Significance
This study reveals a stimulus-driven gradient of lags in functional connectivity on the scales of several seconds during the comprehension of spoken narratives. This narrative-driven information flow proceeds along the cortical processing hierarchy from the early auditory cortex to the language network, then to the default mode network. The hierarchy of processing timescales is thought to be a fundamental organizing principle of the brain. Here, we provide a simple computational model to systematically explore the interplay between the brain’s functional architecture and the temporal structure of natural language inputs. We show that the information flow emerges from hierarchical neural accumulation driven by inputs with structures similar to naturalistic narratives—that is, hierarchically nested structures, which are ubiquitous in real-world contexts.
Keywords: cortical hierarchy, naturalistic stimuli, fMRI, functional connectivity, language processing
Abstract
When listening to spoken narratives, we must integrate information over multiple, concurrent timescales, building up from words to sentences to paragraphs to a coherent narrative. Recent evidence suggests that the brain relies on a chain of hierarchically organized areas with increasing temporal receptive windows to process naturalistic narratives. We hypothesized that the structure of this cortical processing hierarchy should result in an observable sequence of response lags between networks comprising the hierarchy during narrative comprehension. This study uses functional MRI to estimate the response lags between functional networks during narrative comprehension. We use intersubject cross-correlation analysis to capture network connectivity driven by the shared stimulus. We found a fixed temporal sequence of response lags—on the scale of several seconds—starting in early auditory areas, followed by language areas, the attention network, and lastly the default mode network. This gradient is consistent across eight distinct stories but absent in data acquired during rest or using a scrambled story stimulus, supporting our hypothesis that narrative construction gives rise to internetwork lags. Finally, we build a simple computational model for the neural dynamics underlying the construction of nested narrative features. Our simulations illustrate how the gradual accumulation of information within the boundaries of nested linguistic events, accompanied by increased activity at each level of the processing hierarchy, can give rise to the observed lag gradient.
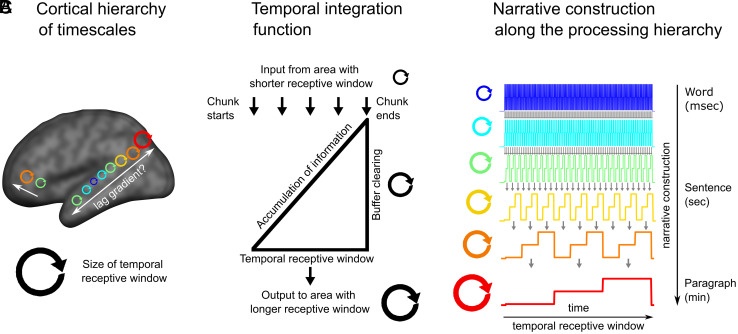
Narratives are composed of nested elements that must be continuously integrated to construct a meaningful whole, building up from words to phrases to sentences to a coherent narrative (1). Recent evidence suggests that the human brain relies on a chain of hierarchically organized brain areas with increasing temporal receptive windows (TRWs) to process this temporally evolving, nested structure (Fig. 1A). This cortical hierarchy was first revealed by studies manipulating the temporal coherence of naturalistic narratives (2, 3). These studies reported a topography of processing timescales where early auditory (AUD) areas respond reliably to rapidly evolving acoustic features, adjacent areas along the superior temporal gyrus respond reliably to information at the word level, and nearby language areas respond reliably only to coherent sentences. Finally, areas at the top of the processing hierarchy in the default mode network (DMN) integrate slower evolving semantic information over many minutes (4).
Fig. 1.
Narrative construction in the hierarchical processing framework. (A) The proposed cortical hierarchy of increasing TRWs (adapted from ref. 5). (B) Each level of the processing hierarchy continuously accumulates information over inputs from the preceding level. For example, phrases built over words are constructed into sentences. The accumulated information is flushed out at structural boundaries. (C) Each level of the processing hierarchy provides the building blocks for the next level, which naturally leads to longer TRWs, corresponding to linguistic units of increasing sizes. This model of narrative construction along the cortical processing hierarchy implies a gradient of response lags across the cortical hierarchy.
This cortical hierarchy of increasing temporal integration windows is thought to be a fundamental organizing principle of the brain (5, 6). The cortical hierarchy of TRWs in humans has been described using fMRI (2, 3, 7, 8) and ECoG (9). Recent work has shown that deep language models also learn a gradient or hierarchy of increasing TRWs (10–12), and that manipulating the temporal coherence of narrative input to a deep language model yields representations closely matching the cortical hierarchy of TRWs in the human brain (13). Furthermore, the cortical hierarchy of TRWs matches the intrinsic processing timescales observed during rest in humans (9, 14, 15) and monkeys (16). This cortical topography also coincides with anatomical and functional gradients such as long-range connectivity and local circuitry (17–19), which have been shown to yield varying TRWs (20, 21).
The proposal that the cortex is organized according to a hierarchy of increasing TRWs implies that each area “chunks” and integrates information at its preferred temporal window and that narrative construction proceeds along the cortical hierarchy. For example, an area that processes phrases receives information from areas that process words (Fig. 1B), which are further transmitted to areas that integrate phrases into sentences. At the end of each phrase, information is rapidly cleared to allow for real-time processing of the next phrase (1, 7). The chunking of information at varying granularity is supported by recent studies that used data-driven methods to detect boundaries as shifts between stable patterns of brain activity (22, 23).
This model of narrative construction (Fig. 1C) predicts a gradient of response lags across the cortical processing hierarchy; namely, shorter temporal lags among adjacent areas along the processing hierarchy than regions further apart in the cortical hierarchy. We provide a computational model to clearly illustrate how the construction of nested narrative features could give rise to the predicted lag gradient. In the current study, we test this prediction by comparing response fluctuations elicited by spoken narratives in different brain areas using lag-correlation. We extract the lag with the peak correlation to estimate interregion temporal differences. To focus on neural responses to linguistic and narrative information, we used intersubject functional connectivity (ISFC) analysis (24, 25). Unlike traditional within-subject functional connectivity (WSFC), ISFC effectively filters out the idiosyncratic fluctuations that drive intrinsic functional correlations within subjects. Isolating stimulus-locked neural activity from intrinsic neural activity allows us to observe the temporal dynamics of narrative construction across the cortical hierarchy. We predicted that ISFC analysis would reveal an interregion lag gradient during the comprehension of intact narrative, but not during scrambled story or rest, which does not involve narrative construction. Finally, our computational model shows how the lag gradient deteriorates with nonnaturalistic inputs.
Results
To test the hypothesis that narrative construction will yield a gradient of response lags across brain regions, we first divided the neural signals into six networks by applying k-means clustering to WSFC measured during rest (SI Appendix, Fig. S1). We labeled these networks based on anatomical correspondence with previously defined functional regions following Simony and colleagues (25), including the AUD, ventral language (vLAN), dorsal language (dLAN), DMN, and attention (ATT) networks, aligning with the previously documented TRW hierarchy (SI Appendix, Fig. S2).
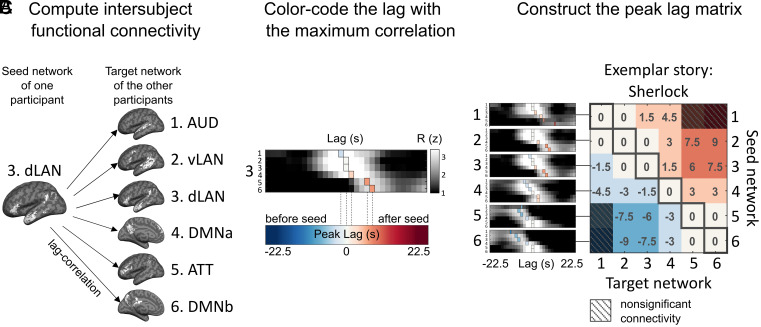
We computed lag-ISFC (i.e. cross-correlation) at varying temporal lags between all pairs of networks (Fig. 2A and SI Appendix, Fig. S3). The lags with maximum ISFC (i.e., “peak lag”) for each seed-target pair were extracted as an index for the temporal gaps in the stimulus-driven processing between each pair of networks. The extracted peak lags were color-coded to construct the network × network peak lag matrix (Fig. 2 B and C). In the following, we describe the observed lag gradient in detail and several control analyses. Finally, we simulated the nested narrative structure and the corresponding brain responses to explore how different integration functions at different timescales could give rise to the observed lag gradient.
Fig. 2.
Construction of the internetwork peak lag matrix. (A) Lag-ISFC (cross-correlation) between seed-target network pairs were computed using the leave-one-subject-out method. The dLAN network is used as an example seed network for illustrative purposes. (B) The matrix depicts ISFC between the dLAN seed and all six target networks at varying lags. The lag with the peak correlation value (colored vertical bars) was extracted and color-coded according to lag. For visualization, the lag-ISFCs were z-scored across lags. (C) The network × network peak lag matrix (P < 0.05, FDR corrected). Warm colors represent peak lags following the seed network, while cool colors represent peak lags preceding the seed network; zeros along the diagonal capture the intranetwork ISC. An example story (“Sherlock”) is shown for illustrative purposes.
Fixed Lag Gradient across Cortical Networks.
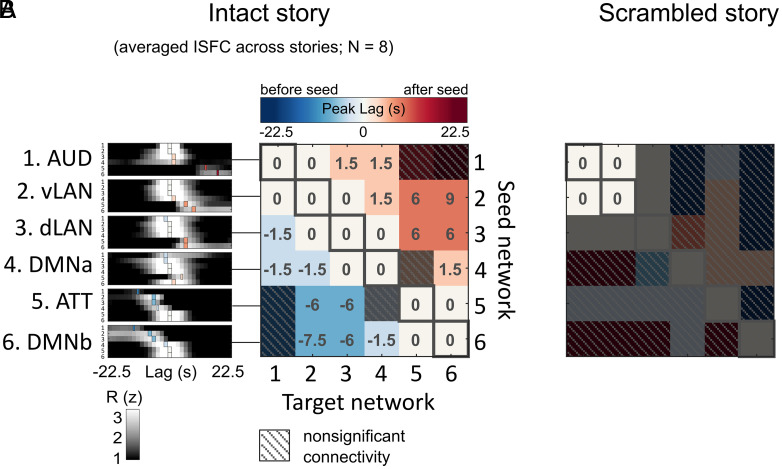
The average lag-ISFC across stories was computed for each seed network (Fig. 3 A, Left). The lag-ISFC between a seed network and the same network in other subjects always peaked at lag 0, reflecting the strong stimulus-locked within-network synchronization reported in the intersubject correlation (ISC) literature (3, 26, 27) (SI Appendix, Fig. S3). Interestingly, however, non-zero peak lags were found between different networks. Relative to a low-level seed, putatively higher level networks showed peak connectivity at increasing lags. For example, the stimulus-induced activity in dLAN lagged 1 TR (1.5 s) behind activity in AUD, whereas the activity in DMNb lagged 4 TRs (6 s) behind activity in dLAN. Importantly, regardless of the choice of seed, the target networks showed peak connectivity in a fixed order progressing through AUD, vLAN, dLAN, DMNa, ATT, and DMNb.
Fig. 3.
The peak lag matrix across eight stories reveals a fixed lag gradient across networks, which is abolished during scrambled narratives and rest. (A) The network × network peak lag matrix is based on the averaged lag-ISFC across eight stories. For visualization, lag-ISFC curves at left were z-scored across lags. (B) Peak lag matrix based on responses to a scrambled story stimulus (scrambled words). Peak lag matrices are thresholded at P < 0.05 (FDR corrected).
To summarize the findings, we color-coded the peak lags and collated them into a peak lag matrix where each row corresponds to a seed network and each column corresponds to a target network (Fig. 3 A, Right and see SI Appendix, Fig. S4 for the lag-ISFC waveforms). The white diagonal indicates a peak at zero lag within each area, reflecting the intranetwork synchronization across subjects (i.e., ISC) (SI Appendix, Fig. S3), while the cool-to-warm color gradient indicates a fixed order of peak lags. For example, the first row shows a white-to-warm gradient, reflecting that when AUD served as the seed, other networks were either synchronized with or followed AUD, but never preceded it. Conversely, the cool-to-white gradient of the last row indicates that all other networks preceded the DMNb seed. The lag gradient can also be observed in individual stories (SI Appendix, Fig. S5), although these patterns are noisier than the averaged results. The lag gradient proceeded in a fixed order across all networks, suggesting that bottom-up narrative construction is reflected in lagged connectivity between stages along the cortical hierarchy from AUD up to DMNb. Similar results were obtained when we defined the networks using the TRW hierarchy (SI Appendix, Fig. S2) or used network masks predefined based on whole-brain functional parcellation of resting-state fMRI data (28) (SI Appendix, Fig. S6).
Temporal Scrambling Abolishes the Lag Gradient.
We hypothesized that the lag gradient reflects the emergence of macroscopic story features (e.g., narrative situations or events) integrated over longer periods of time in higher level cortical networks (22, 23). To support this point, we next used the same procedure to compute the peak lag matrix for a temporally scrambled version of one story (“Pie Man”; as for the results of intact “Pie Man”, please see SI Appendix, Fig. S5). In this dataset, the story stimulus was spliced at the word level and scrambled, thus maintaining similar low-level sensory statistics while abolishing the slower evolving narrative content. The peak lag matrix for the scrambled story revealed synchronized responses at lag 0 both within and between the AUD and vLAN networks, but no significant peaks within or between other networks (Fig. 3B). This reflects low-level speech processing limited to the word level and indicates that disrupting the narrative structure of a story abolishes the temporal propagation of information to higher level cortical areas.
Lag Gradient across Fine-Grained Subnetworks.
To verify that the peak lag gradient could also be observed at a finer spatial scale, we further divided each of the six networks into ten subnetworks, again by applying k-means clustering to resting-state WSFC (k = 10 within each network). The peak lag matrix between the sixty subnetworks was generated using the same methods as in the network analysis (SI Appendix, Fig. S7A). We also visualized the brain map of lags between one selected seed (posterior superior/middle temporal gyrus) and all the target subnetworks (SI Appendix, Fig. S7B). Similar to the network level analysis, the peak lag between the subnetworks revealed a gradient from the early AUD cortex to the language network (AUD association cortex), then to the DMN.
Idiosyncratic Within-Subject Fluctuations Obscure the Lag Gradient.
We next asked whether the internetwork lag gradient could result from intrinsic fluctuations in brain activity previously observed with WSFC (Mitra et al., 2014, 2015, 2016). WSFC did not show a difference between intact stories and other conditions. At the network level, WSFC analyses revealed a strong peak correlation at lag zero within each network but also a peak correlation at lag zero across all networks such that no gradient was observed in either the intact story, the scrambled story, or the resting-state data (SI Appendix, Fig. S8A). At the fine-grained subnetwork level, subtle lags on the scale of −1 to +1 TR (1 TR = 1.5 s) were found in all three conditions without resorting to interpolation (SI Appendix, Fig. S8B), replicating the work by Mitra et al. (2014, 2015; 2016) (29–31) showing interarea lags around −1 to +1 s in resting-state data with WSFC.
The discrepancy may be related to differences in the signals that drive WSFC and ISFC. ISFC isolates stimulus-driven connectivity; in contrast, WSFC is susceptible to idiosyncratic intrinsic signal fluctuations, which propagate across brain areas irrespective of task-related activation (24, 25). Thus, the present result suggests that idiosyncratic, intrinsic fluctuations do not drive the lag gradient observed during narrative comprehension. Furthermore, natural language processing unfolds over timescales slower than the propagation of intrinsic signals revealed by WSFC (29–31). Natural language is processed word by word across sentences and paragraphs, which can unfold over seconds to minutes. Indeed, our ISFC analysis reveals a narrative-driven lag gradient at temporal scales an order of magnitude larger than the intrinsic lags (up to 9 s) (Fig. 3). The narrative-driven lag gradient we report here cannot be explained by factors such as differences in the hemodynamic response, which would be observable using WSFC analyses.
Dominant Bottom-Up Lag Gradient across Networks.
We adopted a method introduced by Mitra and colleagues (31) to discern whether there are multiple parallel lag sequences between networks. We applied principal component analysis (PCA) to the peak lag matrix and examined the cumulative variance accounted for across principal components. Our results revealed that the first principle component explains 88.8% of the variance (SI Appendix, Fig. S9 A and B) in the lag matrix at the coarse level of the cortical networks (Fig. 3A) and 43.0% of the variance in the lag matrix at the fine-grained subnetwork level (SI Appendix, Fig. S7A). These results suggest that there is a single, unidirectional lag gradient across networks.
We visualized the relative lag values from the first principal component of the intersubnetwork lag matrix on a brain map (SI Appendix, Fig. S9C), revealing shorter temporal lags among adjacent areas along the processing hierarchy than regions further apart in the cortical hierarchy.
The Lag Gradient is not Driven by Transient Linguistic Boundary Effects.
Prior work has reported that scene/situation boundaries in naturalistic stimuli elicit transient brain responses that vary across regions (32–37). To test whether this transient effect could drive the gradient observed in our lag matrix, we computed lag-ISFC after regressing out the effects of word, sentence, and paragraph boundaries in two stories with time-stamped annotations. As shown in SI Appendix, Fig. S10, the regression model successfully removed transient effects of the boundaries from the fMRI time series. Critically, however, the lag gradient remained qualitatively similar when accounting for boundaries, indicating that the observed lag gradient does not result from transient responses to linguistic boundaries in the story stimulus.
Reproducing the Lag Gradient by Simulating Narrative Construction.
Narratives have a multilevel nested hierarchical structure (38) and are reported to elicit neural processing at increasingly long timescales along the cortical hierarchy (22, 23). To better understand how the construction of nested narrative features could give rise to the long internetwork lag gradient we observed, with up to 9-s lags, we created a simulation capturing the hierarchically nested temporal structure of real-world narratives and the corresponding hierarchy of cortical responses.
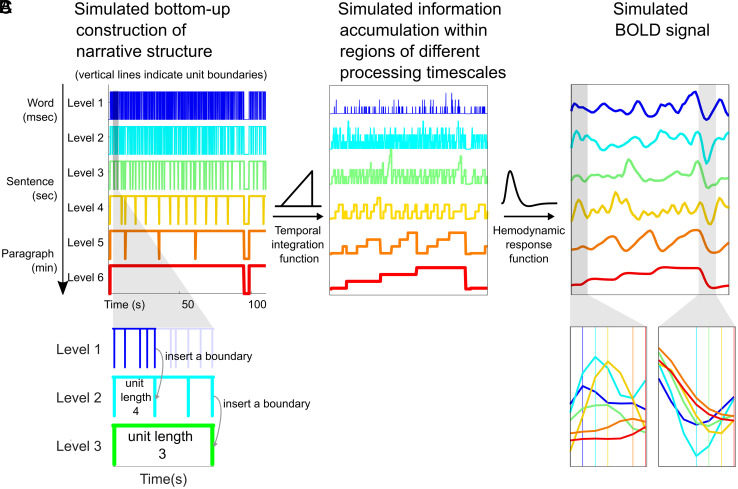
To match the six networks discussed so far, we simulated story features emerging across six distinct timescales, which roughly correspond to words, phrases, sentences, 2 to 3 sentences, and paragraphs (Fig. 4A). To build the first level of a nested structure, we sampled a sequence of 3,000-word durations with replacement from “Sherlock.” Unit boundaries at Level 1 were inserted accordingly. First-level units were integrated into units of the next level with a lognormal distributed unit length (SI Appendix, Fig. S11). For example, if the first Level 2 unit has a unit length of 4, we insert a Level 2 boundary at the time point corresponding to the end of the fourth Level 1 unit, creating a “phrase” of 4 “words.” Second-level units were integrated into the third-level units following the same method. A nested structure of six levels was thus generated. Since paragraphs are often separated in real stories by longer silent periods (SI Appendix, Fig. S12), we inserted pauses at top-level (sixth-level) boundaries. The bottom-up construction of narrative structure gives rise to interlevel alignment and increasing processing timescales at higher levels, as proposed in the hierarchical processing framework (5, 6, 18).
Fig. 4.
Simulating narrative construction and the corresponding brain responses. (A) The construction of the nested narrative structure, simulated by sampling boundary intervals from actual word durations and recursively integrating them to obtain structural boundaries at higher levels. (B) Information accumulation at different levels is generated by a linearly increasing temporal integration function. We postulated that information accumulation is accompanied by increased activity. (C) BOLD responses generated by HRF convolution. This visualization is based on parameters estimated from a spoken story stimulus (SI Appendix, Table S1).
The simulated response amplitudes were generated using a linearly increasing temporal integration function (Fig. 4B), based on prior work showing that information accumulation is accompanied by gradually increasing activation within phrases/sentences (39–44) and paragraphs (32, 35) (a similar sentence/paragraph length effect was also observed in our data; see SI Appendix, Fig. S13). The linearly increasing temporal integration function accumulates activity derived from lower level units within the interval between unit boundaries at the current levels and flushes out the accumulated activity at unit boundaries of the current level. To account for hemodynamic lag in the fMRI signal, we applied a canonical hemodynamic response function (HRF) to the simulated response amplitudes (Fig. 4C). We averaged the interlevel lag-correlations across thirty different simulated structures (equivalent to 30 different stories) and extracted the peak lags. This peak-lag analysis parallels the analysis previously applied to the fMRI data.
The simulation allows us to systematically manipulate the narrative structure and the temporal integration function to reveal the conditions under which the lag gradient emerges. We first performed the simulation with a set of “natural” parameters roughly motivated by the temporal properties of our narrative stimuli and a simple temporal integration function reflecting linear temporal accumulation (SI Appendix, Table S1).
This simple simulation is sufficient to reproduce the internetwork lag gradient observed in the fMRI data (Fig. 5A; as well as the ISFC at lag zero; SI Appendix, Fig. S14). In addition, we also compared the spectral properties of the simulated and real BOLD signals (SI Appendix, Fig. S15). Computing the PSD of the simulated brain responses revealed increased low-frequency power in responses to high-level structures with longer intervals between boundaries. Similarly, stronger low-frequency fluctuations in real BOLD signals were found in high-level regions along the cortical processing hierarchy. We computed the power spectrum voxel by voxel and split the voxels into six clusters of equal size according to their low-frequency power (cumulative power below 0.04 Hz) (SI Appendix, Fig. S15B), replicating Stephens et al. (2013) (15). Networks defined by low-frequency power show a similar topographic gradient as the networks defined by resting-state WSFC, from the AUD areas to DMN, which yields a significant correlation between the two sets of network indices. We then adjusted one parameter at a time to explore the parameter space constrained by natural speech.
Fig. 5.
Simulated peak lag matrix. (A) Simulating the peak lag matrix observed during story-listening fMRI data (Fig. 3A) using parameters derived from a story stimulus (the same parameters as in Fig. 4 and SI Appendix, Table S1). (B) Simulating the lag matrix observed during scrambled story (scrambled words) (Fig. 3B), by setting mean unit length = 1 and unit length variance = 0. (C) Lag matrix from the nonnested structure, created by combining levels extracted from independently generated nested structures, which disrupts the nesting relationship between different levels, similar to the scrambled story, while preserving the spectral properties of individual time series (P < 0.05, FDR correction).
Key Parameters for the Emergence of a Lag Gradient.
Within the bounds of natural speech (SI Appendix, Fig. S16), we observed that the simulated internetwork lag gradient is robust to varying lengths of linguistic/narrative units (mean: 2 to 4; variance: 0.1 to 1; longer length generated longer units, often with the top layers exceeding the length of the simulated story, i.e., 3,000 words). The duration of interparagraph pauses was estimated from two stories (“Sherlock” and “Merlin”; SI Appendix, Fig. S12) (mean length: 1.5 to 4.5 s; pause effect size: 0.01 to 1 SD of simulated activity). We also found that the model, similar to neural responses as observed by Lerner and colleagues (45), was robust to variations in speech rate (0.5 to 1.5, relative to “Sherlock” speech rate). However, the lag gradient deteriorates with parameters outside of the bounds of natural speech, for example, when the interparagraph pause is set to 0 s. We also simulated brain responses to word-scrambled stories by setting mean unit length = 1 and unit length variance = 0. With this setting, word-level units are never integrated into larger units (the units at each level correspond to individual words from the first level). No information integration is involved, resulting in flat activations and eliminating the difference in spectral properties of time series from different levels. No lag gradient is observed in this case (Fig. 5B).
Next, we computed interlevel lag-correlation using simulated responses to different nested structures (similar to responses to different stories), which preserves the spectral properties of individual time series while disrupting their nesting relationship. No significant lag-correlation was found when violating the nested structure of naturalistic narratives (Fig. 5C). In addition to the aforementioned linearly increasing integration function, we also explored several other temporal integration functions. We found that linearly and logarithmically increasing functions both yielded the internetwork lag gradient, but not the symmetric triangular or boxcar functions. The linearly decreasing function resulted in a reversed lag gradient (Fig. 6). These results suggest that the hierarchically nested structure that naturally arises from bottom-up narrative construction and a monotonically increasing integration function are key to the emergence of the lag gradient.
Fig. 6.
Lag matrices generated using different temporal integration functions (P < 0.05, FDR correction). The linearly and logarithmically increasing temporal integration functions yield a simulated peak lag matrix similar to the one observed in fMRI data; the symmetric triangle and boxcar functions, as well as the linearly decreasing function, do not.
Discussion
This study reveals how information is propagated along the cortical processing timescale hierarchy across time (lags) (5). By applying lag-ISFC to a collection of fMRI datasets acquired while subjects listened to spoken stories, we revealed a temporal progression of story-driven brain activity along a cortical hierarchy for narrative comprehension (Fig. 3A). The temporal cascade of cortical responses summarized by the internetwork lag gradient was consistent across stories. Qualitatively similar cortical topographies for the lag gradient were observed using networks defined by the k-means method at both coarse- (Fig. 3A) and fine-grained (SI Appendix, Fig. S7) spatial scales (25, 46), networks defined by TRW index (SI Appendix, Fig. S2), and a predefined functional parcellation (28) (SI Appendix, Fig. S6), suggesting that the lag gradient is robust to different network definitions. The results are in line with the hierarchical processing framework, which proposes a gradual emergence of narrative features of increasing duration and complexity along the processing hierarchy, from early sensory areas into higher order cortical areas (Fig. 1). In support of our interpretation, we found that the lag gradient is absent when the temporal structure of the story is disrupted due to word scrambling (Fig. 3B).
Interestingly, the propagation of linguistic information unfolds over several seconds. As demonstrated by our simulation, these slow timescales are driven by the temporal structure of narratives, in which words are integrated into sentences, which are further integrated into paragraphs and events. This stimulus-locked information flow across brain areas revealed by ISFC was not previously observed because WSFC is driven mainly by intrinsic fluctuations, which propagate at a faster time scale (−1 ~ 1 s) (29–31). The long lags we observed (up to 9 s) (Fig. 3A) cannot be explained by regional variations in neurovascular coupling (47) or transient activity impulses at event boundaries. If the lag gradient only reflects variations in neurovascular coupling across regions, it should be present both when we isolate stimulus-driven activity using ISFC and when we examine idiosyncratic neural responses using WSFC. Instead, however, the lag gradient was detected only with ISFC, but not WSFC (SI Appendix, Fig. S8). In addition, we found that transient event boundaries (32–37) did not account for the lag gradient (SI Appendix, Fig. S10).
Despite the shared gradient pattern in the peak lag matrices, variation across stories is observed (SI Appendix, Fig. S5). Story length, the number of participants, their level of engagement (48, 49), and, as our simulation indicates, the temporal structure of the story—including the length of interparagraph pauses, speech rate, and the mean and variance of linguistic/narrative unit lengths (SI Appendix, Fig. S16)—could all differentially affect the significance of ISFC peaks across stories/datasets. If we have to speculate, the robust ISFC peaks in “Sherlock” might be due to its length (second longest in our stories) and the fact that it is a fast-paced story involving several deaths, violence, drugs, and memorable characters. It has been found that emotional moments in narratives are associated with higher intersubject synchronization (48, 49).
Our simulation provides a simple computational model for how information is integrated within each level of the processing hierarchy and propagates to the next level. This model takes as input the natural temporal structure of the story (including the length of interparagraph pauses, speech rate, and the mean and variance of linguistic/narrative unit lengths) and integrates these units along the processing hierarchy (while accounting for the BOLD HRF) to output simulated fMRI signals. This simulation allows us to parametrically manipulate a wide range of structural narrative features, both within and beyond the bounds of natural speech (SI Appendix, Fig. S16), to reveal the conditions under which the lag gradient emerges: a) a cortical hierarchy of increasing processing timescales (Figs. 1 and 4) (5); b) hierarchically nested linguistic/narrative events of increasing size along the processing hierarchy (Fig. 5 B and C) (22, 23); and c) gradual increasing brain activity, along with information accumulation, within the boundaries of events at each processing level (32, 35, 39–44), combined with a reset of activity (buffer clearing) at event boundaries (7) (see temporal integration function in Figs. 1B and 6).
Our simulation demonstrates that the remarkably large temporal scales of the observed lag gradient in the brain result from the hierarchically nested temporal structure of natural language. In this simple model, information integration at varying granularity (e.g., word, sentence, and paragraph) is sufficient to yield the internetwork lag gradient (Fig. 5) and spectral properties observed in the fMRI data (SI Appendix, Fig. S15). Our model also reflects an effort to bridge the gap between studies of naturalistic narratives and the rich literature based on simpler, well-controlled language stimuli. We show that the complex brain dynamics observed during narrative processing can emerge from a relatively simple temporal integration process found with well-controlled phrases and sentences (39–44).
Importantly, we note that our computational model is not the only one that could generate the predicted lag gradient. We simulated brain responses to narratives of different temporal structures devoid of actual narrative contents. Our aim is to combine separate findings that point to the same cortical hierarchy with the simplest model possible. However, narrative processing is unlikely to be strictly unidirectional (50). The lag gradient only captures the dominant bottom-up process of narrative construction (SI Appendix, Fig. S9). More complex models might be necessary to capture intricate content-/context-sensitive predictive processes. More studies are also needed to examine recurrent or bidirectional connectivity, causal relations between networks, and nonstationary information flow over time.
Our results and simulation indicate that the DMN roughly corresponds to level 6 of the model (which has narrative units on the scale of 200–300 words with the simulation parameter as in SI Appendix, Table S1), supporting the idea that the DMN is the apex of the cortical processing hierarchy, integrating information on the scale of paragraphs and narrative events (3, 4). Since DMN responses track features of the stimulus evolving over long timescales, it is relatively invariant to changes in low-level stimulus properties. For example, stories that preserve the same meanings across forms will evoke similar activity patterns across subjects within the DMN but not in lower level areas of the processing hierarchy. This effect was documented for stories spoken in two different languages (51), stories converted to visual animations (52), and stories told using synonyms (8). These findings suggest that the DMN builds abstract, context-sensitive representations of the situation conveyed by the narrative (4). The current study provides a simple computational model for how different stages of the cortical processing hierarchy sequentially integrate information over time to provide the building blocks for the DMN’s rich representation of situations and narrative events.
Our results are also consistent with reports on the spatiotemporal dynamics of brain responses to naturalistic stimuli. A hierarchically nested spatial activation pattern has been revealed using movie, spoken story, and music stimuli (22, 23, 53). Chien and colleagues (7) reported a gradual alignment of context-specific spatial activation patterns, which was rapidly flushed at event boundaries, similar to the temporal integration function we adopted here. Taken together, the empirical findings, combined with our simulation, indicate that the spatiotemporal neural dynamics reflect the structure of naturalistic, ecologically relevant inputs (6) and that such information is preserved even with the poor temporal resolution of fMRI. Although the current findings are derived from listener–listener coupling, the interregional dynamics may shed light on the lags observed in speaker-listener coupling (54–59). Given a particular seed region in the speaker’s brain, we would expect to observe coupling at differing lags for different target regions in the listener’s brain, and these lags may vary based on the temporal structure of the speaker’s narrative.
Our results demonstrate both the importance of using intersubject methods to isolate stimulus-driven signals and the value of data aggregation. The fact that we obtained nonzero internetwork lag only with ISFC but not WSFC (SI Appendix, Fig. S8) indicates that stimulus-driven network configuration may be masked by the idiosyncratic fluctuations that dominate WSFC analyses (24, 25). Furthermore, although the internetwork lags could be observed within individual stories (SI Appendix, Fig. S5), the gradient pattern is much clearer after aggregating across stories (Fig. 3). Data aggregation is particularly important when using naturalistic stimuli because it is impossible to control the structure of each narrative (e.g., speaking style, duration, complexity, and content) (38, 60–62). With these methods, we are able to reveal the internetwork lag gradient driven by naturalistic narratives, as predicted by the model of shared information flow along the cortical processing hierarchy. Further work will be needed to examine recurrent or bidirectional information flow and to decode the content of narrative representations—specific to each story—as they are transformed along the cortical hierarchy.
Materials and Methods
fMRI Datasets.
This study relied on eight openly available spoken story datasets. Seven datasets were used from the “Narratives” collection (OpenNeuro: https://openneuro.org/datasets/ds002245) (63), including “Sherlock” and “Merlin” (18 participants, 11 females) (57), “The 21st year” (25 participants, 14 females) (64), “Pie Man (PNI)”, “I Knew You Were Black”, “The Man Who Forgot Ray Bradbury”, and “Running from the Bronx (PNI)” (48 participants, 34 females). One dataset was used from Princeton Dataspace: “Pie Man” (36 participants, 25 females) (https://dataspace.princeton.edu/jspui/handle/88435/dsp015d86p269k) (25). Two nonstory datasets were also included as controls: a word-scrambled “Pie Man” (36, participants, 20 females) dataset and a resting-state dataset (36 participants, 15 females) (see the Princeton DataSpace URL above) (25). All datasets were acquired with a TR of 1.5 s.
All participants reported fluency in English and were 18 to 40 y in age. The criteria of participant exclusion have been described in previous studies for “Sherlock”, "Merlin”, "The 21st year”, and “Pie Man.” For “Pie Man (PNI)”, “I Knew You Were Black”, “The Man Who Forgot Ray Bradbury”, and “Running from the Bronx (PNI),” participants with comprehension scores 1.5 standard deviations lower than the group means were excluded. One participant was excluded from “Pie Man (PNI)” for excessive movement (translation along the z-axis exceeding 3 mm).
All participants provided informed, written consent, and the experimental protocol was approved by the institutional review board of Princeton University.
fMRI Preprocessing.
fMRI data were preprocessed using FSL (https://fsl.fmrib.ox.ac.uk/), including slice time correction, motion correction, and high-pass filtering (140-s cutoff). All data were aligned to standard 3 × 3 × 4-mm Montreal Neurological Institute space (MNI152). A gray matter mask was applied.
Functional Networks.
Following Simony and colleagues (25), we defined 6 intrinsic connectivity networks within regions showing reliable responses to spoken stories. Voxels showing top 30% ISC in at least 6 out of the 8 stories were included. Using the k-means method (L1 distance measure), these voxels were clustered according to their group-averaged WSFC with all the voxels during resting. We refer to these functional networks as the AUD, vLAN, dLAN, ATT, and default mode (DMNa and DMNb) networks (SI Appendix, Fig. S1A). To ensure that our results hold for finer grained functional networks, we further divided each of the six networks into ten subnetworks, again by applying k-means clustering to resting-state WSFC (k = 10 within each superordinate network).
To compare these intrinsic functional networks with the TRW hierarchy, we computed the TRW index (i.e., intact > word-scrambled story ISC) following Yeshurun and colleagues (8) for voxels within regions showing reliable responses to spoken stories, using the intact and word-scrambled Pie Man. Six TRW networks were then generated by splitting the TRW indices into six bins by five quantiles (SI Appendix, Fig. S2).
We also include results using networks pre-defined based on whole-brain functional parcellation of resting-state fMRI data (28). The same ISC mask was applied. One predefined network is excluded for encompassing less than 10 voxels in the ISC mask.
WSFC, ISFC, and ISC.
In this study, WSFC refers to within-subject interregion correlation, while ISFC refers to intersubject interregion correlation. ISC refers to a subset of ISFC, namely, ISFC between homologous regions (SI Appendix, Fig. S3). ISFC and ISC were computed using the leave-one-subject-out method, i.e., correlation between the time series from each subject and the average time series of all the other subjects (24).
Before computing the correlation, the first 25 and last 20 volumes of fMRI data were discarded to remove large signal fluctuations at the beginning and end of time course due to signal stabilization and stimulus onset/offset. We then averaged voxelwise time series across voxels within network/region masks and z-scored the resulting time series.
Lag-correlations were computed by circularly shifting the time series such that the non-overlapping edge of the shifted time series was concatenated to the beginning or end. The left-out subject was shifted while the average time series of the other subjects remained stationary. Fisher’s z transformation was applied to the resulting correlation values prior to further statistical analysis.
ISFC Lag Matrix.
We computed the network × network × lag ISFC matrix (SI Appendix, Fig. S3) and extracted the lag with peak ISFC (correlation) value for each network pair (Fig. 2). The peak ISFC value was defined as the maximal ISFC value within the window of lags from −15 to +15 TRs; we required that the peak ISFC be larger than the absolute value of any negative peak and excluded any peaks occurring at the edge of the window.
To obtain the mean ISFC across stories, we applied two statistical tests. Only ISFC that passed both tests were considered significant (Fig. 3A and SI Appendix, Figs. S2D and S6, and lag matrices for the intact story in SI Appendix, Fig. S8). First, we performed a parametric one-tailed one-sample t-test to compare the mean ISFC against zero (N = 8 stories) and corrected for multiple comparisons by controlling the false discovery rate (FDR; 6 seed × 6 target × 31 lags; q < 0.05) (65).
Second, to exclude ISFC peaks that only reflected shared spectral properties, we generated surrogates with the same mean and autocorrelation as the original time series by time-shifting and time-reversing. We computed the correlation between the original seed and time-reversed target with time-shifts of −100 to +100 TRs. The resulting ISFC values were averaged across stories and served as a null distribution. A one-tailed z-test was applied to compare ISFCs within the window of lag −15 to +15 TRs against this null distribution. The FDR method was used to control for multiple comparisons (seed × target × lags; q < 0.05). When assessing ISFC for each story, only this second test was applied, and all possible time-shifts were used to generate the null distribution (Figs. 2C and 3B and SI Appendix, Fig. S5, the lag matrices for the scrambled story and resting-state data in SI Appendix, Figs. S8 and S10B).
PCA of the Lag Matrix.
We examined whether multiple lag sequences similarly contributed to the lag matrix, using the method introduced by Mitra and colleagues (31). We applied PCA to the lag matrix obtained from the averaged ISFC across stories, after transposing the matrix and zero-centering each column. Each principal component represents a pattern of relative lags, in other words, lag sequences. We computed the proportion of overall variance in the lag matrix accounted for by each component in order to determine whether more than one component played an important role.
Word/Sentence/Paragraph Boundary Effect.
To test the transient effect of linguistic boundaries on internetwork lag, we computed the lag-ISFC after regressing out activity impulses at boundaries. A multiple regression model was built for each subject. The dependent variable was the averaged time series of each network, removing the first 25 scans and the last 20 scans as in the ISFC analysis. The regressors included an intercept, the audio envelope, and three sets of finite impulse functions (−5 to +15 TRs relative to boundary onset), corresponding to word, sentence, and paragraph (event) boundaries. We then recomputed lag-ISFC based on the residuals of the regression model.
Word/Sentence/Paragraph Length Effect.
We replicated the sentence length (39–44) and paragraph length (32, 35) effect with the “Sherlock” and “Merlin” datasets, which were collected from the same group of participants. The onsets and offsets of each word, sentence, and paragraph (event) were manually time-stamped. Given the low temporal resolution of fMRI (TR = 1.5 s) and the difficulty of labeling the onset/offset of each syllable, they were estimated by dividing the duration of each word by the number of syllables it contains.
We built individual GLM models that included regressors corresponding to the presence of syllable, word, sentence, and paragraph respectively, accompanied by three parametric modulators: accumulated syllable number within words, accumulated word number within sentences, and accumulated sentence number within paragraphs. These parametric regressors were included to test whether brain activations accumulate toward the end of word/sentence/paragraph; the longer the word/sentence/paragraph the stronger the activations. In addition to the regressors of interest, one regressor was included for speech segments without clear paragraph labels. We did not orthogonalize the regressors to each other.
Effect maps of the three parametric modulators (i.e., word length, sentence length, and paragraph length) from the individual level models of both stories were smoothed with a Gaussian kernel (FWHM = 8 mm) and input to three group-level models to test the word, sentence, and paragraph length effects respectively (flexible factorial design including the main effects of story and participant; P < 0.005, not corrected). We observed sentence and paragraph length effects. Using the same threshold, no word length effect was observed,
Power Spectral Density Analysis.
We performed spectral analyses following Stephens et al. (2013) (15). As for the connectivity analysis, we cropped the first 25 and last 20 scans and z-scored the time series. For each voxel within regions showing reliable responses to spoken stories, the resulting time series was averaged across subjects and normalized across time. The power spectrum of the group-mean time series was estimated using Welch’s method with a Hamming window of width 99 s (66 TRs) and 50% overlap (based on the parameters from Stephens et al. (2013) (15)). The power spectra of individual voxels were averaged across stories. We then split the voxels into six clusters of equal size according to their low-frequency power (cumulative power below 0.04 Hz), following Stephens et al. (2013) (15) (SI Appendix, Fig. S15B). Similar PSD analyses were applied to each level of the simulated BOLD signals. The resulting power spectra were averaged across 30 simulations (SI Appendix, Fig. S15A).
Simulating the Construction of Nested Narrative Structures and the Corresponding BOLD Responses.
To illustrate how information accumulation at different timescales could account for the internetwork lag gradient during story-listening, we simulated the construction of nested narrative structures closely following the statistical structure of real spoken stories and generated BOLD responses at each processing level. To build the first level of a nested structure, we sampled a sequence of 3,000 word durations with replacement from “Sherlock,” which is the longest example of spontaneous speech among our datasets, recorded from a non-professional speaker without rehearsal or script (SI Appendix, Fig. S11). Boundaries between units at the first level were set up accordingly.
Unit length.
First-level units were integrated into units of the next level with a lognormal distributed unit length; e.g. integrating three words into a phrase (unit length = 3) (SI Appendix, Fig. S11). Boundaries between second-level units were inserted accordingly. Second-level units were integrated into the third-level units following the same method. A nested structure of six levels was thus generated.
Temporal integration function.
Postulating that information accumulation is accompanied by increased activity, brain responses within each level of the nested structure were generated as a function of unit length. For example, a linear temporal integration function generates activity [1 2 3] for a “phrase” (i.e., a Level 2 unit) consisting of three “words” (i.e., Level 1 units). The first (word) level integration was computed based on syllable numbers sampled from “Sherlock” along with word durations.
Pause length and pause effect size.
In naturalistic narratives, boundaries between high-level units are often accompanied by silent pauses (SI Appendix, Fig. S12). Therefore, we inserted pauses with normally distributed lengths at the boundaries of the highest level units (SI Appendix, Fig. S11). Activity during the pause period was set as 0.1 SD below the minimum activity of each level.
To account for HRF delay in fMRI signals, we applied the canonical HRF provided by the software SPM (https://www.fil.ion.ucl.ac.uk/spm/) (66) and resampled the output time series from a temporal resolution of 0.001 s to 1.5 s to match the TR in our data. We ran 30 simulations for each set of simulation parameters. Each simulation produced different narrative structures (equivalent to different stories). The peak lag of the mean interlevel correlation across simulations was extracted and thresholded using the same method as in the ISFC analysis (Fig. 2).
To examine whether the simulated and real fMRI signals shared similar power spectra, we also applied the power spectral density analysis to the simulated BOLD responses at each of the six levels and averaged across thirty simulations.
We started with a set of reasonable parameters (SI Appendix, Table S1) (speech rate = 1, relative to “Sherlock”; unit length mean = 3; unit length variance = 0.5; temporal integration function = linearly increasing; mean pause length = 3 s; pause effect size = 0.1 SD of the simulated activity) and explored alternative parameter sets within the bound of natural speech to test whether interlevel lag was robust to parameter changes.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This study was supported by the National Institute of Mental Health (R01-MH112357 and DP1-HD091948).
Author contributions
C.H.C.C., S.A.N., and U.H. designed research; C.H.C.C. and S.A.N. performed research; C.H.C.C. contributed new reagents/analytic tools; C.H.C.C. analyzed data; and C.H.C.C., S.A.N., and U.H. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. K.J.F. is a guest editor invited by the Editorial Board.
Contributor Information
Claire H. C. Chang, Email: claire.hc.chang@gmail.com.
Samuel A. Nastase, Email: sam.nastase@gmail.com.
Data, Materials, and Software Availability
This study relied on eight openly available spoken story datasets. Seven datasets were used from the “Narratives” collection (OpenNeuro: https://openneuro.org/datasets/ds002245) (67), One dataset was used from Princeton Dataspace: “Pie Man” (36 participants, 25 females) (https://dataspace.princeton.edu/jspui/handle/88435/dsp015d86p269k) (68).
Supporting Information
References
- 1.Christiansen M. H., Chater N., The Now-or-Never bottleneck: A fundamental constraint on language. Behav. Brain Sci. 39, e62 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Hasson U., Yang E., Vallines I., Heeger D. J., Rubin N., A hierarchy of temporal receptive windows in human cortex. J. Neurosci. 28, 2539–2550 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerner Y., Honey C. J., Silbert L. J., Hasson U., Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J. Neurosci. 31, 2906–2915 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeshurun Y., Nguyen M., Hasson U., The default mode network: Where the idiosyncratic self meets the shared social world. Nat. Rev. Neurosci. 22, 181–192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasson U., Chen J., Honey C. J., Hierarchical process memory: Memory as an integral component of information processing. Trends Cogn. Sci. 19, 304–313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiebel S. J., Daunizeau J., Friston K. J., A hierarchy of time-scales and the brain. PLoS Comput. Biol. 4, e1000209 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien H. Y. S., Honey C. J., Constructing and forgetting temporal context in the human cerebral cortex. Neuron 106, 675–686.e11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeshurun Y., Nguyen M., Hasson U., Amplification of local changes along the timescale processing hierarchy. Proc. Natl. Acad. Sci. U.S.A. 29, 9475–9480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honey C. J., et al. , Slow cortical dynamics and the accumulation of information over long timescales. Neuron 76, 423–434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters M. E., Neumann M., Zettlemoyer L., Yih W. T., “Dissecting contextual word embeddings: Architecture and representation” in Proceedings of the 2018 Conference on Empirical Methods in Natural Language Processing (Association for Computational Linguistics, Brussels, Belgium, 2018), pp. 1499–1509. [Google Scholar]
- 11.Vig J., Belinkov Y., “Analyzing the structure of attention in a transformer language model” in Proceedings of the 2019 ACL Workshop BlackboxNLP: Analyzing and Interpreting Neural Networks for NLP (Association for Computational Linguistics, Florence, Italy, 2019), pp. 63–7. [Google Scholar]
- 12.Dominey P. F., Narrative event segmentation in the cortical reservoir. PLOS Comput. Biol. 17, e1008993 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caucheteux C., Gramfort A., King J.-R., Model-based analysis of brain activity reveals the hierarchy of language in 305 subjects in Findings of the Association for Computational Linguistics: EMNLP 2021, (Association for Computational Linguistics, Punta Cana, Dominican Republic, 2021), pp. 3635–3644. [Google Scholar]
- 14.Raut R. V., Snyder A. Z., Raichle M. E., Hierarchical dynamics as a macroscopic organizing principle of the human brain. Proc. Natl. Acad. Sci. U.S.A. 117, 20890–20897 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens G. J., Honey C. J., Hasson U., A place for time: The spatiotemporal structure of neural dynamics during natural audition. J. Neurophysiol. 110, 2019–2026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray J. D., et al. , A hierarchy of intrinsic timescales across primate cortex. Nat. Neurosci. 17, 1661–1663 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baria A. T., et al. , Linking human brain local activity fluctuations to structural and functional network architectures. NeuroImage 73, 144–155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Changeux J.-P., Goulas A., Hilgetag C. C., A connectomic hypothesis for the hominization of the brain. Cereb. Cortex 35, 2425–2449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huntenburg J. M., Bazin P. L., Margulies D. S., Large-scale gradients in human cortical organization. Trends Cogn. Sci. 22, 21–31 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri R., Knoblauch K., Gariel M. A., Kennedy H., Wang X.-J., A large-scale circuit mechanism for hierarchical dynamical processing in the primate cortex. Neuron 88, 419–431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demirtaş M., et al. , Hierarchical heterogeneity across human cortex shapes large-scale neural dynamics. Neuron 101, 1181–1194.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldassano C., et al. , Discovering event structure in continuous narrative perception and memory. Neuron 95, 709–721.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geerligs L., et al. , A partially nested cortical hierarchy of neural states underlies event segmentation in the human brain. eLife 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nastase S. A., Gazzola V., Hasson U., Keysers C., Measuring shared responses across subjects using intersubject correlation. Soc. Cogn. Affect. Neurosci. 14, 667–685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simony E., et al. , Dynamic reconfiguration of the default mode network during narrative comprehension. Nat. Commun. 7, 12141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R., Intersubject synchronization of cortical activity during natural vision. Science 303, 1634–1640 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Kauppi J. P., Jääskeläinen I. P., Sams M., Tohka J., Inter-subject correlation of brain hemodynamic responses during watching a movie: Localization in space and frequency. Front. Neuroinformatics 4, 5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen X., Tokoglu F., Papademetris X., Constable R. T. T., Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage 82, 403–415 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitra A., Raichle M. E., How networks communicate: Propagation patterns in spontaneous brain activity. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitra A., Snyder A. Z., Hacker C. D., Raichle M. E., Lag structure in resting-state fMRI. J. Neurophysiol. 111, 2374–2391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitra A., Snyder A. Z., Blazey T., Raichle M. E., Lag threads organize the brain’s intrinsic activity. Proc. Natl. Acad. Sci. U.S.A. 112, E7307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ezzyat Y., Davachi L., What constitutes an episode in episodic memory? Psychol. Sci. 22, 243–252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speer N. K., Zacks J. M., Reynolds J. R., Human brain activity time-locked to narrative event boundaries. Psychol. Sci. 18, 449–455 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Whitney C., et al. , Neural correlates of narrative shifts during auditory story comprehension. NeuroImage 47, 360–366 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Yarkoni T., Speer N. K., Zacks J. M., Neural substrates of narrative comprehension and memory. NeuroImage 41, 1408–1425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zacks J. M., et al. , Human brain activity time-locked to perceptual event boundaries. Nat. Neurosci. 4, 651–655 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Zacks J. M., Speer N. K., Swallow K. M., Maley C. J., The brain’s cutting-room floor: Segmentation of narrative cinema. Front. Hum. Neurosci. 4, 1–15 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willems R. M., Nastase S. A., Milivojevic B., Narratives for neuroscience. Trends Neurosci. 43, 271–273 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Chang C. H. C., Dehaene S., Wu D. H., Kuo W. J., Pallier C., Cortical encoding of linguistic constituent with and without morphosyntactic cues. Cortex 129, 281–295 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Fedorenko E., et al. , Neural correlate of the construction of sentence meaning. Proc. Natl. Acad. Sci. U.S.A. 113, E6256–E6262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giglio L., Ostarek M., Weber K., Hagoort P., Commonalities and asymmetries in the neurobiological infrastructure for language production and comprehension. Cereb. Cortex 32, 1405–1418 (2021), 10.1093/cercor/bhab287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matchin W., Hammerly C., Lau E., The role of the IFG and pSTS in syntactic prediction: Evidence from a parametric study of hierarchical structure in fMRI. Cortex 88, 106–123 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Nelson M. J., et al. , Neurophysiological dynamics of phrase-structure building during sentence processing. Proc. Natl. Acad. Sci. U. S. A. 114, E3669–E3678 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pallier C., Devauchelle A.-D.A., Dehaene S., Cortical representation of the constituent structure of sentences. Proc. Natl. Acad. Sci. U.S.A. 108, 2522–2527 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lerner Y., Honey C. J., Katkov M., Hasson U., Temporal scaling of neural responses to compressed and dilated natural speech. J. Neurophysiol. 111, 2433–2444 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Regev M., et al. , Propagation of information along the cortical hierarchy as a function of attention while reading and listening to stories. Cereb. Cortex 29, 4017–4034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rangaprakash D., Wu G. R., Marinazzo D., Hu X., Deshpande G., Hemodynamic response function (HRF) variability confounds resting-state fMRI functional connectivity. Magn. Reson. Med. 80, 1697–1713 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Nummenmaa L., et al. , Emotions promote social interaction by synchronizing brain activity across individuals. Proc. Natl. Acad. Sci. U.S.A. 109, 9599–9604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nummenmaa L., et al. , Emotional speech synchronizes brains across listeners and engages large-scale dynamic brain networks. NeuroImage 102, 498–509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pickering M. J., Gambi C., Predicting while comprehending language: A theory and review. Psychol. Bull. 144, 1002–1044 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Honey C. J., Thompson C. R., Lerner Y., Hasson U., Not lost in translation: Neural responses shared across languages. J. Neurosci. 32, 15277–15283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen M., Vanderwal T., Hasson U., Shared understanding of narratives is correlated with shared neural responses. NeuroImage 184, 161–170 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams J. A., et al. , High-order areas and auditory cortex both represent the high-level event structure of music. J. Cogn. Neurosci. 34, 699–714 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu L., et al. , The “two-brain” approach reveals the active role of task-deactivated default mode network in speech comprehension. Cereb. Cortex 32, 4869–4884 (2022), 10.1093/cercor/bhab521. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen M., et al. , Teacher–student neural coupling during teaching and learning. Soc. Cogn. Affect. Neurosci. 17, 367–376 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stephens G. J., Silbert L. J., Hasson U., Speaker-listener neural coupling underlies successful communication. Proc. Natl. Acad. Sci. U.S.A. 107, 14425–14430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zadbood A., Chen J., Leong Y. C., Norman K. A., Hasson U., How we transmit memories to other brains: Constructing shared neural representations via communication. Cereb. Cortex 27, 4988–5000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silbert L. J., Honey C. J., Simony E., Poeppel D., Hasson U., Coupled neural systems underlie the production and comprehension of naturalistic narrative speech. Proc. Natl. Acad. Sci. U.S.A. 111, E4687–E4696 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dikker S., Silbert L. J., Hasson U., Zevin J. D., On the same wavelength: Predictable language enhances speaker-listener brain-to-brain synchrony in posterior superior temporal gyrus. J. Neurosci. 34, 6267–6272 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamilton L. S., Huth A. G., The revolution will not be controlled: Natural stimuli in speech neuroscience. Lang. Cogn. Neurosci. 35, 573–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee H., Bellana B., Chen J., What can narratives tell us about the neural bases of human memory? Curr. Opin. Behav. Sci. 32, 111–119 (2020). [Google Scholar]
- 62.Sonkusare S., Breakspear M., Guo C., Naturalistic stimuli in neuroscience: Critically acclaimed. Trends Cogn. Sci. 23, 699–714 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Nastase S. A., et al. , The “Narratives” fMRI dataset for evaluating models of naturalistic language comprehension. Sci. Data 8, 250 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chang C. H. C., Lazaridi C., Yeshurun Y., Norman K. A., Hasson U., Relating the past with the present: Information integration and segregation during ongoing narrative processing. J. Cogn. Neurosci. 33, 1106–1128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 (1995). [Google Scholar]
- 66.Henson R., Friston K., “Convolution models for fMRI” in Statistical Parametric Mapping: The Analysis of Functional Brain Images, Penny W., Friston K., Ashburner J., Kiebel S., Nichols T., Eds. (Academic Press, 2007), pp. 178–192. [Google Scholar]
- 67.Nastase S. A., et al. , Narratives. Open Neuro. https://openneuro.org/datasets/ds002345/versions/1.1.4. Deposited 10 December 2019.
- 68.Simony E., et al. , Dynamic reconfiguration of the default mode network during narrative comprehension. Princeton Dataspace. http://arks.princeton.edu/ark:/88435/dsp015d86p269k. Deposited 18 July 2016. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
This study relied on eight openly available spoken story datasets. Seven datasets were used from the “Narratives” collection (OpenNeuro: https://openneuro.org/datasets/ds002245) (67), One dataset was used from Princeton Dataspace: “Pie Man” (36 participants, 25 females) (https://dataspace.princeton.edu/jspui/handle/88435/dsp015d86p269k) (68).