Abstract
Background
Alcohol use disorders (AUD) are prevalent worldwide. Animal model research on alcohol withdrawal tends to focus on traditional anxiety/stress tests. While this has been essential, heavy alcohol use can trigger adverse withdrawal-related affective states that affect responses to a large variety of life events and stressors. To this end, we show that behaviors in a variety of tasks which differ in task demand and intensity are altered during withdrawal in male and female mice after voluntary alcohol access.
Methods
6-weeks of intermittent two-bottle choice alcohol consumption followed by behavioral tests in male and female mice. Home-cage: low stress baseline environment to measure spontaneous natural behaviors. Open Field: anxiety inducing bright novel environment. Looming disc: arena with a protective hut where mice are exposed to a series of discs that mimic an overhead advancing predator. Robogator-simulated predator task: forces a foraging behavioral choice in the presence of an advancing robot predator that ‘attacks’ when mice are near a food pellet in a large open arena.
Results
A history of alcohol impacted behaviors in these tasks in sex dependent manners. In the home-cage, alcohol induced reductions in digging and heightened stress-coping through an increase in grooming time. In males, increased rearing suggests increased vigilance/exploration in a familiar environment. The open field test revealed an anxiety phenotype in both male and female alcohol mice. Males showed no behavioral alterations to the looming disc task, while female alcohol mice showed greater escape responses compared to water controls, indicative of active stress response behaviors. In males, the robogator task revealed a hesitant/avoidant phenotype in alcohol history mice under increased task demands.
Conclusions
Few drugs have advanced past clinical trials for the treatment of AUD and withdrawal. Understanding how withdrawal alters a variety of behaviors in both males and females that are linked to stress coping can widen our understanding of alcohol abuse and lead us closer to better therapeutics to help individuals with AUD.
Keywords: Alcohol, Withdrawal, Home-cage
Introduction
Alcohol Use Disorders (AUD) affect approximately 14.5 million people in the United States (SAMHSA, 2019). The stress associated with negative affective states during withdrawal is thought to play a role in the resumption of alcohol use and thus in the perpetuance of AUDs (Becker, 2012; Blaine et al., 2019; Blaine and Sinha, 2017; Heilig et al., 2010; Magrys et al., 2013). This ‘dark side of addiction’ (Koob and Le Moal, 2005) has led to extensive work using animal models on how stress drives drinking patterns with a goal of finding targets for treatments that reduce alcohol consumption (Deal et al., 2018; Haun et al., 2022; Koob and Mason, 2016; Lê et al., 2011; Newman et al., 2018). In comparison, relatively little is known about how a history of alcohol use affects stress responses despite the growing evidence in human studies on alcohol-associated alterations to Hypothalamic-Pituitary-Adrenal (HPA) axis physiological and behavioral responses to stress (Blaine et al., 2019; Blaine and Sinha, 2017; Metzger et al., 2018; Sinha et al., 2009). All organisms respond to stress by marshaling physiological responses (i.e., changes in hormones and heart rate) and context-appropriate behaviors (i.e., flight/freeze response to acute stress, exploration in food-seeking, self-grooming in rodents for de-arousal after a stressor) that depend on the stressor, arousal level, and the species (Andrew, 1974; Bracha, 2004; Pankevich and Bale, 2008; Veloso et al., 2016). The behavioral impact of alcohol withdrawal on stress, both generalized and stressor specific, has not been heavily explored.
Recent preclinical studies investigating negative affective states during withdrawal from recurring voluntary alcohol consumption show behavioral effects with considerable variability in findings using traditional stress/anxiety tests (Bloch et al., 2022, 2020; Kliethermes, 2005). This could be due to differences in drinking paradigms that result in distinct intake patterns and overall consumption or the examination of different withdrawal timepoints, which can also affect behavior. Recent analyses, however, have questioned the validity of commonly used behavioral approaches for assessing negative affect in rodents, arguing that their reliance on anthropomorphism undermines their relevance and consistency (Kliethermes, 2005; Lezak et al., 2017). In step with this criticism, elements of behavioral research have moved towards using etho-experimental approaches to behavioral assessment which might prove useful in investigating stress and anxiety-like behaviors in alcohol withdrawal. This includes detailed analysis of spontaneous, natural behavior patterns (i.e. grooming, rearing, digging, locomotion) and ethologically and species relevant behavioral tasks, such as those that drive behavioral choices in the presence of threats or hunger (Blanchard et al., 1993; Blanchard and Blanchard, 1990; Lezak et al., 2017; Richter, 2020). Here we focus on spontaneous home-cage behaviors in mice (Füzesi et al., 2016), as well as two ethologically and species-relevant tests for examining innate defensive behavior: the looming disc (Daviu et al., 2020), and the robogator-simulated predator task (Choi and Kim, 2010), to determine how stress coping and stress response behaviors are altered in mice during forced withdrawal from six weeks intermittent two-bottle choice alcohol consumption.
Materials and Methods
Animals
Group housed male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were delivered at 6–8 weeks of age and maintained on a 12-h reverse dark/light cycle. After 5–7 days habituation to the animal facility, mice were single housed in polycarbonate (GM500, Tecniplast, Italy) Plexiglas cages, given an additional 5–7 days to adjust to the housing conditions, and assigned to the water control or two-bottle choice (2BC) alcohol access group. Subjects were matched for age and weight (N = 98 males: N = 73 females). Unless otherwise specified, mice were given ad libitum access to water and Isopro RMH 3000 chow (LabDiet, St. Louis, MO). Behavioral experiments were conducted during the dark cycle and with the room lights off, and males and females were run separately. The Institutional Animal Care and Use Committee at UNC Chapel Hill approved all experimental procedures, which were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Two-Bottle Choice Alcohol Access
For six weeks, alcohol mice were given 24-hour access to water and a 20% (w/v) alcohol solution (water and 95% ethanol: Pharmaco-AAPER, Brookfield, Connecticut) on Mondays, Wednesdays, and Fridays for a total of 18 alcohol drinking sessions. Both water and alcohol bottles were weighed before and after each 24-hour drinking session, and the averaged value from an empty ‘drip’ cage was subtracted from the daily drinking value. g/kg consumption was determined with this formula: ((grams solution consumed/0.968)*0.2)/(body weight in grams/1000) All mice had unrestricted access to water. Ten male mice used in Experiment 2 had brief interruptions to the alcohol paradigm and only received 16–17 drinking sessions due to COVID-19 related experimental issues.
Experiment 1: Validation of alcohol drinking paradigm
Four hours into the final alcohol drinking session, mice were anesthetized in a small isoflurane chamber, removed from the animal facility, and sacrificed. Trunk blood was collected, and blood samples were centrifuged at 4 °C for 10 min at 3000 RCF for plasma separation. Blood ethanol content (B.E.C.; mg/dL) was analyzed immediately with a 5-μL plasma sample using a Model AM1 Alcohol Analyser (Analox Instruments Ltd., Lunenburg, Massachusetts).
Experiment 2: Effects of alcohol on home-cage and open field behaviors
24–36 hours after the final water or alcohol drinking session, mice were placed on a cart, covered, and transferred to the behavioral testing room. After a minimum of 40-mins acclimation to the room, mice were either left undisturbed in their home-cage (No-Stress) or restrained for 2-hours (Stress). Immediately following the No-Stress or Stress conditions, mice were subjected to Home-cage and Open Field tests.
Restraint Stress
Mice were scruffed and placed in a well-ventilated 50mL conical centrifuge tube (Thermo Scientific, Fisherbrand, Waltham, Massachusetts) for two hours in a dark enclosed space. Crumpled paper towels were placed in between the mouse and the cap of the tube to further restrict movement.
Home-cage
The home-cage was cleared of all items but bedding. No-Stress mice were picked up and placed back in the home-cage and Stress mice were removed from restraint tube and placed back in their home-cage. The cages were then moved to a low light (~60 lux) enclosed space with a webcam placed above to film behavior for 15 minutes.
Open Field
Immediately following the home-cage behavioral test, mice were placed in a white Plexiglas open field (50 × 50 × 25 cm) and allowed to explore the arena for 5 minutes. Light levels in the center of the open field were approximately 300 lux.
DeepLabCut and SimBA
This behavioral experiment was conducted by multiple researchers in separate cohorts. The final male cohort was filmed using higher resolution cameras (C930e Webcam, Logitech, Lausanne, Switzerland) compatible with automatic behavioral analysis pipelines using machine learning. Therefore, we used these videos to characterize the utility of machine learning for the analysis of the most displayed spontaneous home-cage behaviors of digging, rearing, and grooming. The blinded scoring of behavior using BORIS (Friard and Gamba, 2016) as well as the locomotor activity using EthoVision (Noldus Information Technologies, Wageningen, Netherlands) was compared with data derived from Simple Behavioral Analysis (SimBA; https://github.com/sgoldenlab/simba) and DeepLabCut (DLC; https://github.com/DeepLabCut/DLCutils) respectively. We followed the extensive existing GitHub documentation for setup and function of these programs and developed our own tracking DLC (Mathis et al., 2018) project for behavioral pose estimation and used this tracking information for the creation of behavioral classifiers in SimBA (Nilsson et al., 2020), a separate, open source program for identifying specific behaviors.
Experiment 3: Effects of alcohol on the looming disc task
Twice during the final week of water or alcohol access, mice were placed on a covered cart and brought to the testing room on alcohol “off” days. After a minimum of 40-min acclimation to the testing room in the home-cage, mice were habituated to the looming disc environment (a 41 × 19 × 20.5 cm mirrored floor plastic arena with a protective hut (13 × 12 × 10cm) in one corner) for 20-minutes per day. A computer monitor (22er 21.5-inch LED Backlit, HP inc., Palo Alto, California) was placed above the arena with a light grey background that produced an ambient light of approximately 170 lux. 6–8 hours after the final water or alcohol drinking session, mice were acclimated to the testing room and subjected to the looming disc test (Daviu et al., 2020). In females, the video froze, and we lost the data from one loom reaction from an alcohol exposed mouse.
Looming Disc
Mice were given 3 minutes in the looming disc arena for habituation. Following that period, a loom was triggered when the mouse was not in the hut or heading towards the hut. A loom was then triggered an additional 4 times, with at least thirty seconds elapsing between each loom. The loom consists of a 2 cm black disc on a light grey background. The disc is first presented for 3 seconds, and then expands to its full 20cm size over 2 seconds. The 20cm disc stays on the screen for an additional 3 seconds and disappears. A blinded individual analyzed mouse behavior, with the criteria of escape entailing reaching the hut within the 8 second loom presentation and freeze being the absence of movement other than breathing for at least 1 second total during loom presentation.
Experiment 4: Effects of alcohol on the robogator predator task
We began this task three days after the final alcohol or water drinking session. Based on previous experiments using a moving predator-like Lego robot, or robogator (Choi and Kim, 2010), male mice were given chocolate sucrose pellets (Dustless Precision Pellets, Bio-Serv, Flemington, NJ) daily for 3–4 days in their home-cage to reduce neophobia. Mice were then food deprived for approximately 18 hours prior to habituation day and kept at 85% or more of their original weight throughout the entire test period by food restriction. Food was given immediately after the mouse finished each task. Criteria for moving past training to test day was to retrieve the pellets by 300 seconds each. One mouse from the alcohol access group was excluded due to health concerns and one water mouse was excluded for failure to reach the criteria for entering test day. This experiment was conducted in female mice using a different approach with higher stress conditions; thus, only male data is included in this manuscript.
Robogator Test
Habituation –
To associate the arena with food foraging and to habituate and introduce the mice to the nest and field (nest: 8 × 19.5 × 24 inches, field: 40 × 19.5 × 24 inches), mice were first allowed to explore the nest for 10 minutes and then a gate was raised to give access to the field. Mice were then given unrestricted access to the entire arena for an additional 20 minutes. Food pellets were dispersed randomly throughout the arena.
Baseline Training –
For 4 days, mice were restricted in the nest for 1 minute and trained to retrieve a pellet from each of the 30cm, 60cm, and 90cm distances from the nest.
Test Day –
Mice were restricted to the nest for 1 minute and then given access to the arena for 3 minutes to retrieve a pellet in the presence of the robogator (LEGO MINDSTORMS EV3, The Lego Group, Billund, Denmark), which moved forward, snapped its “jaws” 3 times, and moved back when the mouse was approximately 30cm or closer to the food pellet. All mice began with the first trial (pellet was placed 30cm from the nest). Subsequent trials depended on the success of the mouse in retrieving a pellet. If successful, the pellet was moved 15cm further away from the nest (45cm, 60cm, 75cm, 90cm). If a mouse failed, the trial began with the pellet 15cm closer to the nest (15cm, 0cm). Trials continued until the maximum foraging distance (MFD) was determined. The MFD was the final distance from the nest entrance (1cm, 15cm, 30cm, 60cm, 75cm, or 90cm) where each mouse was successful in obtaining a pellet in less than 3 minutes. To account for the time spent obtaining the final pellet, the fear index (MFD / time to pellet) is used as a measurement of hesitancy to pellet. The pellet was moved back to its original spot in the center of the field if it was accidentally moved by the mouse as it responded to the robogator.
Statistics
To determine escalation of alcohol intake and preference, repeated measures (RM) mixed-effects model (as some data points were missing) were conducted followed by post-hoc tests to compare each session to session 1 within each sex. Experiment 1 aimed to test if males and females differed in alcohol intake and BEC during early timepoints of an alcohol session, thus, a two-way ANOVA (Sex × Time) and independent sample t-test (Sex) was conducted respectively. Experiment 2 had three main conditions: Sex (Male vs Female), Alcohol (Alcohol vs Water), and Stress (No-Stress vs Stress). Importantly, the assumption of Homogeneity was violated in home-cage parameters (Levene’s Test for Equality of Variances: Groom, Rear), due to the differences in variance between No-stress and Stress conditions. Due to this violation as well as our main interest in this manuscript to understand how a history of alcohol affects subsequent behaviors, we split up No-Stress and Stress data, and analyzed them using two-way ANOVAs for the conditions of Sex and Alcohol. To explore the relationship between hand-scored and machine learning assisted data, simple linear regressions were performed. Experiment 3 consisted of non-parametric data; thus, Fisher’s exact tests were used to assay loom reactions within each sex. Experiment 4 had one Main condition (Alcohol) and one repeated factor (Training days), thus two-way RM ANOVAs and two-tailed t-tests were conducted when appropriate. We also conducted a survival analysis, an epidemiological statistical method that can be used to understand animal behavior (Asher et al., 2017), on the robogator MFD. A MFD of 1cm −75cm was counted as a ‘death’, while mice foraging up to the final distance of 90cm were counted as ‘surviving’. For all data sets, a p-value of less than 0.05 was considered significant and when post-hoc tests were conducted, Šídák corrected multiple comparisons were performed. When assumption of sphericity was violated, the Geisser-Greenhouse correction was conducted. Data is shown as individual values with mean and standard error of the mean. When individual values are not shown, data is shown as mean and 95% confidence interval. Statistical analyses were conducted using JASP 0.16.1 (JASP Team) and Prism 9.2.0 (GraphPad Software Inc, San Diego, CA).
Results
Experiment 1
Our interest lies in understanding how voluntary alcohol intake affects subsequent behaviors during withdrawal. Therefore, we first explored the six-week intermittent two-bottle choice (2BC) paradigm by examining whether mice escalated their intake and preference for alcohol, if alcohol parameters differed between males and females, and whether this paradigm would induce pharmacologically relevant >80mg/dl binge-level B.E.C.s, which we measured in a subset of mice 4-hours into the final, 18th alcohol session. This timepoint was chosen because an examination of intake during the first 4 hours in the preceding days show that some male alcohol mice do not begin drinking until the 2-hour mark of a 24-hr session (Fig 1D). We first pooled together the daily intake and preference values for every mouse (excluding the 18th session of experiment 1 mice that only drank for 4hrs; N= 52 males and 43 females) and, using a mixed effects model, found a significant main effect of sex and time, as well as an interaction effect, in intake (Fig 1B: Fsex[1,93] = 125.5, p<.001; Ftime[9.094, 831.8] = 9.131, p<.001; FtimeXsex[17,1555] = 3.229, p<.001) and preference for alcohol (Fig 1C: Fsex[1,95] = 12.79, p<.001; Ftime[11.87, 1109] = 34.86, p<.001; FtimeXsex[17,1588] = 2.950, p<.001). Post-hoc tests comparing each sexes session to the initial session indicated an escalation of intake in both males and females, though the effect was larger in females, whose every session except for session 2,3 and 18 (p=.084) was statistically different to session 1, whereas in males, sessions 2,3,4,10,14,17 (p=.060), and 18 (p=.085) did not differ to session 1. The effect of escalation of preference was large for both males and females, as only session 2 and 3 in males and session 2 (p=.051) in females indicated a lack of effect when compared to session 1.
Figure 1:
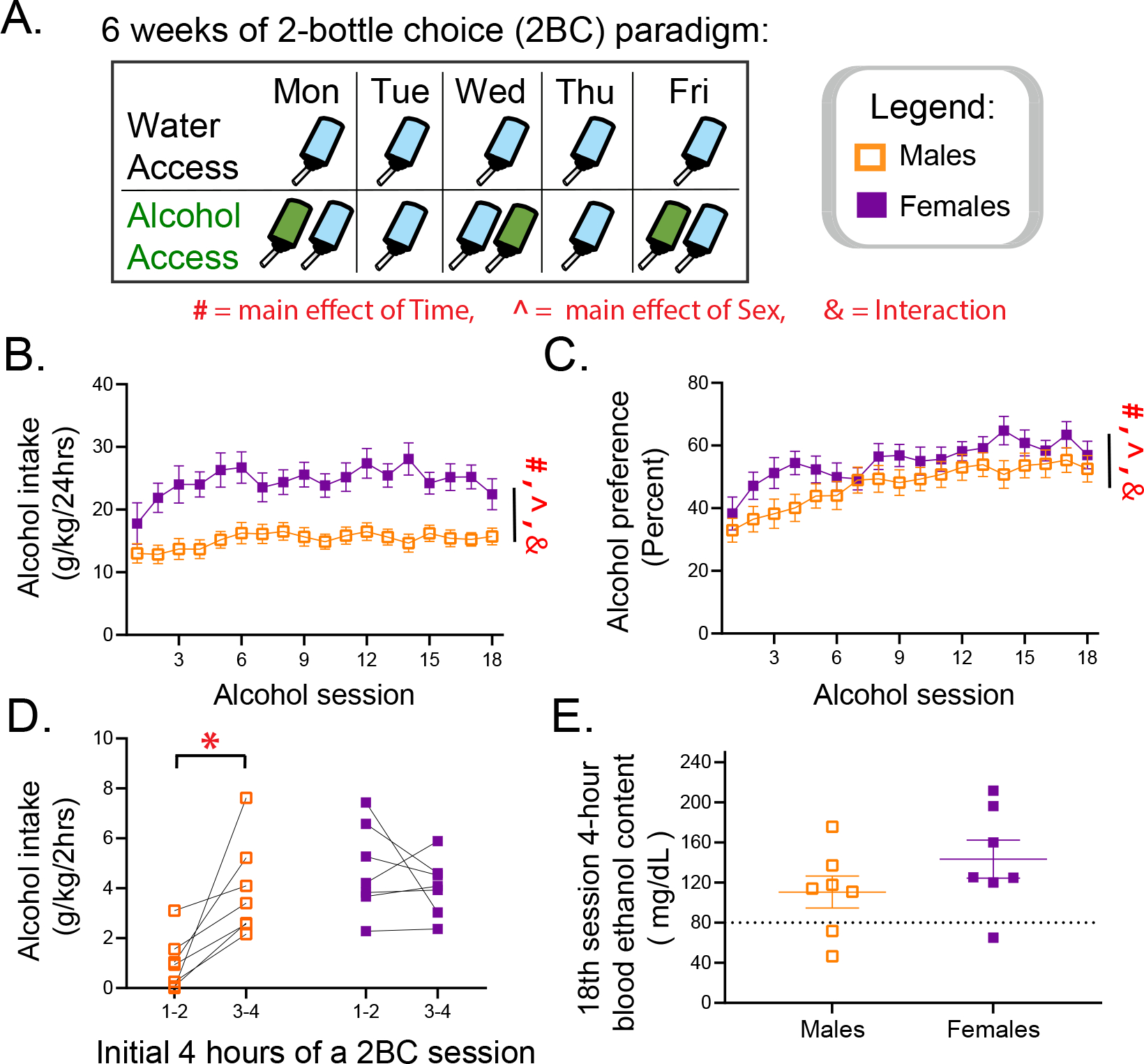
6-week two-bottle choice (2BC) alcohol access with forced 24–48hr withdrawals leads to distinct drinking patters in male and female mice. (A) 2BC schedule. (B) Daily intake and (C) preference over the 18 alcohol sessions for all experimental mice used. Experiment 1: (D) individual mouse 2hr-alcohol intake measurements within the initial 4hrs of a 2BC session and (E) Blood ethanol content four hours into the final, 18th alcohol session. # = Main effect of Time. ^ = Main effect of Sex. & = Interaction. Šídák Post hoc tests: * p<.05.
In a subset of these mice that were used in experiment 1, we observed alcohol intake within 2hr blocks at two early time points (hour 1–2, and hour 3–4) in an alcohol session during the final week of exposure to determine sex effects and mice variability in consumption. We hypothesized mice in the intermittent 2BC paradigm binge drink in the first few hours, particularly in the first 2hrs at the onset of a session. As expected, a main effect of sex emerged in a two-way repeated measures ANOVA, as well as a sex by time interaction (Fig 1D: Fsex[1,12] = 10.81, p<.01; Ftime[1,12] = 3.917, p=.071; FsexXtime[1,12] = 10.18, p<.01). Contrary to our hypothesis, some male mice did not drink in the first two hours, and post-hoc tests indicated that male mice drank more in hour 3–4 than hour 1–2 (Hour 1–2 vs Hour 3–4, p =.007), while female mice drank similar amounts in hour 1–2 than 3–4 (Hour 1–2 vs Hour 3–4, p =.650). Finally, most mice regardless of sex (tSex(12)=1.323, p=.68) attained excessive alcohol consumption levels (Fig 1E), defined as binge drinking (above 80mg/dl B.E.C. levels) in a 4hr alcohol session on the final day of access. Overall, despite large differences in drinking patterns between sexes and variability within subjects (from 46.6 to 211.7 B.E.C. levels achieved), this paradigm does lend itself to excessive alcohol consumption and is effective for testing how voluntary alcohol intake affects the brain and body.
Experiment 2
We sought to determine how behaviors in the home-cage and open field tests were impacted by withdrawal from alcohol as well as immediately following an acute stressor (Fig 2A). Spontaneous home-cage behaviors are altered after stress and may reflect stress coping. These behaviors are observed following a stressor in a familiar home environment rather than during the stressor to determine how preceding behavioral manipulations impact natural behavior patterns in rodents’ post-stress. Thus, observing behaviors in the home cage point to how rodents cope with stress rather than how they react to immediate stressors. In fact, grooming in the home-cage is frequently observed after stress in rodents and is modulated by stressor intensity/arousal levels (Dunn et al., 1987; Fentress, 1988; Fernández-Teruel and Estanislau, 2016; Füzesi et al., 2016). Restraint stress is known to potentiate anxiety-like behaviors during withdrawal from alcohol (Breese et al., 2005, 2004), and is well-characterized to induce physiological and behavioral stress responses (Keim and Sigg, 1976; Paré and Glavin, 1986; Sutherland et al., 2010), thus, a 2hr restraint stress was selected for experiment 2 to determine stress-coping behaviors in the home-cage.
Figure 2:
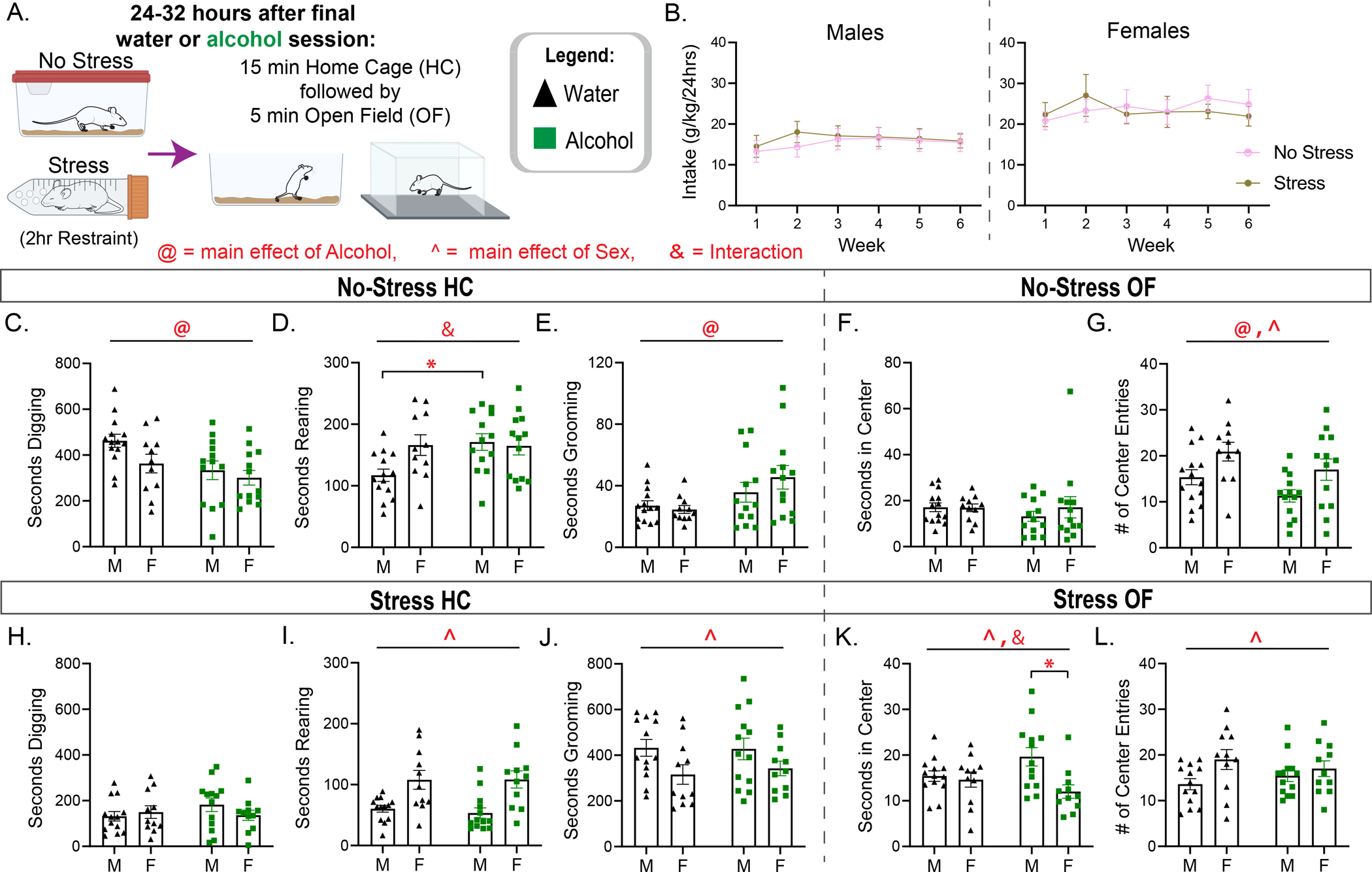
Alcohol withdrawal drives basal alterations to home-cage and open field behaviors in males and females while restraint stress drives alterations to behavior regardless of alcohol history. (A) Experimental design. (B) Alcohol intake in male and female mice. In No-stress mice, Home Cage (HC) test: (C) Seconds spent digging, (D) seconds spent rearing, (E) seconds spent grooming. In No-stress mice, Open Field (OF) test: (F) Seconds spent in the center and (G) number of center entries. In Stress mice, HC test: (H) Seconds spent digging, (I) seconds spent rearing, (J) seconds spent grooming. In Stress mice, OF test: (K) Seconds spent in the center and (L) number of center entries. @ = Main effect of Alcohol. ^ = Main effect of Sex. & = Interaction. Šídák Post hoc tests: * p<.05.
Our main interest in this manuscript is in understanding how alcohol history affects behavior, therefore, we conducted separate analyses on No-stress and Stress mice from experiment 2 to examine alcohol effects within each condition. Analyses in home-cage No-stress mice indicated a main effect of Alcohol on digging time (Fig 2C: Falcohol[1, 47] = 7.220, p=.010), no main effect of Sex (Fsex[1, 47] = 3.437, p=.070), and no interactions (FsexXalcohol[1, 47] = 0.8904, p=.350). There were no main effects for time spent rearing (Fig 2D; Falcohol[1, 47] = 3.744, p=.059; Fsex[1, 47] = 2.448, p=.124), however an interaction emerged (FsexXalcohol[1, 47] = 4.081, p<.05). Post-hoc tests indicated that male water and alcohol exposed mice were statistically distinct, with alcohol males rearing for longer (p = 0.035). Finally, only an Alcohol effect was seen in grooming time (Fig 2E: Falcohol[1, 48] = 7.240, p=.010; Fsex[1, 47] = 0.4306, p=.515; FsexXalcohol[1, 47] = 1.244, p=.270). Alcohol exposure thus drove a reduction in digging and an increase in grooming time regardless of sex and an increase in rearing time in males only. The home-cage effects in No-stress mice were not likely due to differences in locomotion, as there were no main effects of alcohol or interaction effects in distance moved (Supplementary Fig 1A: Falcohol[1, 47] = 1.615, p=.210; FalcoholXsex[1, 47] = 1.974, p=.167), although interestingly, a sex effect emerged showing females move significantly more than males (Fsex[1, 47] = 17.33, p<.001). We next wanted to explore how behaviors are affected in a more widely used test to assess aspects of anxiety-like behavior, the Open Field (OF). There were no effects in the time spent in the center of the OF (Fig 2F), but center entries (Fig 2G) were affected by both Sex (Fsex[1, 47] = 9.153, p<.01) and Alcohol history (Falcohol[1, 47] = 4.573, p<.05). No interactions were seen between alcohol and sex in the OF. Alcohol and Sex both affected the distance moved in the open field test (Supplementary Fig 1B: Falcohol[1, 47] = 8.418, p<.01; Fsex[1, 47] = 5.152, p<.05), with alcohol driving a reduction in locomotion while females in general moved more. Overall, alcohol history drove a reduction in locomotion and center entries regardless of sex. In Stressed mice, alcohol history did not affect digging, rearing or grooming behaviors in the home-cage (Fig 2H–J), while a main effect of Sex emerged for rearing (Fig 2I: Fsex[1, 44] = 21.36, p<.001) and grooming (Fig 2J: Fsex[1, 44] = 6.113, p<.05) time. Alcohol history also did not affect OF behaviors for Stressed mice (Fig 2K–L), while Sex effects once again emerged for both center time (Fig 2K: Fsex[1, 44] = 6.677, p<.05) and center entries (Fig 2L: Fsex[1, 44] = 4.716, p<.05). An interaction effect (Fig 2K: FalcoholXsex[1, 44] = 4.423, p<.05) was also evident in the center time, and post-hoc tests indicated this was driven by a significant difference between alcohol mice, with females spending less time in the center than males (p=.011). A Sex effect also emerged for locomotion in both the home-cage (Fsex[1, 44] = 10.88, p<.01) and OF(Fsex[1, 44] = 10.70 p<.01) tests, with females showing increased locomotion, however, no alcohol or interactions effects were found (Supplementary figure 1C–D). Overall, alcohol-history did not affect how mice coped with restraint stress in the home-cage or the OF, and female mice regardless of alcohol or water history spent more time rearing and less time grooming in the home-cage, while their behaviors in the OF indicated greater time in the center with fewer total center entries.
The Bains group has found significant alterations to home-cage behaviors after a foot-shock stress in male mice (Füzesi et al., 2016). In particular, they found decreased digging time, and increased rearing, grooming and locomotion. To determine how a different stressor, in our case 2hr restraint stress, affects these same behaviors in both males and females, we conducted a separate analysis on water exposed mice only by comparing the No-stress and Stress data in males and females using a two-way ANOVA (Supplementary figure 2). Similarly, to a foot-shock stress, restraint drove reductions in digging time (Supplementary figure 2B; Fstress[1, 45] = 83.85, p<.001), and a drastic increase in grooming time (Supplementary figure 2D; Fstress[1, 45] = 159.8, p<.001). There were no sex or interaction effects in digging behavior (Fsex[1, 45] = 1.878, p=.177; FstressXsex[1,45] = 3.908, p=.054), but males and females did differ in grooming behaviors, as a main effect of sex (Fsex[1, 45] = 4.693, p<.05) and interaction effect (FstressXsex[1,45] = 4.303, p<.05), emerged. On the contrary, rearing (Supplementary figure 2C; Fstress[1, 45] = 22.48, p<.001), and locomotion (Supplementary figure 2E; Fstress[1, 45] = 121.0, p<.001), were reduced after restraint stress. These behaviors were also sensitive to sex, as rearing (Fsex[1, 45] = 15.84, p<.001) and locomotion (Fsex[1, 45] = 15.84, p<.001) had a significant main effect of sex. Overall, restraint stress drastically affects all spontaneous behaviors, but males and females display differences in their natural behavior patterns. Despite stress driving a reduction in rearing and locomotion and an increase in grooming time, females rear and move more frequently than males, while on the whole they groom less often.
In general, the home-cage test indicated that withdrawal from alcohol consumption alone (No-Stress mice) led to alterations to spontaneous baseline behaviors in a familiar environment (Fig 2C–E). Using simple linear regressions to compare DLC/SimBA results to Ethovision (locomotion) and hand-scoring (spontaneous behaviors), we explored the value of using machine learning for analyzing this data (Fig 3). Locomotion data was highly correlated, however, Ethovision data was approximately 3X higher than DLC/SimBA (Fig 3A: R2 = 0.99, F[1, 13] = 699.3, p<.0001). The grooming (Fig 3B: R2 = 0.98, F[1, 13] = 514.1, p<.0001) and rearing (Fig 3C: R2 = 0.93, F[1, 13] = 182.7, p<.0001) DLC/SimBA data was highly correlated to hand-scored data. Interestingly, the digging model did not match the hand scored data as closely as the other two (Fig 3D: R2 = 0.77, F[1, 13] = 42.72, p<.0001), which might reflect the harder start/stop distinction of this behavior in hand-scoring.
Figure 3:
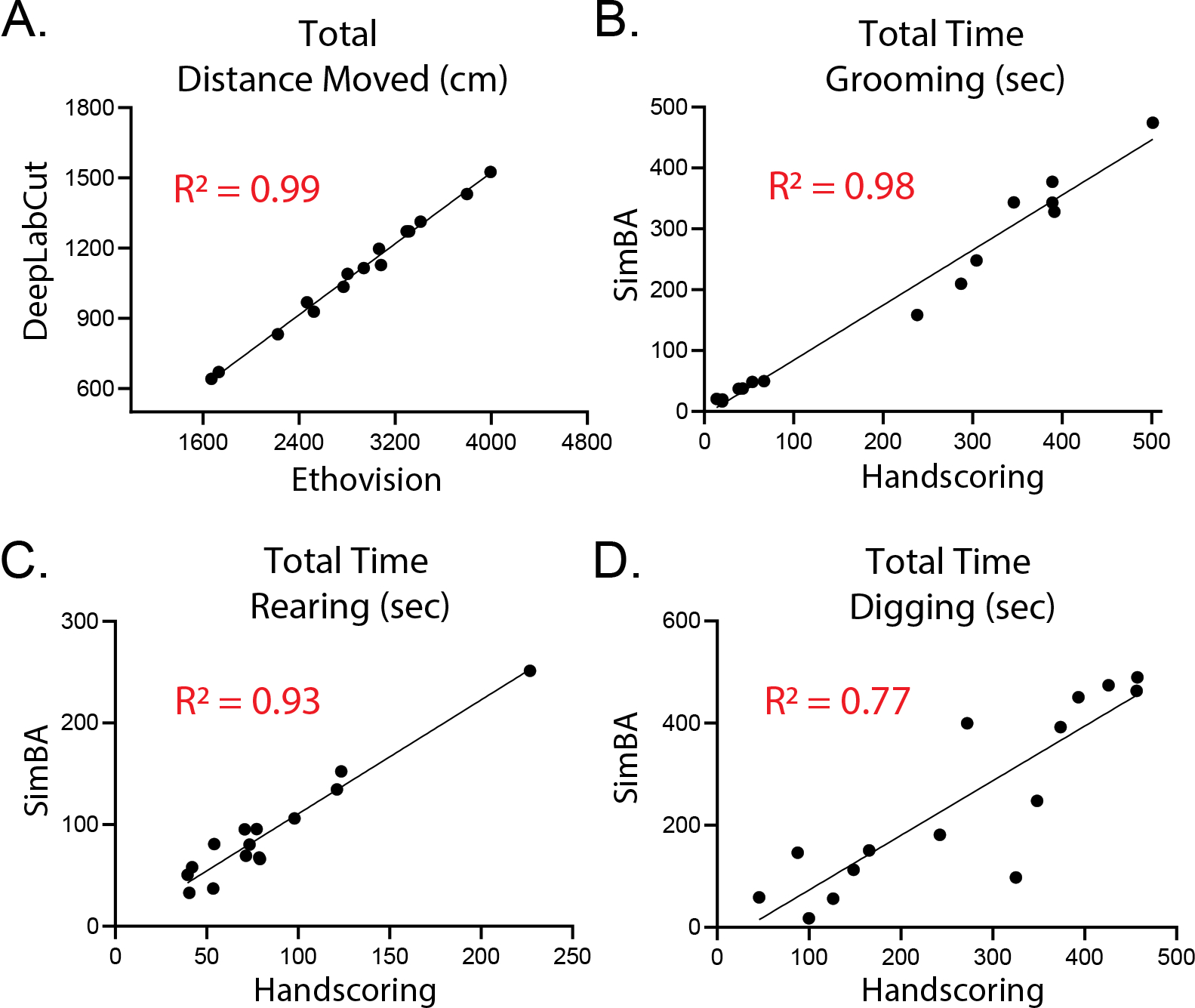
Traditional hand-scoring analysis and machine learning assisted analysis of behaviors are closely aligned. Linear Regression analysis of (A) Ethovision and DeepLabCut analysis of distance moved (A), and hand-score and SimBA analysis of time spent (B) grooming, (C) rearing, and (D) digging.
Experiment 3
Our interests also lie in understanding how long-term alcohol impacts reactions to a ethologically relevant rodent predator task, the looming disc. The looming disc task mimics an overhead predator and elicits a behavioral response. We compared the proportion of active escape, freezing, or neither escape nor freezing responses in the looming disc task (Fig 4A). In males, a Chi-square test indicated no behavioral differences in response to the looms between water and alcohol mice (Fig 4B: X2[1, N = 100] = 4.00, p=.14). However, female alcohol history mice did differ in behavioral responses to this task compared to water controls (Fig 4C: X2[1, N = 99] = 10.45, p<.01). In particular, active escape reactions were seen more in alcohol mice (63% in alcohol vs 38% in water mice), while neither escape nor freezing reactions were more prevalent in water mice (12% vs 40% in water mice).
Figure 4:
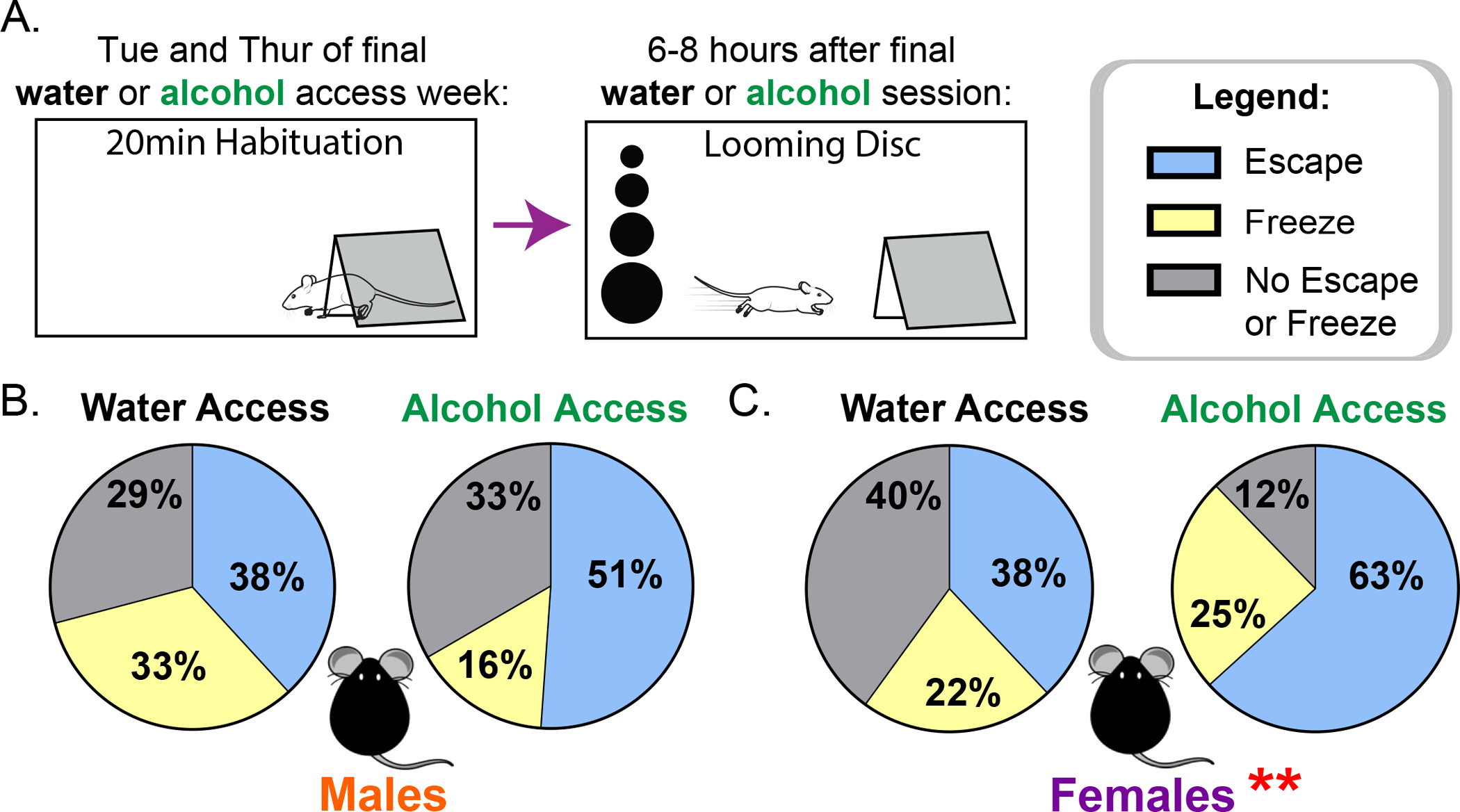
Female alcohol access mice in acute withdrawal escape more often to the looming disc than water access mice, but male alcohol and water mice do not differ in their behavioral reactions to the task. (A) Experimental design. (B) Proportion of Escape (Blue), Freeze (Yellow), and No escape or Freeze (Grey) in male water and alcohol exposed mice. (C) Proportion of Escape (Blue), Freeze (Yellow), and No escape or Freeze (Grey) in female water and alcohol exposed mice. ** p<.01
Experiment 4
To determine if alcohol-exposed mice exhibited altered behavior in a more complex predator task, we examined foraging performance in the presence of a robogator threat during withdrawal. Beginning 7 days after the final drinking session, mice were given a 30-minute habituation session, 4 days of baseline training for pellet foraging, and a final Robogator test (Fig 5A). Mice showed no differences in multiple parameters of the habituation session, as depicted in the heat map for the first 5 minutes in the entire arena (Supplementary fig 3A). Interestingly, a two-way RM ANOVA indicated alcohol access mice showed an overall higher latency to reach the pellet in the first training day (Fig 5B: Falcohol[1, 16] = 7.017, p<.05). Importantly, mice learned the foraging task and behaved equally by training day 4 before being introduced to the Robogator test the following day, suggesting alcohol mice had higher anxiety-like behaviors in the beginning, but the differences in the test were likely not driven by differences in learning the task overall.
Figure 5:
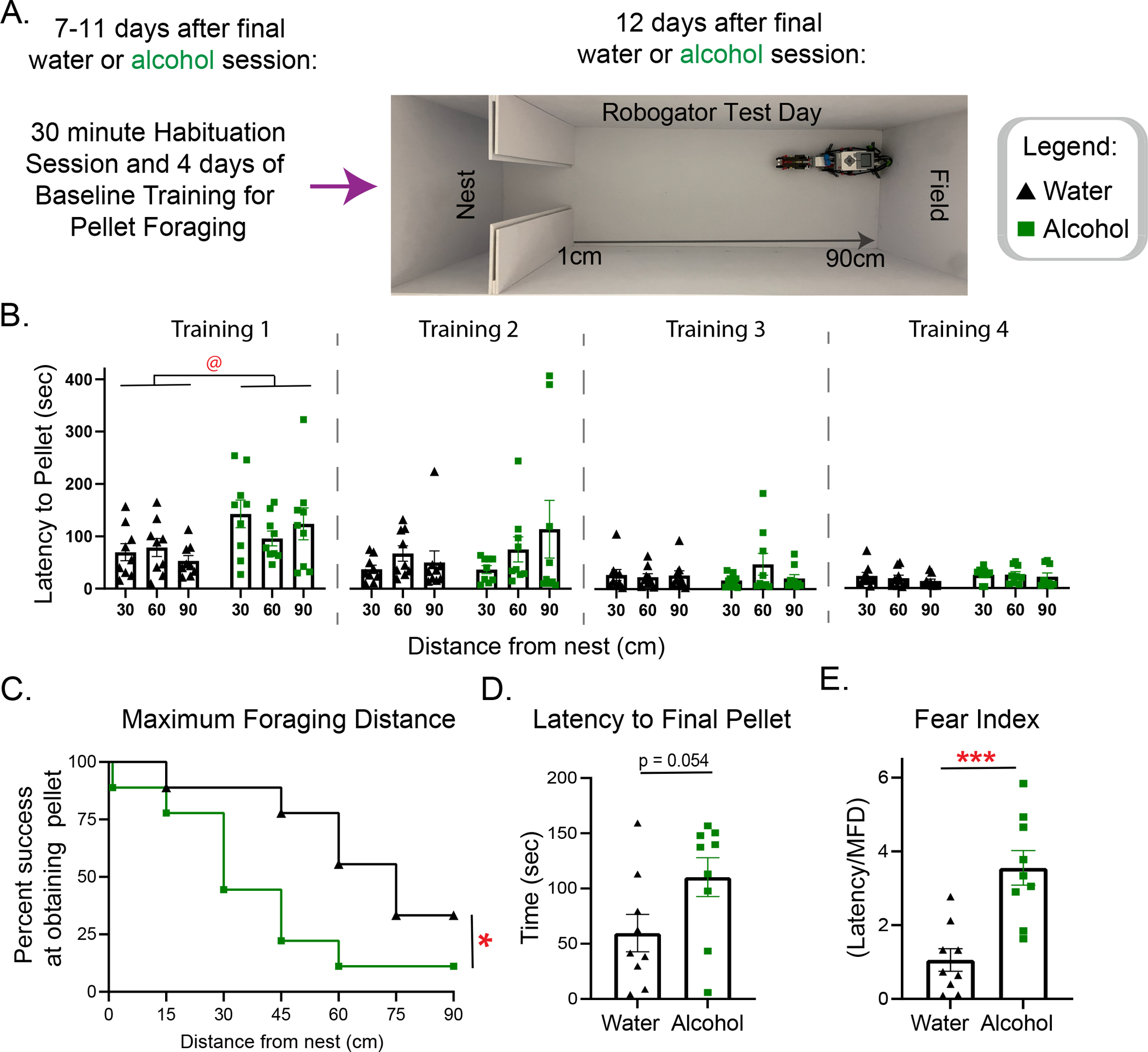
Alcohol access male mice in protracted withdrawal develop a more hesitant approach to foraging in the robogator task than water access mice. (A) Experimental design for the robogator task. (B) Latency to pellet for the 4 training sessions before test day, alcohol mice were slower overall in the first training day. (C) Maximum Foraging Distance (MFD) shown as percent success at obtaining the final pellet at each distance from nest, water access mice achieved farther distances in the presence of the gator than alcohol access mice. (D) Latency to final pellet. (E) Fear index shown as latency over maximum foraging distance, alcohol access mice had higher fear index. @ = main effect of Alcohol. * p<.05, *** p<.001.
A survival analysis was used to determine if there was a difference between groups in the Maximum Foraging Distance (MFD). We found a significant difference between water and alcohol mice, with water mice achieving further distances from the nest (Fig 5C: Log-rank (Mantel-Cox) test, X2[1, N = 18] = 3.970, p<.05). Although we found no differences in the latency to the final pellet (Fig 5D: [t(16)=2.077, p=.054]), when we normalized for the difference in the MFD by using the fear index (distance/latency), we found that alcohol mice scored significantly higher in this index than water mice (Fig 5E: [t(16)=4.462, p<.001). Interestingly, we found no differences in multiple parameters on the only shared trial by all mice, the 30cm first trial (Supplementary fig 3B–E). Overall, a history of alcohol consumption in male mice increased anxiety-like behaviors and the latency of foraging in the presence and absence of a predator-like threat.
Discussion
In this study, we find that six weeks of two-bottle choice alcohol impacts mouse behavior to a home-cage, open field, looming disc, and robogator predator test. While traditional anxiety/stress tests have been valuable in understanding alcohol induced alterations to negative affective behavior, stressors can induce different behavioral responses depending on factors such as stressor intensity and task demands. For instance, an open field test can induce anxiety-like behaviors due to moderately high arousal conditions from a bright light and novel environment (Roth and Katz, 1979). However, a stressor like the looming disc and robogator, which pushes behavioral choices in a novel environment and in the presence of a predator-like threat, can induce a different arousal level and behavioral repertoire than the open field test. The responses to differing stressors may also be sex-specific, with some suggesting that female responses are biased towards stress-induced hyperarousal (Bangasser et al., 2018; Kudielka and Kirschbaum, 2005; Palanza and Parmigiani, 2017). In this manuscript, females also demonstrated a hyperarousal phenotype compared to males and in response to stress. Females in general had greater locomotion and rearing behaviors, increased center entries in the open field, and showed increased escape behaviors in the looming disc during acute withdrawal stress compared to controls. Of note, females are greatly affected by social isolation (Mumtaz et al., 2018; Senst et al., 2016), and this paradigm necessitates extended stress of single housing. Additionally, withdrawal times varied in these tasks, which can affect behaviors differently. Understanding how a history of voluntary alcohol consumption shifts behavioral responses to a variety of tasks can lead to a wider understanding on alcohol induced changes to organisms and the brain.
Two-bottle Choice Alcohol Consumption
Much of the field focuses on forced alcohol models to induce high B.E.C.s, high withdrawal symptoms, and low variability. While this is critical to understanding aspects of severe AUD, many people with AUD have milder consumption patterns and symptomology, and forced alcohol paradigms may be overrepresenting those more extreme cases (Holleran and Winder, 2017). Thus, we first assessed the intermittent two-bottle choice drinking paradigm (2BC; Fig 1A). Overall, both sexes display escalation of intake and preference for alcohol (Fig 1B–C), and experiment 1 showed most mice reach binge level B.E.C.s in a 4hr session, though these vary (Fig 1E). We investigated how total intake and alcohol preference correlated to behaviors in these experiments but found no relationship between alcohol and withdrawal behaviors. However, most mice in this 2BC paradigm drink similar amounts, and only few in a cohort drink very low/large amount. We might need to increase sample sizes to determine relationships between individual differences and alcohol history. Alternatively, this analysis could be done similarly to studies in non-human primates (Grant and Bennett, 2003), and what has been done to study individual differences in voluntary drinking in rodents (Heilig et al., 2019; Leeman et al., 2010) by characterizing between low and high drinkers based on B.E.C. or 4hr short-term intake readings. An important caveat to the intermittent 2BC model is the prolonged social isolation, which is a stressor that particularly affects female rodents (Hermes et al., 2009; Senst et al., 2016). Understanding how other models affect withdrawal related behaviors is vital and can be an important follow up to this study, which only focuses on chronic voluntary consumption of alcohol.
Home-cage and Open Field Behaviors
Experiment 2 explored behavior in water and alcohol exposed mice in a naïve, no-stress, context or following acute restraint stress. We hypothesized that, compared to water No-stressed controls, negative affect related to acute withdrawal from 2BC would lead to increased stress-coping by influencing behaviors (i.e. grooming, digging, and rearing) in the home-cage. Similarly, restraint stress would also increase stress-coping in the home-cage through changes in behavior similar to what has previously been seen immediately after an acute foot shock stress (Füzesi et al., 2016). We aimed to expand the literature on how stress alters natural behavioral patterns, and pain perception is linked to alcohol withdrawal, thus we used another well-characterized stressor, a 2hr restraint stress. Interestingly, suggestive of withdrawal induced stress-coping, naïve, No-stress male and female alcohol-history mice show altered behavioral profiles in the home cage (Fig 2C–E). However, alcohol history did not affect home cage behaviors following acute restraint stress compared to water stressed controls. This might be due to the intensity of a 2hr restraint stress, which overrides behavioral response regardless of previous alcohol history. A milder stressor, and more importantly one that is also ethologically relevant, might lead to distinct behavioral patterns and expand our understanding on what behaviors such as rearing and grooming in the home-cage signify. Overall, sex plays an important role in behavior, as male and female mice displayed different behavioral patterns in the home-cage and open field tests (Fig 2). Much more needs to be understood in relation to what alterations to home-cage behaviors signify and further research into how home-cage behaviors are affected by different alcohol paradigms, stressors, and whether pharmacological treatment with drugs can restore these behaviors can inform the field on the validity of this task. However, it is interesting that acute withdrawal alone impacts behavior similarly to an acute foot-shock stress (Füzesi et al., 2016) through a reduction in digging, an increase in rearing in males, and an increase in grooming time (Fig 2C–E).
In this home-cage context in rodents, rearing behaviors have been associated with increased exploration and vigilance and grooming is thought to be a coping behavior (Dielenberg et al., 2001; Fentress, 1988; Fernández-Teruel and Estanislau, 2016; Hermes et al., 2009; Sturman et al., 2018; Windle et al., 1997). Interestingly, rearing is accompanied by a reliable increase in paraventricular nucleus of the hypothalamus corticotropin releasing hormone (PVNCRH) neuron activity (data not shown) and grooming is driven by optogenetic activation of these neurons (Füzesi et al., 2016). PVNCRH neurons are altered in alcohol exposed rodents and in brain tissue of individuals with heavy alcohol use and are tightly implicated with alcohol (Marty et al., 2020; Sivukhina et al., 2006; Stephens and Wand, 2012), as their activation initiates the HPA-axis stress response system. Alcohol and stress interactions underlie AUDs, and hyperactive basal HPA-axis hormone production is seen in individuals with AUD. The home-cage results suggest a hyperactive stress response in naïve mice, and this test might serve as a good measure of baseline withdrawal affect in rodents. In fact, the home-cage test for the etho-experimental analysis of spontaneous behavior can prove highly useful in regard to alcohol research, as traditional stress/anxiety tests encourage an anxiety-like state and can confound baseline, stress-induced withdrawal behaviors. Examining natural home-cage behaviors reduces external environmental factors that can increase variability, stress, and anxiety (Füzesi et al., 2016; Kliethermes, 2005; Lezak et al., 2017; Richter, 2020; Rojas-Carvajal et al., 2021). Though the home-cage test shows potential for the field, the analysis of these behaviors is extremely time consuming using traditional hand-scoring methods. To this end, we found that machine learning based analysis serves as a robust and reproducible alternative to hand-scoring that can be used for a rapid, unbiased, and robust analysis of these types of behaviors (Fig 3). While there was a curious 3-fold difference in raw values for distance moved between DeepLabCut and Ethovision, DeepLabCut uses multiple body parts to determine movement and placement and might depend on different markers for determining movement than the Ethovision software. An analysis into which is more accurate is warranted. Finally, this unbiased model might lead to better accuracy in behaviors that involve a more abstract start/stop such as digging. A human scorer might get looser with the start/stop distinction, while machine learning analysis follows more strict rules.
Looming Disc Task
Our interests also lie in understanding behavioral responses to immediate/acute stressors following a history of alcohol, particularly in species relevant predator tasks. The animated overhead looming disc, an ethologically relevant stressor that simulates a rapidly approaching overhead predator, has been shown to induce defensive behaviors in mice across many labs (De Franceschi et al., 2016; Shang et al., 2018; Yilmaz and Meister, 2013). At an acute 6–8hr withdrawal timepoint, female, but not male, alcohol-exposed mice showed an increased propensity for active escape responses in the looming disc task (Fig 4). Interestingly, escape responses are sensitive to changes in the activity of PVNCRH neurons. A natural increase in activity precedes the initiation of escape and optogenetic inhibition of these cells decreases escape (Daviu et al., 2020). The propensity for active escape to the looming disc in alcohol-exposed females might indicate increased activity of PVNCRH neurons during task-demanding tests that predispose mice with a history of alcohol use to initiate an active, hyperarousal coping strategy in response to immediate stressors.
Robogator Simulated Predator Task
The robogator is another ethologically relevant task, where a predator-like threat ‘attacks’ mice while they forage for food pellets. This task also allows for the measurement of anxiety-like behaviors, as the baseline training days in the absence of the robogator mirror novelty-suppressed-feeding tests. Additionally, behaviors in this task involve brain regions closely related to anxiety/stress related behaviors, and the anxiolytic drug diazepam has been shown to attenuate risk aversion in a similar moving predator task (Amir et al., 2015; Choi and Kim, 2010; Walters et al., 2019). In 8–12 days forced abstinence, we found that compared to water controls, male alcohol exposed mice showed increased latency to forage in a large arena in the absence of the gator (Fig 5C), as well as increased avoidance in the presence of the robogator (Fig 5D–F). The findings with the robogator mirror those in the open field test at a 24hr withdrawal timepoint, where alcohol-exposed No-stress mice showed some anxiety-like phenotypes (Fig 2G–H). Male alcohol mice also failed to show a more active stress coping strategy in the looming disc task (Fig 4). This avoidance/hesitancy under stress might suggest an underlying, universal phenotype seen after alcohol exposure in male mice. We note that we did run a small pilot group of female mice in a previous version of this task with different arena and lighting settings that is not comparable to the male group. A future study will explore behavioral responses in female mice.
Conclusions
Collectively, we find that a history of alcohol consumption leads to task-sensitive changes in behavior. Mice display different sets of behavioral patterns under varied stress conditions, which may reflect the relative threat level of the assay. For example, the increased rearing at the cost of digging behaviors in male mice in the home-cage may reflect increased arousal and vigilance in a familiar environment. In contrast, the hesitancy in foraging in a brightly lit open environment and the findings with the open field tie more closely to increased anxiety states in these mice under higher arousal conditions. Female mice displayed increased stress coping in the home-cage, as well as increased active coping strategies in the looming disc task, which might suggest stress induced hyperarousal following alcohol history. It will be vital to continue studying how females respond to stress during withdrawal, as most research into negative affect in animal models has been conducted in males, while human research shows sex differences in alcohol and stress interactions during negative affective states (Becker and Koob, 2016; Brady and Sonne, 1999; Hilderbrand and Lasek, 2018; Peltier et al., 2019). Interestingly, behavioral effects in traditional anxiety/stress tests are often not seen in female rodents, which might hint at a need for different tests, such as those that incur heightened arousal or force behavioral choices under stress (Henricks et al., 2017; Jury et al., 2017; Reilly et al., 2009). Alternatively, given that several of these tasks were performed at different points post drinking, it is possible that the length of forced abstinence may impact behavioral performance. An important caveat to our study is that all mice were single housed for 8 or more weeks due to the requirements of the alcohol drinking paradigm. Social isolation itself is a stressor, particularly in females (Hermes et al., 2009; Mumtaz et al., 2018; Senst et al., 2016), which should be considered for the experiments performed in this study.
It is important to continue studying voluntary drinking paradigms, as there may be distinct neural alterations in a voluntary drinking model compared to forced access models. Almost no drug has advanced past clinical trials for treating alcohol withdrawal in recent years, and using new behavioral tests and voluntary drinking paradigms may help expand our understanding of alcohol use and abuse (Heilig et al., 2019). Finding new and efficient treatments could encourage people with AUD to seek treatment earlier in the history of alcohol use, and reduce withdrawal associated negative affective states and the severity of AUD (Finn and Crabbe, 1997; Glass et al., 2017). Ultimately, earlier intervention strategies can aid in harm reduction for overall better health outcomes (Charlet and Heinz, 2017).
Supplementary Material
Acknowledgements
This work was supported by grants from the Howard Hughes Medical Institute James H. Gilliam, Jr. Fellowship for Advanced Study (GT13514) and the National Institute of Health (INIA U01, U24, P60). Special acknowledgements to Dr. Dipanwita Pati and Dr. Melanie Pina for their invaluable mentorship and feedback. Thanks to SciDraw, a free website where people donate their fantastic drawings for others to use, and BioRender.com, which together allowed me to create my figures.
Sources of Support:
Howard Hughes Medical Institute James H. Gilliam Jr. Fellowship for Advanced Study (GT13514) and the National Institute of Health (U01AA020911, U24AA025475, P60AA011605).
Footnotes
Ethics Declarations
The authors declare no competing interests.
Additional Information
N/A
References
- Amir A, Lee S-C, Headley DB, Herzallah MM, Pare D, 2015. Amygdala Signaling during Foraging in a Hazardous Environment. J. Neurosci. 35, 12994–13005. 10.1523/JNEUROSCI.0407-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RJ, 1974. Arousal and the Causation of Behaviour. Behaviour 51, 135–164. 10.1163/156853974X00174 [DOI] [PubMed] [Google Scholar]
- Asher L, Harvey ND, Green M, England GCW, 2017. Application of Survival Analysis and Multistate Modeling to Understand Animal Behavior: Examples from Guide Dogs. Front. Vet. Sci. 4, 116. 10.3389/fvets.2017.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Eck SR, Telenson AM, Salvatore M, 2018. Sex differences in stress regulation of arousal and cognition. Physiol. Behav., The Proceedings of the American University Symposium on Sex Differences: from Neuroscience to the Clinic and Beyond 187, 42–50. 10.1016/j.physbeh.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, 2012. Effects of Alcohol Dependence and Withdrawal on Stress Responsiveness and Alcohol Consumption. Alcohol Res. Curr. Rev. 34, 448–458. [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF, 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacol. Rev. 68, 242–263. 10.1124/pr.115.011163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Nautiyal N, Hart R, Guarnaccia JB, Sinha R, 2019. Craving, cortisol and behavioral alcohol motivation responses to stress and alcohol cue contexts and discrete cues in binge and non-binge drinkers. Addict. Biol. 24, 1096–1108. 10.1111/adb.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Sinha R, 2017. Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology, Alcoholism 122, 136–147. 10.1016/j.neuropharm.2017.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, 1990. Anti-predator defense as models of animal fear and anxiety, in: Fear and Defence, Ettore Majorana International Life Sciences Series, Vol. 8. Harwood Academic Publishers, Amsterdam, Netherlands, pp. 89–108. [Google Scholar]
- Blanchard RJ, Magee L, Veniegas R, Blanchard DC, 1993. Alcohol and anxiety: Ethopharmacological approaches. Prog. Neuropsychopharmacol. Biol. Psychiatry 17, 171–182. 10.1016/0278-5846(93)90041-P [DOI] [PubMed] [Google Scholar]
- Bloch S, Holleran KM, Kash TL, Vazey EM, Rinker JA, Lebonville CL, O’Hara K, Lopez MF, Jones SR, Grant KA, Becker HC, Mulholland PJ, 2022. Assessing negative affect in mice during abstinence from alcohol drinking: Limitations and future challenges. Alcohol 100, 41–56. 10.1016/j.alcohol.2022.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch S, Rinker JA, Marcus MM, Mulholland PJ, 2020. Absence of effects of intermittent access to alcohol on negative affective and anxiety-like behaviors in male and female C57BL/6J mice. Alcohol 88, 91–99. 10.1016/j.alcohol.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha HS, 2004. Freeze, Flight, Fight, Fright, Faint: Adaptationist Perspectives on the Acute Stress Response Spectrum. CNS Spectr. 9, 679–685. 10.1017/S1092852900001954 [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC, 1999. The Role of Stress in Alcohol Use, Alcoholism Treatment, and Relapse. Alcohol Res. Health 23, 263–271. [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH, 2004. Stress Sensitization of Ethanol Withdrawal-Induced Reduction in Social Interaction: Inhibition by CRF-1 and Benzodiazepine Receptor Antagonists and a 5-HT1A-Receptor Agonist. Neuropsychopharmacology 29, 470–482. 10.1038/sj.npp.1300282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M, 2005. Prior Multiple Ethanol Withdrawals Enhance Stress-Induced Anxiety-Like Behavior: Inhibition by CRF1- and Benzodiazepine-Receptor Antagonists and a 5-HT1a-Receptor Agonist. Neuropsychopharmacology 30, 1662–1669. 10.1038/sj.npp.1300706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet K, Heinz A, 2017. Harm reduction—a systematic review on effects of alcohol reduction on physical and mental symptoms. Addict. Biol. 22, 1119–1159. 10.1111/adb.12414 [DOI] [PubMed] [Google Scholar]
- Choi J-S, Kim JJ, 2010. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc. Natl. Acad. Sci. 107, 21773–21777. 10.1073/pnas.1010079108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviu N, Füzesi T, Rosenegger DG, Rasiah NP, Sterley T-L, Peringod G, Bains JS, 2020. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat. Neurosci. 23, 398–410. 10.1038/s41593-020-0591-0 [DOI] [PubMed] [Google Scholar]
- De Franceschi G, Vivattanasarn T, Saleem AB, Solomon SG, 2016. Vision Guides Selection of Freeze or Flight Defense Strategies in Mice. Curr. Biol. 26, 2150–2154. 10.1016/j.cub.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Deal AL, Konstantopoulos JK, Weiner JL, Budygin EA, 2018. Exploring the consequences of social defeat stress and intermittent ethanol drinking on dopamine dynamics in the rat nucleus accumbens. Sci. Rep. 8, 332. 10.1038/s41598-017-18706-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, Carrive P, McGregor IS, 2001. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects11Published on the World Wide Web on 27 February 2001. Brain Res. 897, 228–237. 10.1016/S0006-8993(01)02227-2 [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW, Lai YI, Yachabach TL, 1987. CRF-induced excessive grooming behavior in rats and mice. Peptides 8, 841–844. 10.1016/0196-9781(87)90069-6 [DOI] [PubMed] [Google Scholar]
- Fentress JC, 1988. Expressive Contexts, Fine Structure, and Central Mediation of Rodent Groominga. Ann. N. Y. Acad. Sci. 525, 18–26. 10.1111/j.1749-6632.1988.tb38592.x [DOI] [PubMed] [Google Scholar]
- Fernández-Teruel A, Estanislau C, 2016. Meanings of self-grooming depend on an inverted U-shaped function with aversiveness. Nat. Rev. Neurosci. 17, 591–591. 10.1038/nrn.2016.102 [DOI] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC, 1997. Exploring Alcohol Withdrawal Syndrome. Alcohol Health Res. World 21, 149–156. [PMC free article] [PubMed] [Google Scholar]
- Friard O, Gamba M, 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. 10.1111/2041-210X.12584 [DOI] [Google Scholar]
- Füzesi T, Daviu N, Wamsteeker Cusulin JI, Bonin RP, Bains JS, 2016. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat. Commun. 7, 11937. 10.1038/ncomms11937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JE, Andréasson S, Bradley KA, Finn SW, Williams EC, Bakshi A-S, Gual A, Heather N, Sainz MT, Benegal V, Saitz R, 2017. Rethinking alcohol interventions in health care: a thematic meeting of the International Network on Brief Interventions for Alcohol & Other Drugs (INEBRIA). Addict. Sci. Clin. Pract. 12, 14. 10.1186/s13722-017-0079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Bennett AJ, 2003. Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol. Ther. 100, 235–255. 10.1016/j.pharmthera.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Haun HL, Lebonville CL, Solomon MG, Griffin WC, Lopez MF, Becker HC, 2022. Dynorphin/Kappa Opioid Receptor Activity Within the Extended Amygdala Contributes to Stress-Enhanced Alcohol Drinking in Mice. Biol. Psychiatry. 10.1016/j.biopsych.2022.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Augier E, Pfarr S, Sommer WH, 2019. Developing neuroscience-based treatments for alcohol addiction: A matter of choice? Transl. Psychiatry 9, 1–11. 10.1038/s41398-019-0591-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC, 2010. REVIEW: Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict. Biol. 15, 169–184. 10.1111/j.1369-1600.2009.00194.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks AM, Berger AL, Lugo JM, Baxter-Potter LN, Bieniasz KV, Petrie G, Sticht MA, Hill MN, McLaughlin RJ, 2017. Sex- and hormone-dependent alterations in alcohol withdrawal-induced anxiety and corticolimbic endocannabinoid signaling. Neuropharmacology, A New Dawn in Cannabinoid Neurobiology 124, 121–133. 10.1016/j.neuropharm.2017.05.023 [DOI] [PubMed] [Google Scholar]
- Hermes GL, Delgado B, Tretiakova M, Cavigelli SA, Krausz T, Conzen SD, McClintock MK, 2009. Social isolation dysregulates endocrine and behavioral stress while increasing malignant burden of spontaneous mammary tumors. Proc. Natl. Acad. Sci. 106, 22393–22398. 10.1073/pnas.0910753106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderbrand ER, Lasek AW, 2018. Studying Sex Differences in Animal Models of Addiction: An Emphasis on Alcohol-Related Behaviors. ACS Chem. Neurosci. 9, 1907–1916. 10.1021/acschemneuro.7b00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, Winder DG, 2017. Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav. 16, 8–14. 10.1111/gbb.12338 [DOI] [PubMed] [Google Scholar]
- Jury NJ, DiBerto JF, Kash TL, Holmes A, 2017. Sex differences in the behavioral sequelae of chronic ethanol exposure. Alcohol, Special Issue on Mouse Genetic Models of Alcohol and Stress-Alcohol Interactions 58, 53–60. 10.1016/j.alcohol.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keim KL, Sigg EB, 1976. Physiological and biochemical concomitants of restraint stress in rats. Pharmacol. Biochem. Behav. 4, 289–297. 10.1016/0091-3057(76)90244-6 [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, 2005. Anxiety-like behaviors following chronic ethanol exposure. Neurosci. Biobehav. Rev. 28, 837–850. 10.1016/j.neubiorev.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2005. Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat. Neurosci. 8, 1442–1444. 10.1038/nn1105-1442 [DOI] [PubMed] [Google Scholar]
- Koob GF, Mason BJ, 2016. Existing and Future Drugs for the Treatment of the Dark Side of Addiction. Annu. Rev. Pharmacol. Toxicol. 56, 299–322. 10.1146/annurev-pharmtox-010715-103143 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C, 2005. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 69, 113–132. 10.1016/j.biopsycho.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Lê AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y, 2011. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl.) 218, 89–99. 10.1007/s00213-011-2178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS, 2010. REVIEW: Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict. Biol. 15, 109–124. 10.1111/j.1369-1600.2009.00192.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Missig G, Carlezon WA Jr, 2017. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 19, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrys SA, Olmstead MC, Wynne-Edwards KE, Balodis IM, 2013. Neuroendocrinological responses to alcohol intoxication in healthy males: Relationship with impulsivity, drinking behavior, and subjective effects. Psychophysiology 50, 204–209. 10.1111/psyp.12007 [DOI] [PubMed] [Google Scholar]
- Marty VN, Mulpuri Y, Munier JJ, Spigelman I, 2020. Chronic alcohol disrupts hypothalamic responses to stress by modifying CRF and NMDA receptor function. Neuropharmacology 167, 107991. 10.1016/j.neuropharm.2020.107991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M, 2018. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289. 10.1038/s41593-018-0209-y [DOI] [PubMed] [Google Scholar]
- Metzger IW, Salami T, Carter S, Halliday-Boykins C, Anderson RE, Jernigan MM, Ritchwood T, 2018. African American emerging adults’ experiences with racial discrimination and drinking habits: The moderating roles of perceived stress. Cultur. Divers. Ethnic Minor. Psychol. 24, 489–497. 10.1037/cdp0000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz F, Khan MI, Zubair M, Dehpour AR, 2018. Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomed. Pharmacother. 105, 1205–1222. 10.1016/j.biopha.2018.05.086 [DOI] [PubMed] [Google Scholar]
- Newman EL, Leonard MZ, Arena DT, de Almeida RMM, Miczek KA, 2018. Social defeat stress and escalation of cocaine and alcohol consumption: Focus on CRF. Neurobiol. Stress 9, 151–165. 10.1016/j.ynstr.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson SR, Goodwin NL, Choong JJ, Hwang S, Wright HR, Norville ZC, Tong X, Lin D, Bentzley BS, Eshel N, McLaughlin RJ, Golden SA, 2020. Simple Behavioral Analysis (SimBA) – an open source toolkit for computer classification of complex social behaviors in experimental animals. bioRxiv 2020.04.19.049452. 10.1101/2020.04.19.049452 [DOI] [Google Scholar]
- Palanza P, Parmigiani S, 2017. How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neurosci. Biobehav. Rev., Translational Neuroscience & Mental Disorders: bridging the gap between animal models and the human condition 76, 134–143. 10.1016/j.neubiorev.2017.01.037 [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Bale TL, 2008. Stress and Sex Influences on Food-seeking Behaviors. Obesity 16, 1539–1544. 10.1038/oby.2008.221 [DOI] [PubMed] [Google Scholar]
- Paré WP, Glavin GB, 1986. Restraint stress in biomedical research: A review. Neurosci. Biobehav. Rev. 10, 339–370. 10.1016/0149-7634(86)90017-5 [DOI] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, McKee SA, 2019. Sex differences in stress-related alcohol use. Neurobiol. Stress 10, 100149. 10.1016/j.ynstr.2019.100149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly W, Koirala B, Devaud LL, 2009. Sex Differences in Acoustic Startle Responses and Seizure Thresholds between Ethanol-Withdrawn Male and Female Rats. Alcohol Alcohol 44, 561–566. 10.1093/alcalc/agp049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter SH, 2020. Automated Home-Cage Testing as a Tool to Improve Reproducibility of Behavioral Research? Front. Neurosci. 14. 10.3389/fnins.2020.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, 1996. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol. Clin. Exp. Res. 20, 240–254. 10.1111/j.1530-0277.1996.tb01636.x [DOI] [PubMed] [Google Scholar]
- Rojas-Carvajal M, Quesada-Yamasaki D, Brenes JC, 2021. The cage test as an easy way to screen and evaluate spontaneous activity in preclinical neuroscience studies. MethodsX 8, 101271. 10.1016/j.mex.2021.101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth KA, Katz RJ, 1979. Stress, behavioral arousal, and open field activity--a reexamination of emotionality in the rat. Neurosci. Biobehav. Rev. 3, 247–263. 10.1016/0149-7634(79)90012-5 [DOI] [PubMed] [Google Scholar]
- SAMHSA, 2019. U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. [WWW Document]. URL https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables (accessed 7.12.21). [Google Scholar]
- Senst L, Baimoukhametova D, Sterley T-L, Bains JS, 2016. Sexually dimorphic neuronal responses to social isolation. eLife 5, e18726. 10.7554/eLife.18726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C, Chen Z, Liu A, Li Y, Zhang J, Qu B, Yan F, Zhang Y, Liu W, Liu Z, Guo X, Li D, Wang Y, Cao P, 2018. Divergent midbrain circuits orchestrate escape and freezing responses to looming stimuli in mice. Nat. Commun. 9, 1232. 10.1038/s41467-018-03580-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM, 2009. Enhanced Negative Emotion and Alcohol Craving, and Altered Physiological Responses Following Stress and Cue Exposure in Alcohol Dependent Individuals. Neuropsychopharmacology 34, 1198–1208. 10.1038/npp.2008.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivukhina EV, Dolzhikov AA, Morozov IE, Jirikowski GF, Grinevich V, 2006. Effects of Chronic Alcoholic Disease on Magnocellular and Parvocellular Hypothalamic Neurons in Men. Horm. Metab. Res. 38, 382–390. 10.1055/s-2006-944522 [DOI] [PubMed] [Google Scholar]
- Stephens MAC, Wand G, 2012. Stress and the HPA Axis. Alcohol Res. Curr. Rev. 34, 468–483. [PMC free article] [PubMed] [Google Scholar]
- Sturman O, Germain P-L, Bohacek J, 2018. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress 21, 443–452. 10.1080/10253890.2018.1438405 [DOI] [PubMed] [Google Scholar]
- Sutherland JE, Burian LC, Covault J, Conti LH, 2010. The effect of restraint stress on prepulse inhibition and on corticotropin-releasing factor (CRF) and CRF receptor gene expression in Wistar–Kyoto and Brown Norway rats. Pharmacol. Biochem. Behav. 97, 227–238. 10.1016/j.pbb.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso AWN, Filgueiras GB, Lorenzo P, Estanislau C, 2016. Modulation of grooming behavior in rats by different test situations. Psychol. Neurosci. 9, 91–104. 10.1037/pne0000038 [DOI] [Google Scholar]
- Walters CJ, Jubran J, Sheehan A, Erickson MT, Redish AD, 2019. Avoid-approach conflict behaviors differentially affected by anxiolytics: implications for a computational model of risky decision-making. Psychopharmacology (Berl.) 236, 2513–2525. 10.1007/s00213-019-05197-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD, 1997. Central Oxytocin Administration Reduces Stress-Induced Corticosterone Release and Anxiety Behavior in Rats*. Endocrinology 138, 2829–2834. 10.1210/endo.138.7.5255 [DOI] [PubMed] [Google Scholar]
- Yilmaz M, Meister M, 2013. Rapid innate defensive responses of mice to looming visual stimuli. Curr. Biol. CB 23, 10.1016/j.cub.2013.08.015. 10.1016/j.cub.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


