Abstract
Endometrial cancer (EC) is a common gynecological cancer that endangers women health. Although substantial progresses of EC management have been achieved in recent years, the incidence of EC still remains high. Obesity has been a common phenomenon worldwide that increases the risk of EC. However, the mechanism associating obesity and EC has not been fully understood. Metabolic reprogramming as a remarkable characteristic of EC is currently emerging. As the primary factor of metabolic syndrome, obesity promotes insulin resistance, hyperinsulinemia and hyperglycaemia. This metabolic disorder remodels systemic status, which increases EC risk and is related with poor prognosis. Glucose metabolism in EC cells is complex and mediated by glycolysis and mitochondria to ensure energy requirement. Factors that affect glucose metabolism may have an impact on EC initiation and progression. In this study, we review the glucose metabolic reprogramming of EC not only systemic metabolism but also inherent tumor cell metabolism. In particular, the role of glucose metabolic regulation in malignant properties of EC will be focused. Understanding of metabolic profile and glucose metabolism-associated regulation mechanism in EC may provide novel perspective for treatment.
Keywords: Endometrial cancer, Metabolic syndrome, Glucose metabolic reprogramming, Glycolysis, Mitochondria
Introduction
Endometrial cancer (EC) is one of the most common gynecologic malignancies worldwide. The incidence of EC is rising in recent years especially in young women, which deeply influences reproductive health and imposes heavy social burden. The American Cancer Society estimates that in 2022, there would be 65,950 new cases and 12,550 deaths with EC in the United States, making it the fourth most common cancer and the sixth most common causes of cancer death of women [1]. As the most common subtype of EC, endometrioid carcinoma (type I) is hormone-receptor-positive with less-aggressive characteristic and better prognosis compared with type II. Multiple factors contribute to the development of EC including obesity, metabolic dysregulation, excess estrogen and genetic predisposition [2]. Standard treatment for EC is primary hysterectomy and bilateral salpingo-oophorectomy, whereas lymph node dissection, adjuvant or targeted treatment is conducted according to the clinical pathological and molecular characteristic. Progestin-based conservative treatment of EC is viable for those women who wish to preserve fertility with well-differentiated, clinical stage 1 A, endometrioid EC [3]. Although substantial advances of EC management have been made in recent years, the incidence of EC remains high and our understanding of EC is limited. Filling the gap in this field would contribute to develop novel therapeutic strategies and to optimize the treatment for EC.
Metabolic reprogramming includes the rewiring of glucose, lipid and amino acid, which is essential for cancer cells growth and metastasis. As one of the most essential generation modes of bioenergy, glucose metabolism has been studied extensively especially its role in cancer. This metabolic behavior supplies energy required for the biological events and acts as the initiator for malignant events of cancer cells. Cancer metabolic process is modulated by not only intrinsic cellular metabolism but also systemic metabolic condition [4]. Cancer cells regulate the metabolic mode through diverse pathways to meet the energy demand for the biosynthesis. As one of the most commonly abnormal activated pathways in EC [5], PI3K/Akt signaling network induces tumorigenesis and has various downstream impacts on the metabolic process including glucose utilization, lipid and nucleotide synthesis, which is through not only direct phosphorylating the metabolic enzymes but also regulating them via transcription factors responsible for the gene expression [6]. The tumor microenvironment (TME) forms a specific metabolic environment for cancer cells because of nutrient availability, which causes metabolic dependencies of cancer cells [7]. Increasing evidences have shown that cytokines like interleukin (IL)-2, IL-6, IL-10 and tumor necrosis factorα (TNF-α) act on the downstream mediators that affects cellular metabolism and functions [8–10]. Aberrant increase of serum ILs is associated with more aggressive pathologic features of EC [11, 12], which may partly be caused by the metabolic alteration. Impaired glycolysis and enhanced mitochondrial activity are associated with endometrial hyperplasia in an estrogen receptor (ER) α-dependent way in polycystic ovarian syndrome (PCOS) that might be an early hallmark of EC [13].
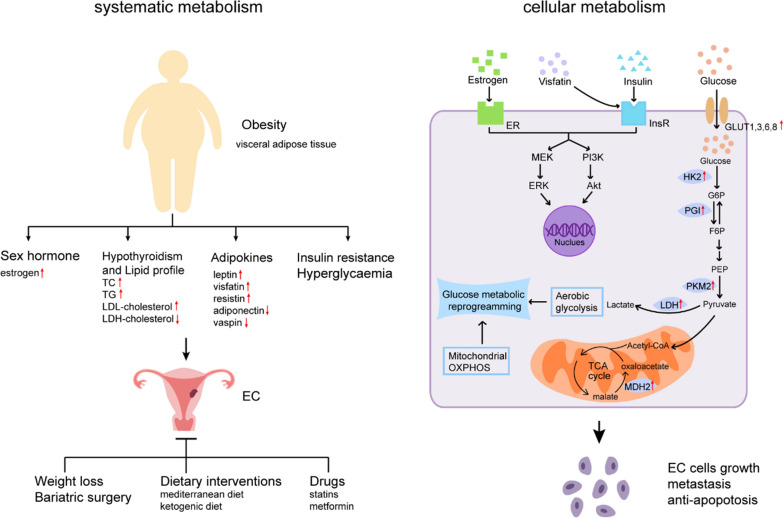
Here, we review recent research advances about the role of glucose metabolism in obesity-associated EC. The role of obesity in EC and its associated systematic and cellular glucose metabolism would be focused. Role of the glucose metabolic regulation in EC tumorigenesis would be discussed in particular (Fig. 1). These perceptions about glucose metabolism would supply a deep understating towards the pathogenic mechanism of EC, thus may provide potentially new therapeutic targets.
Fig. 1.
Glucose metabolic reprogramming in obesity-associated endometrial cancer (EC). Obesity with increased visceral adipose tissue is a remarkable feature of EC and contributes to its tumorigenesis and progression through regulation of both systematic metabolism and cellular metabolism. Obesity leads to multiple risk factors of EC including excessive estrogen, abnormal lipid profile, dysregulated adipokines, insulin resistance and hyperglycaemia. Dyslipidemia exists in EC patients especially in those combined with hypothyroidism, including increased level of total cholesterol (TC), low triglycerides (TG), density lipoprotein (LDL)-cholesterol, and reduced level of high-density lipoprotein (HDL)-cholesterol. Dysregulated adipokines are closely associated with insulin resistance, which is primary pathophysiological basis of EC. Insulin directly or synergistically with estrogen promotes malignancy of EC cells through downstream PI3K/Akt and MEK/ERK signaling pathways. Visfatin has similar effects through activation of insulin receptor (InsR) and its substrate. Glucose enters into EC cells through glucose transporters (GLUT) and rewires cellular glucose metabolism. Increased expression or activity of glycolytic enzymes including hexokinase 2 (HK2), phosphoglucose isomerase (PGI), pyruvate kinase isozymes M2 (PKM2), lactate dehydrogenase (LDH) and mitochondrial enzyme malate dehydrogenase 2 (MDH2) in EC tissues may cause metabolic reprogramming which regulates EC cellular behavior
Metabolic syndrome and EC
Metabolic syndrome is a series of pathological condition characterized by metabolic disorder of glucose, lipid and protein, including diabetes mellitus, obesity, hypertension, cardiovascular and cerebrovascular diseases. EC is closely associated with metabolic syndrome that plays an important role in its development and influence the prognosis of patients. A meta-analysis of 6 studies including 17,772 EC patients and 150,371 healthy controls have shown that women with metabolic syndrome have higher risk of EC (OR 1.62, 95% CI 1.26–2.07) [14]. A case-control study comprising 16,323 EC cases and 100,751 controls have found that the component factor of metabolic syndrome is also correlated with higher risk of EC: overweight/obesity (OR 1.95, 95% CI 1.80–2.11), impaired fasting glucose (OR 1.36, 95% CI 1.30–1.43), hypertension (OR 1.31, 95% CI 1.25–1.36) and high triglycerides (OR 1.13, 95% CI 1.08–1.18) [15]. Metabolic syndrome is closely associated with more aggressive clinicopathological features of EC. The proportion of grade 2–3, stage II–IV, lymph node metastasis, lymph-vascular space invasion or deep-myometrial infiltration is higher in EC patients with metabolic syndrome than those without [16]. A prospective cohort study has reported that metabolic syndrome is associated with worse overall survival (HR 1.98, 95%CI 1.07–3.67) of EC patients [17]. These studies indicate that metabolic syndrome plays a critical role in the development and progression of EC in an epidemiological sense. However, the mechanism of metabolic syndrome in malignancy of EC is still not totally clear. Figuring out how components of metabolic syndrome work in EC may help us understand this causal relation more comprehensively.
Obesity and EC
As the factor of metabolic syndrome, obesity has been reported to be a remarkable risk factor of EC. Compared to women with body mass index (BMI) of 18.5 to 25 kg/m2, symptomatic premenopausal women with BMI more than 25, 30 and 40 were at 3.85, 5.25 and 19.79 times higher risk of EC, respectively [18]. In addition to BMI, other obesity-associated measurements including weight, weight gain, waist circumference, waist-to-hip ratio and hips circumference were correlated with increased risk of EC [19]. The impact of obesity on EC varies at different time points in life course. Compared to early life, obesity in adulthood has a stronger association of EC risk [20, 21]. Every 10-year increase of the adult overweight duration (BMI ≥ 25 kg/m2) has been reported to be correlated with 17% higher risk of postmenopausal EC [22]. Besides baseline BMI, weight gain (per one kg/year increase) during adulthood was also associated with EC (HR 1.14; 95% CI 1.05–1.23) [23]. In addition to the impact of obesity on EC incidence, it also affects the recurrence and survival of EC survivors. One meta-analysis including 46 studies have found that higher BMI (≥ 30 kg/m2) at the diagnosis of EC was correlated with increased recurrence (HR 1.28, 95%CI 1.06–1.56) and all-cause mortality (HR 1.34, 95%CI 1.12–1.59) [24].
Obesity contributes to EC tumorigenesis that may be caused by the dysregulation of hormonal and metabolic homeostasis. Adipose tissue is one important source of estrogen since peripheral aromatization process that converts androgen to estrogen which could cause high level of estrogen in obese women; excessive estrogen condition links obesity and EC development especially in postmenopausal women with increased visceral adipose tissue [25]. Nine BMI-associated single nucleotide polymorphisms (SNPs) variants identified by genome-wide association studies (GWAS) have been found to be related with EC risk, which represent loci in genes including SEC16B/RASAL2, TMEM18, MSRA, SOX6, MTCH2, FTO and MC4R [26]. FTO polymorphisms rs9939609 was reported to be associated with not only metabolic changes in PCOS [27] but also risk of EC [28]. As the m6A demethylase, obesity-associated gene FTO has been reported to rewire glycolytic metabolism through elevation of transcription factors c-Jun, JunB, and C/EBPβ in cancer cells, which further regulates the immune escape and promotes tumor growth [29]. β-estradiol (E2) is the bridge that links obesity and EC progression. E2 enhances FTO expression in EC through PI3K/Akt and MPAK signaling pathways, which then promotes proliferation and invasion of EC cells [30]. Therefore, obesity as the driving factor disrupts systematic metabolism and promotes to EC development. Targeting on obesity may be a promising target for EC therapy.
Obesity and other metabolic diseases in EC
Obesity is commonly associated with endocrine and metabolic disorders like thyroid dysfunctions and cholesterol imbalances. Although it is still unknown whether obesity is the origin or complications of these diseases, current evidences indicate their synergistical involvement in the malignancy and poor outcomes of EC. Hypothyroidism, characterized by increased circulating thyroid-stimulating hormone (TSH) level, is prevalent in people with obesity [31]. One meta-analysis including 22 studies evaluating the relationship of obesity and thyroid diseases have suggested that obesity is associated increased risk of hypothyroidism (OR 1.86, 95% CI 1.63–2.11) [32]. Hypothyroidism is one common morbidity of EC and not only directly affects the endometrium but also interacts with EC-associated risk factors such as metabolic syndrome, PCOS and infertility [33]. One study investigating the impaction of thyroid function on the prognosis of EC patients have found that higher level of pre-therapeutic serum TSH (> 2.5 mU/L) was associated with poor disease-specific survival (HR 2.7, 95%CI 1.1–6.7) [34]. However, one prospective cohort study enrolling 331 EC patients with median follow-up of 35 months have found that women with the history of hypothyroidism had improved overall survival (adjusted HR 0.22, 95%CI 0.06–0.74), cancer-specific survival (adjusted HR 0.21, 95%CI 0.05–0.98) and recurrence-free survival (adjusted HR 0.17, 95%CI 0.04–0.77) than those without [35]. Dyslipidemia is one of the most common metabolic disorders in hypothyroidism including increased level of total cholesterol (TC), low density lipoprotein (LDL), and triglycerides (TG) were significantly higher and reduced high-density lipoprotein (HDL) level compared to euthyroid individuals [36]. Both decreased thyroid hormone (TH) and increased TSH act on lipid metabolism through regulation of production and clearance of cholesterol in the liver [37]. Dyslipidemia was found in EC patients with increased level of TC, TG, LDL-cholesterol and reduced level of HDL-cholesterol [38]. Pretreatment TG/HDL-cholesterol ratio was increased in EC patients compared to controls and high TG/HDL-cholesterol ratio (≥ 1.52) in postmenopausal women could be used as an independent predictor of EC (OR 4.123, 95%CI 2.633–6.457) [39].
Obesity and adipokines in EC
As an endocrine organ, the adipose tissue secretes abundant biological active factors such as adipokines that have a local or systematic impact on multiple physiological process such as lipid and glucose metabolism and insulin sensitivity. Higher levels of leptin, visfatin and resistin have been found in EC patients, which is associated with malignant clinicopathology and poor prognosis [40–43]. As shown in Table 1, these adipocytokines are involved in insulin resistance which may link obesity and its associated malignancy [44–46]. However, several adipokines like adiponectin and vaspin may act as the protective factor against EC through their roles of insulin sensitizing [44, 47]. Various adipocytokines have direct effects on the proliferation and metastasis of EC cells that impacts the risk and prognosis of EC patients [48]. Among them, visfatin is a kind of insulin-like adipokine that plays a crucial role in the metabolism-associated disease especially EC. Emerging evidences in recent decades have shown increased levels of visfatin in overweight/obesity, type 2 diabetes mellitus, metabolic syndrome, cardiovascular diseases and cancers [45, 49–51]. Besides, circulating visfatin level manifested a positive association with insulin resistance [45], which further supports the role of glucose metabolic dysregulation in obesity-associated diseases. Mechanically, visfatin activates insulin receptor (IR) and insulin receptor substrate (IRS)1/2 of EC cells to enhance their proliferation and inhibit apoptosis through PI3K/Akt and MAPK/ERK signaling pathways [52].
Table 1.
Effects of biologically active factors on EC
| Biologically active factors | Roles in EC | References |
|---|---|---|
| Leptin | Involving in insulin resistance | [44] |
| Resistin | Involving in insulin resistance | [46] |
| Visfatin | Activation of insulin receptor and its substrate IRS1/2 to promote proliferation and inhibit apoptosis of EC cells through PI3K/Akt and MAPK/ERK pathways | [52] |
| Insulin |
Promoting EC cells proliferation and invasion, inhibiting apoptosis through PI3K/Akt and MEK/ERK pathway; Synergizing with estrogen to promote type 1 EC progression through activating ER-α and InsR-β respectively; Promoting EC progression through upregulation of TET1-driven GPER expression |
[63] |
| Adiponectin | Protecting against EC through insulin sensitizing | [44, 47] |
| Vaspin | Protecting against EC through insulin sensitizing | [44, 47] |
Obesity and glucose metabolism in EC
Insulin resistance
Obesity promotes inflammation and immune responses in adipose tissue, leading to obesity-associated metabolic dysfunction and insulin resistance locally or systematically [53]. Insulin resistance, as the significant basis of metabolic syndrome, closely links obesity with EC. The prevalence of insulin resistance in EC patients is high as reported to be 66.7% in one prospective study [54]. However, increased BMI is significantly associated with higher risk of insulin resistance and in those women with BMI of 30 kg/m2 or greater, this proportion reaches up to 84% [54]. Insensitivity of target organs to insulin following insulin resistance results in hyperinsulinemia, impaired fasting glucose and could even develop into diabetes mellitus in severe cases. As the pathophysiological basis of metabolic syndrome, insulin resistance is closely correlated with EC. In one meta-analysis of 13 studies including 1562 EC patients and 2526 controls, fasting insulin level was significantly higher in women with EC [55]. Hyperinsulinemia is associated with not only disordered proliferative endometrium as well as endometrial hyperplasia but also type I EC, indicating that it is involved in the development of endometrial hyperplastic lesions and EC [56]. Xue et al. [57, 58] found that increased serum insulin level was the independent risk factors for type I EC. Insulin resistance also indicates worse prognosis of EC patients. Wang et al. [59] have reported that insulin resistance (OR 9.5, 95% CI 3.3–27.0), metabolic syndrome (OR 4.9, 95% CI 1.5–15.5), condition of both insulin resistance and metabolic syndrome (OR 21.0, 95% CI 4.8–92.7) is significantly associated with the recurrence in patients with atypical endometrial hyperplasia (AEH) and early EC, which may help predict the prognosis and optimize the therapeutic strategies for patients with fertility-sparing treatment. In terms of molecular mechanism, insulin is involved in the progression of EC directly or synergistically with other factors. The insulin receptor (InsR) and InsR substrate (IRS)-1 were found to be activated in EC tissues, which was associated with elevated serum insulin in EC [60]. Insulin promotes EC cells proliferation, anti-apoptosis and invasion through PI3K/Akt and MEK/ERK pathway [60, 61]. Insulin and estrogen synergistically promote type 1 EC progression through activating ER-α and InsR-β respectively and their downstream signaling pathways in a crosstalk way [58, 62]. The estrogen sensitivity of EC cells could also be regulated in an insulin-dependent way. Insulin promotes estradiol-driven EC cells proliferation via up-regulating the expression of Ten-eleven-translocation 1 (TET1), which is a DNA hydroxymethylase that could enhance G-protein-coupled estrogen receptor (GPER) expression [63]. GPER expression is strongly increased in tissues from EC patients with insulin resistance and also positively correlates with TET1 expression in EC tissues, which further indicate the role of insulin in the progression of EC mediated by TET1-driven GPER expression [63].
Hyperglycaemia
Since obesity is a main cause of insulin resistance, its subsequent hyperglycaemia furthers involve the development of obesity-associated diseases [64]. Epidemiological studies have shown that hyperglycemia-correlated status increase the incidence of EC, suggesting that there may be a causal relationship between dysregulation of the blood glucose and EC. One Swedish prospective study containing 230,737 women with average follow-up of 11.7 years found that a total of 1070 cases developed EC and increased risk of EC was related to diabetes (HR 1.46, 95% CI 1.09–1.96) and impaired glucose metabolism (HR 1.41, 95% CI 1.08–1.85) [65]. One case-control study including 16,323 patients with EC and 100,751 controls also found that EC risk was associated with impaired fasting glucose (OR 1.36, 95% CI 1.30–1.43) [15]. Liao et al. [66] included 17 prospective studies and 12 retrospective studies for the meta-analysis and confirmed the association between diabetes mellitus and increased risk of EC (RR 1.89, 95%CI 1.46–2.45). Besides, long-term elevated blood glucose may also increase EC risk. Travier et al. [67] found that elevated risk of EC was associated with moderately increase of glycosylated hemoglobin (HbA(1c)) (6–6.9%) (HR 4.05, 95% CI 1.10–14.88) as well as highly increase of HbA(1c) (> or = 7%) (HR 5.07, 95% CI 1.20–21.31).
The role of various concentrations of glucose on EC cells survival has been reported. Han et al. [68] treated EC cells with low glucose (1 mM), normal glucose (5 mM) and high glucose (25 mM) in vitro and found that high glucose promoted malignant phenotype and increase the glucose uptake and glycolytic activity through regulation of AMPK/mTOR/S6 and MAPK pathways. However, how the EC cells uptake high-concentrated glucose and is influenced by hyperglycemia environment? As the essential step of glucose metabolism, the transport of glucose across the plasma membrane mediated by the glucose transporters (GLUT) could achieve the energy supply and regulation of glucose homeostasis. Thus, enhanced expression of specific GLUT may increase the glucose uptake of malignant cells and then promotes tumor progression. In EC tissues, up-regulated GLUT expression has been reported to associate with the clinicopathological features. Increased GLUT3 mRNA level was significantly with elevation of the tumor grade in EC tissues [69]. Compared with benign lesion of endometrium, GLUT1 immunostaining is more remarkable in complex hyperplasia with atypia and in adenocarcinoma, which might be used to distinguish benign and malignant lesions of endometrium [70]. The protein expression of GLUT1 and GLUT8 are higher in uterine papillary serous EC tissues than well-differentiated or poorly-differentiated adenocarcinoma EC tissues [71]. GLUT6 is highly expressed in EC cancerous glandular epithelial cells, whereas not or little expressed in the glandular cells from normal endometrium; suppression of GLUT6 expression could inhibit glycolysis and survival of EC cells in vitro [72].
Aerobic glycolysis
Aerobic glycolysis also named the Warburg effect has linked tumor biological events and metabolism since last century. It refers to the phenomenon that tumors prefer to consume glucose and generate lactate even in presence of oxygen, which may be caused by damaged oxidative respiration [73]. Recent studies about the role of glycolysis in EC mainly focus on aberrant expression of glycolytic genes and enzymes. Glycolysis-associated gene or long noncoding RNA signature in EC has been constructed that could supply the survival prediction of EC patients in clinic [74–76]. As the enzyme that catalyzes the first step in glycolysis, hexokinase 2 (HK2) is upregulated in EC that could be used as the predictor of worse prognosis [77]. Pyruvate kinase (PK) catalyzed phosphoenolpyruvate (PEP) to pyruvate, which is the final rate-limiting step of glycolysis. Four tissue-specific isoforms of PK include PKL, PKR, PKM1, and PKM2, of which PKM2 is up-regulated in most cancer cells involving in metabolic reprogramming [78]. PKM2 has been found to be highly expressed in malignant endometrial lesions associating with poor overall survival (HR for death 3.40, 95% CI 1.35–8.56), whereas low expression of PKM2 in normal endometrium [79]. PKM1 expression is relatively lack in precancerous than noncancerous samples in patients initially diagnosed with complex hyperplasia with atypia through endometrial biopsy, indicating that suppressed expression of PKM1 may predict the progression of endometrial complex hyperplasia with atypia to EC [80]. However, whether the switch from PKM1 to PKM2 promotes the tumorigenesis of EC and the mechanism of this metabolic switch still needs further investigation. PK and phosphoglucose isomerase (PGI) have significantly higher activity in EC tissue than in normal endometrium [81]. Lactate dehydrogenase (LDH) converts pyruvate to lactate, which is the last glycolytic step. The activity of LDH in EC tissue is higher compared with normal uterine endometrium and normal uterine myometrium (1.8 and 2.8 fold, respectively) [82].
Regulation of glycolytic enzymes, transporters and metabolite may have an impact on the survival and progression of EC cells. LncRNA SNHG16 regulated by the transcript factor TFAP2A promotes glycolysis and proliferation of EC cells through miR-490-3p/HK2 axis [83]. HK2 upregulated by upstream lncRNA DLEU2 promotes epithelial-to-mesenchymal transition (EMT) and glycolysis in EC by activating FAK/ERK1/2 signaling [77]. Galloflavin, a novel LDH inhibitor, supresses malignant phenotype through impaired glycolytic metabolism and generation of reactive oxygen species (ROS), which might be a promising agent for EC treatment [84]. Aldehyde dehydrogenase (ALDH), as a marker of cancer stem cells, is increased in EC tissues than normal and hyperplasia endometrium and could reflect the prognosis of patients with endometrial hyperplasia [85]. ALDH-dependent GLUT1 upregulation contributes to the activation of glycolysis and survival of cancer stem cells; the synergistic role of inhibition of ALDH or GLUT1 combined with taxane suppresses the tumorigenesis, which provides a new prospect for EC treatment [86]. Fructose-1,6-bisphosphate (F-1,6-BP), one metabolic intermediate of glycolytic pathway, promotes the generation of ROS and P53-dependent death in EC cells [87]. Genes associated with the regulation of glycolysis may affect biological behavior of EC cells. Proviral insertion in murine lymphomas 2 (PIM2) phosphorylates AMPKα1 to inhibit AMPKα1 kinase activity, which enhances glycolysis and tumor growth in EC [88]. Kinesin family member C1 (KIFC1) enhances glycolysis and survival of EC cells through HMGA1/c-myc pathway [89]. Anterior gradient 2 (AGR2) that is overexpressed in EC tissue has been reported to induce glucose uptake, lactate production and expression of glycolytic enzymes LDHA, phosphoglycerate kinase 1 (PGK1) and HK2, which causes subsequent EC progression through MUC1/HIF-1α pathway [90]. Upregulation of histone deacetylase 1 (HDAC1) also contributes to glycolysis and progression in EC [91].
Mitochondrial regulation
Although Warburg Effect has been the best-characterized metabolic status in tumor, oxidative phosphorylation (OXPHOS) is not inhibited in all types of cancers. It is currently emerging that some cancers including EC are mitochondrial OXPHOS-dependent for the energy need even with active glycolysis [92]. In some cases, OXPHOS has been found to be associated with the prognosis and progression of cancer. Takahashi et al. [93] conducted the Japanese molecular profiling of EC in 85 patients and found that patients with TP53-inactive ECs had poor prognosis and activated OXPHOS, indicating that targeting OXPHOS pathway may be a potential therapeutic strategy for EC. Compared with other subtypes of EC, serous-like ECs presents with substantially increased mitochondrial DNA (mtDNA) copy number [94]. Besides, TP53 mutations, significantly associated with mtDNA abundance, have been found to be enriched in serous-like ECs [94], which implies that mtDNA may involve in the poor prognosis of ECs especially in those with TP53 mutations. The newly constructed OXPHOS-related signature containing ATP5IF1, COX6B1, FOXP3 and NDUFB11 is a great predictive stool for the prognosis of EC, since patients with low-risk score tend to be more immunogenic so that it could stratify patients with EC into different risk groups and optimize the therapy strategies [95].
Malate dehydrogenase (MDH) which catalyzes malate into oxaloacetate is an important NAD-dependent dehydrogenases in the mitochondrial tricarboxylic acid cycle and regulates the metabolism of cancers. MDH2, the isoform of MDH, has been reported to be involved in the development of PTEN-regulated EC [96]. Clinically, MDH2 is overexpressed in the cytoplasm of EC tissues, with a relation to the EC grade. Mechanically, MDH2, co-localizing with PTEN in the cytoplasm, promotes the proliferation and invasion as well as inhibits the apoptosis of EC cells via the suppression of the PTEN expression. Therefore, MDH2 might lead to the disfunction of PTEN and subsequent of the development of EC. In addition to mitochondrial enzyme, genes that mediates mitochondrial energy metabolism may also affect cancer cell survival. COX7RP, one mitochondrial gene coded by the nuclear DNA, involves in the respiratory supercomplex assembly and mitochondrial respiration. Overexpression of COX7RP has been found in EC tissues and could promotes EC cells growth, stabilizes mitochondrial supercomplex assembly and regulate the metabolism in vitro and in vivo [97].
Multiple ways of mitochondrial biology beyond metabolic reprogramming contributes to tumorigenesis such as mitochondrial biogenesis, fission and fusion dynamics [98]. The peroxisome proliferator-activated receptor gamma coactivator 1α(PGC-1α) is an important regulator of mitochondrial biogenesis and oxidative metabolism. It activates nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2) and thus induces the expression of transcription factor A mitochondrial (TFAM), which regulates the mtDNA replication and transcription [99]. The PGC-1α-dependent pathway of mitochondrial biogenesis is upregulated in type I EC, leading to the 2-fold increase of mtDNA content in EC tissue than the proliferative endometrial tissue [100]. Mitochondria are in the dynamic balance between division and fusion, and the disruption of this homeostasis will affect their functions. Dynamin-related protein 1 (Drp1) phosphorylation is increased in EC cells exposed to high glucose, which enhances mitochondrial fission and thus promotes EC cells proliferation, migration and invasion; Drp1 activation is also increased in tissues from EC patients with diabetes than normal endometrial tissues [101]. Besides intracellular mitochondrial movement, recent evidences have indicated that intercellular mitochondrial transfer through tunnelling nanotubes extracellular vesicles and gap junction channels may have potential effects on recipient cells for maintaining body homeostasis and regulating pathological processes [102]. The gap junction protein connexin 43 (Cx43) mediates mitochondrial transfer and is important for intercellular communications [103]. One study reported that Cx43 was weakly expressed in endometrial hyperplasia and EC tissues, suggesting impaired gap junctional intercellular communication in the carcinogenesis of endometrium [104]. Deletion of Cx43 expression in EC cells has been reported to increase EC cellular migration; DNA hypermethylation of GJA1 encoding the protein Cx43 was found in human EC samples which was associated with obesity and in vitro reversal of DNA methylation enhanced intercellular communication and interactions, implying that targeting the activity of gap junction could be used to prevent against obesity-associated EC [105].
The mutations in the fibroblast growth factor receptor 2 (FGFR2) related to poor prognosis have to be found in 12% EC patients with stage I/II and 17% EC patients with stage III/IV [106], indicating that FGFR2 inhibition might be a potential treatment for EC. Packer et al. [107] found that FGFR inhibitors led to FGFR2-mutant EC cell lines death through mitochondrial disfunction as the mitochondrial depolarization, cytochrome c release and mitochondrial respiration damage. IL-24, known as an antitumor gene, may be a potential gene therapeutic target for EC. Overexpression of IL-24 leads to apoptosis via the mitochondrial intrinsic signaling pathway with increased BAX and Cytochrome C as well as decreased BCL-2, Caspase-9 and Caspase-3 [108].
Glucose metabolism-targeted therapies for EC
Weight loss and bariatric surgery
Weight loss is correlated with lower risk and improved survival of EC. One observational study enrolled 36, 794 postmenopausal women (50 to 79 years old) with body weight measured at baseline and year 3, which found that EC risk was reduced in women with weight loss (change ≥ 5%) (HR 0.71, 95% CI 0.54–0.95), especially in obese women with intentional weight loss (HR 0.44, 95% CI 0.25–0.78) [109]. In 875 EC patients, prediagnosis moderate-to-vigorous intensity physical activity (more than 7 h per week) was associated with lower all-cause-5-year mortality (HR 0.57, 95% CI 0.33–0.98) than never or rare exercise, but this correlation was reduced after adjustment for BMI (HR 0.64, 95% CI 0.37–1.12) [110]. In addition to intentional weight loss and physical activity, bariatric surgery has also been reported to reduce the risk of EC. One meta-analysis of 5 studies evaluating the effect of bariatric surgery on EC risk included 113,032 women underwent bariatric surgery as well as 848,864 controls and found that 462 (1.4%) and 11,997 (1.4%) women developed EC respectively (OR 0.317, 95% CI 0.161–0.627) [111]. However, what mechanism contribute to the decreased EC risk in women with weight loss? Firstly, weight loss is associated with changes of sex hormone levels. A prospective study enrolling 106 women with median BMI of 44.5 has found that bariatric surgery reduces the body weight by 32.7% and estradiol level by 35.5% at 1 year after the surgery [112]. Besides, weight loss could improve glucose homeostasis and suppress insulin resistance [113, 114]. Furthermore, weight loss could change the inflammation status with decreased level of C-reactive protein (CRP) and IL-6 [113].
Dietary interventions
Dietary factors have a significant effect in systematic metabolic process and may influence oncogenesis driven by abnormal metabolism. Recent evidences have indicated that interventions targeting dietary patterns and components may reduce risk and improve outcomes of metabolic-associated diseases. Mediterranean diet is a plant-based diet pattern and characterized by high intake of plant foods (fruits, vegetables, breads, whole grains, beans and nuts) and olive oil, moderate intake of wine especially red wine during meals, minimal intake of processed food [115]. Evidences have indicated that mediterranean diet is beneficial for cardiovascular health with reduced incidence of cardiovascular diseases and risk factors including obesity, hypertension, metabolic syndrome and dyslipidaemia, and is also protective against type 2 diabetes and provides better glycaemic control [115]. Since menopause is correlated with weight gain and redistribution of abdominal adipose tissue, increased visceral fat secretes excessive adipocytokines leading to systematic metabolic disorder; therefore, mediterranean diet is recommended for menopausal women to prevent obesity and its associated diseases [116]. Compared with women with low adherence to the mediterranean diet, women with high adherence have a nearly half reduced risk of EC (OR 0.51, 95% CI 0.39–0.86) [117]. In one pooled analysis of three case-control studies including 1411 EC patients and 3668 controls, mediterranean diet is associated with lower risk of EC and the OR for an increment of one component of the mediterranean diet was 0.84 (95% CI 0.80–0.88), and the association was stronger in older women or those who never use oral contraceptive or hormone replacement therapy [118]. However, in one prospective analysis enrolling 84,415 postmenopausal women, there was no association between mediterranean diet and EC risk during follow-up for 13.3 years (HR 0.98, 95% CI: 0.82–1.17) [119].
Ketogenic diet is another dietary pattern that has attracted extensive attention in recent years. It is characterized by a high-fat, adequate-protein and very-low-carbohydrate diet regimen that stimulates metabolic status of fasting leading to the production of ketone bodies [120]. Emerging evidences have indicated that ketogenic diet may be a potential therapeutic strategy for metabolic disorders including epilepsy, type 2 diabetes, obesity, nonalcoholic fatty liver disease, PCOS as well as cancers through reduction of blood glucose and insulin levels, improving insulin sensitivity and regulation of glucose and fat metabolism [120, 121]. Compared with consuming the American Cancer Society (ACS) diet characterized by high-fiber and lower-fat, adherence to ketogenic diet for 12 weeks in ovarian cancer or EC patients was associated with higher physical function score and does not have serious adverse events or affect serum lipid levels, suggesting that short-term ketogenic diet may be a safe approach of dietary intervention for EC patients [122, 123]. In addition, ketogenic diet was reported to be associated with more reduction of visceral fat mass and lower fasting serum insulin than ACS diet in ovarian cancer or EC patients, further indicating the potential therapeutic role of ketogenic diet in EC [124].
Pharmacological treatments
Statins
Statins, also known as 3-hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, exert a potent lipid-lowering effect and thus are used to prevent cardiovascular disease [125]. Through inhibition of the rate-limiting enzyme cholesterol biosynthesis HMG-CoA and subsequent conversion into mevalonate, it reduces the production of cholesterol, increases cellular LDL receptor and promotes the clearance of serum LDL-cholesterol [126]. However, emerging evidences have uncovered pleiotropic effects of statins in cardiovascular protection beyond cholesterol-reduction, which may through plaque stabilization and regression, anti-inflammation effects [126]. In addition to the use in cardiovascular diseases, statins have been paid great attention for their anti-cancer effect through inhibition of cholesterol-associated pathways responsible for cancer cells proliferation [127]. In vitro, simvastatin and metformin synergistically inhibit EC cells viability through inhibition of mTOR pathway, which indicate the potential of statins for EC therapy [128]. Epidemiological studies have indicated that long-term use of statins is associated with the reduction of EC risk [129, 130] and mortality [131]. Statin use contributes to better prognosis of EC patients with increased overall survival and disease-specific survival [132]. In EC patients with hyperlipemia and high-risk histology, statins use has significantly improved their overall survival than those not using statins [133]. However, several populational evidences reported contrary results and did not support anti-cancer role of statins against EC [134, 135]. Since there are bias about study population and results assessment in current studies, the effect of statins in EC may need to be re-evaluated. Further studies should focus on the effect of statins for EC patients with different lipid profile and histological subtypes, which could help exactly identify patients who may benefit from statins [136].
Metformin
Metformin has been widely used as a significant pre-surgical treatment in EC. Metformin is correlated with the reversion of atypical endometrial hyperplasia to normal endometrium [137]. When combined with megestrol acetate, metformin could achieve better prognosis for patients with atypical endometrial hyperplasia than megestrol acetate alone and thus may be a promising fertility-sparing treatment [138]. Besides, metformin is also associated with the reduction of EC risk and could improve the survival of EC patients [137, 139]. In vitro studies have reported that metformin promotes the apoptosis and autophagy and inhibits the proliferation of EC cells through activation of AMPK and subsequent inhibition of mTOR pathway [140, 141]. The PD-L1 expression of EC cells exposed to metformin was reduced through AMPK activation, which further inhibit EC cells growth [142]. In addition to the direct anti-tumor roles, metformin could also inhibit tumor progression through infiltration of tumor-associated macrophages [143] and promotes the M1-like phenotype of tumor-associated macrophages to supress tumor growth and angiogenesis [144]. However, the response to metformin still varies a lot in different patients. One possible cause to that heterogeneity may be hyperglycaemia and hypoxia, which could lead to the switch to glycolysis and reduced metformin response in EC [145]. Targeting on pyruvate dehydrogenase kinase 1 (PDK1) may help solve this dilemma since it could promote the glycolysis of EC cells exposed to long-term high-concentrated glucose; the combination of PDK1 inhibitor JX06 and metformin could inhibit EC cells growth in persistently hyperglycemic environment, which provides the therapeutic prospect for EC patients complicated with diabetes mellitus [146]. Further preclinical investigations are needed to explicit the effect of metformin and potential adjuvant agents in EC therapy, which could help to identify the optimal treatment strategy for EC patients.
Biomaterials-based therapy
Recent development and application of biomaterials and technologies have broadened our insight of the diagnosis and treatment in various diseases [147, 148]. The limits of conventional therapies for cancers accelerate the development of nanotherapies, which have great therapeutic potential for their distinctive features such as increasing the drug therapeutic efficacy and reducing toxicities, targeted delivery of drugs to specific sites and co-delivery of multiple drugs [149]. Multiple nanotechnology platforms like liposomes, albumin nanoparticle and polymeric micelles combining with therapeutic modalities such as chemotherapy, hyperthermia, radiotherapy, gene or RNA interference and immunotherapy have been applied in clinical stage [149]. The oxidative stress amplifying micellar nanoparticles would be a novel anticancer strategy since it been reported to generate ROS and to suppress antioxidant simultaneously, and thus it could make cancer cells more vulnerable to ROS resulting in increased apoptotic cell death and inhibition of tumor growth [150]. Hyaluronic acid (HA)-based hydrogels have shown great potential in drug delivery and targeted cancer therapy since cellular membrane surface HA receptors like CD44 are upregulated in multiple cancer cells [151]. HA-based nanogels loaded with antitumor drugs could be highly internalized by cancer cells overexpressing CD44 and have great inhibition of tumor growth and metastasis [151]. Highly expression of CD44 was reported to be found in 35.4% of EC patients, which was associated with poor overall survival [152]. In vitro study has repeated that HA-functionalized nanomicelles encapsulating suberoylanilide hydroxamic acid (SAHA), one inhibitor of HDAC, increased SAHA delivery and inhibited growth of EC cells expressing CD44 [153]. The application of biomaterials in metabolic-targeting therapy for cancers is still lacking. Current studies mainly focus on the use of versatile platforms with new technologies to combine both metabolic-improving and chemotherapeutic drugs. The co-delivery of metformin and paclitaxel through the folate-modified pH-sensitive micelles leads to higher cytotoxicity and apoptosis against breast cancer cells as well as increased drug uptake and anti-tumor effect in vivo, which could reduce the toxicity of paclitaxel and may be a promising therapy for cancer [154].
Conclusion
Substantial progresses have been achieved in exploration of metabolic changes in EC. Metabolic syndrome is closely associated with the development and progression of EC, in which obesity and insulin resistance are key points that involve in the pathogenesis process. Obesity plays a core role in EC, which affects not only systemic metabolism but also inherent tumor cell metabolism. Tumor cells adjust the metabolism to meet energy requirement of cellular survival and functions, which promotes tumorigenesis and progression. In this study, the metabolic characteristic especially distinctive changes in glycolytic pathway and mitochondrial functions in EC have been systematically summarized. The known evidences of obesity-associated adipocytokines, insulin resistance and metabolic alterations as crucial oncogenic factors of EC have enabled therapeutic strategies of EC targeting systematic or cellular metabolic process. Management of metabolic syndrome through weight loss, lipid- and glucose-lowing is an effective method for EC treatment and prevention. Various studies have assessed the role of treatments including bariatric surgery, statins and metformin on EC, revealing great potential for reduction of EC risk and improving the survival of EC patients. The lifestyle interventions like mediterranean diet are beneficial for patients with obesity, metabolic syndrome and diabetes, and thus could be used as a safe and feasible approach for preventing EC.
However, since the metabolic pathways in tumors are highly plastic, metabolism is reprogrammed not only in glucose but also in other cellular component such as lipid and amino acid. Besides, the metabolic interactional networks among tumor cells and surrounding microenvironment form the unique metabolic condition, which may together contribute to the development and malignant progress of EC. Although obesity-associated adipocytokines, insulin resistance and subsequent metabolic pathways has been proven to promote malignancy of EC, studies on crosstalk of cellular glucose and lipid metabolism as well as immune responses to these metabolic alterations are still lacking. Figuring out the exact metabolic dependency and compensation mechanism along with response of immune microenvironment towards metabolic status may provide new targets for EC therapy. Further studies are required to stratify EC patients with different metabolic status in view of available therapy and to identify personalized treatment regimen.
Acknowledgements
Not applicable.
Author contributions
HPZ conceived the study and drafted the manuscript. FXQ, YHF, ZKW and LHR searched relevant literatures and prepared the figure. WYM and XFX revised the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported by National Nature Science Foundation of China (Grant No. 81972448, 82172626); Tianjin Municipal Science and Technology Bureau, China (Grant No. 21JCYBJC01080); Tianjin Health Commission (Grant No. TJWJ2022XK003); Tianjin Key Medical Discipline (Specialty) Construction Project.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pengzhu Huang, Email: huangpz2019@163.com.
Xiangqin Fan, Email: fanxiangqin1992@tmu.edu.cn.
Hongfei Yu, Email: yuhongfei@tmu.edu.cn.
Kaiwen Zhang, Email: zkw412727@163.com.
Huanrong Li, Email: lihuanrong93@tom.com.
Yingmei Wang, Email: wangyingmei@tmu.edu.cn.
Fengxia Xue, Email: xuefengxia@tmu.edu.cn.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet (London England) 2022;399(10333):1412–28. doi: 10.1016/S0140-6736(22)00323-3. [DOI] [PubMed] [Google Scholar]
- 3.Herrera Cappelletti E, Humann J, Torrejón R, Gambadauro P. Chances of pregnancy and live birth among women undergoing conservative management of early-stage endometrial cancer: a systematic review and meta-analysis. Hum Reprod Update. 2022;28(2):282–95. doi: 10.1093/humupd/dmab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elia I, Haigis MC. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism. Nat Metab. 2021;3(1):21–32. doi: 10.1038/s42255-020-00317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyo S, Nakayama K. Endometrial cancer as a metabolic disease with dysregulated PI3K signaling: shedding light on novel therapeutic strategies. Int J Mol Sci. 2020;21(17). [DOI] [PMC free article] [PubMed]
- 6.Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20(2):74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi J, Wu S, Zhang W, Mischel PS. Targeting cancer’s metabolic co-dependencies: a landscape shaped by genotype and tissue context. Biochim et Biophys Acta Rev Cancer. 2018;1870(1):76–87. doi: 10.1016/j.bbcan.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, et al. The Interleukin-2-mTORc1 kinase Axis defines the signaling, differentiation, and metabolism of T Helper 1 and follicular B helper T cells. Immunity. 2015;43(4):690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumari N, Das A, Bhatt AN. Interleukin-6 confers radio-resistance by inducing akt-mediated glycolysis and reducing mitochondrial damage in cells. J BioChem. 2020;167(3):303–14. doi: 10.1093/jb/mvz091. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Guan D, Huo J, Biswas SK, Huang Y, Yang Y, et al. IL-10 enhances human natural killer cell effector functions via metabolic reprogramming regulated by mTORC1 Signaling. Front Immunol. 2021;12:619195. doi: 10.3389/fimmu.2021.619195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madeddu C, Sanna E, Gramignano G, Tanca L, Cherchi MC, Mola B et al. Correlation of leptin, proinflammatory cytokines and oxidative stress with tumor size and disease stage of endometrioid (Type I) endometrial cancer and review of the underlying mechanisms. Cancers. 2022;14(2). [DOI] [PMC free article] [PubMed]
- 12.Chopra V, Dinh TV, Hannigan EV. Serum levels of interleukins, growth factors and angiogenin in patients with endometrial cancer. J Cancer Res Clin Oncol. 1997;123(3):167–72. doi: 10.1007/BF01214669. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Zhang J, Hu M, Zhang Y, Cui P, Li X, et al. Differential expression patterns of glycolytic enzymes and Mitochondria-Dependent apoptosis in PCOS patients with endometrial hyperplasia, an early Hallmark of Endometrial Cancer, in vivo and the impact of Metformin in Vitro. Int J Biol Sci. 2019;15(3):714–25. doi: 10.7150/ijbs.31425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Du ZH, Qiao JM, Gao S. Association between metabolic syndrome and endometrial cancer risk: a systematic review and meta-analysis of observational studies. Aging. 2020;12(10):9825–39. doi: 10.18632/aging.103247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trabert B, Wentzensen N, Felix AS, Yang HP, Sherman ME, Brinton LA. Metabolic syndrome and risk of endometrial cancer in the united states: a study in the SEER-medicare linked database. Cancer Epidemiol Biomark Prevent. 2015;24(1):261–7. doi: 10.1158/1055-9965.EPI-14-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Li X, Dong Y, Fan Y, Cheng Y, Zhai L, et al. Effects of metabolic syndrome and its components on the prognosis of Endometrial Cancer. Front Endocrinol. 2021;12:780769. doi: 10.3389/fendo.2021.780769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokts-Porietis RL, McNeil J, Nelson G, Courneya KS, Cook LS, Friedenreich CM. Prospective cohort study of metabolic syndrome and endometrial cancer survival. Gynecol Oncol. 2020;158(3):727–33. doi: 10.1016/j.ygyno.2020.06.488. [DOI] [PubMed] [Google Scholar]
- 18.Wise MR, Jordan V, Lagas A, Showell M, Wong N, Lensen S, et al. Obesity and endometrial hyperplasia and cancer in premenopausal women: a systematic review. Am J Obstet Gynecol. 2016;214(6):689. doi: 10.1016/j.ajog.2016.01.175. [DOI] [PubMed] [Google Scholar]
- 19.Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Annal Oncol. 2015;26(8):1635–48. doi: 10.1093/annonc/mdv142. [DOI] [PubMed] [Google Scholar]
- 20.Dougan MM, Hankinson SE, Vivo ID, Tworoger SS, Glynn RJ, Michels KB. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int J Cancer. 2015;137(3):625–37. doi: 10.1002/ijc.29427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byun D, Hong S, Ryu S, Nam Y, Jang H, Cho Y, et al. Early-life body mass index and risks of breast, endometrial, and ovarian cancers: a dose-response meta-analysis of prospective studies. Br J Cancer. 2022;126(4):664–72. doi: 10.1038/s41416-021-01625-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, et al. Duration of adulthood overweight, obesity, and cancer risk in the women’s health initiative: a longitudinal study from the United States. PLoS Med. 2016;13(8):e1002081. doi: 10.1371/journal.pmed.1002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christakoudi S, Pagoni P, Ferrari P, Cross AJ, Tzoulaki I, Muller DC, et al. Weight change in middle adulthood and risk of cancer in the european prospective investigation into Cancer and Nutrition (EPIC) cohort. Int J Cancer. 2021;148(7):1637–51. doi: 10.1002/ijc.33339. [DOI] [PubMed] [Google Scholar]
- 24.Kokts-Porietis RL, Elmrayed S, Brenner DR, Friedenreich CM. Obesity and mortality among endometrial cancer survivors: a systematic review and meta-analysis. Obes reviews: official J Int Association Study Obes. 2021;22(12):e13337. doi: 10.1111/obr.13337. [DOI] [PubMed] [Google Scholar]
- 25.Ding S, Madu CO, Lu Y. The impact of hormonal imbalances associated with obesity on the incidence of endometrial cancer in postmenopausal women. J Cancer. 2020;11(18):5456–65. doi: 10.7150/jca.47580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delahanty RJ, Beeghly-Fadiel A, Xiang YB, Long J, Cai Q, Wen W, et al. Association of obesity-related genetic variants with endometrial cancer risk: a report from the Shanghai Endometrial Cancer Genetics Study. Am J Epidemiol. 2011;174(10):1115–26. doi: 10.1093/aje/kwr233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehr E, Schweighofer N, Möller R, Giuliani A, Pieber TR, Obermayer-Pietsch B. Association of FTO gene with hyperandrogenemia and metabolic parameters in women with polycystic ovary syndrome. Metab Clin Exp. 2010;59(4):575–80. doi: 10.1016/j.metabol.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Huang X, Zhao J, Yang M, Li M, Zheng J. Association between FTO gene polymorphism (rs9939609 T/A) and cancer risk: a meta-analysis. Eur J Cancer Care. 2017;26(5). [DOI] [PubMed]
- 29.Liu Y, Liang G, Xu H, Dong W, Dong Z, Qiu Z, et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metabol. 2021;33(6):1221–33e11. doi: 10.1016/j.cmet.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Zhou D, Lai Y, Liu Y, Tao X, Wang Q, et al. Estrogen induces endometrial cancer cell proliferation and invasion by regulating the fat mass and obesity-associated gene via PI3K/AKT and MAPK signaling pathways. Cancer Lett. 2012;319(1):89–97. doi: 10.1016/j.canlet.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Cordido M, Juiz-Valiña P, Urones P, Sangiao-Alvarellos S, Cordido F. Thyroid function alteration in obesity and the effect of bariatric surgery. J Clin Med. 2022;11(5). [DOI] [PMC free article] [PubMed]
- 32.Song RH, Wang B, Yao QM, Li Q, Jia X, Zhang JA. The impact of obesity on thyroid autoimmunity and dysfunction: a systematic review and meta-analysis. Front Immunol. 2019;10:2349. doi: 10.3389/fimmu.2019.02349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Zhou R, Wang J. Relationship between hypothyroidism and endometrial cancer. Aging Dis. 2019;10(1):190–6. doi: 10.14336/AD.2018.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seebacher V, Hofstetter G, Polterauer S, Reinthaller A, Grimm C, Schwameis R, et al. Does thyroid-stimulating hormone influence the prognosis of patients with endometrial cancer? A multicentre trial. Br J Cancer. 2013;109(1):215–8. doi: 10.1038/bjc.2013.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barr CE, Njoku K, Hotchkies L, Ryan NAJ, Wan YL, Davies DA, et al. Does clinical and biochemical thyroid dysfunction impact on Endometrial Cancer Survival Outcomes? A prospective database study. Cancers. 2021;13:21. doi: 10.3390/cancers13215444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treister-Goltzman Y, Yarza S, Peleg R. Lipid profile in mild subclinical hypothyroidism: systematic review and meta-analysis. Minerva Endocrinol. 2021;46(4):428–40. doi: 10.23736/S2724-6507.20.03197-1. [DOI] [PubMed] [Google Scholar]
- 37.Liu H, Peng D. Update on dyslipidemia in hypothyroidism: the mechanism of dyslipidemia in hypothyroidism. Endocr Connect. 2022;11(2). [DOI] [PMC free article] [PubMed]
- 38.Lindemann K, Vatten LJ, Ellstrøm-Engh M, Eskild A. Serum lipids and endometrial cancer risk: results from the HUNT-II study. Int J Cancer. 2009;124(12):2938–41. doi: 10.1002/ijc.24285. [DOI] [PubMed] [Google Scholar]
- 39.Luo YZ, Yang Z, Qiu YL, Li XH, Qin LQ, Su QS, et al. Pretreatment triglycerides-to-high density lipoprotein cholesterol ratio in postmenopausal women with endometrial cancer. Kaohsiung J Med Sci. 2019;35(5):303–9. doi: 10.1002/kjm2.12033. [DOI] [PubMed] [Google Scholar]
- 40.Tian W, Zhu Y, Wang Y, Teng F, Zhang H, Liu G, et al. Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol Oncol. 2013;129(3):505–12. doi: 10.1016/j.ygyno.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Cymbaluk-Płoska A, Chudecka-Głaz A, Pius-Sadowska E, Sompolska-Rzechuła A, Machaliński B, Menkiszak J. Circulating serum level of visfatin in patients with endometrial Cancer. Biomed Res Int. 2018;2018:8576179. doi: 10.1155/2018/8576179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Liu L, Li C, Ai H. Correlation analysis between the expressions of leptin and its receptor (ObR) and clinicopathology in endometrial cancer. Cancer Biomark A. 2014;14(5):353–9. doi: 10.3233/CBM-140415. [DOI] [PubMed] [Google Scholar]
- 43.Hlavna M, Kohut L, Lipkova J, Bienertova-Vasku J, Dostalova Z, Chovanec J, et al. Relationship of resistin levels with endometrial cancer risk. Neoplasma. 2011;58(2):124–8. doi: 10.4149/neo_2011_02_124. [DOI] [PubMed] [Google Scholar]
- 44.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–4. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diab/Metab Res Rev. 2011;27(6):515–27. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 46.Codoñer-Franch P, Alonso-Iglesias E. Resistin: insulin resistance to malignancy. Clin Chim Acta. 2015;438:46–54. doi: 10.1016/j.cca.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 47.Erdogan S, Sezer S, Baser E, Gun-Eryilmaz O, Gungor T, Uysal S, et al. Evaluating vaspin and adiponectin in postmenopausal women with endometrial cancer. Endocr Relat Cancer. 2013;20(5):669–75. doi: 10.1530/ERC-13-0280. [DOI] [PubMed] [Google Scholar]
- 48.Ray I, Meira LB, Michael A, Ellis PE. Adipocytokines and disease progression in endometrial cancer: a systematic review. Cancer Metastasis Rev. 2022;41(1):211–42. doi: 10.1007/s10555-021-10002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hung AC, Lo S, Hou MF, Lee YC, Tsai CH, Chen YY, et al. Extracellular visfatin-promoted malignant behavior in breast Cancer is mediated through c-Abl and STAT3 activation. Clin Cancer Res. 2016;22(17):4478–90. doi: 10.1158/1078-0432.CCR-15-2704. [DOI] [PubMed] [Google Scholar]
- 50.Vachher M, Arora K, Burman A, Kumar B. NAMPT, GRN, and SERPINE1 signature as predictor of disease progression and survival in gliomas. J Cell Biochem. 2020;121(4):3010–23. doi: 10.1002/jcb.29560. [DOI] [PubMed] [Google Scholar]
- 51.Neubauer K, Misa IB, Diakowska D, Kapturkiewicz B, Gamian A, Krzystek-Korpacka M. Nampt/PBEF/visfatin upregulation in colorectal tumors, mirrored in normal tissue and whole blood of colorectal cancer patients, is associated with metastasis, hypoxia, IL1β, and anemia. Biomed Res Int. 2015;2015:523930. doi: 10.1155/2015/523930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Gao C, Zhang Y, Gao J, Teng F, Tian W, et al. Visfatin stimulates endometrial cancer cell proliferation via activation of PI3K/Akt and MAPK/ERK1/2 signalling pathways. Gynecol Oncol. 2016;143(1):168–78. doi: 10.1016/j.ygyno.2016.07.109. [DOI] [PubMed] [Google Scholar]
- 53.Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circul Res. 2020;126(11):1549–64. doi: 10.1161/CIRCRESAHA.119.315896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burzawa JK, Schmeler KM, Soliman PT, Meyer LA, Bevers MW, Pustilnik TL, et al. Prospective evaluation of insulin resistance among endometrial cancer patients. Am J Obstet Gynecol. 2011;204(4):355e1–7. doi: 10.1016/j.ajog.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez AV, Pasupuleti V, Benites-Zapata VA, Thota P, Deshpande A, Perez-Lopez FR. Insulin resistance and endometrial cancer risk: a systematic review and meta-analysis. Eur J Cancer (Oxford, England: 1990) 2015;51(18):2747–58. doi: 10.1016/j.ejca.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 56.Shan W, Ning C, Luo X, Zhou Q, Gu C, Zhang Z, et al. Hyperinsulinemia is associated with endometrial hyperplasia and disordered proliferative endometrium: a prospective cross-sectional study. Gynecol Oncol. 2014;132(3):606–10. doi: 10.1016/j.ygyno.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Teng F, Ma X, Yu X, Yan Y, Zhao J, Gao J, et al. High serum androgen and insulin concentrations increase the tendency of endometrial carcinoma. J Cancer. 2020;11(19):5656–64. doi: 10.7150/jca.46391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian W, Teng F, Zhao J, Gao J, Gao C, Sun D, et al. Estrogen and insulin synergistically promote type 1 endometrial cancer progression. Cancer Biol Ther. 2017;18(12):1000–10. doi: 10.1080/15384047.2017.1394547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Fan Y, Wang J, Zhou R, Tian L, Wang Y, et al. Insulin resistance and metabolic syndrome increase the risk of Relapse for Fertility preserving treatment in atypical endometrial hyperplasia and early endometrial Cancer patients. Front Oncol. 2021;11:744689. doi: 10.3389/fonc.2021.744689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Hua S, Tian W, Zhang L, Zhao J, Zhang H, et al. Mitogenic and anti-apoptotic effects of insulin in endometrial cancer are phosphatidylinositol 3-kinase/Akt dependent. Gynecol Oncol. 2012;125(3):734–41. doi: 10.1016/j.ygyno.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Zhu Y, Zhang L, Tian W, Hua S, Zhao J, et al. Insulin promotes proliferation, survival, and invasion in endometrial carcinoma by activating the MEK/ERK pathway. Cancer Lett. 2012;322(2):223–31. doi: 10.1016/j.canlet.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 62.Tian W, Teng F, Gao J, Gao C, Liu G, Zhang Y, et al. Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways. Cancer Biol Med. 2019;16(1):55–70. doi: 10.20892/j.issn.2095-3941.2018.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lv QY, Xie BY, Yang BY, Ning CC, Shan WW, Gu C, et al. Increased TET1 expression in inflammatory microenvironment of hyperinsulinemia enhances the response of endometrial cancer to estrogen by epigenetic modulation of GPER. J Cancer. 2017;8(5):894–902. doi: 10.7150/jca.17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009;115(2):86–96. doi: 10.1080/13813450902878054. [DOI] [PubMed] [Google Scholar]
- 65.Lambe M, Wigertz A, Garmo H, Walldius G, Jungner I, Hammar N. Impaired glucose metabolism and diabetes and the risk of breast, endometrial, and ovarian cancer. Cancer Causes Control CCC. 2011;22(8):1163–71. doi: 10.1007/s10552-011-9794-8. [DOI] [PubMed] [Google Scholar]
- 66.Liao C, Zhang D, Mungo C, Tompkins DA, Zeidan AM. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol Oncol. 2014;135(1):163–71. doi: 10.1016/j.ygyno.2014.07.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Travier N, Jeffreys M, Brewer N, Wright CS, Cunningham CW, Hornell J, et al. Association between glycosylated hemoglobin and cancer risk: a New Zealand linkage study. Annal Oncol. 2007;18(8):1414–9. doi: 10.1093/annonc/mdm135. [DOI] [PubMed] [Google Scholar]
- 68.Han J, Zhang L, Guo H, Wysham WZ, Roque DR, Willson AK, et al. Glucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK signaling. Gynecol Oncol. 2015;138(3):668–75. doi: 10.1016/j.ygyno.2015.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, et al. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol Oncol Res POR. 2012;18(3):721–8. doi: 10.1007/s12253-012-9500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashton-Sager A, Paulino AF, Afify AM. GLUT-1 is preferentially expressed in atypical endometrial hyperplasia and endometrial adenocarcinoma. Appl Immunohistochem Mol Morphol AIMM. 2006;14(2):187–92. doi: 10.1097/01.pai.0000162003.43334.c7. [DOI] [PubMed] [Google Scholar]
- 71.Goldman NA, Katz EB, Glenn AS, Weldon RH, Jones JG, Lynch U, et al. GLUT1 and GLUT8 in endometrium and endometrial adenocarcinoma. Mod Pathol. 2006;19(11):1429–36. doi: 10.1038/modpathol.3800656. [DOI] [PubMed] [Google Scholar]
- 72.Byrne FL, Poon IK, Modesitt SC, Tomsig JL, Chow JD, Healy ME, et al. Metabolic vulnerabilities in endometrial cancer. Cancer Res. 2014;74(20):5832–45. doi: 10.1158/0008-5472.CAN-14-0254. [DOI] [PubMed] [Google Scholar]
- 73.Warburg O. On the origin of cancer cells. Science (New York NY) 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 74.Wang ZH, Zhang YZ, Wang YS, Ma XX. Identification of novel cell glycolysis related gene signature predicting survival in patients with endometrial cancer. Cancer Cell Int. 2019;19:296. doi: 10.1186/s12935-019-1001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J, Li S, Feng G, Meng H, Nie S, Sun R, et al. Nine glycolysis-related gene signature predicting the survival of patients with endometrial adenocarcinoma. Cancer Cell Int. 2020;20:183. doi: 10.1186/s12935-020-01264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang Y, Chen J, Ling J, Zhu X, Jiang P, Tang X, et al. Construction of a glycolysis-related long noncoding RNA signature for predicting survival in endometrial cancer. J Cancer. 2021;12(5):1431–44. doi: 10.7150/jca.50413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong P, Xiong Y, Konno Y, Ihira K, Kobayashi N, Yue J, et al. Long non-coding RNA DLEU2 drives EMT and glycolysis in endometrial cancer through HK2 by competitively binding with miR-455 and by modulating the EZH2/miR-181a pathway. J Exp Clin Cancer Res CR. 2021;40(1):216. doi: 10.1186/s13046-021-02018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zahra K, Dey T, Ashish, Mishra SP, Pandey U. Pyruvate kinase M2 and Cancer: the role of PKM2 in promoting Tumorigenesis. Front Oncol. 2020;10:159. doi: 10.3389/fonc.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai YJ, Chou YC, Lin YJ, Yu MH, Ou YC, Chu PW et al. Pyruvate kinase M2 expression: a potential metabolic biomarker to differentiate endometrial precancer and cancer that is associated with poor outcomes in endometrial carcinoma. Int J Environ Res Public Health. 2019;16(23). [DOI] [PMC free article] [PubMed]
- 80.Hosseini Nasab S, Jooya N, Esmaeili A, Zarrin Khameh N, Diaz-Arrastia C, Momeni M. Using pyruvate kinase as a predictor for patient with endometrial cancer having complex hyperplasia with atypia to prevent hysterectomy and preserve fertility: retrospective immunohistochemical study. Reprod Sci (Thousand Oaks, CA) 2018;25(8):1286–91. doi: 10.1177/1933719117741371. [DOI] [PubMed] [Google Scholar]
- 81.Marshall MJ, Goldberg DM, Neal FE, Millar DR. Enzymes of glucose metabolism in carcinoma of the cervix and endometrium of the human uterus. Br J Cancer. 1978;37(6):990–1001. doi: 10.1038/bjc.1978.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simaga S, Abramić M, Osmak M, Babić D, Ilić-Forko J. Total tissue lactate dehydrogenase activity in endometrial carcinoma. Int J Gynecol Cancer. 2008;18(6):1272–8. doi: 10.1111/j.1525-1438.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- 83.Zhang G, Ma A, Jin Y, Pan G, Wang C. LncRNA SNHG16 induced by TFAP2A modulates glycolysis and proliferation of endometrial carcinoma through miR-490-3p/HK2 axis. Am J Transl Res. 2019;11(11):7137–45. [PMC free article] [PubMed] [Google Scholar]
- 84.Han X, Sheng X, Jones HM, Jackson AL, Kilgore J, Stine JE, et al. Evaluation of the anti-tumor effects of lactate dehydrogenase inhibitor galloflavin in endometrial cancer cells. J Hematol Oncol. 2015;8:2. doi: 10.1186/s13045-014-0097-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mah V, Elshimali Y, Chu A, Moatamed NA, Uzzell JP, Tsui J, et al. ALDH1 expression predicts progression of premalignant lesions to cancer in type I endometrial carcinomas. Sci Rep. 2021;11(1):11949. doi: 10.1038/s41598-021-90570-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mori Y, Yamawaki K, Ishiguro T, Yoshihara K, Ueda H, Sato A, et al. ALDH-dependent glycolytic activation mediates stemness and paclitaxel resistance in patient-derived spheroid models of uterine endometrial cancer. Stem Cell Rep. 2019;13(4):730–46. doi: 10.1016/j.stemcr.2019.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costa BP, Nassr MT, Diz FM, Carlessi LP, Fernandes KHA, Nunes FB, et al. Fructose-1,6-bisphosphate induces generation of reactive oxygen species and activation of p53-dependent cell death in human endometrial cancer cells. J Appl Toxicol JAT. 2021;41(7):1050–62. doi: 10.1002/jat.4091. [DOI] [PubMed] [Google Scholar]
- 88.Han X, Ren C, Yang T, Qiao P, Wang L, Jiang A, et al. Negative regulation of AMPKα1 by PIM2 promotes aerobic glycolysis and tumorigenesis in endometrial cancer. Oncogene. 2019;38(38):6537–49. doi: 10.1038/s41388-019-0898-z. [DOI] [PubMed] [Google Scholar]
- 89.Zhou K, Lin J, Dai M, He Y, Xu J, Lin Q. KIFC1 promotes aerobic glycolysis in endometrial cancer cells by regulating the c-myc pathway. J Bioenerg Biomembr. 2021;53(6):703–13. doi: 10.1007/s10863-021-09924-1. [DOI] [PubMed] [Google Scholar]
- 90.Gong W, Ekmu B, Wang X, Lu Y, Wan L. AGR2-induced glucose metabolism facilitated the progression of endometrial carcinoma via enhancing the MUC1/HIF-1α pathway. Hum Cell. 2020;33(3):790–800. doi: 10.1007/s13577-020-00356-4. [DOI] [PubMed] [Google Scholar]
- 91.Wu Q, Zhang W, Liu Y, Huang Y, Wu H, Ma C. Histone deacetylase 1 facilitates aerobic glycolysis and growth of endometrial cancer. Oncol Lett. 2021;22(4):721. doi: 10.3892/ol.2021.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative phosphorylation as an emerging target in cancer therapy. Clin Cancer Res. 2018;24(11):2482–90. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 93.Takahashi N, Hatakeyama K, Nagashima T, Ohshima K, Urakami K, Yamaguchi K, et al. Activation of oxidative phosphorylation in TP53-inactive endometrial carcinomas with a poor prognosis. Int J Gynecol cancer: official J Int Gynecol Cancer Soc. 2021;31(12):1557–63. doi: 10.1136/ijgc-2021-002983. [DOI] [PubMed] [Google Scholar]
- 94.Reznik E, Miller ML, Şenbabaoğlu Y, Riaz N, Sarungbam J, Tickoo SK et al. Mitochondrial DNA copy number variation across human cancers.eLife. 2016;5. [DOI] [PMC free article] [PubMed]
- 95.Liu J, Chen T, Yang M, Zhong Z, Ni S, Yang S, et al. Development of an oxidative phosphorylation-related and Immune microenvironment prognostic signature in uterine corpus endometrial carcinoma. Front Cell Dev Biol. 2021;9:753004. doi: 10.3389/fcell.2021.753004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhuang Y, Xiang J, Bao W, Sun Y, Wang L, Tan M, et al. MDH2 stimulated by estrogen-GPR30 pathway down-regulated PTEN expression promoting the proliferation and invasion of cells in endometrial cancer. Transl Oncol. 2017;10(2):203–10. doi: 10.1016/j.tranon.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ikeda K, Horie-Inoue K, Suzuki T, Hobo R, Nakasato N, Takeda S, et al. Mitochondrial supercomplex assembly promotes breast and endometrial tumorigenesis by metabolic alterations and enhanced hypoxia tolerance. Nat Commun. 2019;10(1):4108. doi: 10.1038/s41467-019-12124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vyas S, Zaganjor E, Haigis MC. Mitochondria and Cancer Cell. 2016;166(3):555–66. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bost F, Kaminski L. The metabolic modulator PGC-1α in cancer. Am J Cancer Res. 2019;9(2):198–211. [PMC free article] [PubMed] [Google Scholar]
- 100.Cormio A, Guerra F, Cormio G, Pesce V, Fracasso F, Loizzi V, et al. The PGC-1alpha-dependent pathway of mitochondrial biogenesis is upregulated in type I endometrial cancer. Biochem Biophys Res Commun. 2009;390(4):1182–5. doi: 10.1016/j.bbrc.2009.10.114. [DOI] [PubMed] [Google Scholar]
- 101.Guo J, Ye F, Jiang X, Guo H, Xie W, Zhang Y, et al. Drp1 mediates high glucose-induced mitochondrial dysfunction and epithelial-mesenchymal transition in endometrial cancer cells. Exp Cell Res. 2020;389(1):111880. doi: 10.1016/j.yexcr.2020.111880. [DOI] [PubMed] [Google Scholar]
- 102.Liu Z, Sun Y, Qi Z, Cao L, Ding S. Mitochondrial transfer/transplantation: an emerging therapeutic approach for multiple diseases. Cell Biosci. 2022;12(1):66. doi: 10.1186/s13578-022-00805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Norris RP. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic. 2021;22(6):174–9. doi: 10.1111/tra.12786. [DOI] [PubMed] [Google Scholar]
- 104.Saito T, Nishimura M, Kudo R, Yamasaki H. Suppressed gap junctional intercellular communication in carcinogenesis of endometrium. Int J Cancer. 2001;93(3):317–23. doi: 10.1002/ijc.1350. [DOI] [PubMed] [Google Scholar]
- 105.Polusani SR, Huang YW, Huang G, Chen CW, Wang CM, Lin LL, et al. Adipokines deregulate cellular communication via epigenetic repression of gap junction loci in obese endometrial cancer. Cancer Res. 2019;79(1):196–208. doi: 10.1158/0008-5472.CAN-18-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeske YW, Ali S, Byron SA, Gao F, Mannel RS, Ghebre RG, et al. FGFR2 mutations are associated with poor outcomes in endometrioid endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2017;145(2):366–73. doi: 10.1016/j.ygyno.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Packer LM, Stehbens SJ, Bonazzi VF, Gunter JH, Ju RJ, Ward M, et al. Bcl-2 inhibitors enhance FGFR inhibitor-induced mitochondrial-dependent cell death in FGFR2-mutant endometrial cancer. Mol Oncol. 2019;13(4):738–56. doi: 10.1002/1878-0261.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao S, Yang Y, Chen S, Bi Y, Huang Q, Wei Z, et al. IL-24 inhibits endometrial cancer cell proliferation by promoting apoptosis through the mitochondrial intrinsic signaling pathway. Biomed Pharmacothera. 2020;124:109831. doi: 10.1016/j.biopha.2020.109831. [DOI] [PubMed] [Google Scholar]
- 109.Luo J, Chlebowski RT, Hendryx M, Rohan T, Wactawski-Wende J, Thomson CA, et al. Intentional weight loss and endometrial Cancer risk. J Clin Oncol. 2017;35(11):1189–93. doi: 10.1200/JCO.2016.70.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arem H, Park Y, Pelser C, Ballard-Barbash R, Irwin ML, Hollenbeck A, et al. Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients. J Natl Cancer Inst. 2013;105(5):342–9. doi: 10.1093/jnci/djs530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28(5):1433–40. doi: 10.1007/s11695-018-3151-x. [DOI] [PubMed] [Google Scholar]
- 112.Sarwer DB, Spitzer JC, Wadden TA, Mitchell JE, Lancaster K, Courcoulas A, et al. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surg. 2014;149(1):26–33. doi: 10.1001/jamasurg.2013.5022. [DOI] [PubMed] [Google Scholar]
- 113.MacKintosh ML, Derbyshire AE, McVey RJ, Bolton J, Nickkho-Amiry M, Higgins CL, et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int J Cancer. 2019;144(3):641–50. doi: 10.1002/ijc.31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Modesitt SC, Hallowell PT, Slack-Davis JK, Michalek RD, Atkins KA, Kelley SL, et al. Women at extreme risk for obesity-related carcinogenesis: baseline endometrial pathology and impact of bariatric surgery on weight, metabolic profiles and quality of life. Gynecol Oncol. 2015;138(2):238–45. doi: 10.1016/j.ygyno.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 115.Guasch-Ferré M, Willett WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. 2021;290(3):549–66. doi: 10.1111/joim.13333. [DOI] [PubMed] [Google Scholar]
- 116.Barrea L, Pugliese G, Laudisio D, Colao A, Savastano S, Muscogiuri G. Mediterranean diet as medical prescription in menopausal women with obesity: a practical guide for nutritionists. Crit Rev Food Sci Nutr. 2021;61(7):1201–11. doi: 10.1080/10408398.2020.1755220. [DOI] [PubMed] [Google Scholar]
- 117.Ricceri F, Giraudo MT, Fasanelli F, Milanese D, Sciannameo V, Fiorini L, et al. Diet and endometrial cancer: a focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial cancer risk. BMC Cancer. 2017;17(1):757. doi: 10.1186/s12885-017-3754-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Filomeno M, Bosetti C, Bidoli E, Levi F, Serraino D, Montella M, et al. Mediterranean diet and risk of endometrial cancer: a pooled analysis of three Italian case–control studies. Br J Cancer. 2015;112(11):1816–21. doi: 10.1038/bjc.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.George SM, Ballard R, Shikany JM, Crane TE, Neuhouser ML. A prospective analysis of diet quality and endometrial cancer among 84,415 postmenopausal women in the Women’s Health Initiative. Ann Epidemiol. 2015;25(10):788–93. doi: 10.1016/j.annepidem.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Thera. 2022;7(1):11. doi: 10.1038/s41392-021-00831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barrea L, Caprio M, Tuccinardi D, Moriconi E, Di Renzo L, Muscogiuri G, et al. Could ketogenic diet “starve” cancer? Emerging evidence. Crit Rev Food Sci Nutr. 2022;62(7):1800–21. doi: 10.1080/10408398.2020.1847030. [DOI] [PubMed] [Google Scholar]
- 122.Cohen CW, Fontaine KR, Arend RC, Gower BA. A ketogenic Diet is acceptable in women with ovarian and endometrial Cancer and has no adverse Effects on blood lipids: a Randomized, Controlled Trial. Nutr Cancer. 2020;72(4):584–94. doi: 10.1080/01635581.2019.1645864. [DOI] [PubMed] [Google Scholar]
- 123.Cohen CW, Fontaine KR, Arend RC, Soleymani T, Gower BA. Favorable effects of a ketogenic diet on physical function, perceived energy, and food cravings in women with ovarian or endometrial cancer: a randomized, controlled trial. Nutrients. 2018;10(9). [DOI] [PMC free article] [PubMed]
- 124.Cohen CW, Fontaine KR, Arend RC, Alvarez RD, Leath CA, III, Huh WK, et al. A ketogenic Diet reduces central obesity and serum insulin in women with ovarian or endometrial Cancer. J Nutr. 2018;148(8):1253–60. doi: 10.1093/jn/nxy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. JAMA. 2022;328(8):746–53. doi: 10.1001/jama.2022.13044. [DOI] [PubMed] [Google Scholar]
- 126.Yu D, Liao JK. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc Res. 2022;118(2):413–23. doi: 10.1093/cvr/cvab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiang W, Hu JW, He XR, Jin WL, He XY. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res CR. 2021;40(1):241. doi: 10.1186/s13046-021-02041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim JS, Turbov J, Rosales R, Thaete LG, Rodriguez GC. Combination simvastatin and metformin synergistically inhibits endometrial cancer cell growth. Gynecol Oncol. 2019;154(2):432–40. doi: 10.1016/j.ygyno.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 129.Jacobs EJ, Newton CC, Thun MJ, Gapstur SM. Long-term use of cholesterol-lowering drugs and cancer incidence in a large United States cohort. Cancer Res. 2011;71(5):1763–71. doi: 10.1158/0008-5472.CAN-10-2953. [DOI] [PubMed] [Google Scholar]
- 130.Lavie O, Pinchev M, Rennert HS, Segev Y, Rennert G. The effect of statins on risk and survival of gynecological malignancies. Gynecol Oncol. 2013;130(3):615–9. doi: 10.1016/j.ygyno.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 131.Sperling CD, Verdoodt F, Kjaer Hansen M, Dehlendorff C, Friis S, Kjaer SK. Statin use and mortality among endometrial cancer patients: a danish nationwide cohort study. Int J Cancer. 2018;143(11):2668–76. doi: 10.1002/ijc.31625. [DOI] [PubMed] [Google Scholar]
- 132.Li J, Liu R, Sun Z, Tang S, Wang L, Liu C, et al. The association between statin use and endometrial cancer survival outcome: a meta-analysis. Medicine. 2018;97(47):e13264. doi: 10.1097/MD.0000000000013264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng CH, Miller CM, Tenney ME, Lee NK, Yamada SD, Hasan Y. Statin use significantly improves overall survival in High-Grade Endometrial Cancer. Int J Gynecol cancer: official J Int Gynecol Cancer Soc. 2016;26(9):1642–9. doi: 10.1097/IGC.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 134.Wang Y, Ren F, Song Z, Chen P, Liu S, Ouyang L. Statin use and the risk of ovarian and endometrial cancers: a meta-analysis. BMC Cancer. 2019;19(1):730. doi: 10.1186/s12885-019-5954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Segev Y, Gemer O, Helpman L, Hag-Yahia N, Eitan R, Raban O, et al. An israeli Gynecologic Oncology Group study of statin use and endometrial cancer prognosis. Int J Gynaecol Obstet. 2020;148(1):79–86. doi: 10.1002/ijgo.12981. [DOI] [PubMed] [Google Scholar]
- 136.Hafizz A, Zin RRM, Aziz NHA, Kampan NC, Shafiee MN. Beyond lipid-lowering: role of statins in endometrial cancer. Mol Biol Rep. 2020;47(10):8199–207. doi: 10.1007/s11033-020-05760-5. [DOI] [PubMed] [Google Scholar]
- 137.Meireles CG, Pereira SA, Valadares LP, Rêgo DF, Simeoni LA, Guerra ENS, et al. Effects of metformin on endometrial cancer: systematic review and meta-analysis. Gynecol Oncol. 2017;147(1):167–80. doi: 10.1016/j.ygyno.2017.07.120. [DOI] [PubMed] [Google Scholar]
- 138.Yang BY, Gulinazi Y, Du Y, Ning CC, Cheng YL, Shan WW, et al. Metformin plus megestrol acetate compared with megestrol acetate alone as fertility-sparing treatment in patients with atypical endometrial hyperplasia and well-differentiated endometrial cancer: a randomised controlled trial. BJOG. 2020;127(7):848–57. doi: 10.1111/1471-0528.16108. [DOI] [PubMed] [Google Scholar]
- 139.Tang YL, Zhu LY, Li Y, Yu J, Wang J, Zeng XX, et al. Metformin use is associated with reduced incidence and improved survival of endometrial cancer: a meta-analysis. Biomed Res Int. 2017;2017:5905384. doi: 10.1155/2017/5905384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takahashi A, Kimura F, Yamanaka A, Takebayashi A, Kita N, Takahashi K, et al. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int. 2014;14:53. doi: 10.1186/1475-2867-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation–implications for a novel treatment strategy. Gynecol Oncol. 2010;116(1):92–8. doi: 10.1016/j.ygyno.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xue J, Li L, Li N, Li F, Qin X, Li T, et al. Metformin suppresses cancer cell growth in endometrial carcinoma by inhibiting PD-L1. Eur J Pharmacol. 2019;859:172541. doi: 10.1016/j.ejphar.2019.172541. [DOI] [PubMed] [Google Scholar]
- 143.Liu Q, Tong D, Liu G, Gao J, Wang LA, Xu J, et al. Metformin inhibits prostate cancer progression by targeting tumor-associated inflammatory infiltration. Clin Cancer Res. 2018;24(22):5622–34. doi: 10.1158/1078-0432.CCR-18-0420. [DOI] [PubMed] [Google Scholar]
- 144.Wang JC, Sun X, Ma Q, Fu GF, Cong LL, Zhang H, et al. Metformin’s antitumour and anti-angiogenic activities are mediated by skewing macrophage polarization. J Cell Mol Med. 2018;22(8):3825–36. doi: 10.1111/jcmm.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sivalingam VN, Latif A, Kitson S, McVey R, Finegan KG, Marshall K, et al. Hypoxia and hyperglycaemia determine why some endometrial tumours fail to respond to metformin. Br J Cancer. 2020;122(1):62–71. doi: 10.1038/s41416-019-0627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang X, Cheng Y, Zhou J, Zhang L, Li X, Wang Z, et al. Targeting cancer metabolism plasticity with JX06 nanoparticles via inhibiting PDK1 combined with metformin for endometrial cancer patients with diabetes. Adv Sci (Weinh) 2022;9(8):e2104472. doi: 10.1002/advs.202104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Eftekhari A, Ahmadian E, Salatin S, Sharifi S, Dizaj SM, Khalilov R, et al. Current analytical approaches in diagnosis of melanoma. Trac-Trends Anal Chem. 2019;116:122–35. [Google Scholar]
- 148.Ahmadian E, Eftekhari A, Kavetskyy T, Khosroushahi AY, Turksoy VA, Khalilov R. Effects of quercetin loaded nanostructured lipid carriers on the paraquat-induced toxicity in human lymphocytes. Pestic Biochem Physiol. 2020;167:104586. doi: 10.1016/j.pestbp.2020.104586. [DOI] [PubMed] [Google Scholar]
- 149.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Park S, Kwon B, Yang W, Han E, Yoo W, Kwon BM, et al. Dual pH-sensitive oxidative stress generating micellar nanoparticles as a novel anticancer therapeutic agent. J Contr Rel. 2014;196:19–27. doi: 10.1016/j.jconrel.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 151.Trombino S, Servidio C, Curcio F, Cassano R. Strategies for hyaluronic acid-based hydrogel design in drug delivery. Pharmaceutics. 2019;11(8). [DOI] [PMC free article] [PubMed]
- 152.Huang HH, Wang YC, Chou YC, Yu MH, Chao TK. The combination of aldehyde dehydrogenase 1 (ALDH1) and CD44 is associated with poor outcomes in endometrial cancer. PLoS ONE. 2018;13(10):e0206685. doi: 10.1371/journal.pone.0206685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Edwards K, Yao S, Pisano S, Feltracco V, Brusehafer K, Samanta S et al. Hyaluronic Acid-functionalized nanomicelles enhance SAHA efficacy in 3D endometrial cancer Models. Cancers. 2021;13(16). [DOI] [PMC free article] [PubMed]
- 154.Xiao Y, Wang S, Zong Q, Yin Z. Co-delivery of Metformin and Paclitaxel Via Folate-Modified pH-Sensitive micelles for enhanced anti-tumor efficacy. AAPS PharmSciTech. 2018;19(5):2395–406. doi: 10.1208/s12249-018-1070-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.