Background
To treat Neisseria gonorrhoeae infection, the Centers for Disease Control and Prevention recommends a single oral dose of cefixime as an alternative to injectable ceftriaxone.
Methods
We conducted a systematic review and meta-analysis to describe the effectiveness of cefixime in treating N. gonorrhoeae infection at 3 different anatomic sites.
We searched PubMed and Embase database to abstract treatment success rates and cefixime dosage/frequency for studies that reported the anatomical site of infection. We included reports published between January 1, 1980, and December 7, 2021. Twenty studies published between 1989 and 2015 were included in our meta-analysis. We calculated pooled treatment success percentages and 95% confidence intervals (CIs) using random-effects models.
Results
Of patients who received a 400-mg single dose of cefixime, 824 of 846 (97%; 95% CI, 96%–98%) patients with urogenital infection, 107 of 112 (97%; 95% CI, 84%–100%) patients with rectal infection, and 202 of 242 (89%; 95% CI, 76%–96%) patients with pharyngeal infection were cured. Of patients who received an 800-mg single dose of cefixime, 295 of 301 (98%; 95% CI, 96%–99%) patients with urogenital infection and 21 of 26 (81%; 95% CI, 61%–92%) patients with pharyngeal infection were cured.
Conclusions
Our meta-analysis found that cefixime is highly effective at treating urogenital infections and less effective at treating pharyngeal infections. We recommend more investigation into the effectiveness of cefixime in treating rectal infections and studying multidose therapy for the cefixime treatment of pharyngeal infection.
Neisseria gonorrhoeae infection is a public health concern worldwide, due in large part to the fact that N. gonorrhoeae has demonstrated unprecedented capacity to develop antibiotic resistance.1 There is evidence of N. gonorrhoeae antimicrobial resistance to all recommended antimicrobial agents.2,3 The Centers for Disease Control and Prevention (CDC) identified antimicrobial-resistant N. gonorrhoeae as 1 of the top 3 ongoing public health threats.4 For those reasons, identifying effective therapies is critical for clinicians, public health professionals, and patients.
Current recommendations for the treatment of gonorrhea rely on ceftriaxone as first-line therapy. In their 2020 European guidelines on the treatment of gonorrhea, the International Union against Sexually Transmitted Infections put forward 2 recommendations. The first International Union against Sexually Transmitted Infections recommendation was dual therapy of ceftriaxone 1 g intramuscularly as a single dose with azithromycin 2 g as a single oral dose. Their second recommendation was monotherapy of ceftriaxone 1 g intramuscularly alone.5 The British Association for Sexual Health and HIV, in their 2018 guidelines on the treatment of infection with N. gonorrhoeae, also recommended ceftriaxone 1 g intramuscularly.6 The CDC's 2020 update on treatment guidelines for gonococcal infection recommended a single intramuscular dose of ceftriaxone 500 mg as first-line therapy to treat gonorrhea.7
In addition, CDC recommends a single oral dose of cefixime 800 mg as an alternative if ceftriaxone is not available.7 The CDC did not recommend cefixime as first-line therapy because it fails to provide the same bactericidal effect as ceftriaxone, because of reduced susceptibility in surveillance data, and because it is unreliable in the treatment of pharyngeal gonorrhea.7 However, concerns have been raised about the availability of ceftriaxone. The ease of one-time oral administration of cefixime may justify the continued use of cefixime in certain settings.8,9
Recent data from the CDC's Gonococcal Isolate Surveillance Project have raised questions about the appropriate dosage of cefixime in the treatment of gonorrhea. Although cefixime minimal inhibitory concentrations rose steadily over the past 30 years, this trend has recently reversed in the United States, Canada, and England.10,11 In addition, the effectiveness of cefixime at various anatomical sites of infection is poorly understood12,13 We performed a systematic review and meta-analysis to compare the effectiveness of 400- and 800-mg single-dose cefixime at treating urogenital, rectal, and pharyngeal gonococcal infections.
MATERIALS AND METHODS
Search Strategy and Selection Criteria
The systematic review was conducted in accordance with PRISMA guidelines.14 We began by searching PubMed (https://pubmed.ncbi.nlm.nih.gov/) using the following search query: (((“Gonorrhea”) AND (“Cefixime”)) AND (“1980/01/01”[Date - Publication]: “2021/12/31”[Date - Publication])) AND (English[Language]) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab] OR open study [tiab]) NOT (animals [mh] NOT humans [mh])). The query restricts our search to articles published between the dates of January 1, 1980, and December 31, 2021.
We performed the same search on Embase using the equivalent search query translated into Embase's syntax. The exact search query used in our Embase search is the following: (“gonorrhea’ AND “cefixime”) AND [1980–2021]/py AND english:la AND ((“randomized controlled trial”:it OR “controlled clinical trial”:it OR “randomized”:ab,ti OR “placebo”:ab,ti OR “drug therapy”:lnk OR “randomly”:ab,ti OR “trial”:ab,ti OR “groups”:ab,ti OR “open study”:ab,ti) NOT (“animals”/exp NOT “humans”/exp)).
We excluded studies that were not in English or studies that were single case reports or case series. We did not encounter any studies investigating multidose therapy with cefixime. We included studies that specified the dose of cefixime administered, the schedule of cefixime administration (e.g., single dose), and the anatomical site of infection by N. gonorrhoeae. We also included studies in which the test of cure was performed by culture or by nucleic acid amplification test. Authors reviewed each article returned by the search query. Determinations on the relevance of each article to our study was determined by the first author and verified by the second. Each study was individually assessed for bias.15 We used Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) to manage the appropriate data from the included articles in performing a meta-analysis on the effectiveness of cefixime in treating gonorrhea at different anatomical sites. Of the articles that met our inclusion criteria and were determined to be relevant to our study, we abstracted the cefixime dose, characteristics of study participants, and the number and percent of treatment successes and failures reported in the articles.
Data Analysis
We compared the effectiveness of cefixime in eliminating N. gonorrhoeae infection at the 3 anatomical sites (urogenital, pharyngeal, and rectal). We performed the analysis separately by dosage (single cefixime dose of 400 or 800 mg). The 95% confidence intervals (CIs) of individual studies were calculated and visualized in forest plots. A logistic normal random-effects model was used to calculate 95% CI and pooled estimates. We calculated and present study-specific proportions with 95% CIs by anatomic site and the pooled treatment success estimates by anatomic site with 95% Wald CIs. Heterogeneity was quantified using the I2 measure where there were greater than 2 degrees of freedom.16 Those analyses were performed using Stata 16 software (College Station, TX).
We conducted an analysis of publication bias using a doi plot and the Luis Furuya-Kanamori (LFK) index using a logit transformation of the cure proportion from each study.17 In addition, we conducted a sensitivity analysis where we excluded the results of each study one at a time to assess the change in the estimates of pooled treatment success.
RESULTS
The search performed on PubMed returned 16 studies. The search on Embase returned an additional 4 studies (Fig. 1). Twenty total studies were included in our meta-analysis (Table 1). We summarized patient characteristics and geographic locations of the included studies (Table 2). Based on the GRADE scale of study quality, 8 studies had a “high” quality of evidence, 10 studies had a “moderate” quality of evidence, and 2 articles had a “low” quality of evidence22 (Table 2). We only encountered studies that reported dosages of 200, 400, or 800 mg. One study found outcomes with a cefixime dosage of 200 mg; we report this study alone but did not include it in our meta-analysis.18 In that study, 93 of 98 (94.9%) patients presenting with uncomplicated urogenital gonococcal infection were cured, 4 of 4 (100.0%) patients presenting with uncomplicated rectal infection were cured, and no pharyngeal infections were included.
Figure 1.
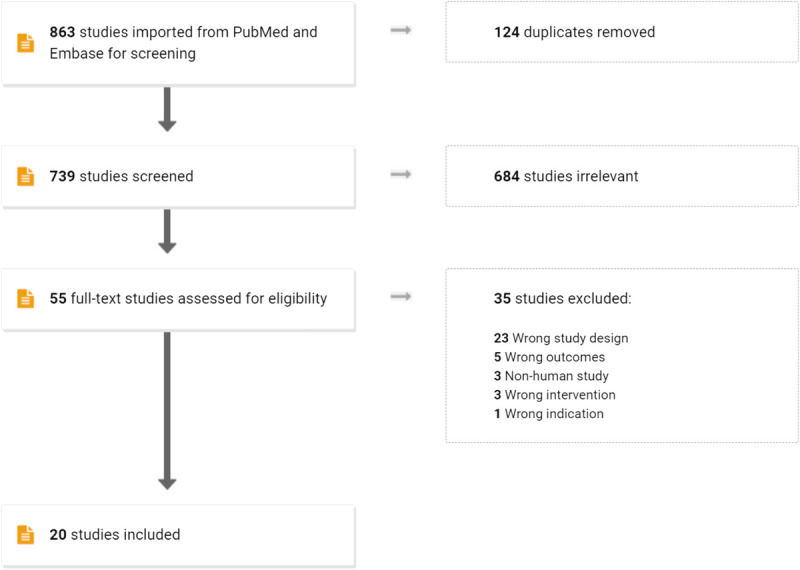
Screening process for the systematic review on PubMed and Embase.
TABLE 1.
Studies of Cefixime Treatment for Neisseria Gonorrhoeae Infection, 1990 to 2015
| Authors | Publication Year | Cefixime Dose, mg | Urogenital | Rectal | Pharyngeal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Successes | Failures | Total | % Success | Successes | Failures | Total | % Success | Successes | Failures | Total | % Success | |||
| Verdon et al.18 | 1993 | 200 | 93 | 5 | 98 | 94.90 | 4 | 0 | 4 | 100.00 | ||||
| Aplasca De Los Reyes et al.31s | 2001 | 400 | 25 | 1 | 26 | 96.15 | ||||||||
| Asbach32s | 1991 | 400 | 43 | 0 | 43 | 100.00 | ||||||||
| Barbee et al.33s | 2013 | 400 | 19 | 5 | 24 | 79.17 | ||||||||
| Gratrix et al.19 | 2013 | 400 | 47 | 18 | 65 | 72.31 | ||||||||
| Handsfield et al.20 | 1991 | 400 | 87 | 4 | 91 | 95.60 | 10 | 10 | 10 | 100.00 | 8 | 0 | 8 | 100.00 |
| Hjelmevoll et al.34s | 2012 | 400 | 25 | 0 | 25 | 100.00 | 1 | 0 | 1 | 100.00 | 1 | 0 | 1 | 100.00 |
| Hook III et al.35s | 1997 | 400 | 145 | 5 | 150 | 96.67 | 3 | 0 | 3 | 100.00 | 10 | 2 | 12 | 83.33 |
| Kuhlwein et al.36s | 1989 | 400 | 30 | 0 | 30 | 100.00 | ||||||||
| McMillan et al.37s | 2007 | 400 | 32 | 0 | 32 | 100.00 | 12 | 0 | 12 | 100.00 | 53 | 0 | 53 | 100.00 |
| Miller Jr.38s | 1997 | 400 | 79 | 4 | 83 | 95.18 | ||||||||
| Moran et al.39s | 1995 | 400 | 8 | 0 | 8 | 100.00 | ||||||||
| Mroczkowski et al.40s | 1997 | 400 | 124 | 1 | 125 | 99.20 | 37 | 1 | 38 | 97.37 | 11 | 5 | 16 | 68.75 |
| Plourde et al.41s | 1992 | 400 | 118 | 3 | 121 | 97.52 | ||||||||
| Portilla et al.42s | 1992 | 400 | 65 | 1 | 66 | 98.48 | ||||||||
| Ramus et al.43s | 2001 | 400 | 44 | 2 | 46 | 95.65 | 16 | 0 | 16 | 100.00 | 6 | 0 | 6 | 100.00 |
| Sathia et al.44s | 2007 | 400 | 14 | 2 | 16 | 87.50 | ||||||||
| Singh et al.21 | 2015 | 400 | 7 | 1 | 8 | 87.50 | 28 | 4 | 32 | 87.50 | 25 | 8 | 33 | 75.76 |
| Dunnett et al.45s | 1992 | 800 | 71 | 2 | 73 | 97.26 | ||||||||
| Handsfield et al.20 | 1991 | 800 | 87 | 1 | 88 | 98.86 | 6 | 0 | 6 | 100.00 | 6 | 1 | 7 | 85.71 |
| Megran et al.46s | 1990 | 800 | 96 | 1 | 97 | 98.97 | ||||||||
| Moran et al.39s | 1995 | 800 | 12 | 3 | 15 | 80.00 | ||||||||
| Portilla et al.42s | 1992 | 800 | 40 | 2 | 42 | 95.24 | ||||||||
| Singh et al.21 | 2015 | 800 | 1 | 0 | 1 | 100.00 | 6 | 0 | 6 | 100.00 | 3 | 1 | 4 | 75.00 |
TABLE 2.
Characteristics of Participants, Geographic Locations, and GRADE Quality Score of Studies Included in the Meta-Analysis of Cefixime for the Treatment of Neisseria Gonorrhoeae Infection, 1990 to 2015
| Study | Participant Sex | Participant Sexual Orientation | Study Location | GRADE Score |
|---|---|---|---|---|
| Aplasca De Los Reyes et al.31s | Female only | Not reported | Manila, Philippines Cebu, Philippines |
High |
| Asbach32s | Female only | Not reported | Remscheid, Germany | High |
| Barbee et al.33s | Male and female | Not reported | Seattle, WA | Moderate |
| Dunnett et al.45s | Male and female | Not reported | Rockford, IL | Low |
| Gratrix et al.19 | Male and female | Heterosexual MSM |
Alberta, Canada | Moderate |
| Handsfield et al.20 | Male and female | Straight Bisexual women |
Seattle, WA Brooklyn, NT Baltimore, MD Denver, CO |
High |
| Hjelmevoll et al.34s | Male and female | Heterosexual MSM |
Oslo, Norway | Moderate |
| Hook III et al.35s | Male and female | Not reported | 10 locations in the United States (cities and states not reported) | High |
| Kuhlwein et al.36s | Male only | Not reported | Germany (city not reported) | Low |
| McMillan et al.37s | Male and female | Heterosexual MSM |
Edinburgh, Scotland, United Kingdom | Moderate |
| Megran et al.46s | Male only | Heterosexual bisexual MSM |
Canada (city not reported) | High |
| Miller Jr.38s | Female only | Not reported | United States (city not reported) | Moderate |
| Moran et al.39s | Male and female | Not reported | Multiple locations globally | Moderate |
| Mroczkowski et al.40s | Female only | Not reported | Baltimore, MD Birmingham, AL Boston, MA Brooklyn, NY Denver, CO Indianapolis, IN New Orleans, LA San Francisco, CA Seattle, WA |
High |
| Plourde et al.41s | Male and female | Heterosexual | Nairobi, Kenya | Moderate |
| Portilla et al.42s | Male and female | Not reported | New Orleans, LA | High |
| Ramus et al.43s | Female only | Not reported | Dallas, TX | High |
| Sathia et al.44s | Not reported | Not reported | London, United Kingdom | Moderate |
| Singh et al.21 | Male and female | Heterosexual MSM |
Calgary, Alberta, Canada Edmonton, Alberta, Canada Vancouver, British Columbia, Canada Ottawa, Ontario, Canada |
Moderate |
| Verdon et al.18 | Male and female | Heterosexual Homosexual |
Denver, CO Seattle, WA |
Moderate |
MSM indicates men who have sex with men.
We found 13 studies that reported outcomes in urogenital infections, 7 studies that reported outcomes in rectal infections, and 11 studies that reported outcomes in pharyngeal infections treated with a 400-mg single dose of cefixime. Of patients who received 400 mg of cefixime, 824 of 846 (97%; 95% CI, 96%–98%) patients with urogenital infection, 107 of 112 (97.0%; 95% CI, 84%–100%) patients with rectal infection, and 202 of 242 (89%; 95% CI, 76%–96%) patients with pharyngeal infection were cured. Figure 2 shows the pooled treatment success proportions calculated from the meta-analysis for studies that used a single 400-mg dose.
Figure 2.
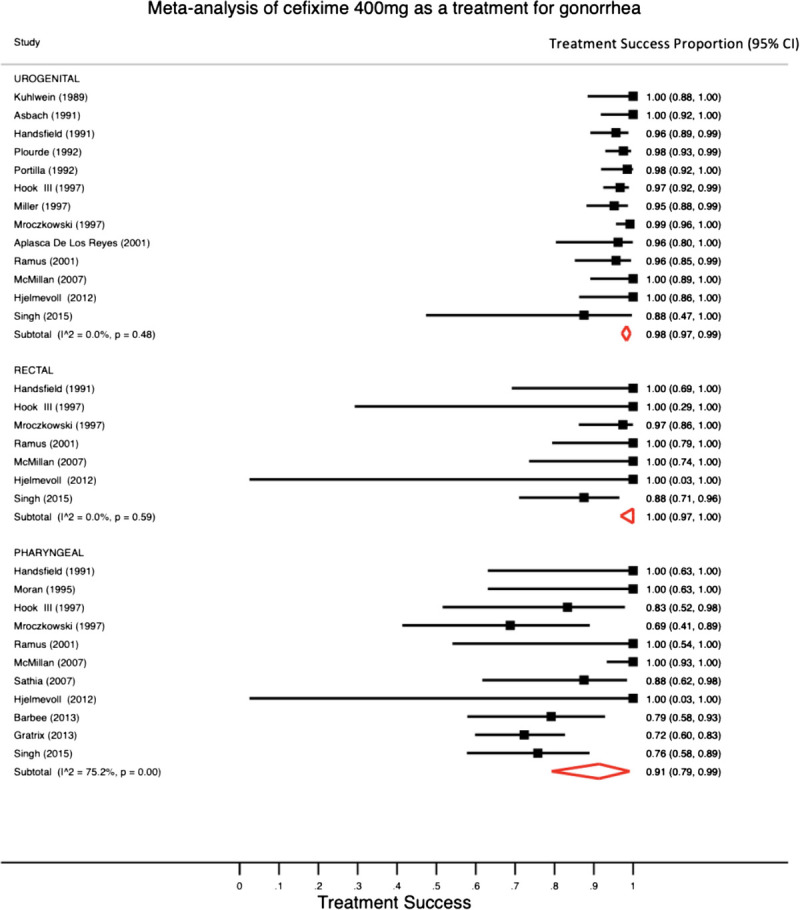
A meta-analysis with pooled estimates and 95% CIs for the single-dose cefixime 400 mg for the treatment of N. gonorrhoeae infection using a random-effects model.
We found 5 studies that reported outcomes of urogenital infections, 2 studies that reported outcomes of rectal infections, and 3 studies that reported outcomes of pharyngeal infections treated with an 800-mg single dose of cefixime. Of patients who received 800 mg of cefixime, 295 of 301 (98%; 95% CI, 96%–99%) patients with urogenital infection and 21 of 26 (81%; 95% CI, 61%–92%) patients with pharyngeal infection were cured. Combining the 2 studies that included rectal infections treated with an 800-mg single dose of cefixime, 12 of 12 patients were cured.20,21 Figure 3 shows the pooled treatment success proportions calculated from the meta-analysis for studies that used a single 800-mg dose. Because there were fewer than 3 studies reporting outcomes of patients with rectal infections treated with 800 mg, heterogeneity could not be assessed with the I2 statistic. Significant intragroup heterogeneity was only observed for the pharyngeal group (I2 statistic = 75.2%, P < 0.001).
Figure 3.
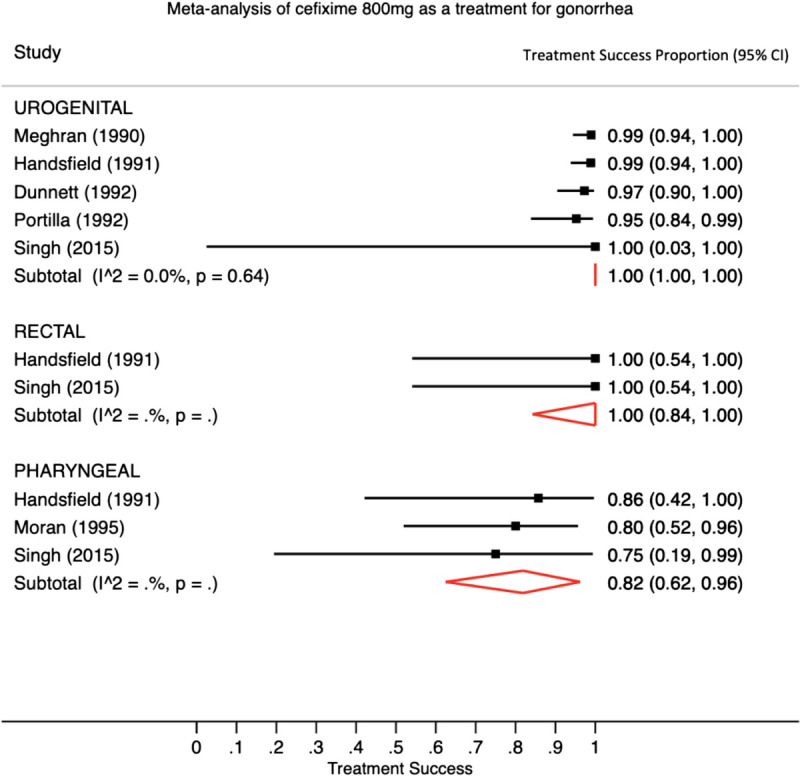
A meta-analysis with pooled estimates and 95% CIs for the single-dose cefixime 800 mg for the treatment of N. gonorrhoeae infection using a random-effects model. Footnote: There is significant heterogeneity for studies demonstrating treatment success for pharyngeal gonorrhea infections. The I2 statistic could not be calculated when there were 3 or fewer studies.
In our analysis of potential publication bias, we found that, for both the 400- and 800-mg dose studies, results showed asymmetry indicating evidence of potential publication bias (LFK index for 400-mg dose studies = −2.04; LFK index for 800 mg does studies = −6.62). The sensitivity analyses that excluded one study at a time found that the pooled treatment success proportion estimates did not change more than 2% except in the case of the pharyngeal results in which we excluded each the McMillan and Gratrix studies. With those exclusions, the 400-mg dose treatment success proportion for pharyngeal infections would be 85% (77%, 92%) and 94% (81%, 100%), without each study respectively.
DISCUSSION
We performed a systematic review and meta-analysis to compare the effectiveness of single-dose oral cefixime in the treatment of urogenital, rectal, and pharyngeal gonococcal infections. Across both dosages of cefixime 400 and 800 mg, cefixime was more effective at treating urogenital infections and less effective at treating pharyngeal infections. More investigation into the effectiveness of cefixime single dose 800 mg in treating rectal infections is warranted.
The difficulty of treating pharyngeal gonorrhea is an ongoing challenge.23 It has been noted that antibiotics have difficulty penetrating pharyngeal mucosa. The particular pharmacokinetic and pharmacodynamic mechanisms are poorly understood.10,24 A multidose regimen of cefixime could theoretically overcome the issue of limited pharyngeal mucosa penetration, but this theory requires further investigation.
Resistance-guided therapy has demonstrated promise in treating gonorrhea.25 Presently, N. gonorrhoeae currently demonstrates widespread ciprofloxacin resistance due to a single point mutation at the serine 91 codon of the GyrA gene. However, not all strains of N. gonorrhoeae exhibit this mutation. Allan-Blitz et al.26 described testing for the presence of the GyrA mutation N. gonorrhoeae to determine whether or not that particular mutation is present to guide therapy. Further studies have demonstrated a high effectiveness of ciprofloxacin in treating gonorrhea when resistance-guided therapy is used.27 Currently, there is no widely available genetic resistance test for cefixime, so resistance-guided therapy is only currently feasible in guiding the use of ciprofloxacin.
We found no other systematic reviews that investigated the effectiveness of cefixime for treatment of gonorrhea by anatomical site. In 2018, Tanvir et al.28 published a systematic review that aimed to characterize the overall effectiveness of a single oral dose of cefixime 400 mg compared with other drugs that are commonly used to treat gonococcal infections. All 8 studies that were included in their final meta-analysis were uncovered by our search query and were included in our meta-analysis. Through their meta-analysis, Tanvir et al. reported an overall success rate greater than 98% for a single oral dose of cefixime 400 mg but did not stratify by anatomic site of infection.
In 2013, Gratrix et al.19 published a retrospective review of pharyngeal gonorrhea treatment failures from clinics located in Alberta, Canada. Their study concluded that cefixime monotherapy is unreliable for the treatment of pharyngeal gonorrhea. Our study lends support to that conclusion, as we found that cefixime is less effective at treating pharyngeal gonococcal infections at both 400- and 800-mg single doses compared with the other 2 anatomical sites we investigated. Although Gratrix et al. found a much lower treatment failure rate when cefixime was combined with azithromycin, the move away from combination therapy due to increasing antimicrobial resistance of N. gonorrhoeae to azithromycin warrants further investigation into longer duration or higher-dose therapy.
Because of the rapid development of antibiotic resistance in N. gonorrhoeae, investigation into alternative therapies for treatment is necessary.1 Our findings suggest that, for urogenital gonococcal infections, cefixime is reliable at treating infection by N. gonorrhoeae. Our findings corroborate the CDC's recommendation of cefixime single dose 800 mg for use in expedited partner therapy in the case of uncomplicated urogenital infection.7 Low-resource settings and other settings in which oral treatment is preferable to treatment by injection make cefixime an attractive choice for providers when considering treatment options for gonococcal infection, especially in settings in which data suggest that local resistance is low.29
There were limitations to our study. First, our study was limited to reports available in English, which left out study reports investigating treatment of gonorrhea with cefixime in other languages. In addition, the analysis performed in our study may have been affected by positive publication bias, whereby studies demonstrating increased failure of cefixime may not have been reported. We did not control for study design. We included all studies that reported the effectiveness of cefixime treatment by anatomical site and did not exclude nonrandomized studies. Furthermore, recent literature has indicated the emergence of increasing cefixime resistance in N. gonorrhoeae in Southeast Asia.10 However, the results from our meta-analyses may not reflect that emerging trend because our search queries did not return any studies reporting on cefixime treatment outcomes by anatomical site from that region. Because of the broad range of publication years included by our search queries, we included several studies that used test of cure by culture. The lower sensitivity of test of cure by culture compared with test of cure by nucleic acid amplification test may have introduced heterogeneity to our study's results.30
Our systematic review and meta-analysis comparing the effectiveness of cefixime at treating urogenital, rectal, and pharyngeal gonococcal infections found that, at both single 400- and single 800-mg doses, cefixime is more effective at treating urogenital infections and less effective at treating pharyngeal infections. We recommend more investigation into the effectiveness of cefixime single dose 800 mg in treating rectal infections and the use of multidose therapy for cefixime treatment of pharyngeal infection.
For further references, please see “Supplemental References,” http://links.lww.com/OLQ/A892.
Footnotes
Conflict of Interest and Sources of Funding: All authors report no conflicts of interest. This study was supported by the National Institutes of Health (grant no. 5R21AI157817-03) and an award from the IDSA Foundation and HIV Medicine Association Grants for Emerging Researcher/Clinician Mentorship (G.E.R.M.) Program 2022. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Contributors: K.J.Y. contributed to literature search, data collection, writing – original draft, writing – review & editing. N.K. accessed and verified the underlying data reported in the manuscript, and contributed to writing – review & editing. C.C.B. contributed to data analysis, data interpretation, and writing – review & editing. J.D.K. contributed to writing – conceptual design and writing – review & editing.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Noah Kojima, Email: nkojima@ucla.edu.
Claire C. Bristow, Email: ccbristow@gmail.com.
Jeffrey D. Klausner, Email: jdklausner@med.usc.edu.
REFERENCES
- 1.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin Microbiol Rev 2014; 27:587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 2012; 7:1401–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolan GA, Sparling PF, Wasserheit JN. The emerging threat of untreatable gonococcal infection. N Engl J Med 2012; 366:485–487. [DOI] [PubMed] [Google Scholar]
- 4.Maldonado NG, Takhar SS. Update on emerging infections: News from the Centers for Disease Control and Prevention. Update to the CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. Ann Emerg Med 2013; 61:91–95. [DOI] [PubMed] [Google Scholar]
- 5.Unemo M Ross J Serwin AB, et al. 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults [published October 29, 2020]. Int J STD AIDS. [DOI] [PubMed] [Google Scholar]
- 6.Fifer H Saunders J Soni S, et al. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int J STD AIDS 2020; 31:4–15. [DOI] [PubMed] [Google Scholar]
- 7.St Cyr S Barbee L Workowski KA, et al. Update to CDC's treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng R Guo J Zhang Y, et al. Impacts of ceftriaxone exposure during pregnancy on maternal gut and placental microbiota and its influence on maternal and offspring immunity in mice. Exp Anim 2021; 70:203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadsworth SJ, Suh B. In vitro displacement of bilirubin by antibiotics and 2-hydroxybenzoylglycine in newborns. Antimicrob Agents Chemother 1988; 32:1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbee LA, St Cyr SB. Management of Neisseria gonorrhoeae in the United States: Summary of evidence from the development of the 2020 gonorrhea treatment recommendations and the 2021 Centers for Disease Control and Prevention sexually transmitted infection treatment guidelines. Clin Infect Dis 2022; 74(Suppl_2):S95–S111. [DOI] [PubMed] [Google Scholar]
- 11.Day MJ Jacobsson S Spiteri G, et al. Significant increase in azithromycin “resistance” and susceptibility to ceftriaxone and cefixime in Neisseria gonorrhoeae isolates in 26 European countries, 2019. BMC Infect Dis 2022; 22:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsson S Cole MJ Spiteri G, et al. Associations between antimicrobial susceptibility/resistance of Neisseria gonorrhoeae isolates in European Union/European economic area and patients' gender, sexual orientation and anatomical site of infection, 2009–2016. BMC Infect Dis 2021; 21:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quilter LAS St Cyr SB Hong J, et al. Antimicrobial susceptibility of urogenital and extragenital Neisseria gonorrhoeae isolates among men who have sex with men: Strengthening the US Response to Resistant Gonorrhea and Enhanced Gonococcal Isolate Surveillance Project, 2018 to 2019. Sex Transm Dis 2021; 48(12S Suppl 2):S111–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ McKenzie JE Bossuyt PM, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Cochrane C. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Book Series. 2nd ed. Hoboken, NJ: Wiley-Blackwell, 2019. p. xxviii, 694 pages; illustrations (some colour). [Google Scholar]
- 16.Higgins JP Thompson SG Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc 2018; 16:195–203. [DOI] [PubMed] [Google Scholar]
- 18.Verdon MS Douglas JM Jr. Wiggins SD, et al. Treatment of uncomplicated gonorrhea with single doses of 200 mg cefixime. Sex Transm Dis 1993; 20:290–293. [DOI] [PubMed] [Google Scholar]
- 19.Gratrix J Bergman J Egan C, et al. Retrospective review of pharyngeal gonorrhea treatment failures in Alberta, Canada. Sex Transm Dis 2013; 40:877–879. [DOI] [PubMed] [Google Scholar]
- 20.Handsfield HH McCormack WM Hook EW 3rd, et al. A comparison of single-dose cefixime with ceftriaxone as treatment for uncomplicated gonorrhea. N Engl J Med 1991; 325:1337–1341. [DOI] [PubMed] [Google Scholar]
- 21.Singh AE Gratrix J Martin I, et al. Gonorrhea treatment failures with oral and injectable expanded spectrum cephalosporin monotherapy vs dual therapy at 4 Canadian sexually transmitted infection clinics, 2010–2013. Sex Transm Dis 2015; 42:331–336. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH Oxman AD Vist G, et al. Grade guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias). J Clin Epidemiol 2011; 64:407–415. [DOI] [PubMed] [Google Scholar]
- 23.Kong FYS, Hocking JS. Treating pharyngeal gonorrhoea continues to remain a challenge. Lancet Infect Dis 2022; 22:573–574. [DOI] [PubMed] [Google Scholar]
- 24.Unemo M. Current and future antimicrobial treatment of gonorrhoea—The rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis 2015; 15:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allan-Blitz LT, Adamson PC, Klausner JD. Resistance-guided therapy for Neisseria gonorrhoeae. Clin Infect Dis 2022; 75:1655–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allan-Blitz LT, Wang X, Klausner JD. Wild-type gyrase a genotype of Neisseria gonorrhoeae predicts in vitro susceptibility to ciprofloxacin: A systematic review of the literature and meta-analysis. Sex Transm Dis 2017; 44:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klausner JD Bristow CC Soge OO, et al. Resistance-guided treatment of gonorrhea: A prospective clinical study. Clin Infect Dis 2021; 73:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanvir SB Qasim SSB Shariq A, et al. Systematic review and meta-analysis on efficacy of cefixime for treating gonococcal infections. Int J Health Sci (Qassim) 2018; 12:90–100. [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Reproductive Health and Research . Who Guidelines for the Treatment of Neisseria gonorrhoeae. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 30.Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and treatment of sexually transmitted infections: A review. JAMA 2022; 327:161–172. [DOI] [PubMed] [Google Scholar]


