Abstract
A three-fold increase in fatal cocaine overdoses during the past decade highlights the critical lack of medications for cocaine use disorders. The brain response to drug cues can predict future drug use; however results have been mixed. We present preliminary evidence that a sustained response to repeated cocaine cues within a single task is a significant predictor of drug-use outcomes. Seventy-three cocaine inpatients were administered a passive-viewing fMRI task, featuring 500ms novel evocative (cocaine, sexual, aversive) and neutral comparator cues in the first half (half1), which were then repeated in the second half (half2). After the baseline scan, patients received eight outpatient treatment weeks with twice-weekly drug screens. Drug use outcome groups were empirically defined based on cocaine-positive or missing urines averaged across the outpatient phase: Good (<40%), Poor (>85%) and Intermediate (INT, between 40–85%) outcomes. Differences of response to initial (half1) and repeated (half2) cues in a priori (cue-reactive) regions were tested between outcome groups (3 [Group] × 2 [Halves] ANOVA). An interaction was found in the brain response to drug (but not sex or aversive) cues, with a significant difference between the Good and Poor outcome groups in half2, driven by a significant decrease in brain response by the Good group, and a sustained brain response by the Poor group, to repeated cocaine cues. The brain response to repeated drug cues may be a useful predictor of future drug use, encouraging future intervention studies to restore a ‘healthy’ (decreasing) response to the repeated presentation of drug cues.
Keywords: Biomarker, Cocaine, Cues, fMRI, Relapse
Introduction
During the past decade, drug overdose deaths have increased substantially, including more than a 3-fold increase in deaths associated with cocaine, largely due to contamination with fentanyl 1. Unfortunately, there are still no FDA-approved medications to treat cocaine use disorder, and rates of relapse remain very high 2. Uncovering neural correlates of drug use would aid development of behavioral and pharmaceutical interventions. Across the past two decades, many imaging studies have characterized the brain response to drug cues (both in our own lab 3–8 and in others 9–15). Fewer have pursued the brain response to drug cues as a potential relapse ‘biomarker’ 16–18, and most of these have been conducted in alcohol and nicotine populations, with far fewer in stimulant or opioid users. Across multiple substance-use disorders, hyperactivity in motivational circuits is linked to worse outcomes 19–26.
Given extensive preclinical research demonstrating that cocaine-associated cues can trigger drug-seeking / reinstatement 27–30, it is perhaps surprising that only two clinical studies have used fMRI to investigate the brain response to drug cues in cocaine patients as it relates to future cocaine use31,32. Kosten et al. (2006) preliminary study in 17 cocaine patients found an association between the brain response in posterior regions (e.g., posterior cingulate gyrus [PCC], superior temporal gyrus [STG]) to cocaine videos with cocaine-use outcomes. Prisciandaro et al. (2013) found a relationship of the brain response to cocaine images in striatal, insula, and visual association areas (e.g., fusiform [FUSI]) between baseline scans and relapse one week later. Worth noting, a third study with stimulant (14 cocaine; 28 methamphetamine) users found the ventral striatum was associated with time to relapse26. The current investigation expands this domain and offers the largest sample size in cocaine patients to date.
The current study offers a novel approach to the investigation of cue-reactivity as a potential relapse biomarker by utilizing very brief (500 msec) evocative (e.g., cocaine, sexual, aversive) cues to probe drug-use vulnerability. This brief stimulus duration allows for “fast” event-related designs that include other evocative cues (e.g., sexual, aversive), enabling the examination of specificity related to drug-cue findings. Furthermore, as dozens of brief cues are presented in the first half of the task and then repeated in the second half, the design offers a natural opportunity for examining the brain response to repeated drug cues. Interestingly, research into several other psychiatric disorders has investigated the brain response to repeated emotional cues and identified a non-decreasing response as a marker of pathology for anxiety 33,34, autism 35,36, and schizophrenia 37. A differential decrease in response to repeated evocative cues may offer a sensitive predictor of future drug use that would otherwise be obscured with the common approach of ‘signal averaging’ across the entire task.
Prior studies reporting on the neural response to drug cues associated with drug-use outcomes have revealed several brain regions of interest (for review, see Moeller and Paulus, 201817). In addition to regions noted above (e.g., striatum, insula, FUSI, STG, PCC), early research found a link between the brain response to drug cues and outcome in the amygdala and thalamus in smokers 19. More recent work has shown the importance of the medial prefrontal cortex, anterior cingulate cortex, and visual areas, among others21,23–26,32. These studies suggest that the ‘outcome-predictive’ brain response to drug cues can be widespread and involve regions that process motivation, reward, attention, self-reflection, and sensory information. Thus, the current analyses utilize this previous research and other studies on cue-reactivity vulnerability 5,8,9,20 to examine several a priori regions of interest. The study’s primary focus is to examine the relationship of both initial and repeated cocaine cues to drug-use outcomes, as the change in brain response within a single task may play a role in differentiation of outcome groups and may also undermine traditional analyses.
Methods
Participants
Seventy-three treatment-seeking cocaine patients participating in imaging studies in our lab from 2004 to 2014 were included in this study. Studies using subsets of the participants have been reported previously4,8,38,39, but none of these previous studies investigated the association of brain response at baseline to future drug use outcomes. Standard eligibility for imaging studies has been described previously 8. Briefly, inclusion criteria were: cocaine dependence (DSM IV) or cocaine-use disorder (DSM V), with reported (smoked) cocaine use on at least 8 of the last 30 days and availability for a 7–10 day inpatient stay. Exclusion criteria included: contraindications for functional magnetic resonance imaging (fMRI); a history of psychosis, head trauma, head injury, seizures, or other organic brain syndrome; medications that affect dopamine transmission; significant cardiovascular, hematologic, hepatic, renal, neurological, and/or endocrine abnormalities. As screened by the Mini-International Neuropsychiatric Interview 40, patients with Axis I psychiatric disorders other than cocaine-use disorder generally were excluded with the following exceptions: nicotine, marijuana, and alcohol-use disorders (not requiring detoxification); current depression associated with cocaine use and/or cessation.
Study Design
Patients were stabilized for 7–10 days in a controlled, inpatient setting in order to diminish potential effects of cocaine intoxication or early cessation symptoms during testing. After stabilization, patients provided baselines measures: addiction severity index, which included prior cocaine use (total years and past 30 days), alcohol use, cannabis use, heroin use, and questions on prior [emotional, physical, or sexual] abuse); Beck’s Depression Index (BDI); and Beck’s Anxiety Index (BAI). Subsequently, they participated in an fMRI scan session. that included a “fast” event-related task to evaluate the brain response to evocative cues (Figure 1). There were two halves of the task, each with 120 target presentations per half. Of these, 72 were stimulus categories (24 cocaine, 24 sexual, 24 aversive), 24 were comparator cues (neutral), and 24 were null images (grey screen with crosshair). In the first half, targets were presented in a quasi-random order (i.e., no more than three of a kind in a sequence), with an interstimulus interval (grey screen with cross-hair) of 1500 msec presented in between each cue. In the second half, the stimulation category cues were repeated in pseudo-random order. Cocaine and neutral cues were from our laboratory archives. Sexual and aversive cues were obtained from the top quartile of “pleasant” and “unpleasant”, respectively, from the International Affective Picture System 41. Length of the task was 8.5 minutes.
Figure 1.
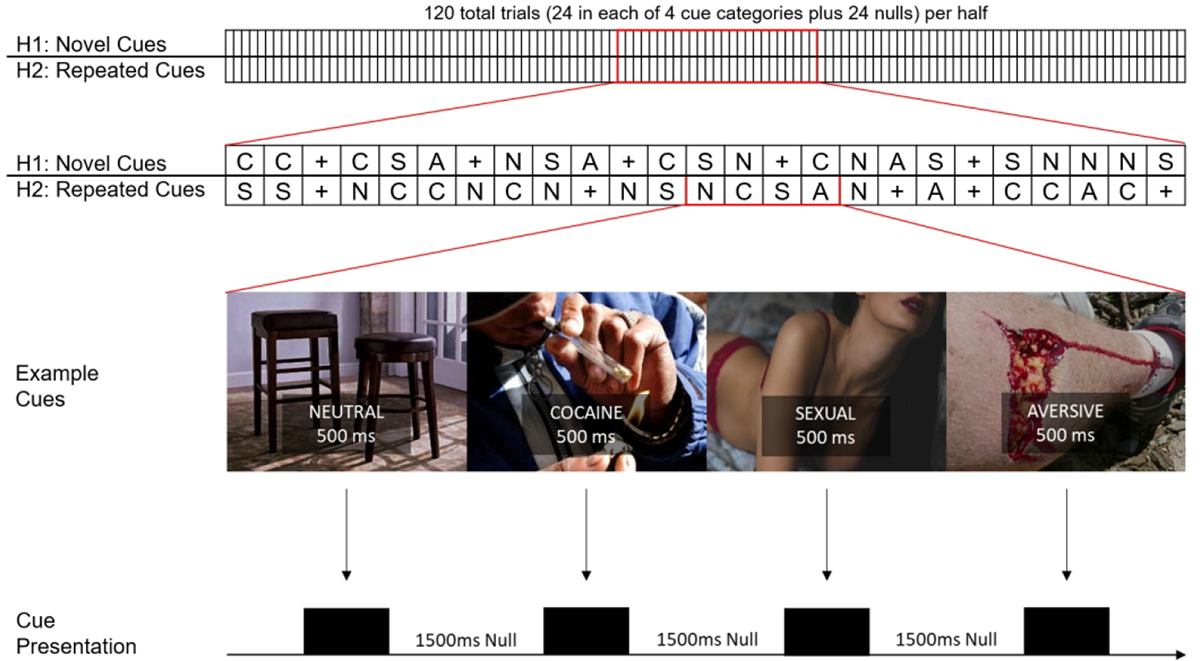
Cue-reactivity task. Top: Representation of the 500 msec cue task in its entirety. There were 120 trials per half, 24 in each of 4 cue categories plus 24 “null” targets (grey screen with cross-hair). Middle Top: Example order of targets in first and second half. Targets were pseudo-randomly presented (no more than three presentations of a target in a row) in the first half, and then that order was quasi-randomized in the second half. N=Neutral, C=Cocaine, A=Aversive, S=Sexual, “+” = Null. Middle Bottom: Examples of actual (neutral, cocaine) cues and cues (sexual, and aversive) that were similar (to IAPS images) used in the task. Bottom: Actual presentation of targets, complete with 1500 msec of ISI (grey screen with crosshair).
Drug-Use Outcomes
After scanning and hospital discharge, patients participated in outpatient treatment, which consisted of at least 8 weeks of twice-weekly, manualized psychosocial treatment (drug counseling and active strategies for coping with craving) and urine samples screened for benzoylecognine (BE), the primary cocaine metabolite. Urine samples with BE values greater than 300 nanograms/ml were considered “cocaine-positive”; values less than 300 ng/ml were considered “cocaine negative”. If participants failed to submit the requested urine sample (usually due to a missed appointment), the missing sample was considered cocaine-positive for the purpose of the outcome analyses. To encourage treatment retention, patients received small travel reimbursements at each visit, along with weekly incentives for attending study appointments.
fMRI Acquisition
Blood-oxygenated-level-dependent (BOLD) images were acquired with a Siemens 3T (Tim Trio) scanner, using an 8-channel head-coil. A 5 min high-resolution 3-D T1-weighted (MPRAGE) structural scan (parameters: repetition time (TR) = 1620 msec; echo time (TE) = 3.97 msec; 160 slices; slice thickness = 1 mm; matrix = 192 × 256; flip angle = 15°) was used for normalization and co-registration purposes. Functional images were acquired via a T2*-weighted single-shot gradient-echo, planar-imaging sequence with the following parameters: TR 2000 msec; TE 30 msec; 33 interleaved slices; slice thickness 3 mm without any gap between adjacent slices; FOV 192 mm; matrix 64 × 64; and flip angle 80°.
Regions of Interest (ROIs)
The focus of the primary analysis was the brain response in anatomically defined a priori regions to novel and repeated evocative cues and its relation to drug-use outcomes. Prior studies have demonstrated that the response in several regions can be predictive of drug-use (e.g., cocaine, nicotine, alcohol, opioid, methamphetamines, etc.) outcomes 19,21,23–26,31,32. These include the anterior (ACC) and posterior cingulate (PCC) cortices, amygdala (AMYG), thalamus (THAL), striatum (STR), orbitofrontal cortex (OFC), superior temporal gyrus (STG), insula (INS), and visual association areas, such as the fusiform gyrus (FUSI). In addition to these areas, other “cue-reactive” regions (e.g., hippocampus [HIPP], parahippocampus [P-HIPP], midbrain [MB]), that our lab has previously found to have a differential response related to risk 42 and vulnerability 8, may also be related to drug-use outcomes in cocaine patients. The regions listed above (n=13) were identified, thresholded (e.g., min 10 or 20 voxels), and binarized via FSL.
Data Analysis
Drug-use outcomes:
The average percent cocaine-positive urines (urine analysis [UA] scores) was calculated for each patient as total positive urines divided by the total opportunities to provide a urine sample (e.g., 1 sample in the first week after discharge and then 2 samples/week for the remainder of treatment, total opportunities = 15). For the primary analysis, hierarchical clustering, using MATLAB (The Mathworks, 2019a) of UA scores, first identified natural cutoffs, creating 7 groups; after which, tertiles were created from the natural cutoffs, with the clinical goal of capturing groups of patients with relatively GOOD, relatively POOR, and intermediate (INT) outcomes. Based on this clustering, three groups were empirically identified: an outcome group with urine samples >85% cocaine positive (Poor, n=34); an outcome group with urine samples ≤40% cocaine-positive (Good, n=15); and an intermediate group (INT, n=24), with outcome scores between the Good and Poor groups (>50% and <81%).
fMRI First-level analyses:
Preprocessing was completed with SPM8 (Wellcome Department of Cognitive Neurology, London, UK), run with MATLAB. It was identical to previous studies 4,7,8, including slice-timing correction, motion correction, temporal filtering, spatial smoothing, co-registration to high-resolution structural images, normalization to Montreal Neurological Institute (MNI) standard space and smoothing with a 8 mm FWHM kernel. Motion statistics ensured inclusion of patients for whom motion did not exceed 2 mm in any plane. A canonical hemodynamic response function with both the first (time) and the second (dispersion) derivatives was fitted to the onset of each event. Six first-level contrasts were defined: cocaine vs. neutral (first [cocaine1-neutral1] and second half [cocaine2-neutral2]); sex vs. neutral (first [sex1-neutral1] and second half [sex2-neutral2]), and aversive vs. neutral (first [aversive1-neutral1] and second half [aversive2-neutral2]).
fMRI Second-level analyses:
Primary: Using SPM8 and Marsbar (marsbar.sourceforge.net), the mean of all ROIs was extracted from the first-level contrasts and were entered into a 3×2 ANOVA (Groups vs. Halves) for each cue-category (e.g., Groups: Good, INT, Poor; Halves: drug1-neutral1, drug2-neutral2). Secondary: If a significant interaction was found in the primary analysis, individual ROIs were entered into a 3×2 ANOVA examining differences between outcome groups and halves for each region in a secondary analysis. Correction for false discovery rate (FDR) was applied, separately, when examining for main effects of group, main effects of halves, interaction of group × halves, and follow-up posthoc tests. MATLAB was used to run all analyses, using built-in functions: anovan (multi-level anovas), fitrm + ranova (repeated measures anovas), ttest2 (post-hoc 2-sample t-tests), ttest (paired t-test); and one custom-made function: fdr_bh (https://www.mathworks.com/matlabcentral/fileexchange/27418-fdr_bh).
Results
Demographics and Health Variables
As shown in Table 1, participants were all males and primarily African American (88%) with an average age of 44.6 years, 12.5 years of education, and 16.9 years of (primarily smoked) cocaine use. The average percentage of cocaine-positive urine samples (UA scores) during the eight weeks of outpatient treatment was 70%. None of the demographic, health, or psychiatric variables (e.g., Beck Depression Index, BDI; Beck Anxiety Index, BAI) differed significantly between groups. There were main effects of group for days of cocaine use in the past 30 days and cannabis use (% yes or no), both of which were highest in the Poor group; differences in cannabis use did not survive correction.
Table 1.
Demographics, Behavioral, Drug-Use Variables by Outcome Group
| Total mean (n=73) |
Good (n=15) |
INT (n=24) |
Poor (n=34) |
p value (ANOVA) |
|
|---|---|---|---|---|---|
|
| |||||
| Age | 44.39 | 42.73 | 46.33 | 43.91 | 0.31 |
|
| |||||
| Education | 12.52 | 12.27 | 12.82 | 12.45 | 0.73 |
|
| |||||
| Addiction Severity | |||||
| Cocaine use (Years) | 17.23 | 16.53 | 18.78 | 16.45 | 0.55 |
| Cocaine Use (30days) | 17.77 | 16.4 | 13.30 | 21.52 | 0.001 |
| n Drug Treatments | 2.99 | 3.00 | 2.77 | 3.12 | 0.91 |
| n Alcohol Treatments | 0.99 | 1.20 | 1.27 | 0.70 | 0.54 |
|
| |||||
| Alcohol Use (Years) | 17.62 | 18.38 | 14.47 | 19.15 | 0.49 |
|
| |||||
| Cannabis Use (%Yes) | 39% (29/73) | 27% (4/15) | 21% (5/24) | 59% (20/34) | 0.008 |
|
| |||||
| Heroin Use (%Yes) | 1% (2/74) | 7% (1/15) | 0% (0/24) | 3% (1/34) | 0.48 |
|
| |||||
| BDI | 10.42 | 7.07 | 10.09 | 12.06 | 0.18 |
|
| |||||
| BAI | 6.49 | 5.93 | 5.86 | 7.16 | 0.72 |
|
| |||||
| Prior Abuse (%Yes) | 55% (40/73) | 50% (7/14) | 63% (15/24) | 51% (20/73) | 0.82 |
fMRI: Primary Analysis
A repeated measures design was used to evaluate the mean brain response of all anatomical ROIs (mean mask) to drug, sexual, and aversive cues, separately, between outcome groups and across task halves (3×2 ANOVA; 3 Groups [Good, INT, Poor]; 2 Halves [Half1, Half2], Figure 2). There was an interaction of Group × Halves in the drug (but not sex or aversive) cue condition (p < 0.05, FDR corrected). Follow-up t-tests (drug-cue condition only) revealed a significant decrease in the Good group from Half1 to Half2 (p < 0.05, FDR corrected), and there was a significant difference between the Good and Poor groups in the second half of the task (p < 0.05, FDR corrected), with the Good group having a lower response to repeated cues compared to the Poor group. Differences between both INT vs. Poor in Half2 and the INT (Half1) vs. INT (Half2) comparison did not survive correction.
Figure 2.
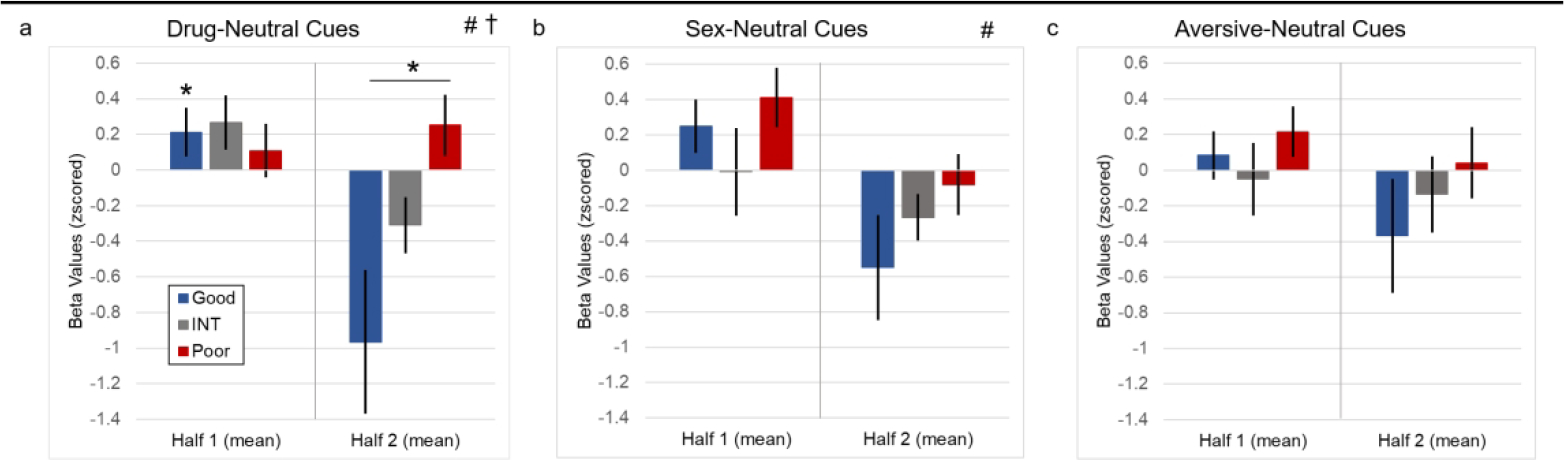
Mean brain response (via a priori regions, see Methods) to evocative (drug, sexual, and aversive) minus neutral cues displayed by outcome group across task halves. a) Mean to drug (-neutral) cues. There was an interaction of group × halves and a main effect of halves. There was a significant difference between the Good vs. Poor outcome groups in Half2 and a significant decrease in responding in the Good group from Half1 to Half2. b) Mean response to sex (-neutral) cues. There was a main effect of halves, in that all outcome groups generally had a decrease from Half1 to Half2. c) Mean response to aversive (-neutral) cues. No significant results were found. † significant interaction (FDR corrected, p < 0.05). # significant main effect of halves (FDR corrected, p < 0.05). * significant difference, post-hoc t-test (FDR corrected, p < 0.05). Good: outcome group with less than 40% positive cocaine urines in outpatient. Poor: outcome group with more than 85% positive cocaine urines in outpatient. INT: outcome group with percent positive urines in between Good and Poor outcome groups.
fMRI: Secondary Analysis
A priori regions of interest (aROIs) that composed the mean brain response to drug cues in the primary analysis (see above) were examined separately with 3×2 ANOVAs (Group [Good, INT, Poor] × Halves [Half1, Half2], Figure 3). There was a main effect of Halves in the AMYG, HIPP, FUSI, PCC, MB, and P-HIPP (all: p < 0.05, FDR corrected). There was a significant interaction in the FUSI (p < 0.05, FDR corrected). Main effects of group in the AMYG and PCC did not survive correction. Post-hoc t-tests revealed between-group and within-task differences. Specifically, Good and Poor groups differed significantly in the second half, with the Poor group having a higher response compared to the Good group in the AMYG, FUSI, and P-HIPP (all p < 0.05, FDR corrected). In addition, the Good group had a lower response to repeated cues (cocaine2-neutral2) compared to novel cues (cocaine1-neutral1) in the AMYG, FUSI, MB, and P-HIPP (all p < 0.05, FDR corrected). Interactions in the PCC and STG did not survive correction, Several between-group differences (Good vs Poor [Half2]: MB [p = 0.08], P-HIPP [p = 0.05]) and within-task differences (Half1 vs. Half2, Good and INT: MB [p = 0.08]) did not survive FDR correction. See supplemental figure (SF1) for results from all 13 ROIs.
Figure 3.
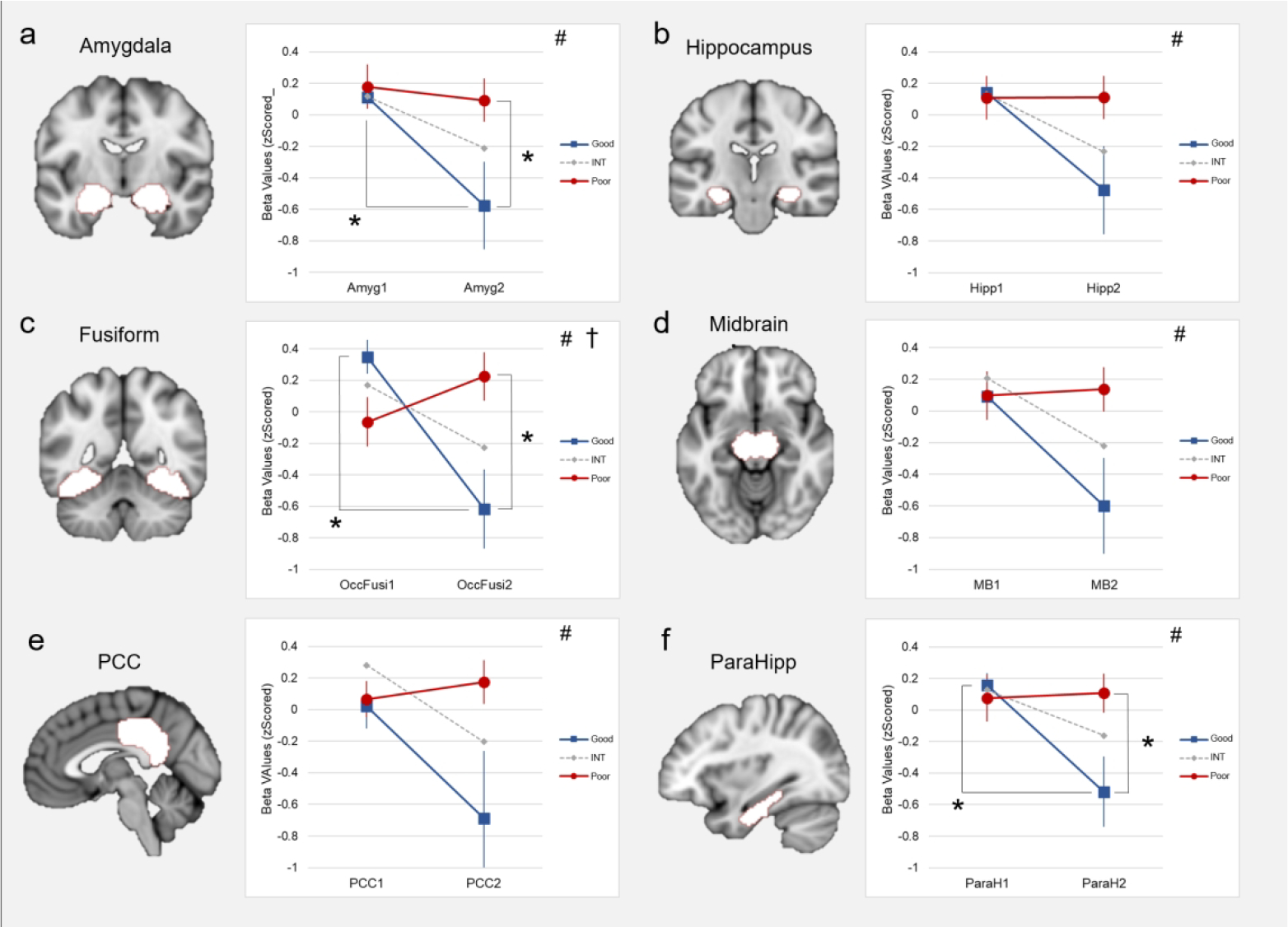
Changing dynamics of cue-response related to outcome groups and task halves in a priori regions that had significant results from ANOVA 3×2 (Group × Halves). Results in these regions similarly the Poor outcome group (red) with a sustained response from Half1 to Half2, the Good outcome group (blue) with a decreasing response from Half1 to Half2, and the INT group (grey) decreasing but ending somewhere between the Good and Poor groups. a) A main effect of halves was found in the amygdala, with post-hoc tests revealing differences between Good vs Poor groups in Half2 and a significant decrease from Half1 to Half2 in the Good outcome group. b) A main effect of halves was found in the hippocampus, but no significant post-hoc results. c) A significant main effect of halves and a Group × Halves interaction was found in the fusiform, with post-hoc tests revealing differences between Good vs Poor groups in Half2 and a significant decrease from Half1 to Half2 in the Good outcome group. d) A main effect of halves was found in the midbrain, but no significant post-hoc results. e) A main effect of halves was found in the post-cingulate cortex (PCC), but no significant post-hoc results. f) A main effect of halves was found in the parahippocampus, with post-hoc tests revealing a significant decrease from Half1 to Half2 in the Good group and a near-significant result (p = 0.05) between Good vs Poor outcome groups in Half2. † significant interaction (FDR corrected, p < 0.05). # significant main effect of halves (FDR corrected, p < 0.05). * significant difference, post-hoc t-test (FDR corrected, p < 0.05).
Discussion
This investigation in individuals with severe cocaine use disorder examined the relationship of the brain response to 500 msec brief, evocative cues with drug use outcomes (percent positive urines) obtained during 8 weeks of outpatient treatment. The primary analysis showed a significant interaction of the mean brain response (from a priori regions) between outcome groups (Good, INT, and Poor) to novel vs. repeated drug (but not sex or aversive) cues. Specifically, the group with a decreased brain response to repeated drug cues had better drug-use outcomes (Good group); in contrast, the group with a sustained response to repeated drug cues had worse drug-use outcomes (Poor group). Secondary analyses revealed the brain regions primarily driving these results, specifically the Good group significantly reduced responding to repeated cues in the amygdala (AMYG), visual association areas (e.g., fusiform, FUSI), and the parahippocampus (PARAH). This reduction, along with a sustained response to repeated cues, in the Poor group, resulted in significant differences between the Good and Poor groups in these regions. Similar differences in the midbrain (MB) did not survive correction (p = 0.08, see supplemental figure [SF1] for non-significant results). While the INT group generally exhibited a decrease to repeated cues, none of these differences either reached significance or survived FDR correction.
The results showing a relationship of the differential response to repeated drug cues in motivational, emotional, and sensory regions with drug-use outcomes suggest that the temporal approach used in this investigation may capture the multi-dimensional nature of the brain response, and its connection to real-world drug use. Additionally, the temporal approach (analyzing the task halves separately) revealed outcome relationships for the second half of the task – even though results for each task half was necessarily based on fewer data points. This underscores the potential importance of temporal effects within similar paradigms: the temporal effects may not only be strong enough to countervail a reduction in statistical power – they could actually be obscured by the common averaging of (twice as many) data points across the entire task. Indeed, the main effect of group (whole task results) in the drug-cue condition did not survive correction.
Intriguingly, the change in reactivity to cocaine cues from the first to second half of the task also showed a strong alignment with clinical outcome. As described, a small group of individuals with a decreased response to cues across the task halves had better drug use outcomes (Good group). Conversely, individuals with a pattern of sustained or increased responding (across the task halves) showed worse drug use outcomes (Poor group), and this pattern was evidenced by the majority of the cocaine patients. Using this metric of change is novel for cue studies and is a natural follow-on to examining the two task halves separately. Both approaches may reveal effects – and relationships to clinical outcome – that may be missed otherwise.
While results showed specificity of significant between-group differences in the brain response to repeated drug (but not sex or aversive) cues, the Good outcome group showed consistent decreases in brain response to all evocative cues, and the other groups were inconsistent. For instance, the INT group had a decreased response to repeated drug cues (but not sex or aversive), and the Poor group had a decreased response to repeated sexual cues (but not drug or aversive), suggesting the Good group exhibited a “normal” response to repeated cues eliciting an emotional response, while the INT and Poor groups did not.
The present results may be interpreted in the context of prior research on conditioned drug cues, extinction, and drug-cue exposure therapies43, possibly linked to drug seeking44. where researchers have demonstrated individual differences in extinction learning 45,46. Another possibility is the results could be related to research on the emotional response to repeated (non-drug) cues52, where a decrease in brain activation to repeated cues is the common, adaptive response of a healthy brain 47. Researchers have found evidence of individual differences in the changing brain response to repeated stimuli, in which a “maladaptive”, sustained (e.g., non-decreasing) brain response has been associated with pathology33–37, such as anxiety, autism, and schizophrenia.
The present results may inform and expand on additional areas of research. The brain response to drug cues (well-matched to the individual’s drug use history) offers an objective response in reward and motivational circuits that has been replicated across several drug classes3–15. However, most of this work has used a ‘magnitude’ measure for examining primary contrasts (e.g., drug vs. neutral) and for group comparisons – rather than temporal changes within the task. To our knowledge, the present study would be the first to find individual differences of a sustained (e.g., non-decreasing) brain response to evocative cues in a population with cocaine use (and possibly any substance use) disorder, wherein the sustained response is tied to more drug use.
In the current design, the observed non-decreasing response to drug cues (in the Poor outcome group) could reflect either a ‘failure of habituation’ or a ‘failure of extinction’, or both, and these of course are not mutually exclusive. The pattern of results – significant group × half interactions for the drug cue condition, but not for the aversive or sexual cue condition, does argue for the importance of extinction processes. Simplified designs that explicitly study both conditioning and extinction to drug-related cues, and that explicitly examine habituation to novel stimuli – can help parse the processes underlying the sustained brain response. Though not the focus of the study, worth noting is that specificity of results (between outcome groups) for repeated drug (and not sex or aversive) cues could be interpreted through the lens of research investigating the differential response to emotional cues (e.g., devaluation of natural cues)48,49.
While the exact mechanism of action and theoretical frame for the present results will benefit from further study, the extent of the group differences in the brain response to repeated cues between clinical outcome groups is clear. Significant differences occurred in several regions including AMYG; P-HIPP; MB, and FUSI. A recent investigation, utilizing connectome-based predictive modeling, found that large-scale networks were predictive of outcome 50. This recent evidence along with the present results suggest a potential biomarker developing treatments: a network of brain regions that could be targeted to facilitate reduction of brain response to repeated, evocative images. Future prospective studies could guide patients with temporal “sustained” cue responding toward interventions (pharmacotherapy, behavior, or neurostimulation) to normalize this (e.g., to restore ‘healthy’ extinction), with the goal of improved clinical outcomes
There are some limitations to the current study, suggesting areas for follow-on research. First, the majority of the participants were African-American males; studies in women and in other ethnicities will be useful to demonstrate generalizability. Further, as with many clinical outcome studies in the substance-use disorder population, treatment retention was often poor, even with incentives. Scaled, increasing incentives for study participation might improve future retention, and thus the number of available urines, for linking brain response to clinical outcomes.
Conclusions
Even though there are no FDA-approved medications for stimulant-use disorders, accruing evidence suggests that brain measures can reveal targets to speed the development of therapeutics. In the present study, in individuals with cocaine-use disorders, a sustained, non-decreasing brain response to repeated drug cues was associated with worse drug-use outcomes, while a decreasing brain response to repeated drug cues was associated with better drug-use outcomes. The evidence suggests the dynamics of cue-reactivity may themselves constitute a biomarkers of relapse vulnerability. The current study in those struggling with stimulant use also adds to the wider literature highlighting these dynamics both as a potential indicator of pathology, and as a meaningful clinical target for therapeutic intervention.
Supplementary Material
Footnotes
Financial Interests of Conflicts of Interest: None
Bibliography
The data that support the findings of this study are available from the corresponding author upon reasonable request.
- 1.CDC. Multiple Cause of Death: 1999–2016. Center for Disease Control, National Center for Health Statistics; 2017. http://wonder.cdc.gov [Google Scholar]
- 2.Sinha R New Findings on Biological Factors Predicting Addiction Relapse Vulnerability. Curr Psychiatry Rep. 2011;13(5):398. doi: 10.1007/s11920-011-0224-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic Activation During Cue-Induced Cocaine Craving. Am J Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Childress AR, Ehrman RN, Wang Z, et al. Prelude to Passion: Limbic Activation by “Unseen” Drug and Sexual Cues. PLOS ONE. 2008;3(1):e1506. doi: 10.1371/journal.pone.0001506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franklin TR, Wang Z, Wang J, et al. Limbic Activation to Cigarette Smoking Cues Independent of Nicotine Withdrawal: A Perfusion fMRI Study. Neuropsychopharmacology. 2007;32(11):2301–2309. doi: 10.1038/sj.npp.1301371 [DOI] [PubMed] [Google Scholar]
- 6.Langleben DD, Ruparel K, Elman I, et al. Acute Effect of Methadone Maintenance Dose on Brain fMRI Response to Heroin-Related Cues. Am J Psychiatry. 2008;165(3):390–394. doi: 10.1176/appi.ajp.2007.07010070 [DOI] [PubMed] [Google Scholar]
- 7.Wetherill RR, Childress AR, Jagannathan K, et al. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology (Berl). 2014;231(7):1397–1407. doi: 10.1007/s00213-0133342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regier PS, Monge ZA, Franklin TR, et al. Emotional, physical and sexual abuse are associated with a heightened limbic response to cocaine cues. Addict Biol. Published online September 22, 2016. doi: 10.1111/adb.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkow ND, Wang G-J, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci Off J Soc Neurosci. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70(8):785–793. doi: 10.1016/j.biopsych.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33(7):1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- 12.Bonson KR, Grant SJ, Contoreggi CS, et al. Neural Systems and Cue-Induced Cocaine Craving. 2002;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2 [DOI] [PubMed] [Google Scholar]
- 13.Grant S, London ED, Newlin DB, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93(21):12040–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo D, Lacadie CM, Tuit K, Hong K-I, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70(7):727–739. doi: 10.1001/jamapsychiatry.2013.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaine SK, Wemm S, Fogelman N, et al. Association of Prefrontal-Striatal Functional Pathology With Alcohol Abstinence Days at Treatment Initiation and Heavy Drinking After Treatment Initiation. Am J Psychiatry. 2020;177(11):1048–1059. doi: 10.1176/appi.ajp.2020.19070703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courtney KE, Schacht JP, Hutchison K, Roche DJO, Ray LA. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. 2016;21(1):3–22. doi: 10.1111/adb.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moeller SJ, Paulus MP. Toward biomarkers of the addicted human brain: Using neuroimaging to predict relapse and sustained abstinence in substance use disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(Pt B):143–154. doi: 10.1016/j.pnpbp.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moningka H, Lichenstein S, Worhunsky PD, DeVito EE, Scheinost D, Yip SW. Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2019;44(2):259–273. doi: 10.1038/s41386-018-0232-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. IMAGING STUDY: Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12(3–4):503–512. doi: 10.1111/j.1369-1600.2007.00075.x [DOI] [PubMed] [Google Scholar]
- 20.Janes AC, Pizzagalli DA, Richardt S, et al. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biol Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck A, Wüstenberg T, Genauck A, et al. Effect of Brain Structure, Brain Function, and Brain Connectivity on Relapse in Alcohol-Dependent Patients. Arch Gen Psychiatry. 2012;69(8):842–852. doi: 10.1001/archgenpsychiatry.2011.2026 [DOI] [PubMed] [Google Scholar]
- 22.Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude – An activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50(7):1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 23.Versace F, Engelmann JM, Robinson JD, et al. Prequit fMRI Responses to Pleasant Cues and Cigarette-Related Cues Predict Smoking Cessation Outcome. Nicotine Tob Res. 2014;16(6):697–708. doi: 10.1093/ntr/ntt214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Li W, Wang H, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: an event-related functional magnetic resonance imaging study. Addict Biol. 2015;20(5):968–978. doi: 10.1111/adb.12182 [DOI] [PubMed] [Google Scholar]
- 25.Reinhard I, Leménager T, Fauth-Bühler M, et al. A comparison of region-of-interest measures for extracting whole brain data using survival analysis in alcoholism as an example. J Neurosci Methods. 2015;242:58–64. doi: 10.1016/j.jneumeth.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 26.MacNiven KH, Jensen ELS, Borg N, Padula CB, Humphreys K, Knutson B. Association of Neural Responses to Drug Cues With Subsequent Relapse to Stimulant Use. JAMA Netw Open. 2018;1(8):e186466–e186466. doi: 10.1001/jamanetworkopen.2018.6466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71(3):517–529. doi: 10.1016/S0091-3057(01)00682-7 [DOI] [PubMed] [Google Scholar]
- 28.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl). 2003;168(1–2):3–20. doi: 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- 29.Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc B Biol Sci. 2008;363(1507):3233–3243. doi: 10.1098/rstb.2008.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namba MD, Tomek SE, Olive MF, Beckmann JS, Gipson CD. The Winding Road to Relapse: Forging a New Understanding of Cue-Induced Reinstatement Models and Their Associated Neural Mechanisms.Front Behav Neurosci. 2018;12. doi: 10.3389/fnbeh.2018.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosten TR, Scanley BE, Tucker KA, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2006;31(3):644–650. doi: 10.1038/sj.npp.1300851 [DOI] [PubMed] [Google Scholar]
- 32.Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Brady KT. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend. 2013;131(1–2):44–49. doi: 10.1016/j.drugalcdep.2013.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ousdal OT, Andreassen OA, Server A, Jensen J. Increased amygdala and visual cortex activity and functional connectivity towards stimulus novelty is associated with state anxiety. PloS One. 2014;9(4):e96146. doi: 10.1371/journal.pone.0096146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avery SN, Blackford JU. Slow to warm up: the role of habituation in social fear. Soc Cogn Affect Neurosci. 2016;11(11):1832–1840. doi: 10.1093/scan/nsw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52(1):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinhans NM, Richards T, Greenson J, Dawson G, Aylward E. Altered Dynamics of the fMRI Response to Faces in Individuals with Autism. J Autism Dev Disord. 2016;46(1):232–241. doi: 10.1007/s10803-015-2565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams LE, Blackford JU, Luksik A, Gauthier I, Heckers S. Reduced habituation in patients with schizophrenia. Schizophr Res. 2013;151(1–3):124–132. doi: 10.1016/j.schres.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young KA, Franklin TR, Roberts DCS, et al. Nipping Cue Reactivity in the Bud: Baclofen Prevents Limbic Activation Elicited by Subliminal Drug Cues. J Neurosci. 2014;34(14):5038–5043. doi: 10.1523/JNEUROSCI.4977-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam SCB, Wang Z, Li Y, et al. Wavelet-transformed temporal cerebral blood flow signals during attempted inhibition of cue-induced cocaine craving distinguish prognostic phenotypes. Drug Alcohol Depend. 2013;128(1):140–147. doi: 10.1016/j.drugalcdep.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–33. [PubMed] [Google Scholar]
- 41.Lang PJ, Bradley MM, Cuthbert BN, others. International affective picture system (IAPS): Instruction manual and affective ratings. Cent Res Psychophysiol Univ Fla. Published online 1999. [Google Scholar]
- 42.Regier PS, Teitelman AM, Jagannathan K, et al. Women at Greater Sexual Risk for STIs/HIV Have a Lower Mesolimbic and Affective Bias Response to Sexual Stimuli. Front Behav Neurosci. 2020;13. doi: 10.3389/fnbeh.2019.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunsmoor JE, Niv Y, Daw N, Phelps EA. Rethinking Extinction. Neuron. 2015;88(1):47–63. doi: 10.1016/j.neuron.2015.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broos N, Diergaarde L, Schoffelmeer AN, Pattij T, De Vries TJ. Trait Impulsive Choice Predicts Resistance to Extinction and Propensity to Relapse to Cocaine Seeking: A Bidirectional Investigation. Neuropsychopharmacology. 2012;37(6):1377–1386. doi: 10.1038/npp.2011.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bush DEA, Sotres-Bayon F, LeDoux JE. Individual differences in fear: Isolating fear reactivity and fear recovery phenotypes. J Trauma Stress. 2007;20(4):413–422. doi: 10.1002/jts.20261 [DOI] [PubMed] [Google Scholar]
- 46.Lonsdorf TB, Kalisch R. A review on experimental and clinical genetic associations studies on fear conditioning, extinction and cognitive-behavioral treatment. Transl Psychiatry. 2011;1(9):e41. doi: 10.1038/tp.2011.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plichta MM, Grimm O, Morgen K, et al. Amygdala habituation: A reliable fMRI phenotype. NeuroImage. 2014;103:383–390. doi: 10.1016/j.neuroimage.2014.09.059 [DOI] [PubMed] [Google Scholar]
- 48.Versace F, Engelmann JM, Jackson EF, et al. Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers. Eur J Neurosci. 2011;34(12):20542063. doi: 10.1111/j.1460-9568.2011.07915.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral, and cigarette-related stimuli in smokers. Addict Biol. 2011;16(2):296–307. doi: 10.1111/j.1369-1600.2010.00273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yip SW, Scheinost D, Potenza MN, Carroll KM. Connectome-Based Prediction of Cocaine Abstinence. Am J Psychiatry. 2019;176(2):156–164. doi: 10.1176/appi.ajp.2018.17101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


