Multiple sclerosis (MS) is an autoimmune disease that affects nearly 1 million people in the United States, leading to significant morbidity and mortality. It affects the central nervous system (CNS) and is characterized by demyelinating lesions and axonal damage. Although a number of drugs have been approved for the treatment of MS, current treatments are unable to prevent the long-term decline of the CNS function and have side effects related to their general impact on the patient’s immune system. The development of tolerogenic vaccines, vaccines that induce tolerance to specific agents, for MS has been the focus of considerable research, as this approach promises to avoid nonspecific immune suppression while enabling a broadly useful, inexpensive, and relatively simple therapy. A report by Kwiatkowski et al. (1) in this edition of PNAS describes a biomaterials-based strategy to induce tolerance to a peptide frequently used as a model antigen in MS research. This biomaterial vaccine significantly alters numerous immune cell populations in both the vaccine-draining lymph nodes and CNS and, strikingly, is capable of reversing paralysis in a murine model of advanced disease.
There is no cure for MS and few therapeutic options exist, highlighting the complexity and difficulty of treating this disease. Research into the treatment of MS has focused on similar strategies as other autoimmune disorders, which can largely be divided into approaches for either broad immunosuppression or immune tolerance. A wide range of immune cell populations have been implicated in MS, including T cells, B cells, and phagocytes such as macrophages and microglia (2). The pathology is characterized by increased permeability of the blood–brain barrier for these activated immune cells, resulting in upregulation of diverse chemokine receptors, expression of proinflammatory cytokines, and direct cytotoxicity mediated by T cells, macrophages, and microglia (3). These responses result in the destruction of the myelin sheath via a rate, severity, and mechanism that depend on the stage of disease progression (4).
Broad immunosuppression thus offers opportunities to prevent this highly complex inflammatory process, the details of which are still under active investigation. Immunosuppressants employed for treatment of MS include corticosteroids to decrease inflammation, immunomodulatory molecules such as interferon β, and anti-α4-integrin monoclonal antibodies to limit immune cell trafficking, as well as anti-CD52, anti-CD25, and anti-CD20 monoclonals to directly deplete immune cell subsets (5). But long-term immunosuppression is not desirable, as it is associated with higher rates of cancer, susceptibility to opportunistic infection, and decreased efficacy of vaccination (6). These side effects have plagued the utility of immunosuppressants in cell and organ transplantation, rheumatoid arthritis, and diverse autoimmune disorders in addition to MS.
The alternative strategy, antigen-specific immune tolerance, presents a sustainable solution that would require only a short treatment regimen. Here, the immune system is trained to accept, or tolerize, specific self-antigens driving the inflammatory responses. Unlike broad immunosuppression that nonspecifically hinders immune responses systemically, tolerance selectively inhibits the key activated T cells contributing to responses against relevant autoantigens, allowing the immune system to otherwise function unimpeded. This mechanism is primarily mediated by regulatory T cells, which are activated by tolerogenic antigen-presenting cells (APCs) that include dendritic cells (DCs), monocytes and macrophages among others. This process requires the controlled exposure of immune cells to select autoantigens while simultaneously promoting a tolerizing immune microenvironment, the latter of which typically involves the promotion of tolerogenic APCs with antiinflammatory therapeutics like vitamin D3 (VD3) and rapamycin (7, 8). Although the autoantigens responsible for MS remain under active investigation, several potential culprits associated with the myelin sheath have been identified, such as myelin oligodendrocyte glycoprotein (MOG) and myelin basic protein (MBP). But achieving antigen-specific immune tolerance has remained an exceptionally difficult feat to achieve, representing a “holy grail” of sorts for the treatment of autoimmune disorders.
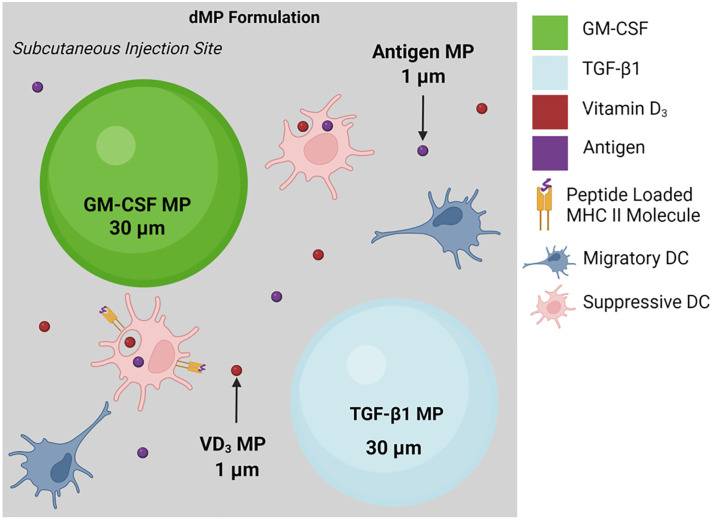
Nanoscale and macroscale biomaterials have emerged as versatile tools to enhance tolerogenic strategies and have been previously employed in the treatment of MS (5). These materials present versatile and customizable options for controlling the release rate and cellular targets of therapy, both of which are particularly critical for attaining lasting immune modulation (9). Seminal work from a team led by Stephen Miller and Lonnie Shea demonstrated that the development and progression of MS could be inhibited in a preclinical experimental autoimmune encephalomyelitis (EAE) mouse model via administration of poly(lactic-co-glycolic acid) (PLGA) microparticles encapsulating MBP-derived peptides (10). Building upon these findings, Kwiatkowski et al. (1) present a novel combinatorial materials strategy that takes advantage of both extracellular and intracellular immune modulation to therapeutically treat established MS and reverse hind limb paralysis in an advanced EAE model. Four distinct PLGA microparticle formulations with two distinct sizes were employed (Fig. 1): two large nonphagocytosable 50-µm particles and two smaller phagocytosable 1-µm variants. The two nonphagocytosable microparticles separately encapsulated GM-CSF and TGF-β1 for sustained recruitment of DCs. The two phagocytosable MPs contained either VD3 or MOG to generate tolerogenic DC phenotypes capable of eliciting antigen-specific responses. Through a materials-based approach, both the innate and adaptive autoimmune responses were thus advantageously engaged for antigen-specific tolerance, which was capable of reversing MS-induced paralysis without broad systemic immunosuppression.
Fig. 1.
Schematic of the dual microparticle (dMP) formulations that were loaded with four distinct immunomodulatory agents (1).
“A report by Kwiatkowski et al. in this edition of PNAS describes a biomaterials-based strategy to induce tolerance to a peptide frequently used as a model antigen in MS research.”
Kwiatkowski et al. (1) demonstrated both reversal of paralysis that results from advanced disease in a murine model of MS and a dramatic alteration in the infiltration and gene expression of immune cells infiltrating the CNS. These findings are consistent with previous work by this research laboratory that demonstrated that this treatment could prevent EAE progression. Both sets of studies utilized EAE as an animal model for MS, as it replicates a number of mechanisms mediating MS and the resulting pathology (11). While the specific antigen(s) mediating MS is unclear, EAE is triggered via immunization against a model antigen using appropriate adjuvants. Following treatment of diseased animals, the infiltration of immune cells in the CNS was significantly reduced along with various inflammatory cytokines established to be both drivers for immune cell recruitment and relevant to Th1 and Th17 T cells, which play key roles in the pathogenesis of EAE. Notably, T cells were reduced in the CNS of treated animals, and these are believed to be the main mediators of MS. However, these effects were only found when the antigen used to trigger EAE was included in the treatment; inclusion of a nonrelevant antigen had little impact. These findings, combined with an improvement in the level of demyelination and number of lesions, demonstrate the treatment impacts key hallmarks of MS.
Importantly, single-cell analysis of immune cells in the draining lymph node for the treatment site, and the CNS, revealed significant changes in gene expression that were largely parallel in the two anatomic sites. In general, both APCs and B cells exhibited decreased expression of genes related to antigen presentation pathways. This was paralleled by diminished expression of genes related to T cell receptor signaling in CD4 and CD8 T cells. Altogether, these findings suggest a diminished ability of immune cells in treated mice to both present and respond to antigens that could maintain and further disease progression, and are consistent with the current hypothesis regarding the mechanisms underlying MS. Strikingly, the efficacy of treatment was diminished when studies were performed in mice lacking B cells. This may seem contradictory to current MS treatment, as the only therapy that slows disease progression involves B cell depletion (12). The findings of this publication suggest that B cells play a role not only in disease progression but also in immune reversal. This possibility is supported by previous research demonstrating that B cell-deficient mice fail to recover spontaneously in an adoptive transfer model of MS, in contrast to normal mice (13). However, one must be cautious about drawing conclusions regarding human disease from animal studies.
These results provide supportive insights into the realization of therapeutic tolerance in MS and may be applicable to the treatment of additional autoimmune disorders. The cellular and biochemical mechanisms behind autoimmunity are poorly understood, complex, and disease specific, which has necessitated the use of immunosuppressants with broad immunomodulatory and, unfortunately, adverse effects. As we gain further knowledge into the specific cell populations and molecular pathways contributing to the pathology of autoimmune disease, tools and strategies that can specify the kinetics and targets of delivered agents will allow controlled immunomodulation while minimizing side effects. Furthermore, the benefit of multiplex signaling to direct complex biological processes has been demonstrated for many other biomedical applications, such as enhanced healing of chronic wounds (14). To this end, Kwiatkowski et al. (1) demonstrate the versatility, customization, and vast potential of materials-based approaches for the synergistic delivery of multiple therapeutic molecules, each possessing distinct biochemical functions and rates of release. By simultaneously directing both innate and adaptive immune responses, their dMP strategy holds promise for achieving antigen-specific tolerance as a viable alternative to immunosuppression.
Acknowledgments
Author contributions
E.A.S. and D.J.M. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
See companion article, “Treatment with an antigen-specific dual microparticle system reverses advanced multiple sclerosis in mice,” 10.1073/pnas.2205417119.
References
- 1.Kwiatkowski A. J., et al. , Treatment with an antigen-specific dual microparticle system reverses advanced multiple sclerosis in mice. Proc. Natl. Acad. Sci. U.S.A. 119, e2205417119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dendrou C. A., Fugger L., Friese M. A., Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Frohman E. M., Racke M. K., Raine C. S., Multiple sclerosis–the plaque and its pathogenesis. N Engl. J. Med. 354, 942–955 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Filippi M., et al. , Multiple sclerosis. Nat. Rev. Dis. Primers 4, 43 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Kwiatkowski A. J., Stewart J. M., Cho J. J., Avram D., Keselowsky B. G., Nano and microparticle emerging strategies for treatment of autoimmune diseases: Multiple sclerosis and type 1 diabetes. Adv. Healthc. Mater 9, e2000164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee A., et al. , Efficacy of covid-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. Bmj 376, e068632 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke J. A., et al. , Subcutaneous nanotherapy repurposes the immunosuppressive mechanism of rapamycin to enhance allogeneic islet graft viability. Nat. Nanotechnol. 17, 319–330 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi S., et al. , Surface engineered polymersomes for enhanced modulation of dendritic cells during cardiovascular immunotherapy. Adv. Funct. Mater. 29, 1904399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott E. A., Karabin N. B., Augsornworawat P., Overcoming immune dysregulation with immunoengineered nanobiomaterials. Annu. Rev. Biomed. Eng. 19, 57–84 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Getts D. R., et al. , Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 30, 1217–1224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birmpili D., Charmarke Askar I., Bigaut K., Bagnard D., The translatability of multiple sclerosis animal models for biomarkers discovery and their clinical use. Int. J. Mol. Sci. 23, 11532 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ancau M., Berthele A., Hemmer B., CD20 monoclonal antibodies for the treatment of multiple sclerosis: Up-to-date. Expert Opin. Biol. Ther. 19, 829–843 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Zhao M. L., Fritz R. B., Acute and relapsing experimental autoimmune encephalomyelitis in IL-4- and alpha/beta T cell-deficient C57BL/6 mice. J. Neuroimmunol. 87, 171–178 (1998). [DOI] [PubMed] [Google Scholar]
- 14.White M. J. V., Briquez P. S., White D. A. V., Hubbell J. A., VEGF-A, PDGF-BB and HB-EGF engineered for promiscuous super affinity to the extracellular matrix improve wound healing in a model of type 1 diabetes. npj. Regener. Med. 6, 76 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]