Abstract
Background:
Patellar maltracking is widely accepted as an underlying mechanism of patellofemoral pain. However, methodological differences in the literature hinder our ability to generate a universal quantitative definition of pathological patellofemoral kinematics (patellar maltracking) in patellofemoral pain, leaving us unable to determine the etiology of patellofemoral pain.
Purpose:
Systematically review the literature to provide evidence regarding the influence of confounding variables on patellofemoral kinematics.
Study Design:
Systematic review and random effects meta-analysis of control-case studies.
Methods:
A literature search of case-control studies that evaluated patellofemoral kinematics at or near full extension and were written in English was conducted using Embase, PubMed, Scopus, and Web of Science up to September 2019. Cases were defined as patients with patellofemoral pain. Studies were eliminated if they lacked quantitative findings; had a primary aim to assess therapy efficacy; or included participants with osteoarthritis and/or previous trauma, pathology, or surgery. A quality assessment checklist was employed to evaluate each study. Meta-analyses were conducted to determine the influence of confounding variables on measures of patellofemoral kinematics.
Results:
Forty studies met the selection criteria, with quality scores ranging from 13% to 81%. Demographic characteristics, data acquisition, and measurement methods were the primary sources of methodological variability. Active quadriceps significantly increased lateral shift (standardized mean difference [SMD]shift = 0.33, P = .0102) and lateral tilt (SMDtilt = 0.43, P = .006) maltracking. Individuals with pain secondary to dislocation had greater effect sizes for lateral maltracking than those with isolated patellofemoral pain (ΔSMDshift = 0.71, P = .0071; ΔSMDtilt = 1.38, P = .0055).
Conclusion:
This review exposes large methodological variability across the literature, which not only hinders the generalization of results, but ultimately mitigates our understanding of the underlying mechanism of patellofemoral pain. Although our meta-analyses support the diagnostic value of maltracking in patellofemoral pain, the numerous distinct methods for measuring maltracking and the limited control for cofounding variables across the literature prohibit defining a single quantitative profile. Compliance with specific standards for anatomic and outcome measures must be addressed by the scientific and clinical community to establish methodological uniformity in this field.
Keywords: patellofemoral joint, maltracking, kinematics, dislocation
INTRODUCTION
Patellofemoral pain (PFP) is a common condition encountered in orthopaedic practice,10 with an annual prevalence in the general population of 23% in adults and 29% in adolescents.63 In the absence of structural injury or previous trauma, this anterior knee pain appears related to neuromechanical factors within the patellofemoral joint.16 One widely accepted hypothesis is that a force imbalance around the knee leads to abnormal patellofemoral position (malalignment) and pathological patellofemoral kinematics (patellar maltracking) during functional activities.52,56 This maltracking leads to elevated mechanical stress on the subchondral bone through altered patellofemoral contact, thereby causing pain.17 Although maltracking in patients with PFP has been extensively studied, conflicting results have raised doubt regarding maltracking’s role in the etiology of PFP.5,7,18,38
The divergent patellar maltracking results likely arise from methodological differences across studies,30,79 including differences in patient characteristics (eg, age, comorbidities, sex), activities studied (eg, open vs closed chain exercise), image analysis techniques (eg, dynamic magnetic resonance imaging, static radiographs), and outcome measures selected to represent patellofemoral kinematics (eg, congruence angle vs lateral patellar displacement). Based on these differences, comparisons among studies are typically confined to general qualitative discussions, leaving us unable to generate a universal quantitative definition of patellar maltracking in PFP. The few studies exploring the influence of covariates in the assessment of patellofemoral maltracking (eg, patient position,1 muscle participation,3 knee angle6,58,74) suggested that the experimental paradigm employed influences the study conclusions. For example, Becher and colleagues1 demonstrated an increased patellar tilt angle (patient group only) and an increase in bisect offset (patient and control cohorts) when comparing measures acquired in an upright posture with active quadriceps but not when measured in a supine position with relaxed musculature. The authors attributed the differences to the posture. However, the effect of quadriceps activity was not explored. By not controlling for potential confounding variables, such studies add confusion in regard to which factors are indeed influencing patellofemoral maltracking. The methodological differences within and across studies diminish the potential diagnostic value of maltracking in PFP.
We conducted this systematic review to provide a comprehensive understanding of the influence that confounding variables have on quantifying patellar maltracking patterns in individuals with PFP. This systematic review used meta-analysis and meta-regression to provide evidence of the confounding variables’ influence on axial measures of patellofemoral position/kinematics across studies. In doing so, we hoped to support the development of a standard framework for the assessment of the patellar alignment/kinematics. We asked 3 primary questions: (1) Does quadriceps activity increase the ability to detect maltracking? (2) Are patients with isolated PFP (PFP_iso) kinematically unique from patients with PFP secondary to dislocation (PFP_dis)? (3) Do other variables (eg, patient position, activities studied, image techniques) influence the measured values of maltracking? Integral to our meta-analysis exploring the influence of confounding variable was the following overarching question: Is patellar maltracking associated with PFP? The purpose of the study was not to determine whether one methodology, imaging modality, or outcome variable is superior when investigating patellofemoral alignment/tracking but to explore the sources of variability across the literature in an attempt to provide clarity and unity for future studies.
METHODS
Search Strategy and Eligibility Criteria
This review followed the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses). The search string was developed by 1 author (J.W.), a clinical informationist at the National Institutes of Health Library with 14 years of experience, using input from all authors. The search of the literature was conducted in July 2018 and then updated in September 2019 with the following databases: Embase, PubMed, Scopus, and Web of Science. The search strategy included 3 keyword strings and their associated range of terms (Appendix Table A1, available in the online version of this article). We included all studies irrespective of publication year, but limited inclusion to full-text English-language publications. The updated search was limited to publications published after 2017 that were not included in the original search.
This preliminary database was screened per the predetermined eligibility criteria. A study was included into the final review only if (1) it evaluated patellofemoral alignment and/or tracking in individuals with idiopathic PFP or pain secondary to subluxation, dislocation of the patella, or recurrent patellofemoral instability and (2) the results were reported at or near full extension (≤10° of knee flexion). A study was eliminated if (1) its participants had previous leg trauma, pathology, or surgery or displayed signs of osteoarthritis; (2) it did not quantitatively report the main findings or evaluate controls; and (3) its primary aim was to assess the therapy or treatment efficacy. Two reviewers (C.G. and C.N.F.) independently screened titles and abstracts to identify the initial study database. Finally, full-text manuscripts were screened according to the inclusion and exclusion criteria for final inclusion. A third reviewer (J.M.) was consulted when a consensus could not be reached.
Data Extraction and Quality Assessment
Data from all studies in the final database were extracted and tabulated. The potential sources of intra- and inter-study variability30,79 were identified during data extraction. These sources fell into 3 main categories: demographics, study design, and outcome measurement techniques. As the influence of knee angle on the patellar position is well established,6,58,74 this source of variability was not explored. Instead, for each included study, outcomes for patellofemoral shift and tilt were extracted for the patient (case) and asymptomatic (control) groups with the knee in full extension (≤10°). As part of the extraction process, the patient cohort was defined as PFP_iso, PFP_dis, or mixed (PFP_mix). A patient group was defined as being PFP_iso if the recruitment criteria clearly excluded individuals with history of dislocation, instability, or subluxation. If a study recruited only individuals with history of patellofemoral instability (primary dislocation, recurrent dislocation, subluxation, or signs of instability), then the patient group was defined as PFP_dis. Last, the patient group was defined as PFP_mix if neither of the aforementioned 2 criteria was met or if indications within the study demonstrated the partial presence of individuals with instability, dislocation, or history of subluxation.
A quality assessment checklist of 15 questions adopted from a validated quality assessment tool by Downs and Black12 was employed to evaluate each study (Appendix Table A2, available online). Questions were rated 1 point for yes and 0 for no or unable to assess, except Q4, which was given a value of 2 for yes, 1 for partial, and 0 for no. Scores were converted to percentage of the maximum score (16 points).
Statistical Analysis
A separate meta-analysis for muscle effect was performed first to determine whether to include all the studies in the meta-analysis or only those that required the active quadriceps. Next, using subgroup meta-analysis, we explored whether the maltracking profile was unique in studies focused solely on individuals with PFP_iso, relative to studies that focused on individuals with PFP_dis. Following this, if possible, the other covariates of interest were explored with a meta-regression (Figure 1).
Figure 1.
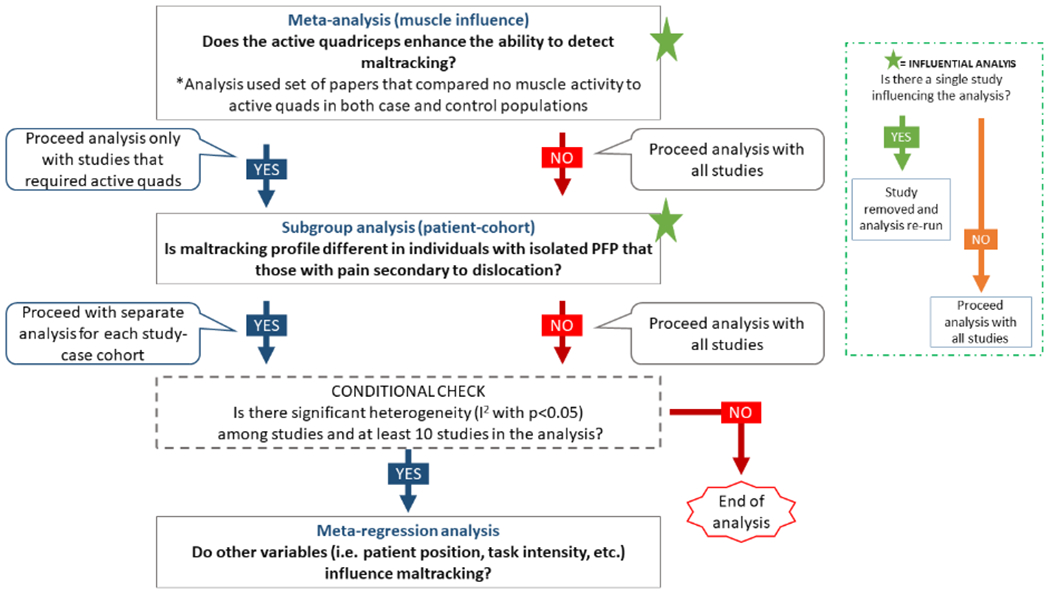
Schematic representation of the statistical analysis pathway. The research questions are shown in bold letters and placed at the stage in which the statistical analysis would provide answers to them.
All meta-analyses were conducted in R (v 3.6.0; RStudio)46,48 with the functions meta53 and metafor.72 As there were 2 primary outcome variables (patellofemoral shift and tilt), 2 independent meta-analyses were run for each study question. When a single study reported patellofemoral shift and/or tilt with >1 outcome measure,6,47 the outcome measure with the lowest effect size was used. For multiple studies from the same research group that included or potentially shared the same population, the most recent study was used for comparison across studies. Because studies used different metrics/scales to measure patellar shift or tilt, calculation of effect size for each study was performed through standardized mean difference (SMD). SMD was calculated with Hedges g,26 defined as the mean difference between groups in each study divided by the study’s pooled standard deviation, producing a unitless index enabling comparison across studies (Equation 1):
| Equation 1 |
where M1 = mean value for patients (group1), M2 = mean value for controls (group2), and SDpooled = pooled standard deviation of group1 and group2 (Equation 2):
| Equation 2 |
where n1 = cohort size for group1, n2 = cohort size for group2, SD1 = standard deviation for group1, and SD2 = standard deviation for group2.
The SMD was entered into the meta-analysis weighted by the inverse inter-study variance (Sidik-Jonkman estimator for tau-squared).62 I2 identified the percentage of variation across studies attributed to heterogeneity rather than chance.27 Outcomes were reported as the SMD and 95% CI. An influential analysis based on a leave-one-out design was undertaken to identify if a single influential study significantly altered the heterogeneity. If such an influencer was found, this study was removed, and the meta-analysis was rerun.
We conducted the meta-analysis with random effects to explore whether quadriceps activity during patellofemoral alignment/tracking acquisition enhances the ability to diagnose malalignment/maltracking in patients with PFP (PFP_iso, PFP_dis, and PFP_mixed).52 This separate meta-analysis of muscle effect on patellar profile included only studies that assessed the same individuals with and without participation of the quadriceps musculature for both cases and controls. Based on equation 1, M1 and M2 were the difference in shift/tilt between states (active vs passive quadriceps) for the control group (group1) and the patient group (group2), whereas SD1 and SD2 were the pooled standard deviation of the active and passive states for each group. If this analysis revealed that muscle activity influenced patellar maltracking, we moved forward with the main analysis using only studies that required active quadriceps. If this was not the case, we moved forward using all studies (Figure 1).
Next, we explored if PFP_iso has a distinct pathomechanism from PFP_dis,9 by adding a subgroup analysis to a meta-analysis with random effects. For this analysis, M1 and M2 (equation 1) were the mean values for shit/tilt for the control (group1) and patient cohort (group2). In addition, this analysis tested if maltracking was significant in each patient population. If a significant distinction in effect size (SMD) between populations was found, meta-regression analyses were performed separately for studies of PFP_iso versus PFP_dis, with the PFP_mix studies being removed at this juncture. These final meta-regressions were run only if a minimum of 10 studies were available for analysis (the smallest number of studies recommended for meta-regression)27 and significant heterogeneity remained (I2).
RESULTS
After removal of duplicates, 4204 publications remained, with 487 belonging to the updated search (Figure 2). Applying the study selection criteria removed 4155 articles, leaving 49 for inclusion. During the data extraction process, 9 additional studies were excluded because of the presence of osteoarthritis in the study population that was not previously recognized. Thus, in total, the 2 searches produced 40 articles for the review.
Figure 2.
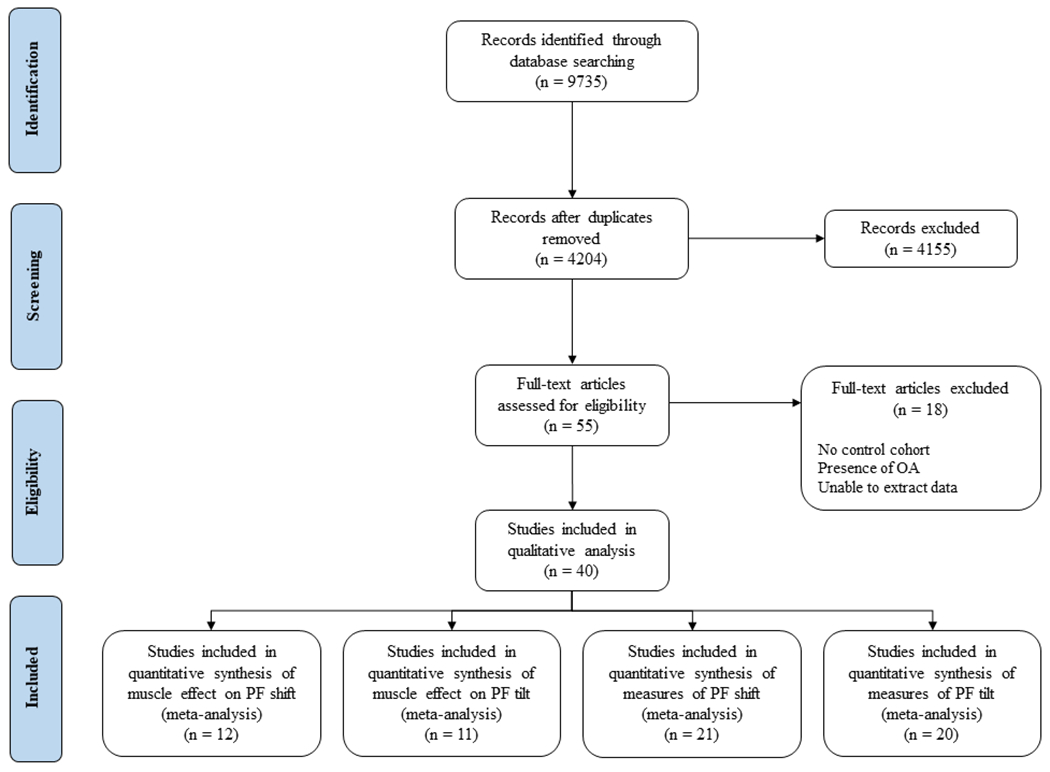
Study selection process for the systematic review and meta-analysis. OA, osteoarthritis; PF, patellofemoral.
Quality Assessment and Study Characteristics
The study quality mean score ± SD was 52% ± 18%, with a range of 13% to 81% (Appendix Table A2, available online). No study received a score of 2 (yes) for Q4, as the distribution of principal confounders was not fully described. Thirty studies received a score of 1 (partial) for Q41, 4, 6–8, 14, 15, 18, 19, 21, 24, 25, 28, 33, 35, 38, 42– 44, 47, 49, 50, 56, 57, 65, 67– 69, 73, 77. Unable to assess (U) was frequently indicated for questions regarding the main outcome measures’ accuracy (Q11) and the recruitment timeline (Q12), as they were scored affirmatively for 13 (27%)1, 4, 21, 33, 35, 38, 42, 44, 49, 50, 56, 71, 73 and 4 (10%)41,47,49,77studies, respectively. An attempt to blind researchers measuring the primary outcomes (Q14) was mentioned in only 4 studies (10%).14, 15, 21, 57
The survey of the literature revealed 9 main sources of methodological variability in the 3 main categories (Table 1): demographic characteristics (age, sex, study case [PFP_iso, PFP_dis, PFP_mix]), data acquisition (imaging modality and condition [static vs dynamic], muscle activity, contraction intensity, patient position), and measurement methods (outcome measurement to quantify patellofemoral shift and tilt and analysis dimensionality: 2- vs 3-dimensional [3D]). As knee angle influences patellofemoral kinematics,6,58,74 we reported information regarding whether a study cited an anatomic definition of knee angle. Height, weight, and body mass index were not listed in the extraction table, because they were often not provided. The mean cohort age ranged across studies from 13 to 36 years old. The majority of studies focused on adult populations, with only 2 studies focused solely on adolescents.7,47 Several studies used a mixed or potentially mixed population of adolescent and adults. As for sex, the majority of studies excluded males. Only 1 study focused on males in isolation.38 Differences between sexes were assessed by only 2 studies,28,42 with conflicting results. Out of the 40 studies included in this review, 21 evaluated PFP_iso4, 7, 8, 14, 15, 18, 19, 21, 23, 25, 35, 38, 42–44, 52, 65, 68, 69, 73, 74; 10 evaluated PFP_dis (ie, history of dislocation, “instability,” or “subluxation”)1, 6, 28, 33, 41, 45, 47, 51, 75, 76; 1 study included 2 isolated patient populations;67 and 8 allowed for a patient cohort of mixed diagnoses (PFP_iso and PFP_dis). 3,24,49,50,56,57,71,77 As highlighted by the quality assessment (Appendix Table A1, Q11, available online), two-thirds of the studies did not effectively describe the kinematic measures used. Surprisingly, knee angle was the measurement most often neglected, with only 10 studies7, 8, 15, 21, 42, 43, 56, 57, 73, 74 providing an anatomic definition for it. Four studies6,44,47,77 did not measure it; 10 studies1, 18, 19, 33, 49–51, 65, 67, 68 used a locating device or goniometer to measure knee angle, but did not provide an anatomic definition; and the remaining 163, 4, 14, 23–25, 28, 35, 38, 41, 45, 52, 69, 71, 75, 76 failed to report how the knee angle was measured.
TABLE 1:
Characteristics of Included Studies
| Demographics a | Data Acquisition b | Measurement Methods c | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Control | Case | Imaging | Metrics | ||||||||||||
|
| |||||||||||||||
| Study: Author (Year) | Patient Cohort | No. | Age, y | No. | Age. y | % Fem | Mode | Condition | Muscle Activity | Task Intensity | Patient Position | ML Shift | ML Tilt | 2D/3D Analysis | Knee Angle |
| Becher (2017)1 | PFP_dis | 22.3 ± 2.7 | 16 | 21.3 ± 2.7 | 9 | MR | Static | Isom and Rel | UD | S&U | BSO1* | PTA1 * | 2D (m) | UD^ | |
| Biedert (1997)3 | PFP_mix | 8 | NR | 35 | 29.8 | 77.1 | CT | Static | Isom and Rel | UD | S | LPD1, PLCI | LPFA | 2D (s) | UD |
| Bolgla (2019)4 | PFP_iso | 12 | 24.3 ± 1.1 | 18 | 24.7 ± 3.4 | 100 | US | Static | Rel | S | PPO | NA | 2D (s) | UD | |
| Brossmann (1993)6 | PFP_dis | 15 | 29.5 | 13 | 23.2 | 100 | MR | Dynamic | Isok | LW | S | BSO2*, LPD2 * | PTA2 * | 2D (m) | NM |
| Carlson (2017)7 | PFP_iso | 13 | 14.2 ± 1.0 | 12 | 14.1 ± 1.1 | 100 | MR | Dynamic | Isok | LW | S | PFD* | PTA2* | 3D | D |
| Carlson (2017)8 | PFP_iso | 38 | 27.4 ± 9.4 | 32 | 28.0 ± 7.9 | 75 | MR | Dynamic | Isok | LW | S | PFD* | PTA2* | 3D | D |
| Erkocak (2016)14 | PFP_iso | 40 | 20-40 | 35 | 20-40 | 54.3 | CT | Static | Isom and Rel | UD | S | LPD4 | LPT | 2D (s) | UD |
| Esfandiarpour (2018)15 | PFP_iso | 10 | 25.0 ± 7.7 | 8 | 29.7 ± 10.6 | 100 | Fluoro | Dynamic | Isok and Rel | BW | Lunge | 3D Shift5 | 3D Tilt5 | 3D | D |
| Felicio (2011)18 | PFP_iso | 20 | 21.5 ± 2.2 | 19 | 23.5 ± 3.2 | 100 | MR | Static | Isom | MIVC | S | BSO2 | PTA1 | 2D (s) | UD^ |
| Felicio (2012)19 | PFP_iso | 20 | 21.5 ± 2.2 | 19 | 23.5 ± 3.2 | 100 | MR | Static | Isom | MIVC | S | BSO2 | PTA1 | 2D (s) | UD^ |
| Freedman (2013)21 | PFP_iso | 26 | 25.3 ± 7.7 | 26 | 25.9 ± 11.1 | 73.1 | MR | Dynamic | Isok and Rel | LW | S | BSO3*, PFD* | PTA2* | 2D (m) | D |
| Grelsamer (2008)23 | PFP_iso | 51 | NR | 30 | NR | NR | CT | Static | Rel | S | NA | PTA1 | 2D (s) | UD | |
| Guzzanti (1994)24 | PFP_mix | 20 | 14 | 27 | 14 | 77.8 | CT | Static | Isom and Rel | MIVC | S | CA | PTA2 | 2D (s) | UD |
| Harper (1995)25 | PFP_iso | 10 | 26 | 10 | 28 | 80 | CT | Static | Isom and Rel | UD | S | CA | LPFA | 2D (s) | UD |
| Inoue (1988)28 | PFP_dis | 30 | 22.7 | 50 | 19 | 86 | CT | Static | Rel | S | CA | LPFA | 2D (s) | UD | |
| Kujala (1989)33 | PFP_dis | 10 | 24.5 ± 3.9 | 11 | 26.7 ± 6.0 | 100 | MR | Static | Rel | S | CA, LPS | LPFA, LPT | 2D (s) | UD^ | |
| Lau (2016)35 | PFP_iso | 6 | 28.6 ± 2.8 | 10 | 32.3 ± 5.2 | 80 | MR | Static | Isom | 25% BW | S | 3D Shift3 | 3D Tilt3 | 3D | UD |
| MacIntyre (2006)38 | PFP_iso | 20 | 30.4 ± 8.4 | 20 | 36.0 ± 8.2 | 35 | MR | Static | Isom | 152 N | S | 3D Shift1 | 3D Tilt1 | 3D | UD |
| Nietosvaara (1993)41 | PFP_dis | 30 | 12.9 | 20 | 13.9 | 75 | US | Static | Isom | UD | S | LD | NA | 2D (s) | UD |
| Pal (2011)42 | PFP_iso | 15 | 28.2 ± 3.9 | 40 | 28.9 ± 4.6 | 47.5 | MR | Static | Isom | 90% BW | U | BSO2 | PTA1 | 2D (s) | D |
| Pal (2012)43 | PFP_iso | 15 | 28.4 ± 3.8 | 39 | 30.9 ± 5.8 | 53.8 | MR | Static | Isom | 90% BW | U | BSO2 | PTA1 | 2D (s) | D |
| Powers (2000)44 | PFP_iso | 12 | 29.1 ± 5.0 | 23 | 26.8 ± 8.5 | 100 | MR | Static | Isot | 15% BW | P | BSO1 or BSO2 | PTA1 | 2D (s) | NM |
| Prakash (2016)45 | PFP_dis | 87 | 25.6 | 48 | 23.4 | 73.8 | CT | Static | Rel | S | BSO2*, CA | LPFA, PTA1 | 2D (m) | UD | |
| Regalado (2013)47 | PFP_dis | 10 | 13.0 ± 2.0 | 29 | 14.0 ± 1.0 | 75.9 | MR | Static | Isom | LW | S | BSO2, LPD2 | PTA2 | 2D (s) | NM |
| Salsich (2007)49 | PFP_mix | 21 | 23.2 ± 4.1 | 21 | 26.8 ± 7.7 | 76.2 | MR | Static | Isom | UD | S | BSO2 | PTA1 | 2D (s) | UD^ |
| Salsich (2013)50 | PFP_mix | 29 | 24.2 ± 4.5 | 27 | 27.0 ± 7.1 | 77.8 | MR | Static | Isom | UD | S | BSO2 | PTA1 | 2D (s) | UD^ |
| Sasaki (1986)51 | PFP_dis | 12 | NR | 20 | 20.0 | NR | CT | Static | Isom and Rel | UD | S | LS | LPT | 2D (s) | UD^ |
| Schutzer (1986)52 | PFP_iso | 10 | 16-34 | 24 | 19 | 91.7 | CT | Static | Isom and Rel | MVIC | LD | CA | PTA2 | 2D (s) | UD |
| Sheehan (2009)56 | PFP_mix | 20 | 26.7 ± 9.4 | 16 | 28.6 ± 10.3 | 81.3 | MR | Static | Isok | LW | S | PFD* | PTA2* | 3D | D |
| Sheehan (2009)57 | PFP_mix | 28 | 26.3 ± 8.9 | 19 | 28.7 ± 11.4 | 84.2 | MR | Static | Isok | LW | S | PFD* | PTA2* | 3D | D |
| Souza (2010)65 | PFP_iso | 15 | 30.8 ± 8.9 | 15 | 29.1 ± 4.2 | 100 | MR | Static | Isom | BW | U | BSO1 or BSO2 | PTA1 | 2D (s) | UD^ |
| Taşkiran (1998)67 | PFP_iso | 9 | 23.0 | 10/8 | 31.0 | 100 | CT | Static | Isom and Rel | MIVC | S | CA | PTA1 | 2D (s) | UD^ |
| PFP_dis | 8 | 23.0 | 87.5 | ||||||||||||
| Teng (2014)68 | PFP_iso | 18 | 26.2 ± 5.4 | 18 | 28.3 ± 6.9 | 100 | MR | Static | Isom | 25% BW | S | BSO2 | PTA1 | 2D (s) | UD^ |
| Thomeé (1995)69 | PFP_iso | 20 | 22.0 ± 3.0 | 40 | 20.0 ± 3.0 | 100 | CT | Static | Rel | S | CA, subluxation | PTA2 | 2D (u) | UD | |
| Türkmen (2018)71 | PFP_mix | 50 | 33.7 ± 7.1 | 54 | 34.5 ± 7.1 | 70.4 | MR | Static | Rel | S | PTA2 | 2D (u) | UD | ||
| Wilson (2009)73 | PFP_iso | 10 | 26.7 ± 7.7 | 9 | 30.2 ± 12.5 | 77.8 | MAS | Dynamic | Isok | BW | U | 3D Shift2 | 3D Tilt2 | 3D | D |
| Witonski (1999)74 | PFP_iso | 10 | 19.4 | 10 | 18.8 | 100 | MR | Static | Isom and Rel | UD | S | CA | PTA2 | 2D (s) | D |
| Yamada (2017)76 | PFP_dis | 10 | 24.0 | 56 | 22.0 | 83.9 | MR | Static | Rel | S | BSO2*, 3D Shift4 | PTA1*, 3D Tilt4 | 3D | UD | |
| Yamada (2018)75 | PFP_dis | 10 | 24.0 | 56 | 22.0 | 83.9 | MR | Static | Rel | S | BSO2*, 3D Shift4 | PTA1*, 3D Tilt4 | 3D | UD | |
| Yang (2007)77 | PFP_mix | 20 | 28.6 ± 4.7 | 23 | 22.3 ± 5.4 | 78.3 | MR | Static | Isom and Rel | LW | S | BSO2*, LPD3* | PTA2 * | 2D (m) | NM |
Patient cohort: PFP_dis, history of patellofemoral instability (dislocation, subluxation, or signs of instability); PFP_iso, isolated patellofemoral pain; PFP_mix, mixed comorbidities in the PFP cohort. No.: number of participants in specified cohort. Age: reported as mean, mean ± SD, range, or not reported (NR). % Fem: percentage of females in the pain cohort.
Mode: CT, computed tomography; MAS, motion acquisition system; MR, magnetic resonance; NR, not reported; US, ultrasound. Muscle Activity: Isok, isokinetic activation; Isom, isometric activation; Isot, isotonic activation; Rel, relaxed. Task intensity: BW, body weight; LW, lower leg weight; MVIC, maximum voluntary isometric contraction; N, newton; UD, unable to determine. Patient position: LD, lateral decubitus; P, prone; S, supine; U, upright.
Metrics: 3D Shift, patellar shift defined with a 3-dimensional patellofemoral model; 3D Tilt, patellar tilt defined with a 3-dimensional patellofemoral model; BSO, bisect offset; CA, congruence angle; LD, lateral distance; LPD, lateral patellar displacement; LPFA, lateral patellofemoral angle; LPT, lateral patellar tilt; LS, lateral shift; NA, not applicable; PFD, patellofemoral displacement; PLCI, patella–lateral condyle index; PTA, patellar tilt angle. A number is added to the metric if multiple studies defined the same metric name with different anatomic landmarks. 2D/3D analysis: 2D (m), metric uses multiple 2-dimensional images; 2D (s), metric is 2-dimensional with a single axial image; 3D, a 3-dimensional model is used to define metric. Knee angle: D, method defined; NM, not measured; UD, unable to determine; UD^, use of a measuring device (eg, goniometer) with no further description.
Multiple images were used for measuring the outcome (Appendix Table A3, available online).
For the patellofemoral alignment and tracking outcome measures, 22 distinct methods were found for measuring medial-lateral (ML) shift, defined as patellar displacement relative to the femur (Figure 3). For example, patellar ML shift was reported as a pure length measurement (lateral patellar displacement, axial linear patellar displacement), as an index (bisect offset index, patella–lateral condyle index), or with proxies (congruence angle, patella offset position). Similarly, 11 distinct methods for measuring patellar ML tilt were reported (Figure 4). Although many studies used the same nomenclature, the actual measurement differed across studies because of differences in referential landmarks. In 23 studies,3, 4, 14, 18, 19, 23–25, 28, 33, 41–44, 47, 49–52, 65, 67, 68, 74 a single axial image was used to measure patellar position. The use of multiple axial slices was adopted by 5 other studies, 1,6,21,45,77 while 10 studies7, 8, 15, 35, 38, 56, 57, 73, 75, 76 adopted 3-dimensional techniques for consistency in bony reference landmarks (Table 1).69,71
Figure 3.
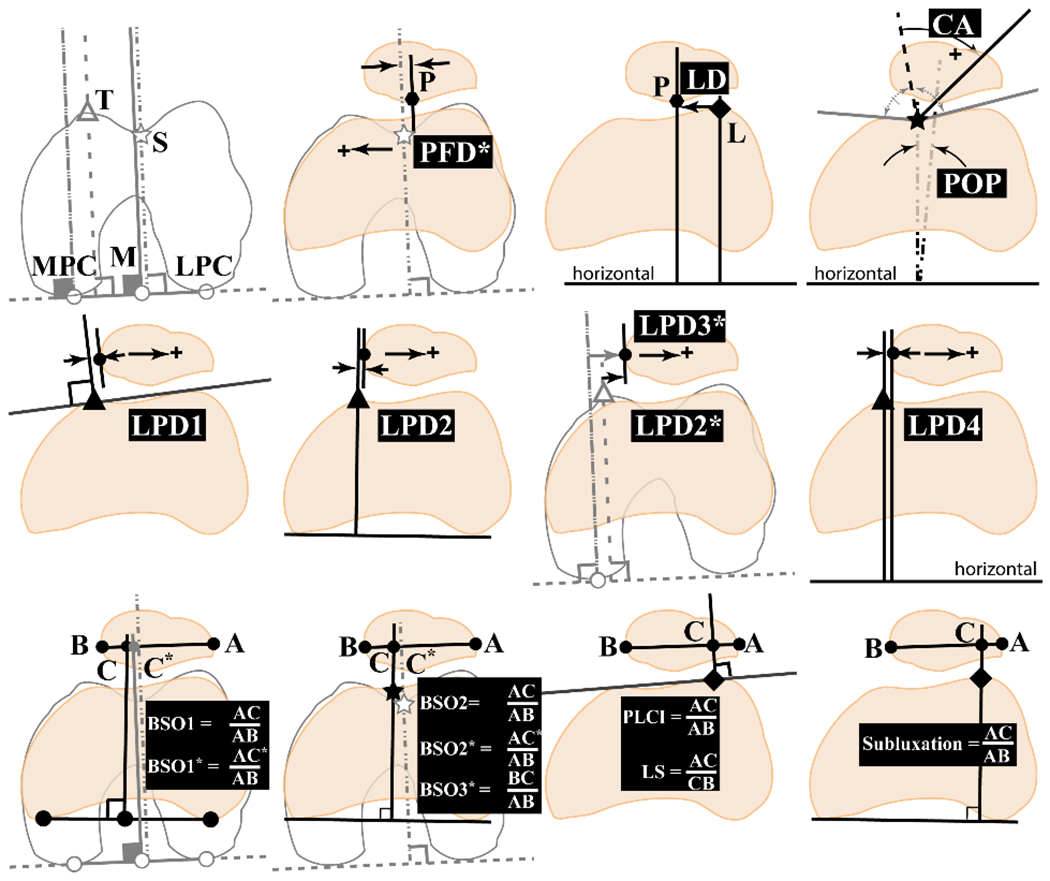
Twenty-two patellar shift outcome metrics from studies within the review. Metrics: BSO, bisect offset (BSO1,66 BSO1*,1 BSO2,44 BSO2*,6 BSO3*21); CA, congruence angle40; LD, lateral displacement41; LPD, lateral patellar displacement (LPD1,36 LPD2,48 LPD2*,6 LPD3*,77 LPD414); LS, lateral shift51; PFD*, patellar femoral displacement56; PLCI, patella–lateral condyle index32; POP, patella offset position4; subluxation.69 The five 3-dimensional (3D) model metrics are not included (3D Shift1,38 3D Shift2,73 3D Shift3,35 3D Shift4,76 3D Shift514). Top and middle rows: metrics measuring linear distance between patellofemoral bony landmarks, with the exception of CA and POP, which are measured in degrees. Bottom row: metrics with ratios. Referential landmarks: white symbols and gray lines are obtained at the widest femoral section. Solid symbols and black lines depict references obtained at the mid-patellar image. First row image 1: M, the bisect of line MPC-LPC; MPC/LPC, the medial/lateral femoral posterior condyles; S (star), the deepest point in femoral sulcus; T (triangle), the most anterior aspect of the medial trochlea. First row image 2: L (diamond), the most anterior aspect of the lateral trochlea; P, the most posterior point of the patella. Bottom row: B/A, the most medial/lateral points on the patella; C, the point where vertical reference line crosses line B-A. The citation associated with each metric is the earliest published definition of that metric. The asterisk (*) denotes the use of multiplane reference points. A number is added to the metric if multiple studies defined the same metric name through different anatomic landmarks. A written description of all metrics is provided (Appendix Table A3, available online).
Figure 4.
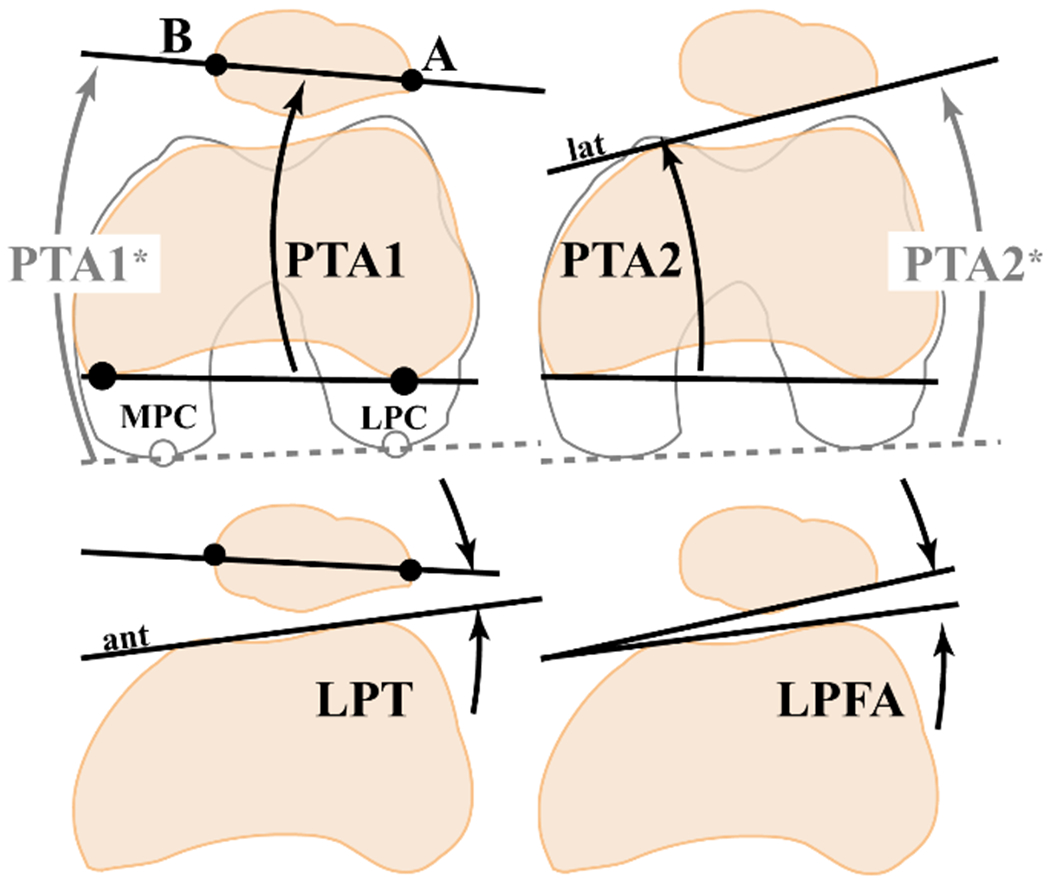
Eleven patellar tilt outcome measurements from studies within the review. Metrics: LPFA, lateral patellofemoral angle37; LPT, lateral patellar tilt11; PTA, patella tilt angle (PTA1,22 PTA1*,1 PTA2,39 PTA2*59). The five 3-dimensional (3D) methods (3D Tilt1,38 3D Tilt2,73 3D Tilt3,35 3D Tilt4,76 3D Tilt515) are not included. Referential landmarks: Solid lines and symbols are obtained at the mid-patellar image, while the dashed gray line is obtained at the widest femoral section. MPC/LPC, the medial/lateral femoral posterior condyles. A and B, the most lateral and medial patellar points. Ant, the line connecting the 2 most anterior points of the femoral condyle. Lat, the lateral posterior patellar edge. The reference associated with each metric is the earliest published definition of that metric. The asterisk (*) denotes the use of multiplane reference points. A number is added to the metric if multiple studies defined the same metric name through different anatomic landmarks. A written description of all metrics is provided (Appendix Table A4, available online).
Statistical Analyses
Active quadriceps during data acquisition significantly increased lateral maltracking for shift and tilt (SMDshift = 0.33, P = .0102; SMDtilt = 0.43, P = .006), based on 121, 6, 14, 15, 21, 24, 25, 41, 51, 67, 74, 77 and 111, 6, 14, 15, 21, 24, 25, 51, 67, 74, 77 of the 40 studies, respectively, within the review. No single study was found to influence the results (Figure 5). Thus, in subsequent analyses, only studies with active quadriceps were included.
Figure 5.
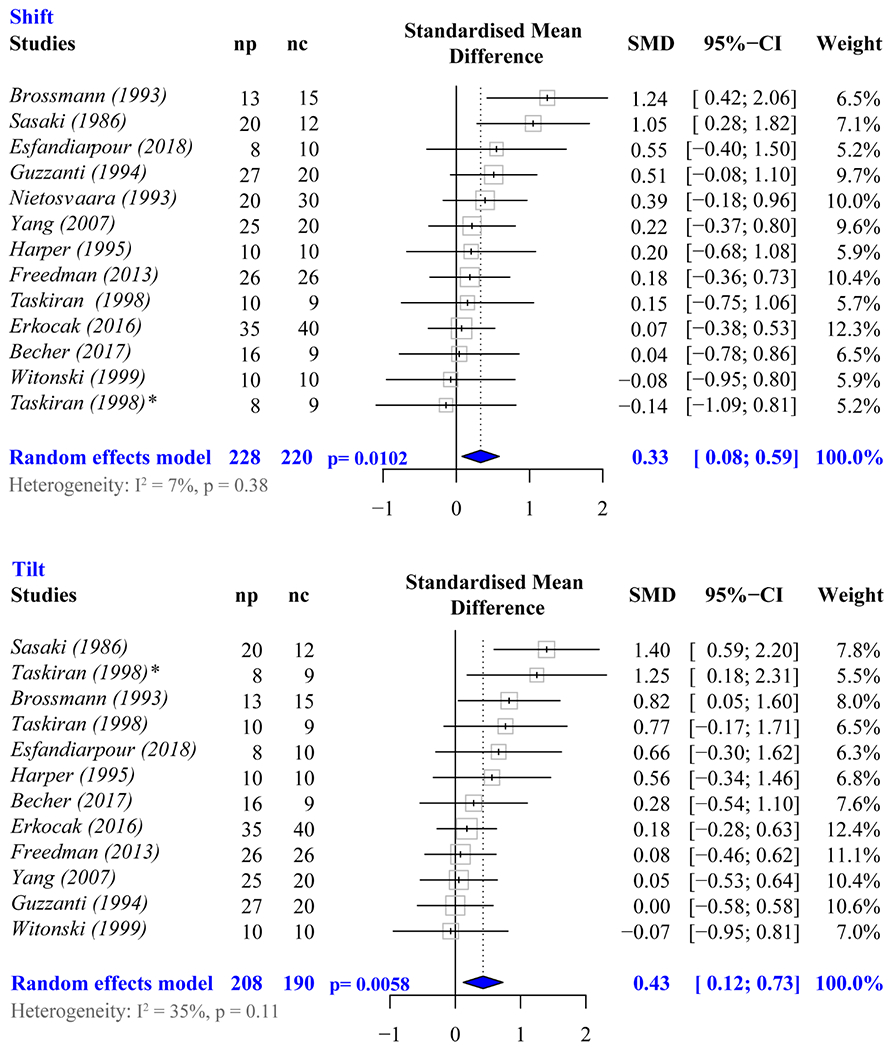
Meta-analysis forest plots demonstrating the effect of the active muscle on measures of shift (top) and tilt (bottom). Diamonds represent the pooled effect size (standardized mean difference [SMD]) and 95% CI. nc, number in control cohort; np, number in patient cohort. *The study by Taskirin et al67 evaluated 2 patient groups (isolated PFP and PFP secondary to instability. The asterisk (*) indicates the results comparing the control group to the latter patient group. The non-asterisk reference are the results comparing the group with isolated PFP to controls.
A total of 21 studies1, 6–8, 14, 15, 18, 24, 25, 38, 41, 43, 44, 47, 50, 51, 65, 67, 68, 73, 74 were suitable for the subgroup meta-analysis based on patient cohort, as these were the only studies requiring active quadriceps participation. One of these 21 studies67 had 2 case groups (PFP_iso and PFP_dis), and another41 did not evaluate patellar tilt. Thus, 22 and 21 comparisons were used in the meta-analysis for shift and tilt maltracking (Figures 6 and 7). Significant differences among groups (PFP_iso, PFP_dis, and PFP_mix) were found in both subgroup analyses (ML shift, P = .024; ML tilt, P = .027). A post hoc analysis revealed that individuals with PFP_dis have a distinctively greater effect size for lateral maltracking than those with PFP_iso (ΔSMDshift = 0.71, P = .0071; ΔSMDtilt = 1.38, P = .0055). Comparisons between studies with PFP_mix and those with PFP_dis revealed significant differences in effect size for lateral tilt (ΔSMDtilt = 1.19, P = .029), but not for shift (ΔSMDshift = −0.21, P = .80). No differences in effect size for lateral maltracking were found between PFP_mix and PFP_iso (ΔSMDshift = 0.51, P = .52; ΔSMDtilt = 0.14, P = .59).
Figure 6.
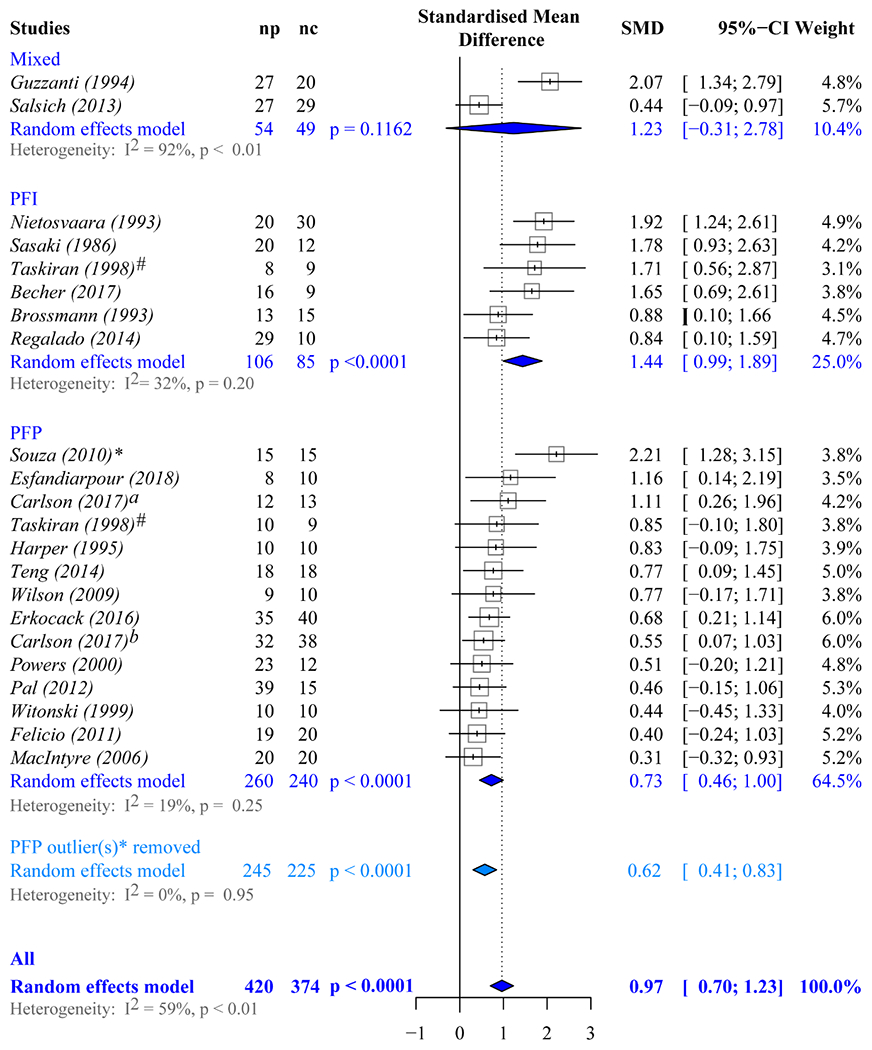
Meta-analysis forest plots used to evaluate if lateral shift maltracking is significant in each cohort. Diamonds represent the pooled effect size (standardized mean difference [SMD]) and 95% CI. Mixed, studies in which the patient cohort contains individuals with patellofemoral pain and patellofemoral instability; nc, the number in the control cohort; np, the number in the patient cohort; PFI, studies in which the patient cohort contains only individuals with patellofemoral instability; PFP, studies in which the patient cohort contains only individuals with isolated patellofemoral pain. *The asterisk denotes the significant single-study effect of the Souza et al65 study on the overall estimates for lateral shift maltracking for the PFP cohort. Thus, the random effects model results for the studies focused on PFP are listed twice. The first (dark grey) are based on all 14 PFP studies listed. The second (light grey) are based on the remaining 13 studies once the Souza et al study was removed. #The Taskirin et al67 study is listed twice, as it evaluated patients with isolated PFP relative to controls, as well as patients with PFP secondary to instability relative to controls. aCarlson et al.7 bCarlson et al.8
Figure 7.
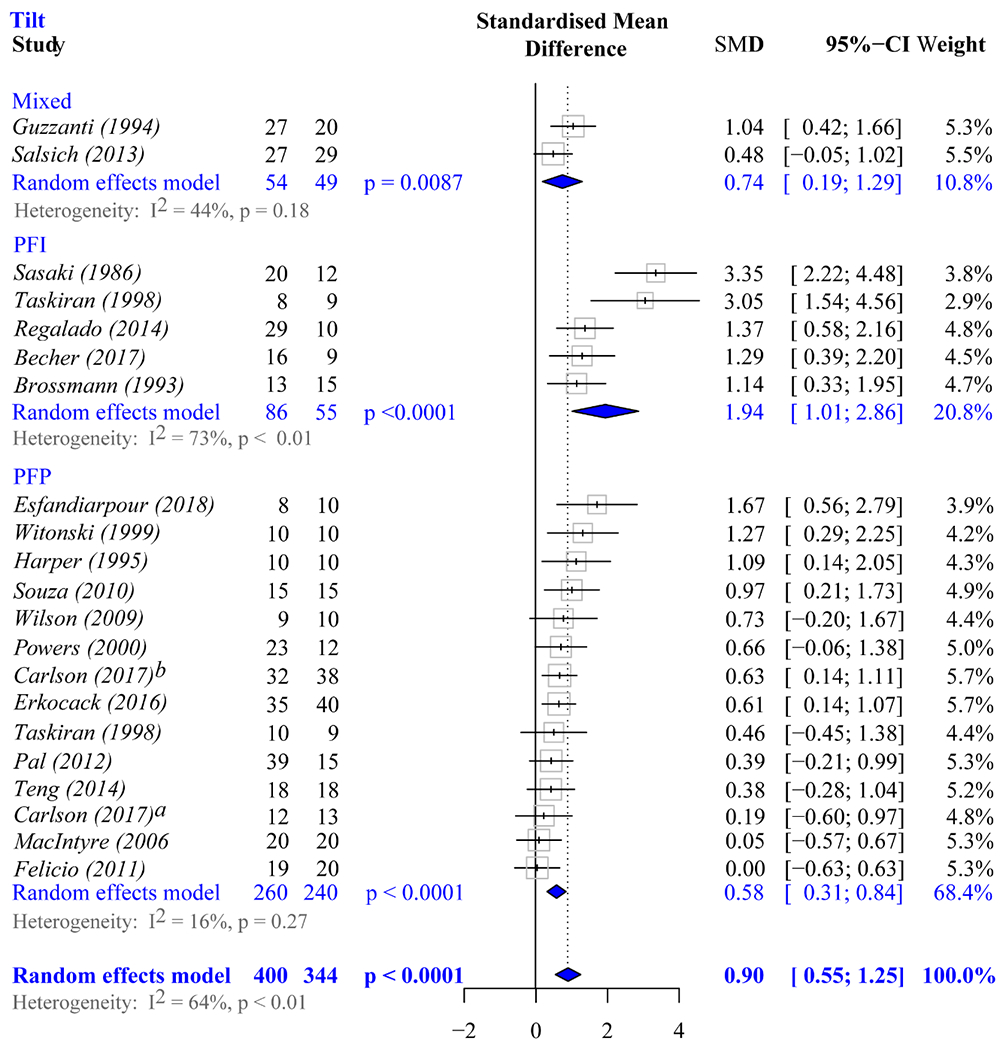
Meta-analysis forest plots used to evaluate if lateral tilt maltracking is significant in each cohort. Diamonds represent the pooled effect size (standardized mean difference [SMD]) and 95% CI. Mixed, studies in which the patient cohort contains individuals with patellofemoral pain and patellofemoral instability; PFI, studies in which the patient cohort contains only individuals with patellofemoral instability; PFP, studies in which the patient cohort contains only individuals with isolated patellofemoral pain. aCarlson et al.7 bCarlson et al.8
When the estimates of shift and tilt were then explored separately, as stratified by patient cohort and by removing studies with PFP_mix populations,24,50 lateral shift maltracking and lateral tilt maltracking were significantly associated with PFP in both subgroups (Figures 6 and 7). No single study significantly influenced the overall lateral tilt results within any subgroup (PFP_iso or PFP_dis). However, a single study65 significantly influenced the overall estimates for lateral shift maltracking in the subgroup of studies of PFP_iso. After this study was removed, the adjusted effect size was attenuated, but remained significant (SMDshift, 0.62; 95% CI, 0.41-0.83; P < .0001).
Meta-regression analyses investigating covariate influence in estimates of lateral maltracking in shift and tilt were not performed after grouping of studies by patient cohort and subsequent removal of influential studies, as the resultant heterogeneity among studies with iso_PFP became nonsignificant70 (I2 = 0%, P = .95, for ML shift; I2 = 16%, P = .27, for ML tilt). Furthermore, the subgroup of studies focused on PFP_dis fell below 10 studies.27 Thus, the influence of other covariates (eg, patient position, activities studied, image techniques) identified by this review could not be investigated.
DISCUSSION
This systematic review expands our clinical and research understanding of the etiology of PFP by providing quantitative evidence of the specific confounding variables’ influence, or lack thereof, on our ability to distinguish patellofemoral kinematics measured in patients with PFP from the kinematics measured in asymptomatic controls. This sets it apart from past reviews that attempted to generate a normative patellofemoral kinematic profile across studies30,79 or sought to define which factors are most associated with patellofemoral pain (eg, sulcus angle, tibiofemoral alignment, bisect offset).13,33 Irrespective of inter-study differences, this review presents strong evidence that lateral patellar shift and tilt maltracking are significantly associated with PFP_iso and PFP_dis. Furthermore, it provides quantitative evidence that assessing patellar maltracking during activities requiring active quadriceps improves our ability to diagnose maltracking. Last, this review adds much-needed quantitative evidence supporting a clear clinical distinction between patients with PFP_iso and those with PFP_dis. This not only fosters an evidence-based rationale for future study design, but exposes key clinical issues. Although our significant findings do not prove cause and effect, they do support the hypothesis that altered muscle forces leading to maltracking are integral to the etiology of PFP. The findings highlight the necessity to treat PFP_iso and PFP_dis as distinct pathologies and to advance interventions uniquely targeted for each patient cohort. Most important, this review clearly demonstrates the need to develop reporting standards for patellar maltracking.
Based on 12 studies1, 6, 14, 15, 21, 24, 25, 41, 51, 67, 74, 77 representing 225 patients and 220 controls, evaluating patellofemoral kinematics with active quadriceps enhances our ability to detect patellar maltracking. Active quadriceps increased lateral tracking in patients and controls, but this increase was significantly larger in the patient cohorts.51 The larger effect of quadriceps activity on maltracking in all patients, irrespective of population cohort, demonstrates the importance of controlling for this variable in future studies. For example, Becher et al1 and Kim et al31 attributed increased lateral tracking in controls and patients to the upright, relative to the supine, position. Based on the current results, the difference in quadriceps (active-upright vs passive-supine) likely accounted for this variability. Clinically, the finding of increased maltracking with active quadriceps activity emphasizes the role of imbalanced muscle forces in the etiology of PFP55 and the importance of evaluating patellofemoral kinematics under active quadriceps conditions.
Although the tendency to evaluate patellofemoral kinematics during active quadriceps is supported by the current results, the type (static or dynamic) and intensity (maximal and submaximal voluntary contractions) of muscle contraction vary considerably between and within studies.1,49 Vasti muscle stiffness changes at different rates in response to increments in contraction intensity.2 Thus, while submaximal intensities may reveal imbalanced forces between the vasti, maximal isometric contraction may not. Consequently, the exercise intensity during assessment may influence the resultant patellar profile, and this parameter should be explored as a potential confounding variable in future studies.
The quantitative distinction in maltracking between individuals with PFP_iso (260 patients and 240 controls) and patients with PFP_dis (106 patients and 85 controls) clearly demonstrates that future research must carefully control for comorbidity (ie, any concomitant condition other than idiopathic PFP). Although the presence of comorbidities is typically controlled for in PFP research, mixed populations were used as recently as 5 years ago,50 and dislocation history is not often clearly defined as an inclusion/exclusion criterion. Thus, future study designs must include clear comorbidity inclusion/exclusion criteria that are fully explained in the manuscript. Furthermore, most studies including patients with a dislocation history do not distinguish if this history is a single traumatic event or a recurrent event. Patients with these 2 unique histories likely have distinct kinematic profiles, but this meta-analysis is unable to tease this out, owing to a lack of clarity in the literature. Clinically, the distinction in kinematics based on the specific patellofemoral pathological condition has implications regarding the selection of the most appropriate treatment intervention. Further stratification of the patient populations could arise from investigation of homogeneous subgroups with shared characteristics. The presence of subgroups with the PFP_iso and PFP_dis cohorts could explain the large variability found within studies for estimates of lateral maltracking (Figures 4 and 5). In addition, patients within unique subgroups may have distinct etiologies for PFP,54,55 which would suggest the need to stratify interventional strategies based on subgrouping patients.
In agreement with previous reviews,13,30 this study identifies several sources of methodological variability, beyond muscle activity and patient cohort, ranging from demographic characteristics to data acquisition to measurement methods. Given the lack of statistical heterogeneity (ie, inter-study differences in effect size) across studies, once these first 2 variables are controlled, the remaining sources cannot be explored with meta-regression. This is not to say that these remaining variables do not influence the estimates of maltracking.29 Instead, the likely intertwined interaction among the numerous sources of methodological variability potentially masks their influence on maltracking estimates. Thus, expanded work is needed not only to understand how these factors influence PFP, but more important, to understand if and how treatment needs to be tailored on the basis of patient demographics.
The stark inter-study lateral maltracking differences in individuals with PFP, purely based on the metric used, create blind spots in our ability to establish a universal definition of normative and pathological tracking. On average, for every 2 studies, there is a unique measure of shift. Furthermore, use of a single axial image (typically, a mid-patellar image; 23 of 40 studies) (Table 1) to quantify patellar position results in unwanted variance and bias across cohorts owing to inter- and intra-individual differences in the femoral level captured.61 For some studies,45,65,76 the definition of shift is altered if alta and/or dysplasia is present, resulting in one measure being used primarily for the control group (Figure 3, BSO2) and another for the patient group (Figure 3, BSO1). For studies using only the mid-patellar image for analysis, the femoral reference changes for every knee angle evaluated. Thus, changes in metrics with knee angle may also be due to alterations in the bony references used.60,61 This illustrates the importance of using 3D coordinates to identify referential landmarks to foster consistency within and across patients.76 Last, with no common bony landmarks to offset differences among kinematic metrics, generalization across studies becomes impossible. To highlight these points, patellar position was measured in 3 individuals via 3 patellofemoral shift metrics (Figure 8). P3 has the most lateralized patella with BSO2*, but the most medialized patella with PFD*, resulting from the long lateral patellar nose.20 Similarly, P2 has the most lateralized patella with PFD*, but the most medialized patella with BSO2* given the small symmetric patella. The interpretation of ML shift changes across metrics owing to LPD2* and BSO2* sensitivity to bone shape size and shape. Recent work demonstrated a larger patella in patients with PFP_iso64 and a smaller patella in patients with PFP_dis.78 This would indicate that a measure such as LPD2* would be more lateral in the PFP_dis cohort, as compared with the PFP_iso cohort, even if the congruence (PFD*) was identical.
Figure 8.
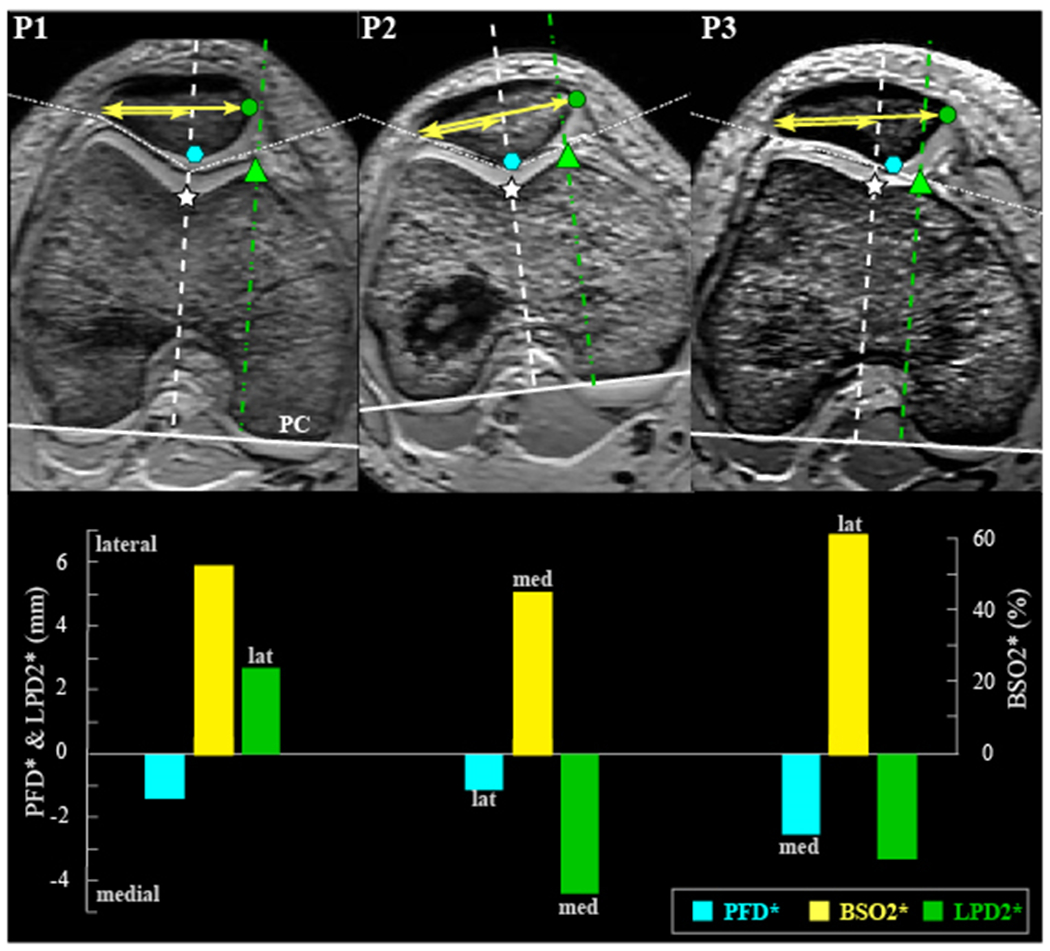
Comparing 3 measurement methods for medial-lateral (ML) shift. Top row: Images for 3 control participants from our database (pixel size is not constant across participants). The images containing the femoral epicondylar width (widest femur) and the midpatella (defined as the inferior-to-superior midpoint of the posterior cartilage on the sagittal image containing the posterior ridge) were cropped together (white dotted line) without changing their relative medial-lateral or anterior-posterior position. The posterior condyle (PC) defines the lateral direction. For all 3 participants, the left side of the image is lateral. Bottom row: Graph of lateral shift for 3 participants per the metrics of PFD* (patellofemoral distance): distance from white star to blue octagon. BSO2* (bisect offset), ratio of the shorter to longer yellow lines. LPD2* (lateral displacement), distance from green triangle to green circle (Appendix Table A3, available online).
Although the purpose of this study was not to determine whether one methodology is superior for investigating patellofemoral alignment/tracking, the numerous distinct methods for measuring patellar shift and tilt (Figures 3 and 4) inhibit our understanding of PFP. As such, for the field to move forward, standardized metrics are needed. This review clearly demonstrates that metrics of ML shift and ML tilt must be acquired via a multiplane method (ie, landmarks defined with 3D coordinates). This will improve accuracy and precision relative to identifying the referential landmarks in a single axial image.61 In terms of specific metrics, we recommend using PFD* or 3Dshift1*, as both relate to the congruency of the joint and are insensitive to alterations in patellofemoral shape and size. For tilt, PTA1* holds no distinct advantage over PTA2*, and both enable comparison with previous literature.
This review’s greatest strength is its thorough examination of the literature with quantitative analyses focused on how variability in study recruitment, design, and methods affects assessing patellar maltracking. The reasonably large number of studies included within these meta-analyses attests to the validity of the conclusions drawn. Nonetheless, this review is not without limitations. As mentioned earlier, because of the varying study designs and assessment protocols within and across studies, the influence of certain potential confounders could not be teased out. As such, further evidence is needed to address the potential moderating influence of demographic characteristics (eg, age, sex, weight, physical activity), study design (eg, static vs dynamic, upright vs supine), and type of muscle activity (eg, maximum isometric vs isokinetic) on patellofemoral kinematics. Sixty-five percent (26 of 40) of the included studies had at least 1 cohort with <20 participants. Although studies with such small population sizes potentially introduce publication bias, we chose not to restrict manuscript selection based on cohort size, in an effort to represent the field as broadly as possibly. Thus, future research can be improved by defining demographic characteristics within the methods, including larger cohorts, and by blinding the researchers to the participant’s cohort. In addition, study measures need to be precisely defined within the article for clarity. For example, “full knee extension” can have unique definitions across studies, and kinematic metrics (eg, lateral patellar displacement and bisect offset) often have varying definitions across the literature (Figure 3).
In conclusion, this review exposes large methodological variability across the literature, which not only hinders the generalization of results, but ultimately mitigates our understanding of the underlying mechanism of PFP. Although our meta-analyses support the diagnostic value of maltracking in PFP, the numerous distinct methods for measuring maltracking prohibit a single quantitative maltracking profile from being defined. Requiring active quadriceps during assessment clearly enhances our ability to diagnose patellar maltracking. The meta-analyses highlight the importance of study participant selection by exposing statistically unique maltracking profiles between individuals with PFP_iso and those with PFP_dis. However, intrastudy variability in maltracking estimates supports the need for further subgrouping of these 2 patient populations. This will not only provide further explanation regarding the etiology of PFP_iso and PFP_dis, but also guide treatment intervention development. Finally, compliance with specific standards for anatomic and outcome measures must be addressed by the scientific and clinical community to establish methodological uniformity in this field.
Supplementary Material
ACKNOWLEDGMENT
All data for the current study were collected at the Clinical Center of the National Institutes of Health. The authors thank the National Institutes of Health Clinical Center Radiology Department for its technical support this work. C.N.F. acknowledges funding support through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH, and generous contributions from the Doris Duke Charitable Foundation, Genentech, American Association for Dental Research, the Colgate-Palmolive Company, Elsevier, alumni of student research programs, and other individual supporters via the Foundation for the National Institutes of Health. For a complete list, visit https://fnih.org/what-we-do/current-education-and-training-programs/mrsp. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
REFERENCES
- 1.Becher C, Fleischer B, Rase M, et al. Effects of upright weight bearing and the knee flexion angle on patellofemoral indices using magnetic resonance imaging in patients with patellofemoral instability. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2405–2413. [DOI] [PubMed] [Google Scholar]
- 2.Bensamoun SF, Ringleb SI, Littrell L, et al. Determination of thigh muscle stiffness using magnetic resonance elastography. J Magnetic Resonance Imaging. 2006;23(2):242–247. [DOI] [PubMed] [Google Scholar]
- 3.Biedert RM, Gruhl C. Axial computed tomography of the patellofemoral joint with and without quadriceps contraction. Arch Orthop Trauma Surg. 1997;116(1-2):77–82. [DOI] [PubMed] [Google Scholar]
- 4.Bolgla LA, Gordon R, Sloan G, Pretlow LG, Lyon M, Fulzele S. Comparison of patella alignment and cartilage biomarkers in young adult females with and without patellofemoral pain: a pilot study. Int J Sports Phys Ther. 2019;14(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- 5.Brazaitis A, Tamošiūnas A, Tutkuvienė J, Gocentas A. Predictors of patellofemoral pain applying full weight bearing kinematic MRI. Central Eur J Sport Sci Med. 2015;12(4):43–51. [Google Scholar]
- 6.Brossmann J, Muhle C, Schroder C, et al. Patellar tracking patterns during active and passive knee extension: evaluation with motion-triggered cine MR imaging. Radiology. 1993;187(1):205–212. [DOI] [PubMed] [Google Scholar]
- 7.Carlson VR, Boden BP, Sheehan FT. Patellofemoral kinematics and tibial tuberosity-trochlear groove distances in female adolescents with patellofemoral pain. Am J Sports Med. 2017;45(5):1102–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson VR, Sheehan FT, Shen A, Yao L, Jackson JN, Boden BP. The relationship of static tibial tubercle-trochlear groove measurement and dynamic patellar tracking. Am J Sports Med. 2017;45(8):1856–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crossley KM, Stefanik JJ, Selfe J, et al. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br J Sports Med. 2016;50(14):839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeHaven KE, Lintner DM. Athletic injuries: comparison by age, sport, and gender. Am J Sports Med. 1986;14(3):218–224. [DOI] [PubMed] [Google Scholar]
- 11.Delgado-Martins H The bicondylo-patellar angle as a measure of patellar tilting. Arch Orthop Trauma Surg. 1980;96(4):303–304. [DOI] [PubMed] [Google Scholar]
- 12.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew BT, Redmond AC, Smith TO, Penny F, Conaghan PG. Which patellofemoral joint imaging features are associated with patellofemoral pain? Systematic review and meta-analysis. Osteoarthritis Cartilage. 2016;24(2):224–236. [DOI] [PubMed] [Google Scholar]
- 14.Erkocak OF, Altan E, Altintas M, Turkmen F, Aydin BK, Bayar A. Lower extremity rotational deformities and patellofemoral alignment parameters in patients with anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 2016;24(9):3011–3020. [DOI] [PubMed] [Google Scholar]
- 15.Esfandiarpour F, Lebrun CM, Dhillon S, Boulanger P. In-vivo patellar tracking in individuals with patellofemoral pain and healthy individuals. J Orthop Res. Published online February 28, 2018. doi: 10.1002/jor.23887 [DOI] [PubMed] [Google Scholar]
- 16.Fairbank JC, Pynsent PB, van Poortvliet JA, Phillips H. Mechanical factors in the incidence of knee pain in adolescents and young adults. J Bone Joint Surg Br. 1984;66(5):685–693. [DOI] [PubMed] [Google Scholar]
- 17.Farrokhi S, Keyak JH, Powers CM. Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthritis Cartilage. 2011;19(3):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felicio LR, Baffa ADP, Liporacci RF, Saad MC, De Oliveira AS, Bevilaqua-Grossi D. Analysis of patellar stabilizers muscles and patellar kinematics in anterior knee pain subjects. J Electromyogr Kinesiol. 2011;21(1):148–153. [DOI] [PubMed] [Google Scholar]
- 19.Felicio LR, Saad MC, Liporaci RF, Baffa Ado P, dos Santos AC, Bevilaqua-Grossi D. Correlation between trochlear groove depth and patellar position during open and closed kinetic chain exercises in subjects with anterior knee pain. J Appl Biomech. 2012;28(3):335–342. [DOI] [PubMed] [Google Scholar]
- 20.Fick CN, Grant C, Sheehan FT. Patellofemoral pain in adolescents: understanding patellofemoral morphology and its relationship to maltracking. Am J Sports Med. 2020;48(2):341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman BR, Sheehan FT. Predicting three-dimensional patellofemoral kinematics from static imaging-based alignment measures. J Orthop Res. 2013;31(3):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fulkerson JP, Schutzer SF, Ramsby GR, Bernstein RA. Computerized tomography of the patellofemoral joint before and after lateral release or realignment. Arthroscopy. 1987;3(1):19–24. [DOI] [PubMed] [Google Scholar]
- 23.Grelsamer RP, Weinstein CH, Gould J, Dubey A. Patellar tilt: the physical examination correlates with MR imaging. Knee. 2008;15(1):3–8. [DOI] [PubMed] [Google Scholar]
- 24.Guzzanti V, Gigante A, Di Lazzaro A, Fabbriciani C. Patellofemoral malalignment in adolescents: computerized tomographic assessment with or without quadriceps contraction. Am J Sports Med. 1994;22(1):55–60. [DOI] [PubMed] [Google Scholar]
- 25.Harper WM, McCaskie AW, Harding ML, Finlay DBL. Anterior knee pain: the use of computerized tomography to assess the results of tibial tubercle transfer. Knee. 1995;2(4):207–210. [Google Scholar]
- 26.Hedges LV, Olkin I. Statistical Methods for Meta-analysis. Academic Press; 2014. [Google Scholar]
- 27.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23(11):1663–1682. [DOI] [PubMed] [Google Scholar]
- 28.Inoue M, Shino K, Hirose H, Horibe S, Ono K. Subluxation of the patella: computed tomography analysis of patellofemoral congruence. J Bone Joint Surg Am. 1988;70(9):1331–1337. [PubMed] [Google Scholar]
- 29.Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katchburian MV, Bull AM, Shih Y-F, Heatley FW, Amis AA. Measurement of patellar tracking: assessment and analysis of the literature. Clin Orthop Relat Res. 2003;412:241–259. [DOI] [PubMed] [Google Scholar]
- 31.Kim T-H, Sobti A, Lee S-H, Lee J-S, Oh K-J. The effects of weight-bearing conditions on patellofemoral indices in individuals without and with patellofemoral pain syndrome. Skeletal Radiol. 2013;43(2):157–164. [DOI] [PubMed] [Google Scholar]
- 32.Kujala UM, Kormano M, Österman K, et al. Magnetic resonance imaging analysis of patellofemoral congruity in females. Clin J Sport Med. 1992;2(1):21–26. [Google Scholar]
- 33.Kujala UM, Osterman K, Kormano M, Nelimarkka O, Hurme M, Taimela S. Patellofemoral relationships in recurrent patellar dislocation. J Bone Joint Surg Br. 1989;71(5):788–792. [DOI] [PubMed] [Google Scholar]
- 34.Lankhorst NE, Bierma-Zeinstra SM, van Middelkoop M. Factors associated with patellofemoral pain syndrome: a systematic review. Br J Sports Med. 2013;47(4):193–206. [DOI] [PubMed] [Google Scholar]
- 35.Lau BC, Thuillier DU, Pedoia V, et al. Inter- and intra-rater reliability of patellofemoral kinematic and contact area quantification by fast spin echo MRI and correlation with cartilage health by quantitative T1rho MRI. Knee. 2016;23(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurin CA, Dussault R, Levesque HP. The tangential x-ray investigation of the patellofemoral joint: x-ray technique, diagnostic criteria and their interpretation. Clin Orthop Relat Res. 1979;144:16–26. [PubMed] [Google Scholar]
- 37.Laurin CA, Levesque HP, Dussault R, Labelle H, Peides JP. The abnormal lateral patellofemoral angle: a diagnostic roentgenographic sign of recurrent patellar subluxation. J Bone Joint Surg Am. 1978;60(1):55–60. [PubMed] [Google Scholar]
- 38.MacIntyre NJ, Hill NA, Fellows RA, Ellis RE, Wilson DR. Patellofemoral joint kinematics in individuals with and without patellofemoral pain syndrome. J Bone Joint Surg Am. 2006;88(12):2596–2605. [DOI] [PubMed] [Google Scholar]
- 39.Martinez S, Korobkin M, Fondren FB, Hedlund LW, Goldner JL. Computed tomography of the normal patellofemoral joint. Invest Radiol. 1983;18(3):249–253. [DOI] [PubMed] [Google Scholar]
- 40.Merchant AC, Mercer RL, Jacobsen RH, Cool CR. Roentgenographic analysis of patellofemoral congruence. J Bone Joint Surg. 1974;56(7):1391–1396. [PubMed] [Google Scholar]
- 41.Nietosvaara AY, Aalto KA. Ultrasonographic evaluation of patellar tracking in children. Clin Orthop Relat Res. 1993;297:62–64. [PubMed] [Google Scholar]
- 42.Pal S, Besier TF, Draper CE, et al. Patellar tilt correlates with vastus lateralis: vastus medialis activation ratio in maltracking patellofemoral pain patients. J Orthop Res. 2012;30(6):927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pal S, Draper CE, Fredericson M, et al. Patellar maltracking correlates with vastus medialis activation delay in patellofemoral pain patients. Am J Sports Med. 2011;39(3):590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers CM. Patellar kinematics, part II: the influence of the depth of the trochlear groove in subjects with and without patellofemoral pain. Phys Ther. 2000;80(10):965–978. [PubMed] [Google Scholar]
- 45.Prakash J, Seon JK, Woo SH, Jin C, Song EK. Comparison of radiological parameters between normal and patellar dislocation groups in Korean population: a rotational profile CT-based study. Knee Surg Relat Res. 2016;28(4):302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R: A Language and Environment for Statistical Computing. Version 3.6.0. R Foundation for Statistical Computing; 2019. [Google Scholar]
- 47.Regalado G, Lintula H, Eskelinen M, et al. Dynamic KINE-MRI in patellofemoral instability in adolescents. Knee Surg Sports Traumatol Arthrosc. 2013;22(11):2795–2802. [DOI] [PubMed] [Google Scholar]
- 48.RStudio, ed. Integrated Development Environment for R. RStudio; 2018. [Google Scholar]
- 49.Salsich GB, Perman WH. Patellofemoral joint contact area is influenced by tibiofemoral rotation alignment in individuals who have patellofemoral pain. J Orthop Sports Phys Ther. 2007;37(9):521–528. [DOI] [PubMed] [Google Scholar]
- 50.Salsich GB, Perman WH. Tibiofemoral and patellofemoral mechanics are altered at small knee flexion angles in people with patellofemoral pain. J Sci Med Sport. 2013;16(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sasaki T, Yagi T. Subluxation of the patella: investigation by computerized tomography. Int Orthop. 1986;10(2):115–120. [PubMed] [Google Scholar]
- 52.Schutzer SF, Ramsby GR, Fulkerson JP. The evaluation of patellofemoral pain using computerized tomography: a preliminary study. Clin Orthop Relat Res. 1986;204:286–293. [PubMed] [Google Scholar]
- 53.Schwarzer G meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 54.Selfe J, Janssen J, Callaghan M, et al. Are there three main subgroups within the patellofemoral pain population? A detailed characterisation study of 127 patients to help develop targeted intervention (TIPPs). Br J Sports Med. 2016;50(14):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheehan FT, Borotikar BS, Behnam AJ, Alter KE. Alterations in in vivo knee joint kinematics following a femoral nerve branch block of the vastus medialis: implications for patellofemoral pain syndrome. Clin Biomech (Bristol, Avon). 2012;27(6):525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheehan FT, Derasari A, Brindle TJ, Alter KE. Understanding patellofemoral pain with maltracking in the presence of joint laxity: complete 3D in vivo patellofemoral and tibiofemoral kinematics. J Orthop Res. 2009;27(5):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheehan FT, Derasari A, Fine KM, Brindle TJ, Alter KE. Q-angle and J-sign: indicative of maltracking subgroups in patellofemoral pain. Clin Orthop Relat Res. 2009;468(1):266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheehan FT, Zajac FE, Drace JE. In vivo tracking of the human patella using cine phase contrast magnetic resonance imaging. J Biomech Eng. 1999;121(6):650–656. [DOI] [PubMed] [Google Scholar]
- 59.Sheehan FT, Zajac FE, Drace JE. Using cine phase contrast magnetic resonance imaging to non-invasively study in vivo knee dynamics. J Biomech. 1998;31(1):21–26. [DOI] [PubMed] [Google Scholar]
- 60.Shibanuma N, Sheehan FT, Lipsky PE, Stanhope SJ. Sensitivity of femoral orientation estimates to condylar surface and MR image plane location. J Magnetic Resonance Imaging. 2004;20(2):300–305. [DOI] [PubMed] [Google Scholar]
- 61.Shibanuma N, Sheehan FT, Stanhope SJ. Limb positioning is critical for defining patellofemoral alignment and femoral shape. Clin Orthop Relat Res. 2005;434:198–206. [DOI] [PubMed] [Google Scholar]
- 62.Sidik K, Jonkman JN. Robust variance estimation for random effects meta-analysis. Computational Statistics and Data Analysis. 2006;50(12):3681–3701. [Google Scholar]
- 63.Smith BE, Selfe J, Thacker D, et al. Incidence and prevalence of patellofemoral pain: a systematic review and meta-analysis. Plos One. 2018;13(1):e0190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith RM, Boden BP, Sheehan FT. Increased patellar volume/width and decreased femoral trochlear width are associated with adolescent patellofemoral pain. Clin Orthop Relat Res. 2018;476(12):2334–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Souza RB, Draper CE, Fredericson M, Powers CM. Femur rotation and patellofemoral joint kinematics: a weight-bearing magnetic resonance imaging analysis. J Orthop Sports Phys Ther. 2010;40(5):277–285. [DOI] [PubMed] [Google Scholar]
- 66.Stanford W, Phelan J, Kathol MH, et al. Patellofemoral joint motion: evaluation by ultrafast computed tomography. Skeletal Radiol. 1988;17(7):487–492. [DOI] [PubMed] [Google Scholar]
- 67.Taşkiran E, Dinedurga Z, Yagiz A, Uludag B, Ertekin C, Lok V. Effect of the vastus medialis obliquus on the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 1998;6(3):173–180. [DOI] [PubMed] [Google Scholar]
- 68.Teng H-L, Chen Yu-Jen, Powers CM. Predictors of patellar alignment during weight bearing: an examination of patellar height and trochlear geometry. Knee. 2014;21(1):142–146. [DOI] [PubMed] [Google Scholar]
- 69.Thomeé R, Renstrom P, Karlsson J, Grimby G. Patellofemoral pain syndrome in young women: I. A clinical analysis of alignment, pain parameters, common symptoms and functional activity level. Scand J Med Sci Sports. 1995;5(4):237–244. [PubMed] [Google Scholar]
- 70.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Statistics in medicine. 2002;21(11):1559–1573. [DOI] [PubMed] [Google Scholar]
- 71.Türkmen İ, Işık Y. Association between patellofemoral congruence and patellofemoral chondropathy in patients with anterior knee pain: a T2 mapping knee MRI study. Eklem Hastalik Cerrahisi. 2018;29(2):93–99. [DOI] [PubMed] [Google Scholar]
- 72.Viechtbauer W Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36(3):48. [Google Scholar]
- 73.Wilson NA. In vivo noninvasive evaluation of abnormal patellar tracking during squatting in patients with patellofemoral pain. J Bone Joint Surg Am. 2009;91(3):558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witonski D, Goraj B. Patellar motion analyzed by kinematic and dynamic axial magnetic resonance imaging in patients with anterior knee pain syndrome. Arch Orthop Trauma Surg. 1999;119(1-2):46–49. [DOI] [PubMed] [Google Scholar]
- 75.Yamada Y, Toritsuka Y, Horibe S, et al. Patellar instability can be classified into four types based on patellar movement with knee flexion: a three-dimensional computer model analysis. J ISAKOS. 2018;3(6):328–335. [Google Scholar]
- 76.Yamada Y, Toritsuka Y, Nakamura N, et al. Correlation of 3D shift and 3D tilt of the patella in patients with recurrent dislocation of the patella and healthy volunteers: an in vivo analysis based on 3-dimensional computer models. Am J Sports Med. 2017;45(13):3111–3118. [DOI] [PubMed] [Google Scholar]
- 77.Yang JH, Demarchi GTS, Garms E, et al. Avaliação quantitativa das forças laterais da patela: ressonância magnética estática e cinemática. Radiologia Brasileira. 2007;40:223–229. [Google Scholar]
- 78.Yılmaz B, Çiçek ED, Şirin E, Özdemir G, Karakuş Ö, Muratlı HH. A magnetic resonance imaging study of abnormalities of the patella and patellar tendon that predispose children to acute patellofemoral dislocation. Clin Imaging. 2017;42:83–87. [DOI] [PubMed] [Google Scholar]
- 79.Yu Z, Yao J, Wang X, et al. Research methods and progress of patellofemoral joint kinematics: a review. J Healthc Eng. 2019;2019:9159267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


