2021.1888184. [DOI] [PubMed] [Google Scholar]
- 31.Koo J, Auletta JJ, Hartley DM, et al. Secondary impact of the coronavirus disease 19 pandemic on patients and the cellular therapy healthcare ecosystem. Transplant Cell Ther. 2022;28(11):737–746. doi: 10.1016/j.jtct.2022.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed N, Fitzmaurice S, Morey C, et al. Telehealth to increase access to transplant survivorship care for allogeneic stem cell transplant recipients regardless of distance to transplant center or neighborhood income. J Clin Oncol. 2021;39(15_suppl) e13615-e13615. [Google Scholar]
- 33.Song Q, Bates B, Shao YR, et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the national COVID cohort collaborative. J Clin Oncol. 2022;40(13):1414–1427. doi: 10.1200/JCO.21.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies R, Mowbray F, Martin AF, Smith LE, Rubin GJ. A systematic review of observational methods used to quantify personal protective behaviours among members of the public during the COVID-19 pandemic, and the concordance between observational and self-report measures in infectious disease health protection. BMC Public Health. 2022;22(1):1436–1438. doi: 10.1186/s12889-022-13819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant. 2013;47(4):2025–2047. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.