Abstract
Purpose:
Intravitreal injections of anti-vascular endothelial growth factors (anti-VEGF) are the current standard of care for patients with choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD). There is a growing subset of patients that does not respond to anti-VEGF monotherapy treatment. Some patients, however, do respond to a combination therapy of corticosteroids and anti-VEGF. This treatment requires monthly/bimonthly injections of anti-VEGF and semi-annual injections of corticosteroid. A drug delivery system (DDS) that simultaneously releases multiple drugs could benefit these patients by reducing the number of injections. The purpose of this study was to characterize the simultaneous release of aflibercept and dexamethasone from a biodegradable microparticle- and nanoparticle-hydrogel DDS.
Methods:
Dexamethasone-loaded nanoparticles and aflibercept-loaded microparticles were created using modified single- and double-emulsion techniques, respectively. Then, microparticles and nanoparticles were embedded into a thermoresponsive, biodegradable poly(ethylene glycol)-co-(L-lactic acid) diacrylate (PEG-PLLA-DA)-N-isopropylacrylamide (NIPAAm) hydrogel DDS. Drug release studies and characterization of DDS were conducted with varying doses of microparticles and nanoparticles.
Results:
The combination aflibercept-loaded microparticle- and dexamethasone-loaded nanoparticle- hydrogel (Combo-DDS) achieved a total release time of 224 days. Small decreases were seen in swelling ratio and equilibrium water content for Combo-DDS compared to monotherapy aflibercept-loaded microparticle-hydrogel DDS (AFL-DDS) and monotherapy dexamethasone-loaded nanoparticle-hydrogel DDS (DEX-DDS). Bioactivity of aflibercept was maintained in Combo-DDS compared to AFL-DDS.
Conclusions:
The Combo-DDS was able to extend and control the release of both aflibercept and dexamethasone simultaneously from a single DDS. This may eliminate the need for separate dosing regiments of anti-VEGF and corticosteroids for wet AMD patients.
Keywords: controlled release, anti-VEGF, corticosteroid, drug delivery system, neovascularization
Introduction
Age-related macular degeneration (AMD) is the leading cause of vision loss in the elderly population 1–2. Wet AMD, which accounts for 10% of AMD cases, is characterized by abnormal blood vessel growth in the macula 3. These immature blood vessels cause leakage, hemorrhage, and retinal damage leading to rapid and severe vision loss 4–5. Clinically, the current standard of care for patients with choroidal neovascularization (CNV) secondary to wet AMD is monthly or bimonthly intravitreal (IVT) injections of anti-vascular endothelial growth factor (VEGF) 6–7. Anti-VEGFs used to treat wet AMD include bevacizumab (Avanstin®, off-label, Genentech, San Francisco, CA, USA), ranibizumab (Lucentis®, Genentech), and aflibercept (EYLEA®, Regeneron Pharmaceuticals, Tarrytown, NY, USA).
While this treatment has proven to be safe and effective, about 45% of wet AMD patients do not fully respond to monotherapy anti-VEGF treatment 8. Some studies, however, have shown that these patients respond to a combination therapy of corticosteroids and anti-VEGF 9–12. Like many retinal diseases, it has been demonstrated that the progression of CNV involves both angiogenesis and inflammatory pathways 13–19. The addition of corticosteroids addresses the inflammatory pathway of CNV that is not targeted by anti-VEGF. Dexamethasone, a steroid that helps reduce inflammation, has shown potential in maintaining visual acuity and reducing central retinal thickness when delivered in combination with monthly or bimonthly IVT injections of anti-VEGF 12,17. Dexamethasone must be delivered, however, as an implant due to its low solubility and short vitreous half-life of 3.48 hours 20. Thus, this combination treatment requires separate dosing regiments for each drug: monthly or bimonthly bolus IVT injections of anti-VEGF and semi-annual IVT injections of a dexamethasone implant such as Ozurdex® (Allergan Inc., Irvine, CA, USA).
A major limitation of anti-VEGF treatment is the need for monthly or bimonthly bolus IVT injections due to rapid clearance and short vitreous half-lives 21. The known risks of complications associated with repeated IVT injections include retinal detachment, endophthalmitis, IVT hemorrhage, and cataract 4–5. Additionally, the frequency of treatment places a significant burden on patients and healthcare systems. Lastly, bolus injections have suboptimal pharmacokinetics by reaching peak drug concentration immediately after administration that is rapidly cleared and is then followed by subtherapeutic concentrations between doses that allows for further disease progression 22.
Many drug delivery systems (DDS) have been developed to extend the release of anti-VEGF to address these limitations 23. Some examples of successful DDSs include polymer based micro- and nano-particles, hydrogels, cell-based therapeutics, and composite systems 24. Recently, Susvimo™ (Genentech), a port delivery system that releases ranibizumab for six months, received FDA approval for the treatment of wet AMD after its successful results in the Archway phase 3 trial 25–26. The port system must be surgically implanted, but then only requires refills every six months 26. Additionally, our laboratory has developed a composite microparticle-hydrogel DDS to continually release anti-VEGF for six months 27–32. In this system, aflibercept-loaded poly(ethylene glycol)-co-(L-lactic acid) (PLGA) microparticles are embedded into a thermoresponsive, biodegradable poly(ethylene glycol)-co-(L-lactic acid) diacrylate (PEG-PLLA-DA)-N-isopropylacrylamide (NIPAAm) hydrogel. It has shown treatment efficacy and safety in a laser-induced CNV model in rodents and non-human primates 31–32.
By encapsulating dexamethasone into PLGA particles and then embedding into this composite system, a single DDS would administer both aflibercept and dexamethasone, therefore eliminating the need for separate dosing regiments. By simultaneously releasing aflibercept and dexamethasone in a controlled manner for six months, a more advantageous pharmacokinetic profile can be achieved. It would also result in the reduction of the total number of IVT injections, reducing the risks of complications associated with repeated IVT injections and the socioeconomic burden on patients and healthcare systems.
The purpose of this study was to characterize the extended and controlled dual release of dexamethasone-loaded PLGA nanoparticles and aflibercept-loaded PLGA microparticles from a single thermoresponsive, biodegradable poly(ethylene glycol)-co-(L-lactic acid) diacrylate-N-isopropylacrylamide hydrogel DDS in vitro. Optimal release kinetics for dexamethasone were determined by varying the loading dose of dexamethasone-loaded nanoparticles in the DDS. The effects of nanoparticle loading dose on drug release kinetics, swelling ratio, and equilibrium water content were investigated. Additionally, in vitro bioactivity of aflibercept was evaluated after microparticle fabrication and after release from the DDS.
Methods
Fabrication of DDS
Dexamethasone (Sigma-Aldrich, St. Louis, MO) was encapsulated into PLGA (75:25, MW 4–15 kDa) nanoparticles by a modified single-emulsion, solvent evaporation technique 33–34. Dexamethasone and PLGA were dissolved in dichloromethane (DCM) to create the oil phase, and polyvinyl alcohol (PVA) was used as the water phase. The oil-in-water emulsion (o/w) was created by sonication at 100 watts for 3.5 minutes. After the solvent was allowed to evaporate, dexamethasone loaded-nanoparticles (DEX-np) were collected by centrifugation, washed with deionized (DI) water at least three times, lyophilized, and stored at 4°C.
Similarly, aflibercept (Eylea®, Regeneron, Tarrytown, NY) was encapsulated into PLGA (75:25) microspheres by a modified double-emulsion, solvent evaporation technique 29–30,34. The first emulsion (w1/o) was created by vortex, then immediately added to polyvinyl alcohol phase (w2) to create a double emulsion (w1/0/w2) by vortex. Excipients were added to the inner aqueous phase (w1) for protein stabilization and to the oil phase (o) to act as a buffer 29. After solvent evaporation, aflibercept loaded-microparticles (AFL-mp) were collected by centrifugation, washed with DI water at least three times, lyophilized, and stored at 4°C.
Thermoresponsive, biodegradable PEG-PLLA-DA/NIPAAm hydrogels were synthesized by free radical polymerization, described elsewhere 27,35. NIPAAm (350 mM), N-tert-butylacrylamide (50 mM), ammonium persulfate (13 mM), and PEG-PLLA-DA (2 mM) were dissolved into 1xPBS (pH 7.4) to prepare the hydrogel precursor solution 30. Varying loading doses of microparticles (0mg/ml and 20mg/ml) and nanoparticles (0mg/ml, 20mg/ml, 40 mg/ml, 60mg/ml, and 80mg/ml) were suspended into the solution to create the composite DDS. N,N,N’,N’-tetramethylethylenediamine (168 mM, pH 7.4) was added to the hydrogel precursor solutions to initiate polymerization. Hydrogels were placed on ice for 30 minutes while reaction proceeded to create nanoparticle- and microparticle-hydrogel DDS. DDS was then collected, washed with DI water five times, and stored at 4°C.
Aflibercept Radiolabeling
Aflibercept was radiolabeled with iodine-125 using iodination beads (Pierce, Rockford, IL, USA) for all characterization and release kinetic studies. To remove any unbound, free iodine, radiolabeled aflibercept was dialyzed against DI water using a dialysis cassette for 48 hours. Then radiolabeled aflibercept was collected, lyophilized, and dissolved in PBS to create a 40 mg/ml stock solution. Radioactivity was measured using a gamma counter (Cobra-II, Auto-Gamma, Packard Instrument Co., Meriden, CT).
Encapsulation Efficiencies and Characterization
Encapsulation efficiency (E.E.) for microparticles and nanoparticles was defined as the percent of drug within the particles relative to the theoretical loading amount of drug. Similarly, E.E. of particles into the hydrogel was defined as the percent of particles in the hydrogel relative to the theoretical loading amount of particles. The size distribution of dexamethasone-loaded nanoparticles was determined using Nanoparticle Tracking Analysis (NanoTracking v 3.0, NanoSight LM10, Malvern, UK). For dexamethasone, E.E. into nanoparticles was determined by using reverse phase HPLC as previously reported 36. Nanoparticle encapsulation into the hydrogel could not be directly measured due to experimental limitation.
For aflibercept-loaded microparticles, particle size was determined by capturing images with a light microscope and using ImageJ Software (National Institutes of Health, Bethesda, MD). Radioactivity before and after fabrication of microparticles and hydrogel DDS were used to determine the E.E.’s for aflibercept.
In Vitro Drug Release Studies
In vitro release profiles of both dexamethasone and aflibercept from composite DDS were conducted using a separation method described elsewhere 37. Three preparations of composite DDS were made for release studies: (1) monotherapy aflibercept-loaded microparticle-hydrogel DDS (AFL-DDS), (2) monotherapy dexamethasone-loaded nanoparticle-hydrogel DDS (DEX-DDS) and (3) combination aflibercept-loaded microparticle- and dexamethasone-loaded nanoparticle- hydrogel DDS (Combo-DDS). Each DDS was prepared in 1ml volumes and incubated at 37°C in 1.5 ml of PBS (1x, pH 7.4). At predetermined time points, 1ml of supernatant was removed for analysis and replaced with 1 ml of fresh PBS.
Dexamethasone release studies were conducted using a NanoDrop™ OneC (Thermo-Fischer Scientific Inc., MA, USA), a microvolume UV-spectrophotometer. To determine optimal release kinetics of dexamethasone, release studies for DEX-DDS consisted of 20, 40, 60 or 80 mg/ml dexamethasone-loaded nanoparticles (DEX-np). Similarly, release studies for Combo-DDS consisted of 20 mg/ml DEX-np with 20 mg/ml of aflibercept-loaded microparticles (AFL-mp).
For aflibercept release profiles, radiolabeled aflibercept was used to conduct release studies using a gamma counter. The AFL-DDS previously developed by our laboratory showed treatment efficacy with a microparticle loading dose of 20 mg/ml 31. Thus, AFL-DDS and Combo-DDS consisted of 20 mg/ml AFL-mp, and 20 mg/ml AFL-mp and 20 mg/ml of DEX-np, respectively.
Temperature Dependent Swelling Ratios and Equilibrium Water Content
Due to the thermoresponsive nature of the hydrogel, swelling ratios and equilibrium water content (EWC) at varying temperatures were measured. After polymerization, hydrogels were incubated at 22°C overnight to allow for equilibration. Wet weight (Wwet) of hydrogels were measured from 22°C to 46°C after 30 minutes of incubation at each temperature. Hydrogels were then lyophilized to determine dry weight (Wdry). The swelling ratio (Q) was defined using the following equation:
| (1) |
Similarly, the equilibrium water content (EWC) was defined as:
| (2) |
EWC for each DDS was determined by averaging the values at each temperature.
Bioactivity
First, bioactivity of aflibercept after microparticle formulation was evaluated. The primary emulsification (w1/o) was simulated to determine the effects of the organic phase (DCM) on aflibercept stability. The inner water phase (w1) was created by dissolving 100 μl of aflibercept stock solution (40mg/ml) in PBS with 12% BSA (w/v%). Then it was incorporated into the organic phase at a 1:5 v/v water-to-organic phase ratio. The emulsification was created by stirring at 3200 rpm for 90s to evaluate water/organic solvent interface effect. In order to extract aflibercept from the organic phase, 4 ml of PBS was added, and then the mixture was stirred for 2 min. Then the mixture was centrifuged at 5000 rpm for 10 min to accelerate phase separation (total o/w ratio was 1:8.2). The aqueous phase was collected and used to evaluate the recovered amount and stability of aflibercept by enzyme linked immunosorbent assay (ELISA). As a control, PBS was used in place of the organic phase and the same procedure was conducted.
Next, bioactivity of aflibercept released from DDS was determined. Aflibercept-loaded DDS showed significant inhibition of VEGF-induced human umbilical vascular endothelial cell (HUEVC) proliferation compared to blank DDS at all timepoints 28. This indicates that bioactivity of aflibercept remains throughout the entire release time. To determine the bioactivity of aflibercept from Combo-DDS, HUVECs were cultured, trypsinized, and seeded into 96-well plates at 5000 cells per well in growth medium (Lonza Inc., Allendale, NJ, USA). After the cells had grown to ~80% confluence (~48 hours), cell growth was arrested by washing the plates twice with PBS (1x, pH 7.4) and then adding 50 μL of basal growth media (Lonza Inc., Allendale, NJ, USA) to each well. To achieve VEGF-induced proliferation, 25 μL of 10 ng/mL exogenous VEGF (Lonza) was added to each well. Then, 25 μL of a release sample of anti-VEGF (aflibercept) was added.
A solution containing a tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS) and an electron coupling reagent (phenazine ethosulfate) (MTS assay, CellTiter 96® Aqueous One Solution Reagent; Promega, Madison, WI, USA) was used according to the manufacturer’s protocol to determine cell proliferation and cytotoxicity after exposure to the release samples for two days. According to the MTS assay protocol, cell proliferation is proportional to the optical density (OD) of a sample when read using a spectrophotometer at 490 nm 1 to 4 hours after adding the MTS reagent. Cell proliferation was normalized relative to wells receiving only exogenous 10 ng/mL VEGF (negative control). Positive controls consisted of 25 μL of a clinical dose of drug (40 mg/mL aflibercept) added to wells containing 25 μL 10 ng/mL VEGF.
Statistical Analysis
All values are reported as mean ± standard error and in all graphs, error bars represent standard error. All statistical analysis between experiment groups was performed using Student’s t-test and any p value less than 0.05 considered to be significant.
Results
Characterization of Particles
AFL-mp and DEX-np were successfully fabricated. AFL-mp had an average diameter of 6.9 ± 0.4 μm and encapsulation efficiency of drug into microparticle of 66.6 ± 1.4% (n=3), which agrees with previously reported values 27,30. Dexamethasone nanoparticles had an average diameter of 138.9 ± 6.2 nm and encapsulation efficiency of drug into nanoparticles of 91.6 ± 4.2% (n=10).
In Vitro Release Studies
Release Profiles of Dexamethasone
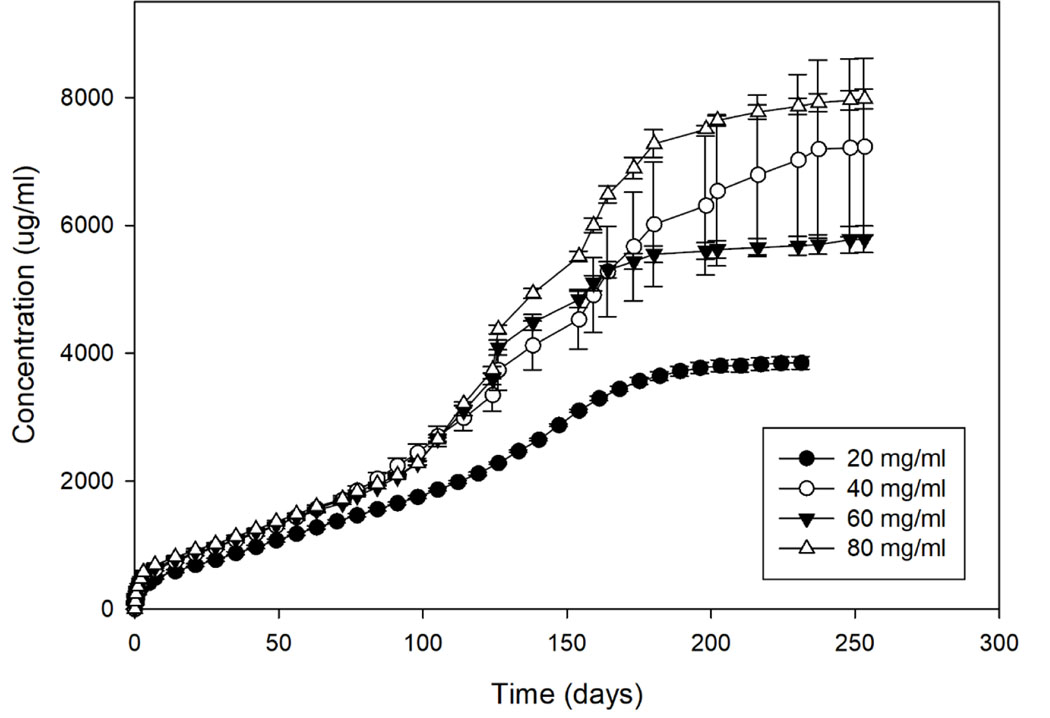
Cumulative releases of dexamethasone from DEX-DDS can be seen in Figure 1. All loading doses of DEX-np were successfully embedded into DDS and maintained drug release for at least 200 days. Loading doses of 20, 40, 60 and 80 mg/ml had similar release rates of 16.0, 21.1, 19.4, and 19.6 ug/day, respectively, within the first 100 days (Table 1). While the 20 mg/ml continued at a similar release rate of 18.4 ug/day, the higher loading doses of DEX-np had a drastic increase in release rate after day 100. The release rates after 100 days increased to 32.2, 27.0, and 64.3 ug/day for 40, 60 and 80 mg/ml, respectively.
Figure 1.
In vitro release of dexamethasone from DEX-DDS with 20, 40, 60, and 80 mg/ml loading dose of dexamethasone-loaded nanoparticles. Error bars represent standard error (n=3).
Table 1.
Release Characteristics of Dexamethasone From DEX-DDS.
| Concentration of DEX-np (mg/ml) | Total drug released (μg) | Initial burst (first 24 hours) (μg) | Release rate first 100 days (μg per day) | Release rate after 100 days (μg per day) | Release time achieved (days) |
|---|---|---|---|---|---|
| 20 | 3850.8 ± 99.8 | 244.5 ± 17.0 | 16.0 (R2 = 0.97) | 18.4 (R2 = 0.93) | 224 |
| 40 | 7230.9 ± 1385.8 | 294.9 ± 12.8 | 21.1 (R2 = 0.97) | 32.0 (R2 = 0.96) | 253 |
| 60 | 5784.3 ± 208.7 | 323.8 ± 3.2 | 19.4 (R2 = 0.96) | 27.0 (R2 = 0.87) | 253 |
| 80 | 7980.3 ± 154.4 | 348.9 ± 6.5 | 19.6 (R2 = 0.96) | 64.3 (R2 = 0.99) | 253 |
Data represented by mean ± SEM (n=3).
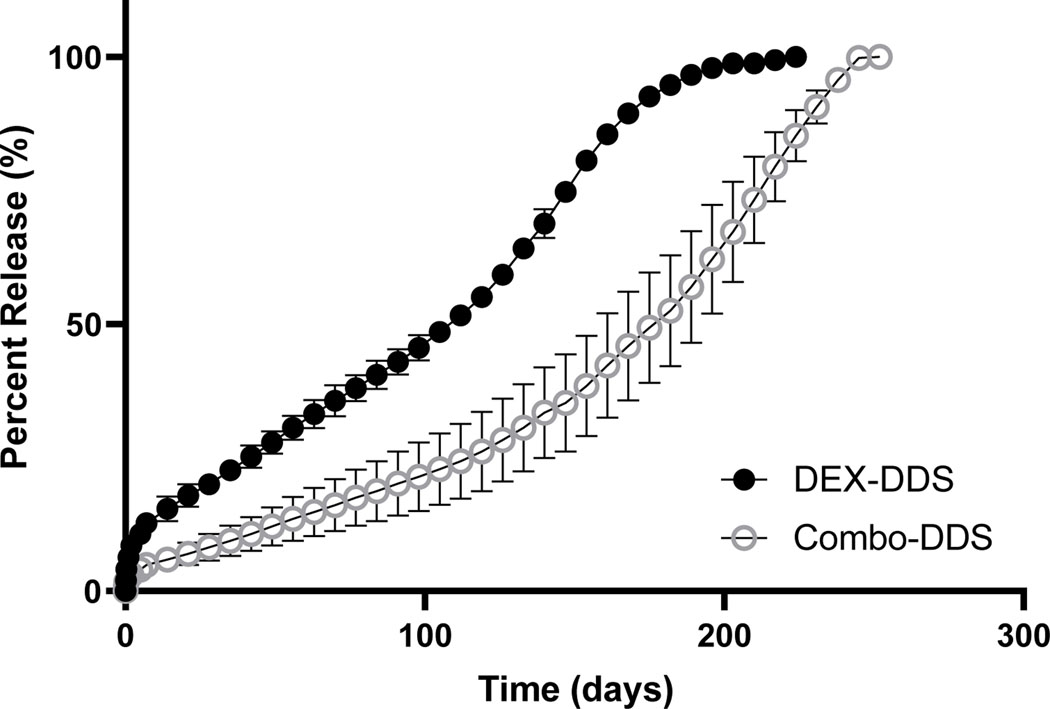
The 20 mg/ml loading dose had the most consistency and control of release, and therefore was used for DEX-np dose in the Combo-DDS for all other experiments. As seen in Figure 2, the addition of AFL-mp into the DDS did not significantly change the releases kinetic or characterization of dexamethasone release. DEX-DDS had initial burst, release rate after 7 days, release time and total drug released of 244.5 ± 17.0 ug, 17.6 ug/day (R2 = 0.98), 224 days, and 3850.8 ± 99.8 ug, respectively. Similarly, Combo-DDS had initial burst, release rate after 7 days, release time and total drug released of 217.8 ± 2.5 ug, 22.9 ug/day (R2 = 0.97), 252 days, and 5764.8 ± 3.2 ug, respectively (Table 2). No significant differences were seen in initial burst, release rate, or total drug released for DEX-DDS and Combo-DDS. However, Combo-DDS was able to further reduce the initial burst and increase the total release time and amount of dexamethasone released.
Figure 2.
Cumulative releases of dexamethasone from DEX-DDS and Combo-DDS. Error bars represent standard error (n=3).
Table 2.
Dexamethasone Release Characteristics from DDS.
| Formulation | Estimated Total Drug Load (ug) | Initial Burst (first 24 hours) (%) | Release Rate after 7 days (ug/day) | Release time achieved (days) |
|---|---|---|---|---|
| DEX-DDS | 3850.8 ± 99.8 | 6.3 ± 0.4 | 17.6 (R2 = 0.98) | 224 |
| Combo-DDS | 5764.8 ± 3.2 | 2.4 ± 0.6 | 22.9 (R2 = 0.97) | 224 |
Data represented by mean ± SEM (n=3).
Release Profiles of Aflibercept
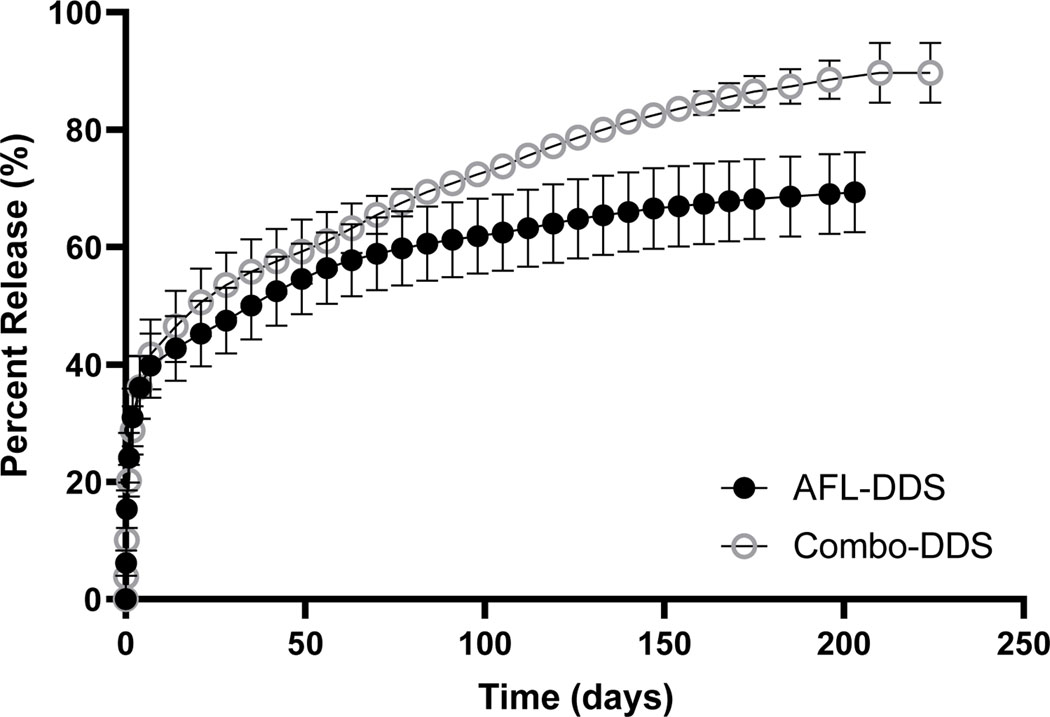
Figure 3 shows the cumulative releases of aflibercept microparticles (20 mg/ml) from both AFL-DDS and Combo-DDS. The release of aflibercept from AFL-DDS had similar characteristics as previously reported [30]. Table 3 shows the release characteristics of aflibercept from AFL-DDS and Combo-DDS. AFL-DDS achieved a total aflibercept drug load of 277.9 ± 8.4 ug and initial burst of 66.7 ± 11.2 ug. Similarly, Combo-DDS achieved a total aflibercept drug load of 267.4 ± 2.2 ug and initial burst of 54.2 ± 7.7 ug. There were no significant differences between AFL-DSS and Combo-DDS for initial burst or total aflibercept drug load (p > 0.2). After 7 days, AFL-DDS and Combo-DDS achieved release rates of 0.37 and 0.58 ug/day, respectively. Combo-DDS was able to achieve a longer release time of 224 days compared to AFL-DDS release time of 203 days. Similarly, Combo-DDS achieved a higher final percent of aflibercept released (89.7 ± 5.1) compared to AFL-DDS (69.4 ± 6.8).
Figure 3.
Cumulative releases of aflibercept from AFL-DDS and Combo-DDS. Error bars represent standard error (n=3).
Table 3.
Aflibercept Release Characteristics from DDS.
| Formulation | Estimated Total Drug Load (ug) | Initial Burst (first 24 hours) (%) | E.E. of Particles into Hydrogel (%) | Release Rate after 7 days (ug/day) | Release time achieved (days) | Final Percent Released (%) |
|---|---|---|---|---|---|---|
| AFL-DDS | 277.9 ± 8.4 | 24.1 ± 4.3 | 74.9 ± 2.5 | 0.37 (R2 = 0.88) | 203 | 69.4 ± 6.8 |
| Combo-DDS | 267.4 ± 2.2 | 20.2 ± 2.7 | 75.6 ± 0.4 | 0.58 (R2 = 0.95) | 224 | 89.7 ± 5.1 |
Data represented by mean ± SEM (n=3).
Temperature-Dependent Swelling Ratios and Equilibrium Water Content
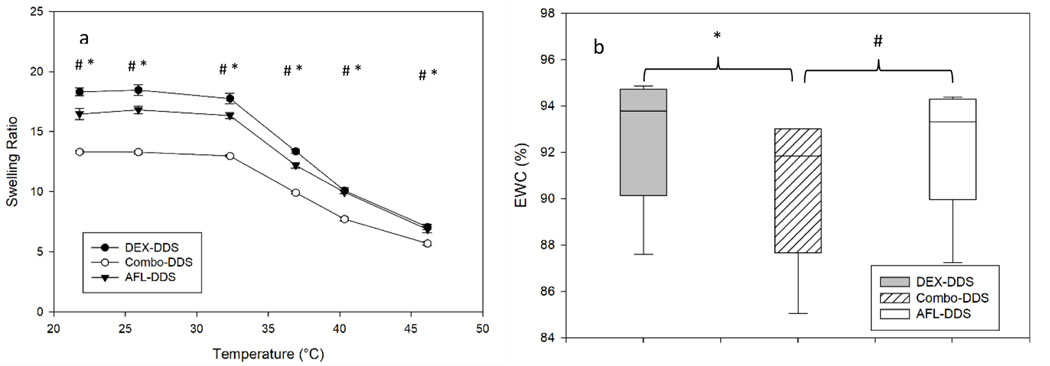
Figure 4a shows the swelling ratios and equilibrium water content (EWC) for DEX-DDS, AFL-DDS, and Combo-DDS. At room temperature (21.8°C), swelling ratios for DEX-DDS, AFL-DDS, and Combo- DDS were 18.3 ± 0.3, 16.5 ± 0.5, and 13.3 ± 0.1, respectively. Similar decreasing trends were seen for all DDSs from 22 to 46°C. Above physiological temperature (42°C), swelling ratios for DEX-DDS, AFL-DDS, and Combo-DDS were 7.1 ± 0.2, 6.9 ± 0.3, and 5.7 ± 0.2, respectively. Statistically significant differences were found between Combo-DDS and AFL-DDS (p < 0.05), in addition to Combo-DDS and DEX-DDS (p < 0.05), at each temperature. EWC for DEX-DDS, AFL-DDS, and Combo-DDS hydrogels were 93.2 ± 2.6, 93.6 ± 2.6, and 91.7 ± 2.9, see Figure 5b. A statistically significant difference was found between the EWC of Combo-DDS and AFL-DDS (p = 0.035). No other significant differences were seen in EWC.
Figure 4.
Swelling ratios (a) and equilibrium water content (b) for dexamethasone, aflibercept and combination DDSs at varying temperatures. “DEX-DDS” had 20 mg/ml of dexamethasone nanoparticles, “AFL-DDS” had 20 mg/ml of aflibercept microparticles, and “Combo-DDS” had 20 mg/ml of dexamethasone nanoparticles and 20 mg/ml of aflibercept microparticles. Error bars represent standard error (n=3). Statistically significant difference (p < 0.05) between DEX-DDS and Combo-DDS is indicated by * and between AFL-DDS and Combo-DDS is indicated by #.
Figure 5.
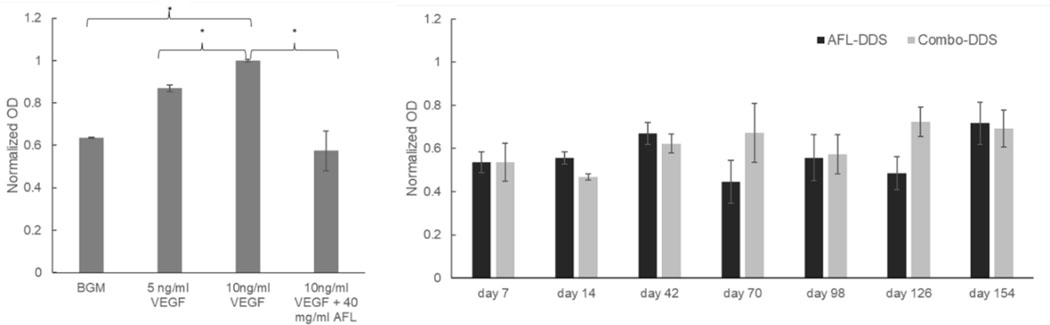
Bioactivity of dexamethasone- and aflibercept-loaded DDS (Combo-DDS), and aflibercept-loaded DDS (AFL DDS). Statistically significant differences between groups are indicated by * (p < 0.05). Error bars represent standard error (n=3).
Bioactivity of Aflibercept
In order to evaluate aflibercept bioactivity after exposure to organic solvent (DCM), the primary emulsification was simulated, and ELISA was conducted with the recovered aflibercept. When compared to the control (PBS), aflibercept maintained 91.0 ± 3.2% of its bioactivity (n=3). No significant differences were seen with exposure to DCM compared to exposure to PBS (p = 0.38). Additionally, the bioactivity of aflibercept released from DDS was evaluated. Proportional increases in HUVEC proliferation were observed with varying amounts of VEGF (positive controls) compared to negative control (BGM) (Figure 5a). The addition of a clinical dose of aflibercept (40mg/ml) showed a significant reduction of VEGF-induced proliferation compared to positive controls (p < 0.05). The addition of aflibercept did not show any significant difference compared to negative control (p > 0.62). Significant inhibition of VEGF-induced proliferation was observed at each timepoint throughout the release period of AFL-DDS and Combo-DDS (Figure 5b). This is shown by the reduction in normalized OD for all drug-treated HUVECs compared to the controls. No significant differences were seen between AFL-DDS and Combo-DDS.
Discussion
In this study, dexamethasone-loaded nanoparticles and aflibercept-loaded microparticles were successfully embedded and released from a biodegradable, thermoresponsive hydrogel drug delivery system for ~200 days. DEX-np were created using a modified single-emulsion technique, resulting in PLGA nanoparticles averaging 138.9 ± 6.2 nm in diameter with drug encapsulation efficiency of 91.6 ± 4.2%. Similarly, AFL-mp were created using a modified double-emulsion technique, which resulted in PLGA microparticles averaging 6.9 ± 0.4 μm in diameter and a drug encapsulation efficiency of 66.6 ± 1.4%. Long-term release of dexamethasone was achieved by the degradable nanoparticle-hydrogel DDS. Additionally, combining DEX-np and AFL-mp did not significantly change drug release kinetics compared to their single releases. The Combo-DDS in this study has the potential to replace the standard treatment of monthly/bimonthly IVT injections of anti-VEGF and semi-annual IVT injections of dexamethasone implant for non-responsive patients.
While some optimization of dexamethasone encapsulation into PLGA particles has been done, consistency in DEX-np fabrication remains a challenge due to the poor water solubility (0.16 mg/ml) 38 and crystallization of drug 33. Variability in drug encapsulation leads to differences in final drug-to-polymer ratio, which may explain why there is not a direct relationship between increase in nanoparticle load and increase in total drug release. Meaning, different loading doses of DEX-np could result in similar amounts of total drug released since loading dose is based on weight of particles not weight of drug. Further optimization of dexamethasone encapsulation into PLGA nanoparticles could achieve more consistent drug release.
All loading doses of DEX-np (20, 40, 60 and 80 mg/ml) were investigated to determine release kinetics and characterization from 1 ml of DDS. Similar release rates were observed within the first 100 days of all releases. After 100 days, 20 mg/ml loading dose maintained its release rate, while the higher loading doses had more drastic increases in release rate (Table 1). We speculate that as the hydrogel degrades and loses its integrity, release rates increase since the hydrogel acts as less of a barrier, which would be compounded at higher doses. When scaled to a clinical injection volume of 50 μl, all loading doses of DEX-np would maintain drug levels that have been shown to suppress inflammation intravitreally (0.15–4.00 μg/ml) 39–44, and therefore would all be viable options. There are some associated risks, however, with long-term dexamethasone use. In the GENEVA study, there was a significant occurrence of cataract progression in retinal vein occlusion (RVO) patients after only 12 months of receiving a DEX implant 45. Similarly, increases in intraocular pressure (IOP) were found in RVO patients after two injections of DEX 45. To minimize any risk of continuous DEX treatment, the lowest loading dose of DEX-np (20mg/ml) was used for Combo-DDS. When embedded in combination, no significant changes in characterization or the release kinetics of dexamethasone or aflibercept were observed compared to their individual single releases (Figures 2 and 3).
The thermal transition behavior of PEG-PLLA-DA/NIPAAm hydrogels with micro- and nanoparticles was evaluated from 22 to 46°C. DEX-DDS, AFL-DDS and Combo-DDS had an inverse relationship between temperature and swelling ratio (Figure 4a), which is similar to previously reported trends 29. Since hydrogel mesh size is proportional to swelling ratio, these results indicate that the hydrogel mesh size would decrease at physiological temperature and allow for a more controlled drug release in vivo. As for EWC, all DDSs had relatively high values (Figure 4b), which is advantageous since hydrogels with high water content are generally more biocompatible in medical applications 46. Differences in swelling ratio and EWC were seen when comparing Combo-DDS to both DEX-DDS and AFL-DDS (p < 0.05), which may be due to the increase in total dry weight compared to AFL-DDS and DEX-DDS. The Combo-DDS has double the total weight of microparticles and nanoparticles than the monotherapy DDSs, which in turn would yield a higher dry weight. This would account for the decreases in swelling ratio and EWC seen in the Combo-DDS since both are directly calculated using dry weight.
For protein encapsulation using a double emulsion technique, the organic solvent has the greatest influence on bioactivity due to denaturation and aggregation of protein at the interface 47–49. Therefore, simulation of the primary emulsion (w1/o) was used to evaluate changes in bioactivity due to the microparticle formulation process. No change in aflibercept bioactivity was seen with the presence of BSA (12% w/v). Previous reports have shown that albumin is the most protective additive in the primary emulsion 49–51. It is thought that albumin occupies the solvent/water interface, which prevents therapeutic proteins from contacting the interface 50–53.
Dexamethasone loaded-nanoparticles can be incorporated into the aflibercept-loaded microparticle-hydrogel DDS without significantly altering the bioactivity of released aflibercept. No significant differences were seen in the bioactivity of aflibercept from AFL-DDS or Combo-DDS (Figure 5). Previous studies have shown that dexamethasone does not inhibit VEGF-induced proliferation in vitro 9,54, and therefore, no differences in aflibercept bioactivity would be expected between AFL-DDS and Combo-DDS. The similarities in bioactivity of AFL indicate that there are no antagonistic drug-drug interactions from Combo-DDS, thus allowing for combination therapy DDS rather than two monotherapies.
By modifying the particle-hydrogel DDS, this system can effectively control and extend drug release for a range of drugs, from hydrophobic steroids to hydrophilic proteins. This study shows that simultaneous release of drugs can be achieved without significant changes to release kinetics or characterization. The nanoparticle- and microparticle-hydrogel DDS has proven to be an effective method for simultaneously releasing dexamethasone and aflibercept for six months. Not only would this DDS eliminate the need for separate dosing regiments of anti-VEGF and corticosteroids for wet AMD patients, but it would also reduce the annual number of IVT injections, lower the socioeconomic burden on patients and healthcare systems, and allow for more favorable release kinetics.
Acknowledgements
This work was supported by the NIH under Grant EY029298.
Footnotes
Disclosure Statement
Kayla M. Rudeen: none
Wenqiang Liu: none
William F. Mieler: none
Jennifer J. Kang-Mieler: US Patent
Data availability statement
The data that support the findings of this study are available from the corresponding author, JJKM, upon reasonable request.
References:
- 1.Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, Parodi MB, Pesudovs K, Price H, White RA, et al. Preval. and causes of vis. loss in high-income ctries. and in East. and Central Eur.: 1990–2010. Br. J. of Ophthalmol 2014; 98(5):629–38. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Glob. preval. of age-relat. macular degener. and dis. burd. proj. for 2020 and 2040: a syst. rev. and meta-analysis. The Lancet Glob. Health 2014;2(2): e106–16. [DOI] [PubMed] [Google Scholar]
- 3.Foundation AMD. Dry vs wet age-relat. macular degener 2017. [accessed 2021 November 8]. http://www.macular.org/dry-vs-wet-macular-degeneration
- 4.Ghasemi Falavarjani K, Nguyen QD. Advers. events and complicat. assoc. with intravitreal inject. of anti-VEGF agents: a rev. of lit. Eye. 2013;27(7):787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyman GA, Lad EM, Moshfeghi DM. Intravitreal inject. of ther. agents. Retina. 2009;29(7):875–912. [DOI] [PubMed] [Google Scholar]
- 6.Park YG, Rhu HW, Kang S, Roh YJ. New approach of anti-VEGF agents for age-relat. macular degener. J. of Ophthalmol 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovach JL, Schwartz SG, Flynn HW, Scott IU . Anti-VEGF treat. strateg. for wet AMD. J. of Ophthalmol 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) ther. of choroidal neovasc. lesions. British J. of Ophthalmol 2007;91(10):1318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Jiang J, Chen W, Jiang H, Zhang Z, Sun X. Target. deliv. of low-dose dexamethasone using PCL–PEG micelles for eff. treat. of rheum. arthritis. J. of Control. Release 2016; 230:64–72. [DOI] [PubMed] [Google Scholar]
- 10.Al-Khersan H, Hussain RM, Ciulla TA, Dugel PU. Innov. ther. for neovasc. age-relat. macular degener. Expert Opin. on Pharmacother 2019; 20(15):1879–91. [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Zhao J, Sun X. Resistance to anti-VEGF ther. in neovasc. age-relat. macular degener.: a compr. rev. Drug Design, Dev. and Ther 2016; 10:1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaya C, Zandi S, Pfister IB, Gerhardt C, Garweg JG. Adding a corticosteroid or switch. to another anti-VEGF in insufficiently responsive wet age-relat. macular degener. Clin. Ophthalmol (Auckland, NZ). 2019; 13:2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflamm. in the pathog. of age-relat. macular degener. Surv. of Ophthalmol 2006; 51(2):137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Soloway P, Ryan SJ, Miller JW. The pathog. of choroidal neovasc. in patients with age-relat. macular degener. Mol Vis. 1999; 5(11):34–8. [PubMed] [Google Scholar]
- 15.London NJ, Chiang A, Haller JA. The dexamethasone drug deliv. syst.: indic. and evidence. Adv. in Ther 2011; 28(5):351–66. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary V, Barbosa J, Lam WC, Mak M, Mavrikakis E. Ozurdex in age-relat. macular degener as adjun. to ranibizumab (The OARA Study). Can. J. of Ophthalmol 2016; 51(4):302–5. [DOI] [PubMed] [Google Scholar]
- 17.Todorich B, Thanos A, Yonekawa Y, Mane G, Hasbrook M, Thomas BJ, Woodward MA, Williams GA, Capone A Jr, Wolfe JD, Faia LJ. Simultaneous dexamethasone intravitreal implant and anti-VEGF ther. for neovasc. age-relat. macular degener. resist. to anti-VEGF monother. J. of Vitreoretin. Dis 2017; 1(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuo J, Grob S, Zhang K, Chan CC. Genet. of immunol. and inflamm. compon. in age-relat. macular degener. Ocul. Immunol. and Inflamm 2012; 20(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement compon. C3a and C5a promot. choroidal neovasc. Proc. of the National Acad. of Sciences 2006; 103(7):2328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak HW, D’Amico DJ. Evaluation of the retin. toxic. and pharmacokinet. of dexamethasone after intravitreal inject. Arch. of Ophthalmol 1992; 110(2):259–66. [DOI] [PubMed] [Google Scholar]
- 21.Kim LA, D’Amore PA. A brief history of anti-VEGF for the treat. of ocul. angiogenesis. The Am. J. of pathol 2012; 181(2):376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Sanharawi M, Kowalczuk L, Touchard E, Omri S, De Kozak Y, Behar-Cohen F. Protein deliv. for retin. dis.: from basic consid. to clin. appl. Prog. in Retin. and Eye Res 2010; 29(6):443–65. [DOI] [PubMed] [Google Scholar]
- 23.Herrero-Vanrell R, Bravo-Osuna I, Andrés-Guerrero V, Vicario-de-la-Torre M, Molina-Martínez IT. The potential of using biodegrad. microspheres in retin. dis. and other intraocul. pathol. Prog. in Retin. and Eye Res 2014; 42:27–43. [DOI] [PubMed] [Google Scholar]
- 24.Kang-Mieler JJ, Dosmar E, Liu W, Mieler WF. Ext. ocul. drug deliv. syst.s for the anterior and posterior segments: biomater. options and appl. Expert Opin. on Drug Deliv 2017; 14(5):611–20. [DOI] [PubMed] [Google Scholar]
- 25.Holekamp NM, Campochiaro PA, Chang MA, Miller D, Pieramici D, Adamis AP, Brittain C, Evans E, Kaufman D, Maass KF, Patel S. Archway Randomized Phase 3 Trial of the Port Deliv. Syst. with Ranibizumab for Neovasc. Age-Relat. Macular Degener. Ophthalmol. 2021. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Parachuri N, Kumar N, Kuppermann BD, Bandello F. The Port Deliv. Syst. with ranibizumab—journey of mitigating vitreous hemorrhage. Eye. 2021:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osswald CR, Kang-Mieler JJ. Control. and ext. release of a model protein from a microsphere-hydrogel drug deliv. syst. Annals of Biomed. Engineering 2015;43(11):2609–17. [DOI] [PubMed] [Google Scholar]
- 28.Osswald CR, Kang-Mieler JJ. Control. and ext. in vitro release of bioact. Anti-vasc. endothel. growth factors from a microsphere-hydrogel drug deliv. syst. Curr. Eye Res 2016; 41(9):1216–22. [DOI] [PubMed] [Google Scholar]
- 29.Liu W, Borrell MA, Venerus DC, Mieler WF, Kang-Mieler JJ. Charact. of biodegrad. microsphere-hydrogel ocul. drug deliv. syst. for Control. and ext. release of ranibizumab. Transl. Vis. Science & technol 2019; 8(1):12-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Lee BS, Mieler WF, Kang-Mieler JJ. Biodegrad. microsphere-hydrogel ocul. drug deliv. syst. for Control. and ext. release of bioact. aflibercept in vitro. Curr. Eye Res 2019; 44(3):264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Tawakol AP, Rudeen KM, Mieler WF, Kang-Mieler JJ. Treat. effic. and biocompat. of a biodegrad. aflibercept-loaded microsphere-hydrogel drug deliv. syst. Transl. Vis. Science & Technol. 2020; 9(11):13-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S, Kang-Mieler JJ, Liu W, Wang Z, Yiu G, Teixeira LB, Mieler WF, Thomasy SM. Safety and biocompat. of aflibercept-loaded microsphere thermo-responsive hydrogel drug deliv. syst. in a nonhum. primate model. Transl. Vis. Science & Technol. 2020; 9(3):30-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gómez-Gaete C, Tsapis N, Besnard M, Bochot A, Fattal E. Encapsulation of dexamethasone into biodegrad. polym. nanoparticles. Int. J. of Pharm 2007; 331(2):153–9. [DOI] [PubMed] [Google Scholar]
- 34.Herrero-Vanrell R, Bravo-Osuna I, Andrés-Guerrero V, Vicario-de-la-Torre M, Molina-Martínez IT. The potential of using biodegrad. microspheres in retin. dis. and other intraocul. pathol. Prog. in Retin. and Eye Res 2014; 42:27–43. [DOI] [PubMed] [Google Scholar]
- 35.Drapala PW, Jiang B, Chi YC, Mieler WF, Brey EM, Kang-Mieler JJ, Pérez-Luna VH. The effect of glutathione as chain transf. agent in PNIPAAm-based thermo-responsive hydrogels for control. release of proteins. Pharm. Res 2014; 31(3):742–53. [DOI] [PubMed] [Google Scholar]
- 36.Hickey T, Kreutzer D, Burgess DJ, Moussy F. Dexamethasone/PLGA microspheres for contin. deliv. of an anti-inflamm. drug for implant. med. devices. Biomater. 2002; 23(7):1649–56. [DOI] [PubMed] [Google Scholar]
- 37.Giteau A, Venier-Julienne MC, Aubert-Pouëssel A, Benoit JP. How to achieve sustain. and complet. protein release from PLGA-based microparticles?. Int. J. of Pharm 2008; 350(1–2):14–26. [DOI] [PubMed] [Google Scholar]
- 38.Cao J, Naeem M, Noh JK, Lee EH, Yoo JW. Dexamethasone phosphate-loaded folate-conjugated polym. nanoparticles for selective deliv. to act. macrophages and suppression of inflamm. responses. Macromol. Res 2015; 23(5):485–92. [Google Scholar]
- 39.Arya SK, Wong-Staal F, Gallo RC. Dexamethasone-mediated inhib. of hum. T cell growth factor and gamma-interferon messenger RNA. The J. of Immunol 1984; 133(1):273–6. [PubMed] [Google Scholar]
- 40.Culpepper JA, Lee F. Regul. of IL 3 expr. by glucocorticoids in cloned murine T lymph. The J. of Immunol 1985. Nov 1;135(5):3191–7. [PubMed] [Google Scholar]
- 41.Lewis GD, Campbell WB, Johnson AR. Inhib. of prostaglandin synth. by glucocorticoids in hum. endothel. cells. Endocrinol. 1986; 119(1):62–9. [DOI] [PubMed] [Google Scholar]
- 42.Grabstein K, Dower S, Gillis S, Urdal D, Larsen A. Expr. of interleukin 2, interferon-gamma, and the IL 2 receptor by hum. peripheral blood lymph. The J. of Immunol 1986; 136(12):4503–8. [PubMed] [Google Scholar]
- 43.Knudsen PJ, Dinarello CA, Strom TB. Glucocorticoids inhib. transcr. and post-transcr. expr. of interleukin 1 in U937 cells. The J. of Immunol 1987; 139(12):4129–34. [PubMed] [Google Scholar]
- 44.Lee SW, Tsou AP, Chan H, Thomas J, Petrie K, Eugui EM, Allison AC. Glucocorticoids sel. Inhib. the transc. of the interleukin 1 beta gene and decrease the stab. of interleukin 1 beta mRNA. Proc. of the National Acad. of Sciences 1988; 85(4):1204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jiao J, Li XY, Whitcup SM. Dexamethasone intravitreal implant in patients with macular edema relat. to branch or central retin. vein occlusion: twelve-mon. study results. Ophthalmol. 2011; 118(12):2453–60. [DOI] [PubMed] [Google Scholar]
- 46.Katime I, Novoa R, Zuluaga F. Swelling kinet. and release studies of theophylline and aminophylline from acrylic acid/n-alkyl methacrylate hydrogels. Euro. Polym. J 2001; 37(7):1465–71. [Google Scholar]
- 47.Sah H Protein instab. toward organic solvent/water emuls.: implic. for protein microencapsul. into microspheres. PDA J. of Pharm. Science and Technol 1999; 53(1):3–10. [PubMed] [Google Scholar]
- 48.Li X, Zhang Y, Yan R, Jia W, Yuan M, Deng X, Huang Z. Influ. of process parameters on the protein stab. encapsulated in poly-DL-lactide–poly (ethylene glycol) microspheres. J. of Control. Release 2000; 68(1):41–52. [DOI] [PubMed] [Google Scholar]
- 49.Varshochian R, Jeddi-Tehrani M, Mahmoudi AR, Khoshayand MR, Atyabi F, Sabzevari A, Esfahani MR, Dinarvand R. The prot. effect of albumin on bevacizumab activity and stab. in PLGA nanoparticles intend. for retin. and choroidal neovasc. treat. Euro. J. of Pharm. Sciences 2013; 50(3–4):341–52. [DOI] [PubMed] [Google Scholar]
- 50.He J, Feng M, Zhou X, Ma S, Jiang Y, Wang Y, Zhang H. Stab. and encapsulation of recomb. hum. erythropoietin into PLGA microspheres using hum. serum albumin as a stabilizer. Int. J. of Pharm 2011; 416(1):69–76. [DOI] [PubMed] [Google Scholar]
- 51.Bilati U, Allémann E, Doelker E. Strateg. approaches for overcoming peptide and protein instab. within biodegrad. nano-and microparticles. Euro. J. of Pharm. and Biopharm 2005. Apr 1;59(3):375–88. [DOI] [PubMed] [Google Scholar]
- 52.van de Weert M, Hoechstetter J, Hennink WE, Crommelin DJ. The effect of a water/organic solvent interface on the struct. stab. of lysozyme. J. of Control. Release 2000; 68(3):351–9. [DOI] [PubMed] [Google Scholar]
- 53.van de Weert M, Hennink WE, Jiskoot W. Protein instab. in poly (lactic-co-glycolic acid) microparticles. Pharm. Res 2000; 17(10):1159–67. [DOI] [PubMed] [Google Scholar]
- 54.Won JY, Kim J, Gao G, Kim J, Jang J, Park YH, Cho DW. 3D printing of drug-loaded multi-shell rods for local deliv. of bevacizumab and dexamethasone: A synerg. ther. for retin. vasc. dis. Acta Biomater. 2020; 116:174–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, JJKM, upon reasonable request.