Abstract
Background
Patients with cancer are at risk of multidrug-resistant bacteria colonization, but association of colonization with in-hospital mortality and one-year survival has not been established in critically ill patients with cancer.
Methods
Using logistic and Cox-regression analyses adjusted for confounders, in adult patients admitted at intensive care unit (ICU) with active cancer, we evaluate the association of colonization by carbapenem-resistant Gram-negative bacteria or vancomycin-resistant enterococci with in-hospital mortality and one-year survival.
Results
We included 714 patients and among them 140 were colonized (19.6%). Colonized patients more frequently came from ward, had longer hospital length of stay before ICU admission, had unplanned ICU admission, had worse performance status, higher predicted mortality upon ICU admission, and more hematological malignancies than patients without colonization. None of the patients presented conversion of colonization to infection by the same bacteria during hospital stay, but 20.7% presented conversion to infection after hospital discharge. Colonized patients had a higher in-hospital mortality compared to patients without colonization (44.3 vs. 33.4%; p < 0.01), but adjusting for confounders, colonization was not associated with in-hospital mortality [Odds ratio = 1.03 (0.77–1.99)]. Additionally, adjusting for confounders, colonization was not associated with one-year survival [Hazard ratio = 1.10 (0.87–1.40)].
Conclusions
Adult critically ill patients with active cancer and colonized by carbapenem-resistant Gram-negative bacteria or vancomycin-resistant enterococci active cancer have a worse health status compared to patients without colonization. However, adjusting for confounders, colonization by carbapenem-resistant Gram-negative bacteria or vancomycin-resistant enterococci are not associated with in-hospital mortality and one-year survival.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-023-01214-2.
Keywords: Drug resistance, Multiple, Neoplasms, Intensive care units, Vancomycin-resistant enterococci, Carbapenem-resistant gram-negative bacteria, Hospital mortality, Survival analysis
Background
Multidrug-resistance bacteria (MDRB) are a leading health public threat, with millions of deaths annually attributed to MDRB infection [1]. MDRB colonization is the main risk factor for progression to future MDRB infection [2, 3].
Patients with cancer are considered a population at risk of MDRB colonization because prior hospitalization and antimicrobial treatment are risk factors for colonization [4], and these factors are frequently presented in patients with cancer [5]. Colonization by carbapenem-resistant Gram-negative (CR-GrN) bacteria and vancomycin-resistant enterococci (VRE) are particularly worrisome because these bacteria are associated with worse outcomes compared to other MDRB, such as extended spectrum beta-lactamase producing enterobacteria [6].
Patients with cancer are a growing population in intensive care units (ICU), making up approximately 30% of the ICU occupancy [7, 8]. The association of MDRB colonization with increased in-hospital mortality has been established in general populations of critically ill patients [9–11], but not in critically ill patients with cancer. Moreover, the association of MDRB colonization with one-year survival is unknown in patients with cancer. If MDRB colonization is associated with worse in-hospital outcomes or lower one-year survival, strategies for decolonization of selected patients, close monitoring or antibiotic stewardship have the potential for improve in-hospital outcomes and increase long-term survival.
In patients with active cancer admitted at ICU, we evaluated the association of colonization by CR-GrN bacteria or VRE with in-hospital mortality. We also evaluated the association of colonization with one-year survival.
Methods
We designed a retrospective study including patients from the five ICUs of the AC Camargo Cancer Center (São Paulo–Brazil).
Patients
For epidemiological surveillance, at the beginning of May 2017, the Hospital Infection and Prevention Control Department ordered a once-a-week rectal swab for routine screening of CR-GrN bacteria and VRE. Briefly, two separate rectal swabs were collected. For VRE detection, one swab was plated on ChromID® VRE (bioMérieux, Brazil). For carbapenem-resistant enterobacterales and acinetobacter detection, the other swab was plated on CHROMagar mSuperCarba (Plastlabor, Brazil). Both plates were incubated in air at 35 ± 1 °C for a maximum of 48 h. If colonies were observed, identification was carried out using the Microflex mass spectrometry system (Bruker, Germany) (further details on Additional file 1).
Every Tuesday, all patients admitted for more than 48 h in the ICU were screened, excluding patients at end-of-life care. There was no routine screening for MDRB colonization outside ICU.
Between May 2017 and May 2019, we included all adult patients (≥ 18 years old), with active solid tumors or hematologic malignancies screened for CR-GrN bacteria and VRE during ICU stay. If a patient had multiple ICU admissions, only the first was considered. However, if a patient had multiple ICU admissions during the same hospital admission, and not the first, but a subsequent admission revealed CR-GrN bacteria or VRE colonization, that was the admission included in the study.
Data collection
Upon ICU admission, patient’s age and sex, days in hospital before ICU admission, Simplified Acute Physiology Score (SAPS 3) [12]; Eastern Cooperative Oncology Group (ECOG) performance status [13]; type of cancer (nonmetastatic or metastatic solid tumors, hematologic malignancies); ICU architectural design (single-bed room or multibed room), type of ICU admission (planned or unplanned); and source of ICU admission (emergency room, ward or operating room) were recorded.
We recorded the result of the rectal swab as follow: negative, positive for CR-GrN bacteria, positive for VRE; or positive for both bacteria. We also recorded the ICU and hospital length of stay.
In patients colonized by CR-GrN bacteria or VRE, we evaluated the conversion of colonization to clinical infection by the same bacteria up until one-year after hospital discharge. The conversion was confirmed when the same CR-GrN bacteria or VRE initially identified in the rectal swab and with the same antibiotic-resistance profile was isolated in any culture and the antibiotic regimen was initiated or changed accordingly. The conversion detection was performed by two investigators that independently examined the electronic health record and microbiological laboratory results.
The main outcomes of the study were in-hospital mortality and one-year survival. There were not missing values.
Data analysis
Categorical and continuous data were presented as absolute values (percentages) and median (25–75% interquartile range), respectively. Categorical variables were compared using the Chi-square test or Fisher’s exact test, as appropriate. Continuous variables were compared with the Mann–Whitney test.
In-hospital mortality
A logistic regression model was used to evaluate the association of colonization with in-hospital mortality. We used directed acyclic graph to identify confounders [14]. We identified the following confounders: age, performance status, SAPS 3 score, type of ICU admission and type of cancer (Additional file 1: Fig. S1).
Odds ratio (OR) with 95% confidence interval was used to measure the association of colonization with in-hospital mortality. We presented the unadjusted and adjusted odds ratios.
One-year survival
We used the multivariate Cox proportional hazards regression to compare the survival curve adjusted for confounders identified using directed acyclic graph. We identified the following confounders: age, performance status, and type of cancer (Additional file 1: Fig. S2). The time zero of the one-year follow-up period was the day of rectal swab screening.
Hazard ratio (HR) with 95% confidence interval was used to measure the association of colonization with one-year survival. We presented the unadjusted and adjusted hazard ratios.
Statistical analyses were performed using SPSS software (Version 23.0. Armonk, NY: IBM Corp). The directed acyclic graphs were created using the browser-based environment DAGitty [15]. We followed the recommendations of the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology). statement that guides the report of observational studies [16] and the guidance for control of confounding and reporting of results in causal inference studies from editors of respiratory, sleep, and critical care journals [14]. P values ≤ 0.05 were considered significant.
Results
3737 patients were admitted during the study period, 733 (19.6%) were elected to colonization screening and 714 patients were included in the study (Fig. 1). Only two patients had their second ICU admission included in the analyses and not the first admission. Both patients had a first negative rectal swab at first ICU admission, but few days later were readmitted at ICU and had a positive rectal swab.
Fig. 1.
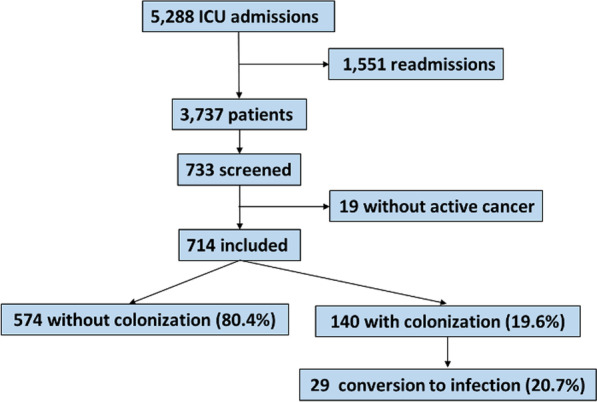
Flowchart of the study
One hundred and forty patients (19.6%) were colonized. Fifty patients (35.7%) were colonized by VRE, 73 (52.1%) by CR-GrN bacteria and 17 (12.2%) by both bacteria.
Upon ICU admission, patients with colonization more frequently came from ward, had longer hospital length of stay before ICU admission had unplanned ICU admission, worse performance status, higher predicted mortality upon ICU admission (SAPS3), and more hematological malignancies compared to patients without colonization (Table 1).
Table 1.
Characteristics upon ICU admission of patients with cancer colonized by carbapenem-resistant Gram-negative bacteria, vancomycin-resistant enterococci or both
| Variable | Total (n = 714) |
Without colonization (n = 574) |
With colonization (n = 140) |
P |
|---|---|---|---|---|
| Age (years) | 64 (53–73) | 64 (53–73) | 66 (54–74) | 0.71 |
| Male | 389 (54.5%) | 79 (56.4%) | 310 (54.0%) | 0.61 |
| Type ICU admission | < 0.01 | |||
| Planned | 201 (28.2%) | 182 (31.7%) | 19 (13.6%) | |
| Unplanned | 513 (71.8%) | 392 (68.3%) | 121 (86.4%) | |
| Before ICU admission | < 0.01 | |||
| Operating room | 243 (34.0%) | 214 (37.3%) | 29 (20.7%) | |
| Emergency room | 181 (25.4%) | 151 (26.3%) | 30 (21.4%) | |
| Ward | 290 (40.6%) | 209 (36.4%) | 81 (57.9%) | |
| ICU design | 0.51 | |||
| Single-bed room | 316 (44.3%) | 258 (44.9%) | 58 (41.4%) | |
| Multibed room | 398 (55.7%) | 316 (55.1%) | 82 (58.6%) | |
| Performance status (ECOG) | < 0.01 | |||
| 0–1 | 409 (57.3%) | 353 (61.5%) | 56 (40.0%) | |
| 2 | 159 (22.3%) | 122 (21.3%) | 37 (26.4%) | |
| 3–4 | 146 (20.4%) | 99 (17.2%) | 47 (33.6%) | |
| SAPS 3 | 63 (53–72) | 62 (51–72) | 68 (59–79) | < 0.01 |
| Type of cancer | 0.02 | |||
| Solid tumor | 623 (87.3%) | 510 (88.9%) | 113 (80.7%) | |
| Hematological | 91 (12.7%) | 64 (11.1%) | 27 (19.3%) | |
| Metastatic tumor | 277 (44.5%) | 228 (44.8%) | 49 (43.6%) | 0.85 |
| Days in hospital before ICU | 1 (0–9) | 1 (0–7) | 3 (1–16) | < 0.01 |
| Days in ICU before screening | 3 (2–5) | 3 (2–5) | 3 (2–5) | 0.98 |
| ICU length of stay (days) | 6 (4–10) | 6 (4–9) | 8 (5–12) | < 0.01 |
| Hospital length of stay(days) (days) | 22 (13–38) | 21 (13–35) | 28 (17–53) | < 0.01 |
Categorical data are presented as median (25–75% interquartile range). Continuous data were presented as absolute values (percentages)
ICU Intensive Care Unit, planned ICU admission postoperative elective surgery, unplanned ICU admission medical reason or postoperative emergency surgery, ECOG Eastern Cooperative Oncology Group, SAPS3 Simplified Acute Physiology Score (SAPS 3), Days in ICU before screening ICU length of stay before colonization screening with rectal swabs
In-hospital mortality
No one of the colonized patients presented conversion to infection during the hospital stay. Patients with colonization had a higher in-hospital mortality compared to patients without colonization (44.3 vs. 33.4%; p < 0.01) [unadjusted OR = 1.58 (1.09–2.30)]. However, after adjusting for age, performance status, SAPS 3 score, type of ICU admission and type of cancer, colonization was not associated with in-hospital mortality [adjusted OR = 1.03 (0.77–1.99)].
One-year survival
Among the 140 colonized patients, 29 (20.7%) presented conversion of colonization to infection. The conversion rate to infection was 27.4% (20/73) for patients colonized by CR-GrN, 8.0% (4/50) for patients colonized by VRE, and 29.4% (5/17) for patients colonized by both bacteria. The most frequent infections sites were urinary tract (31.0%), respiratory tract (20.7%), intrabdominal (17.2%) and bacteremia (13.8%). The interval between colonization diagnosis and infection was 27 days (15–60). Upon ICU admission, the characteristics of the colonized patients that converted or not infection were similar (Table 2).
Table 2.
Characteristics upon ICU admission of colonized patients with active cancer with or without conversion to infection
| Variable | Total (n = 140) |
Colonization without infection (n = 111) |
Colonization with infection (n = 29) |
P |
|---|---|---|---|---|
| Age (years) | 66 (54–74) | 67 (55–74) | 61 (48–75) | 0.21 |
| Male | 79 (56.4%) | 63 (56.8%) | 16 (55.2%) | 0.99 |
| Type ICU admission | 0.23 | |||
| Planned | 19 (13.6%) | 13 (11.7%) | 6 (20.7%) | |
| Unplanned | 121 (86.4%) | 98 (88.3%) | 23 (79.3%) | |
| Before ICU admission | 0.08 | |||
| Operating room | 29 (20.7%) | 19 (17.1%) | 10 (34.5%) | |
| Emergency room | 30 (21.4%) | 23 (20.7%) | 07 (24.1%) | |
| Ward | 81 (57.9%) | 69 (62.2%) | 12 (41.4%) | |
| ICU design | 0.68 | |||
| Single-bed room | 58 (41.4%) | 45 (40.5%) | 13 (44.8%) | |
| Multibed room | 58 (41.4%) | 45 (40.5%) | 13 (44.8%) | |
| Performance status (ECOG) | 0.75 | |||
| 0–1 | 56 (40.0%) | 46 (41.4%) | 10 (34.5%) | |
| 2 | 37 (26.4%) | 28 (25.2%) | 9 (31.0%) | |
| 3–4 | 47 (33.6%) | 37 (33.3%) | 10 (34.5%) | |
| SAPS 3 | 68 (56–79) | 68 (58–80) | 62 (50–72) | 0.08 |
| Type of cancer | 0.99 | |||
| Solid tumor | 113 (80.7%) | 89 (80.2%) | 24 (82.8%) | |
| Hematological | 27 (19.3%) | 22 (19.8%) | 05 (17.2%) | |
| Metastatic tumor | 61 (43.6%) | 46 (41.4%) | 15 (51.7%) | 0.40 |
Categorical data are presented as median (25–75% interquartile range). Continuous data were presented as absolute values (percentages)
ICU Intensive Care Unit, planned ICU admission postoperative elective surgery, unplanned ICU admission medical reason or postoperative emergency surgery, ECOG Eastern Cooperative Oncology Group, SAPS3 Simplified Acute Physiology Score (SAPS 3)
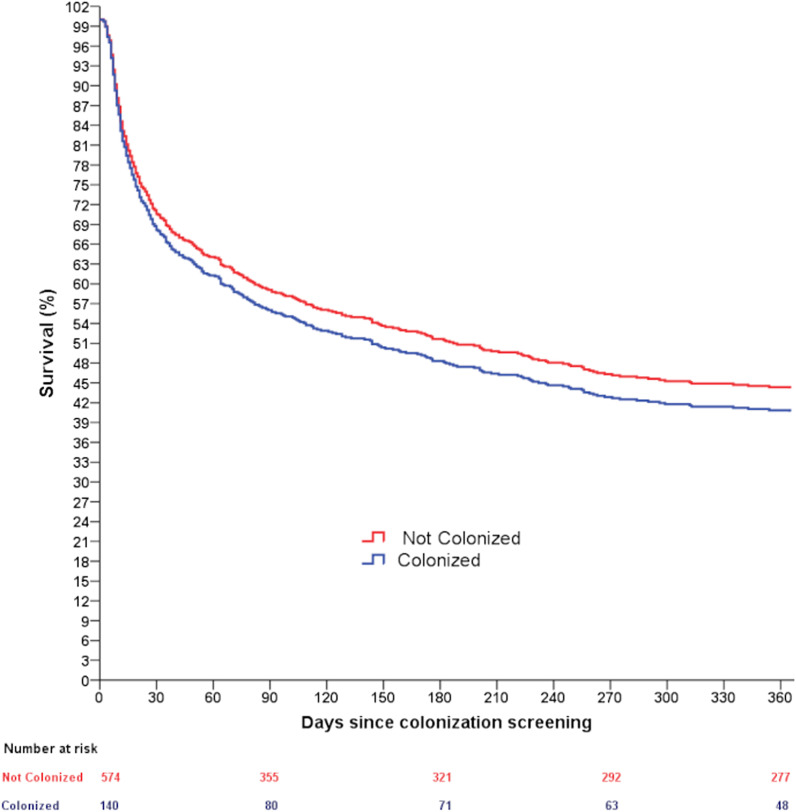
Two hundred and ninety-seven (51.7%) patients without colonization and 92 (65.7%) patients with colonization died during the one-year follow-up period [unadjusted HR = 1.38 (1.09–1.74)]. However, adjusting for confounders, colonization was not associated with lower one-year survival compared to patients without colonization [adjusted HR = 1.10 (0.87–1.40)] (Fig. 2).
Fig. 2.
Survival curves of patients with (blue) or without (red) colonization by carbapenem-resistant Gram-negative bacteria or vancomycin-resistant enterococci
Discussion
In patients with active cancer admitted at ICU, we showed that colonization by CR-GrN bacteria or VRE was not associated with in-hospital mortality and one-year survival.
In our study, the prevalence of colonization among patients with cancer admitted at ICU was 19.6%. The comparison of MDRB prevalence among the studies is difficult because the studies evaluated different MDRB and populations. Evaluating a general critically ill patient population, Nseir et al. [4] showed a 13% prevalence of colonization or infection at ICU admission, while Masse et al. [3] showed that 34% of patients had colonization or infection during ICU stay. Among patients in hematological institutions, Cattaneo et al. [6] showed that 6.5% had colonization. Forcina et al. [17] showed that 16.9% of patients had colonization by multidrug-resistant Gram-negative bacteria before an allogenic hematopoietic stem cell transplantation. Although the comparison is troublesome, the MDRB prevalence in our study is of the same magnitude of other studies.
In our study, the in-hospital mortality of patients with colonization was higher compared to patients without colonization. However, the comparison is not straightforward because patients with colonization had worse performance status, longer hospital length of stay before ICU admission, more unplanned ICU admission, higher SAPS 3 score, and more hematological malignancies. All these variables have been strongly associated with higher in-hospital mortality [8, 18–20]. Adjusting for the identified confounders, colonization was not associated with in-hospital mortality.
Our results showed that the conversion to infection was higher among patients colonized by CR-GrN compared to patients colonized by VRE. Likewise, Cattaneo and colleagues showed a higher percentage of conversion to infection among patients colonized by CR-GrN compared to patients colonized by VRE. The authors evaluated patients with cancer and the conversion from colonization to bloodstream infection was 14.1% for patients colonized by CR-GrN bacteria compared to 11.1% for patients colonized by VRE [6]. Liss et al. [21] showed a conversion rate of 2.0% for patients with cancer colonized by VRE, while Girmenia et al. [22] showed that 25.8% of autologous stem cell transplant and 39.2% of allogenic stem cell transplant patients presented a conversion from CR-GrN colonization to infection. The difference in conversion rates probably reflects the variability in bacteria virulence and patients’ immunological competence.
Adjusting for confounders, we also showed that colonization was not associated with one-year survival, corroborating the knowledge that cancer-associated variables are more associated with long-term survival than other clinical variables [21] Upon ICU admission, the characteristics of the colonized patients that converted or not to infection over one-year was similar. Therefore, either the causes of conversion of colonization to infection were not recorded upon ICU admission or were present only after the colonization diagnosis.
We believe that our study has strengths worth mentioning. We evaluated a population that had not been previously evaluated, patients with cancer demanding ICU admission. We recorded short-term (in-hospital mortality) and long-term (one-year survival) outcomes of patients with or without CR-GrN or VRE colonization. One clinical implication of the present study is that patients with cancer and poor health status should be considered to MDRB screening. Another implication is that oncologists should not consider MDRB colonization in the short or long-term prognostication of patients with cancer. However, the impact of conversion to infection on short or long-term survival needs further investigation.
Our study has limitations that shall be mentioned. First, it was a single- center study and the results could be different in ICUs with different population and care. Our results are limited to patients colonized with CR-GrN or VRE. We did not record detailed information about the immunosuppression status, such as recent use of chemotherapy. However, active cancer is already considered immunosuppression. We only screened 19.6% of patients admitted to ICU because due to economical constraints, we decided to screen only on Tuesdays. The results could be different if we had screened all patients because patients with shorter stay had a lower chance of sampling than those with longer stay, resulting in overrepresentation of patients with longer stay. Finally, the conversion of colonization to infection was considered using phenotypically and not genotypically identical bacteria.
Conclusions
In conclusion, adult critically ill patients with active cancer and colonized by carbapenem-resistant Gram-negative bacteria or vancomycin-resistant enterococci active cancer have a worse health status compared to patients without colonization. However, adjusting for confounders, colonization by carbapenem-resistant Gram-negative bacteria or vancomycin-resistant enterococci are not associated with in-hospital mortality and one-year survival.
Supplementary Information
Additional file 1. Supplementary figures.
Abbreviations
- CR-GrN
Carbapenem-resistant gram-negative bacteria
- ECOG
Eastern cooperative oncology group
- HR
Hazard ratio
- ICU
Intensive care unit
- MDRB
Multidrug-resistance bacteria
- OR
Odds ratio
- SAPS 3
Simplified acute physiology score
- STROBE
Strengthening the reporting of observational studies in epidemiology
- VRE
Vancomycin-resistant enterococci
Author contributions
GMDM, APAP and APNJ: (1) acquisition of data, analysis and interpretation of data; (2) drafting the article critically for important intellectual content; and (3) final approval of the version to be published. ILAFeS: (1) interpretation of data; (2) revising the article critically for important intellectual content; and (3) final approval of the version to be published. PC: (1) conception and design of the study, analysis and interpretation of data; (2) drafting the article critically for important intellectual content; and (3) final approval of the version to be published. All authors read and approved the final manuscript.
Funding
This work was supported by Intensive Care Unit Department of AC Camargo Cancer Center. The authors declare that no funds, Grants, or other support were received during the preparation of this manuscript.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This is an observational study. The local ethics committees approved this study (CAAE: 86761718.0.0000.5432) and waived the need for informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng WP, Chen YC, Chen SY, Chen SY, Chang SC. Risk for subsequent infection and mortality after hospitalization among patients with multidrug-resistant gram-negative bacteria colonization or infection. Antimicrob Resist Infect Control. 2018;7:93. doi: 10.1186/s13756-018-0388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masse J, Elkalioubie A, Blazejewski C, Ledoux G, Wallet F, Poissy J, et al. Colonization pressure as a risk factor of ICU-acquired multidrug resistant bacteria: a prospective observational study. Eur J Clin Microbiol Infect Dis. 2017;36:797–805. doi: 10.1007/s10096-016-2863-x. [DOI] [PubMed] [Google Scholar]
- 4.Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16:902–908. doi: 10.1111/j.1469-0691.2009.03027.x. [DOI] [PubMed] [Google Scholar]
- 5.Numico G, Zanelli C, Ippoliti R, Rossi M, Traverso E, Antonuzzo A, et al. The hospital care of patients with cancer: a retrospective analysis of the characteristics of their hospital stay in comparison with other medical conditions. Eur J Cancer. 2020;139:99–106. doi: 10.1016/j.ejca.2020.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Cattaneo C, Di Blasi R, Skert C, Candoni A, Martino B, Di Renzo N, et al. Bloodstream infections in haematological cancer patients colonized by multidrug-resistant bacteria. Ann Hematol. 2018;97:1717–1726. doi: 10.1007/s00277-018-3341-6. [DOI] [PubMed] [Google Scholar]
- 7.Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent JL. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13:R15. doi: 10.1186/cc7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zampieri FG, Romano TG, Salluh JIF, Taniguchi LU, Mendes PV, Nassar AP, et al. Trends in clinical profiles, organ support use and outcomes of patients with cancer requiring unplanned ICU admission: a multicenter cohort study. Intensive Care Med. 2021;47:170–179. doi: 10.1007/s00134-020-06184-2. [DOI] [PubMed] [Google Scholar]
- 9.Barbier F, Pommier C, Essaied W, Garrouste-Orgeas M, Schwebel C, Ruckly S, et al. Colonization and infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in ICU patients: what impact on outcomes and carbapenem exposure? J Antimicrob Chemother. 2016;71:1088–1097. doi: 10.1093/jac/dkv423. [DOI] [PubMed] [Google Scholar]
- 10.Detsis M, Karanika S, Mylonakis E. ICU acquisition rate, risk factors, and clinical significance of digestive tract colonization with extended-spectrum beta-lactamase-producing enterobacteriaceae: a systematic review and meta-analysis. Crit Care Med. 2017;45:705–714. doi: 10.1097/CCM.0000000000002253. [DOI] [PubMed] [Google Scholar]
- 11.Dautzenberg MJD, Wekesa AN, Gniadkowski M, Antoniadou A, Giamarellou H, Petrikkos GL, et al. The association between colonization with carbapenemase-producing enterobacteriaceae and overall ICU mortality: an observational cohort study. Crit Care Med. 2015;43:1170–1177. doi: 10.1097/CCM.0000000000001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno RP, Metnitz PGH, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3 - From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden CPP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 15.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: the R package “dagitty”. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Forcina A, Lorentino F, Marasco V, Oltolini C, Marcatti M, Greco R, et al. Clinical impact of pretransplant multidrug-resistant gram-negative colonization in autologous and allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2018;24:1476–1482. doi: 10.1016/j.bbmt.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Soares M, Silva UVA, Teles JMM, Silva E, Caruso P, Lobo SMA, et al. Validation of four prognostic scores in patients with cancer admitted to Brazilian intensive care units: results from a prospective multicenter study. Intensive Care Med. 2010;36:1188–1195. doi: 10.1007/s00134-010-1807-7. [DOI] [PubMed] [Google Scholar]
- 19.Soares M, Caruso P, Silva E, Teles JMM, Lobo SMA, Friedman G, et al. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med. 2010;38:9–15. doi: 10.1097/CCM.0b013e3181c0349e. [DOI] [PubMed] [Google Scholar]
- 20.Oeyen SG, Benoit DD, Annemans L, Depuydt PO, Van Belle SJ, Troisi RI, et al. Long-term outcomes and quality of life in critically ill patients with hematological or solid malignancies: a single center study. Intensive Care Med. 2013;39:889–898. doi: 10.1007/s00134-012-2791-x. [DOI] [PubMed] [Google Scholar]
- 21.Liss BJ, Vehreschild JJ, Cornely OA, Hallek M, Fätkenheuer G, Wisplinghoff H, et al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 2012;40:613–619. doi: 10.1007/s15010-012-0269-y. [DOI] [PubMed] [Google Scholar]
- 22.Girmenia C, Rossolini GM, Piciocchi A, Bertaina A, Pisapia G, Pastore D, et al. Infections by carbapenem-resistant Klebsiella pneumoniae in SCT recipients: a nationwide retrospective survey from Italy. Bone Marrow Transplant. 2015;50:282–288. doi: 10.1038/bmt.2014.231. [DOI] [PubMed] [Google Scholar]
- 23.Junior APN, da Silva M, Trevisani BB, Bettim PC. Long-term mortality in very old patients with cancer admitted to intensive care unit: a retrospective cohort study. J Geriatr Oncol. 2021;12(1):106–111. doi: 10.1016/j.jgo.2020.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary figures.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.