Introduction:
Excipients, are inactive ingredients of drugs that are not under the same regulation as the parent drug and are used to stabilize, preserve, or enhance the pharmacokinetics, bioavailability of the active ingredients and palatability of the preparation. In the event of a reaction to a particular drug, it is typically assumed that the reaction is due to the active ingredient, and for many drugs and drug classes that assumption is correct. However, many excipients are also potential allergens, and should not be overlooked in patients with a compatible medical history (Table 1).
Table 1:
Clinical history where excipient allergy should be considered
| Excipient | History/Presentation |
|---|---|
| Any Excipient in General |
|
| PEG (Macrogol) |
|
| Polysorbate 80 |
|
| Poloxamers |
|
| Carboxymethylcellulose (Carmellose, croscarmellose, E466) |
|
| Mannitol |
|
| Povidone-Iodine |
|
| Gelatin |
|
| Alpha-gal |
|
Excipient allergy is overall rare and hence often missed or misdiagnosed due to lack of awareness of the need to carefully review the drug ingredients. In addition, some excipients also have an unearned reputation for being “inert” that may cause them to be dismissed from a suspect line-up. For these reasons, excipient allergy can often be overlooked by everyone involved, despite a history of life-threatening reactions.
For the patient, excipient allergy can be frightening and potentially disruptive to healthcare delivery. It may present as multiple reactions to structurally unrelated drugs, as reactions to only some formulations or doses of a specific drug, or as reactions to drugs not usually associated with allergy such as laxatives and injectable corticosteroids. Due to variations in drug formulation that have arisen across different national healthcare systems and pharmacopoeias, the same allergy may also present with reactions to different drugs, in different geographical settings.
While the underlying mechanism of anaphylaxis is not apparent in all cases of immediate excipient hypersensitivity, IgE mediated anaphylaxis does appear to be the most likely and has been reported for the allergens selected in this review, which are: Polyethylene glycols, polysorbates, carboxymethylcellulose, mannitol, povidone, protamine, gelatin, and galactose-alpha, 1,3, galactose (alpha-gal) (Figure 1). We have also included poloxamers, due to multicenter observations from the authors that these compounds may have cross reactivity with PEGs. While we have focused on excipients to which anaphylaxis has been demonstrated repeatedly or to their potentially cross-reactive substances, this review does not exclude the possibility that other excipients with rare allergic potential (such as tromethamine1 or hypromellose2) could emerge over time. As our knowledge grows, evidence for the allergenicity of an excipient should ideally pass through a sequence of initial reports, validation, and confirmation of the mechanism.
Figure 1:

Key allergenic excipients covered in this review
Polyethylene containing excipients
These excipients share the common structural feature of repeating ethylene oxide units, and include polyethylene glycols, polysorbates, and poloxamers. (Figure 2)
Figure 2:

Key polyethylene containing excipients: the polyethylene glycols, poloxamers, and polysorbates.
Polyethylene glycols (PEGs) (Macrogols)
Polyethylene glycols (PEGs) also called macrogols, are polymers of repeating ethylene oxide units, in which the number of ethylene repeats, each weighing 44 g/mol, determines the molecular weight of the compound. The term macrogol was initially chosen to refer to the PEG used as an excipient in drugs and devices to differentiate from PEG used as an excipient in cosmetic products. The nomenclature of PEGs is based on either the number of repeating units (e.g. PEG 76, as typically used in topical products), or the molecular weight 76 × 44 g/mol = PEG 3350 typically used in pharmaceutical products.3 Understanding this structural concept is crucial, because a wide variety of excipients in current use also contain chains of repeating ethylene of various lengths, such as PEG sorbitans (polysorbates), poloxamers, PEG castor oils (cremophor), and others. Cross-reactivity amongst polyethylene compounds is currently the most important unknown for PEG allergy, affecting our ability to advise patients on what truly needs to be avoided.
PEGs have many properties and are found in a wide variety of products. They are used as surface coating and pill binders in tablets, as the active ingredient in laxatives and can be conjugated directly to an active ingredient to prolong or potentiate the active ingredient’s mechanism of action (PEGylation). On a publicly available database of FDA regulated products4 a focused search for PEG 3350 content (on August 17th, 2021) finds 1,434 products available in the United States of which laxatives, bowel preparations, film coated tablets, topical gels, and parenteral steroids are typical representatives. Although not strictly speaking an excipient, PEG-lipid compounds are utilized in mRNA technologies to construct the lipid nanoparticle carrier system. The intent is to stabilize the construct and protect those lipid microspheres from complement activation. The mRNA vaccines against COVID-19 are the first widely used vaccines utilizing this technology.
Poloxamers
Poloxamers, like other PEG derivatives, are commonly used in pharmaceutical and cosmetic products as surfactants, stabilizers, and solubilizers.23 Poloxamers are copolymers arranged in a triblock structure formed by a hydrophobic central chain of polypropylene glycol surrounded by two hydrophilic chains of PEG23 (Figure 2). While there are more than 50 poloxamers with a similar chemical structure, they differ in their molecular weight (MW) due to the variable number of polypropylene glycol and PEG units. Therefore, each type of poloxamer has a different hydrophilic-lipophilic balance. Poloxamer 188 and poloxamer 407 are the most prevalent due to their great solubility in water.24 MW vary from 1,100 to 14,000 g/mol. The generic term “poloxamer” is commonly followed by a numerical value of three digits: the first two digits, multiplied × 100, indicates the MW of the hydrophobic core of propylene glycol, and the last digit, multiplied × 10 gives the percentage of the hydrophilic PEG content. Poloxamer 188 contains roughly 80% ethylene oxide repeats by weight, with two 75-repeat polyethylene chains (~6600 g/mol out of an average 8400g/mol) and poloxamer 407 contains around 70% ethylene oxide repeats by weight, with two 98-repeat polyethylene chains (~8,624 g/mol out of an average 12,600 g/mol)25
Polysorbates
Polysorbates are surfactants commonly used as emulsifiers, solubilizers and stabilizers in cosmetic and pharmaceutical products as well as food agents.28 Polysorbate 80 has been used in vaccines and biologic pharmaceutical drugs for years, though only one case of anaphylaxis linked with polysorbate 80 in a vaccine had ever been reported prior to this becoming a concern during the COVID pandemic,29,30 suggesting it is a rare allergen. Polysorbates are derived from pegylated sorbitan esterified with a lipophilic group of fatty acids; e.g. lauric acid in the case of polysorbate 20 or in the case of polysorbate 80 - oleic acid.28 (Figure 2) The generic term “polysorbate” is followed by a number, e.g. 20, 40, 60 or 80. This number represents not the number of repeating ethylene oxide units but qualitatively the lipophilic group associated with the pegylated sorbitan portion. Polysorbates such as polysorbate 80 have 20 to 24 PEG units linked to the lipophilic group of fatty acids with a PEG MW between 880 g/mol and 1056 g/mol.
Non-polyethylene derived excipients:
Carboxymethylcellulose (E466 – Europe, Carmellose/Croscarmellose – Australia and Asia)
Carboxymethylcellulose (CMC) is an organic polysaccharide compound derived from cellulose via an alkali-catalyzed reaction with chlorocetic acid.34 CMC is found as an ingredient in film coated tablets, ophthalmic drops, oral suspensions, foods, and as a stabilizer for some injectable, parenteral drugs. It is confusing for patients and physicians alike that different regions have different names for carboxymethylcellulose.
Mannitol
Mannitol is a sugar occurring naturally in many plants, fruits, vegetables and fungi. Medical uses include inducing osmotic diuresis, reducing raised intracranial or intraocular pressure and as an inhalational irritant used in bronchial inhalational challenge to assess airway hyperreactivity. It is used as a food additive labelled E421 according to the European Directive on food additives,39 and as a low calorie sweetener due to poor intestinal absorption.40 Mannitol is also used as an inactive ingredient in a wide variety of tablets where it functions as a sweetener, diluent, tonicity agent and a bulking agent. In addition, it is used in anticoagulant blood bags as part of the red cell preservative.4
Povidone
Povidone is also known as polyvinylpyrrolidone, PVP, or when used in food, E1201 according to the European Directive on food additives. It is a hydrophilic polymer originally developed as a plasma expander. Povidone is currently used in medications as a synthetic vehicle for suspension and dispersal of drugs, in other tablets as a binder and disintegrator, as povidone-iodine in skin disinfectants used before surgery and wound antiseptic solutions, as a lubricant in ophthalmic solutions (including eye drops and contact lenses), in cosmetic products, and as a food additive.4 A common myth of patients and health care providers is cross-reactivity between povidone and radiocontrast dyes. There is no relationship.
Protamine
Protamine is a highly cationic peptide historically derived from salmon spermatozoa, but now produced primarily through recombinant technologies. Protamine is administered as an active agent for heparin reversal after cardiac bypass surgery. It is also used in various preparations of intermediate duration insulins (such as neutral protamine Hagedorn ”NPH” insulin) as an excipient that slows insulin absorption, but not in newer fast or long-acting insulins.4,49 Protamine is not used as an excipient outside of insulin preparations.4
Gelatin
Gelatin is an animal-derived product made by denaturing collagen from mammal/fish skins and bones using heat and dilute acids or bases. As an excipient, its intended role is to stabilize the active ingredients, or to comprise the bulk of an oral drug containing tablet, capsule, or chewable candy/wine gum formulations. As an active ingredient, gelatin has use in topically and intraoperatively applied hemostatic drugs.58 Gelatin is found in topical gelatin-based hemostatic products containing porcine59–62 or bovine61–64 gelatin. It is also found in gelatin-containing vaccines.29
Alpha-gal
Galactose-alpha-1,3 galactose (alpha-gal) is a disaccharide added by alpha 1,3 galactosyl transferase to the surface of non-primate mammalian cell membranes and cellular synthesized peptides. Because many drugs contain mammalian derived active or inactive ingredients, alpha-gal can be considered an unintended excipient allergen.
Alpha-gal allergy is found in many countries all over the world and is related to the bite of endemic ticks that produce sensitization due to the presence of alpha-gal in their salivary glands and gut.69,70 Patients may (but will not always) recall an inflamed tick bite in the weeks or months preceding the onset of their alpha-gal symptoms and can also occasionally experience recall urticaria at the site of the bite during a subsequent reaction to alpha-gal.71 In terms of drugs, alpha-gal allergen is found in products with mammal derived ingredients or manufacturing processes using mammalian cell lines, such as cetuximab,72 infliximab,73 porcine derived pancrelipase,74 heparin,75 MMR vaccine,66 varicella vaccine,17, bovine collagen,76,77 gelatin capsules,78 and gelatin-based colloid plasma expanders.65 Varicella zoster vaccine was previously found to contain alpha-gal,67 in a version that is no longer commercially available in the United States. Due to use of mammalian ingredients and mammalian cell lines in pharmaceutical manufacturing processes, and a large number of affected patients in endemic areas, there is currently an unmet need to evaluate drugs more widely for this allergen in both the pre-approval and post-marketing phases.
Overall Management of Excipient Allergy:
In general, patients with a proven excipient allergy need to be adequately educated on the name of their allergen, its potential cross-reactivities, how to read medication and food labels including the presence of different nomenclature used in different countries (e.g. CMC), how to risk-assess and identify their target allergen within a product, and how to engage their families and healthcare providers in keeping them safe. Epinephrine autoinjectors are recommended for excipients that are difficult to avoid, and when the index reaction is severe. Wallet cards or medical alert bracelets can protect patients from unintentional medical exposures in the event of altered mental status. Key times at which a patient is at highest risk of exposure include healthcare encounters with new providers or interactions in which new medications will be given (the operating theater, provider from a new specialty becoming involved in the patient’s care, during hospital admissions). Consultation with the allergist in these situations can reduce patient and non-allergy provider anxiety, while improving safety.
Limitations and Barriers to Optimal Excipient Allergy Management:
When considering the need to test for an excipient allergy, there are some key elements and limitations that currently need to be accounted for.
Firstly, the differences across healthcare systems are myriad. The primary challenge in even evaluating the possibility of an excipient allergy is knowing how to access the necessary information in a drug’s product information and to identify ingredients most likely to be allergenic. Once the ingredient list has been located, it is then important to remember that requirements to declare the drug’s inactive ingredients, amounts and molecular weights (especially with PEGs) may vary across national boundaries. Another barrier at this stage is that there can be many synonyms and cross-reactive compounds (for example: PEG is often called macrogol and the poloxamers and polysorbates also contain PEGs of varying lengths). Therefore, it is important to determine the different aliases by which an excipient can be labelled. Because generic drugs containing the same active ingredient are frequently manufactured by multiple competing companies, it is also crucial to recognize that inactive ingredients may vary at the level of the manufacturer or even different doses of the same drug preparation. All these barriers are most easily overcome by the use of comprehensive databases on approved drug formulation, that can be accessible to the practitioner. In future directions, access to such databases should be standardized within national healthcare systems, with the possibility of transnational databases of drug formulations over time.
Secondly, it is important to consider the characteristics of the patient being tested, along with the limitations of the testing modality being utilized. True excipient allergy patients are rare but patients reporting “allergy” to multiple drugs are common. Standardized skin test protocols and excipient panels are useful in the authors’ experience, and continuously being refined. Further information on non-irritant concentrations will evolve with time and experience. However, it is also difficult to set up an excipient panel in a small clinic/office. Many practices may not deem that such a panel is needed as the number of positive tests will be very low, while the requirement to perform such testing in specialized centers is higher. Toward that end, skin testing protocols/panels that are validated and readily available are needed in the future. In the meantime, it is recommended that future publications to the literature also provide data on skin testing results from healthy controls. We have provided such data, when available, for test protocols suggested in this paper (Table 3).
Table 3:
Quantity/Concentrations of Excipients Used in the Literature and by Authors for Allergy Testing
| Excipient | Notes on Testing | SPT | Intradermal | Blood testing modalities | Oral Challenge in Skin Test Negative Patients |
|---|---|---|---|---|---|
| Polyethylene derived compounds | |||||
| Polyethylene glycols of various molecular weights (300–20,000) |
|
|
|
||
| Polyethylene glycol 3350 (using commercially available OTC products) |
|
|
Challenges using Gaviscon (alginic acid) double action tablets (20,000)9 Open titrated challenges using PEG 335013 850 mg oral challenge, using 5ml of 170mg/ml concentration has been used by authors (Krantz, Stone) after 1st dose mRNA vaccine anaphylaxis, prior to 2nd dose vaccine attempt.84 Note: common side effect of sticky mouth sensation. |
||
| Methylprednisolone acetate (PEG 3350 containing) |
|
|
|||
| Poloxamer 407 |
|
|
|||
| Poloxamer 188 |
|
|
|||
| Polysorbate 80 |
|
|
|||
| Triamcinolone acetonide (contains polysorbate 80) |
|
|
|||
| Non-polyethylene derived compounds | |||||
| Carboxymethylcellulose |
|
|
|
|
|
| Triamcinolone acetonide (contains carboxymethylcellulose) |
|
|
|||
| Mannitol |
|
|
|
|
|
| Povidone |
|
|
|
|
|
| Protamine |
|
|
|
|
|
| Gelatin |
|
|
|
|
|
| Galactose-alpha-1,3- galactose |
|
|
|
||
Thirdly, there are not many excipient allergies for which ex vivo testing approaches are commercially available or widely utilized, alpha-gal and gelatin allergy being the key exceptions. While scientific reports have shown promise in this area for further excipients, there is a need for scalable collaborations between excipient allergy researchers and the laboratory testing industry to meet this need for confirmatory tests. In future directions, testing modalities that can screen drugs for key allergens prior to market approval or as a post-marketing safety step will be invaluable. A hope is that in the future, standardized excipient allergy quantification would lead to comprehensive databases that clearly reveal the presence, molecular weight (if applicable) and quantity of key allergens in the drug.
Conclusions:
In conclusion, excipient allergies are uncommon but underrecognized reactions that are potentially fatal. This is due largely to a limited awareness of these ingredients as allergens, the challenges in ascertaining drug ingredients within and across healthcare systems, the ubiquity of many of these ingredients in otherwise innocuous or important drugs and the variable nature of allergic responses within sensitized individuals. To answer these challenges, management should focus on increased awareness and recognition, clearly confirming the diagnosis, educating patients and other healthcare providers about their allergy, teaching patients how to search ingredient lists, and careful avoidance in collaboration with their providers. Knowledge about the sensitization pathways, epidemiology and host risk is paramount and international collaboration to obtain large cohorts to study will be essential. The work of such collaborations should focus on standardized skin testing, validation of confirmatory ex vivo tests, and encourage readily available access to national drug formularies with information on both active and inactive ingredients.
Figure 3.

Skin prick test results in patient diagnosed two weeks after anaphylaxis to a depot steroid injection containing PEG 3350. Note that SPT is negative to PEG 6000 and lower MW, but positive to polysorbate 80, poloxamer 407 and PEG 20.000 at 0.01% and 0.1% solution. Patient consent obtained.
Figure 4:

Positive intradermal skin testing to carboxymethylcellulose (Carb), in a patient with anaphylaxis to triamcinolone injection containing carboxymethylcellulose (T), with otherwise negative corticosteroid testing. Patient consent obtained.
Figure 5:
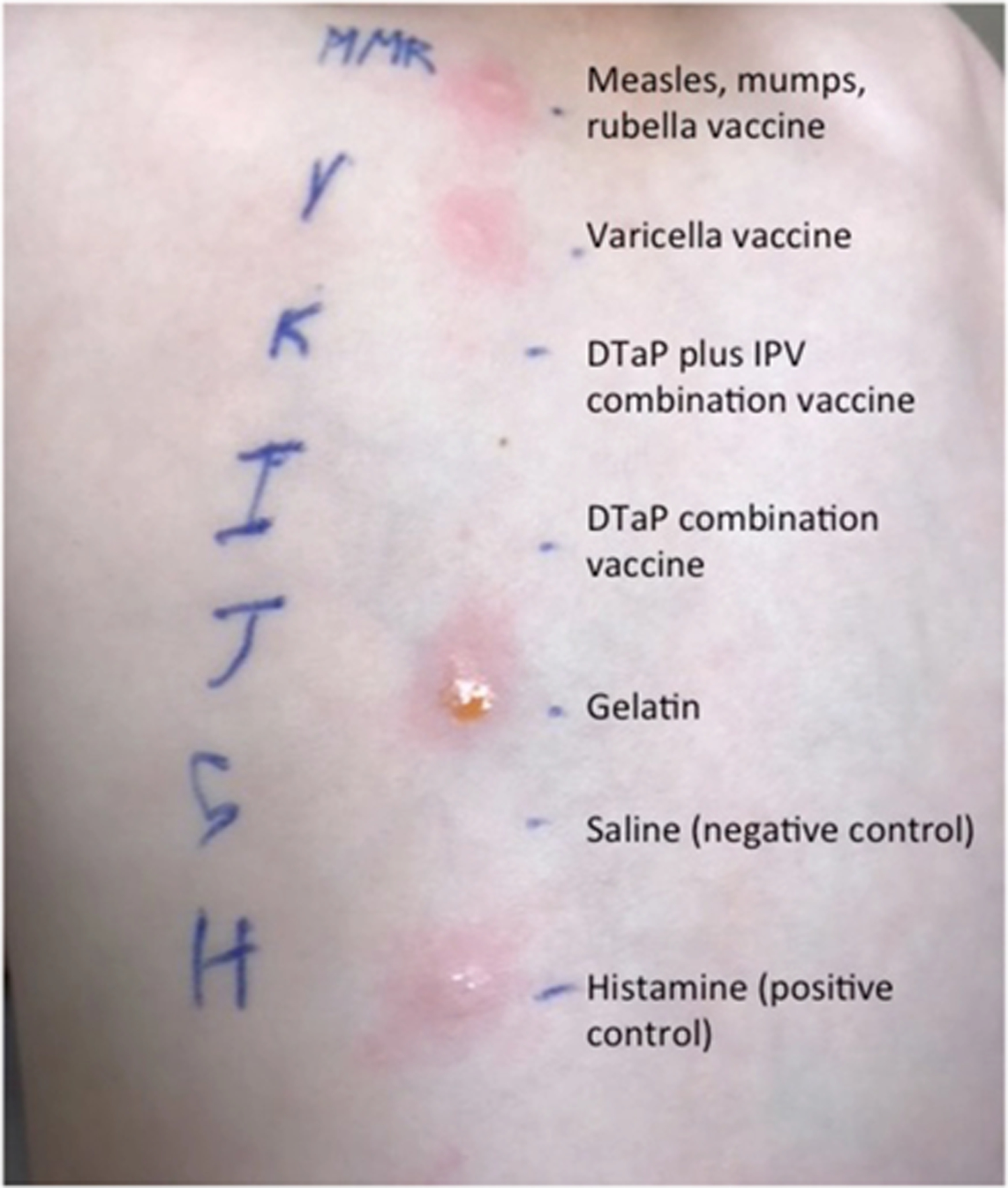
Positive vaccine and gelatin skin prick testing in a patient with alpha-gal allergy and a reaction to gelatin containing vaccines. From Stone et al., J Allergy Clin Immunol Pract66
Table 2:
Overview of Allergy to Key Excipients
| Excipient | Presentation | Knowns/Unknowns | Management of Proven Allergy |
|---|---|---|---|
| Polyethylene glycol derived compounds | |||
| Polyethylene Glycols (Macrogols) | The most common presentations of PEG allergy are with immediate type reactions including anaphylaxis to PEG containing bowel preparations or laxatives,3,5–9 or after injected corticosteroids containing PEG as an excipient.10–16
Patients may also present with PEG allergy after multiple drug reactions to oral tablets9,16 or effervescing oral products containing high molecular weight PEGs, such as PEG 4000–20,000 range.17 Some rarer presentations include anaphylaxis to intravenous PEGylated liposomal echocardiogram contrast,18 medroxyprogesterone acetate injections (excipient PEG 3350),9,19 and ultrasound gels in contact with mucous membranes (PEG 8000).20 PEGs contained in implantable devices are potentially of concern. |
|
|
| Poloxamers | Anaphylaxis to poloxamer has been described in the literature in two cases with anaphylaxis to poloxamer 238 in radiopharmaceuticals (1 case confirmed with skin testing).26,27 Among 17 patients diagnosed with allergy to PEG (skin prick test positive to PEG 3000 and/or PEG 6000 and a clinical history of an allergic reaction to a PEG-containing product) at the Allergy Clinic at Gentofte Hospital, Denmark, 15/17 patients were positive to poloxamer 407 (PEG MW 4444 g/mol) at time of diagnosis. Of these, 3/15 patients have had anaphylaxis to a poloxamer-containing pharmaceutical prior to diagnosis, in one case presenting as a perioperative cardiac arrest after insertion of poloxamer 407 containing bone cement during hand surgery.16 Similarly, all 4 PEG allergic patients (PEG 3350, PEG 8000) tested to poloxamer 407 by SPT at Vanderbilt University Medical Center, have tested positive (unpublished observations). |
|
|
| Polysorbates | Rarely, immediate hypersensitivity to polysorbates has been described in the literature in monoclonal antibodies, disinfectant solutions, intraarticular depot-steroids such as methylprednisolone acetate and triamcinolone acetonide, dexamethasone-lidocaine preparations for intramuscular and intraarticular injection and subcutaneously injected erythropoietin.31–33 |
|
|
| Non-polyethylene derived compounds | |||
| Carboxy-methylcellulose (CMC) | Skin test proven anaphylactic reactions have most commonly been associated with parenteral drugs and oral suspensions containing a large amount of CMC, for example to injectable corticosteroids or to oral barium sulfate contrast suspensions.17,35,36 The majority of reported cases have shown oral tolerance for CMC despite anaphylaxis on parenteral exposure. Rare cases of anaphylaxis to ice creams37 and popsicles/ice lollies38 due to CMC has also been reported. |
|
|
| Mannitol | Most case reports of anaphylaxis to mannitol describe reactions to intravenous infusion of 20% mannitol as treatment for raised intracranial pressure and cerebral edema.41 However only a few cases have been confirmed on allergy testing so the level of certainty is low. A more recent report describes two patients who presented with anaphylaxis to intravenous paracetamol containing mannitol as an excipient,42 while another report described a patient with anaphylaxis after oral consumption of effervescing paracetamol tablets containing mannitol and to a mannitol based artificial sweetener.39 This highlights, that patients may react to mannitol as a “hidden” excipient. The natural occurrence of mannitol in many plants increases the risk of anaphylaxis on ingestion of certain foods in sensitized individuals, cases of anaphylaxis to pomegranate and cultivated mushrooms have been described.41 |
|
|
| Povidone | Povidone anaphylaxis has been described after intraarticular injections of corticosteroids43 oral tablets44, facial creams44, eyedrops45 and topical povidone-iodine swabs for wound antiseptics46. |
|
|
| Protamine | Anaphylaxis to protamine typically presents at reversal of heparin-anticoagulation during open-heart surgery, or with insulin injections.50 In one case series of 3 patients who developed anaphylaxis during cardiac surgery, all three patients were taking long-acting insulins containing protamine as an excipient which was the likely source of allergic sensitisation.51 Previous authors had also suggested that sensitization to protamine occurs through to insulin injections.52,53 The incidence of allergic or anaphylactic reactions to protamine in patients on protamine containing insulins is reported to be 0.6% to 2%.54,55 |
|
|
| Gelatin | A typical gelatin allergy patient will present with reactions that are rapid in onset and overtly related to gelatin such as gelatin containing foods (marshmallows, wine gums/gummy bears, foods molded within gelatin) or medications (vaccinations, capsules, intraoperative hemostatics or gelatin based colloids.65 The pattern of sensitization and time to reaction is thought to be more like a typical food allergy and unrelated to tick bites, unless alpha-gal allergy is an underlying reason/contributing factor for the gelatin allergy. |
|
|
| Alpha-gal | A typical alpha-gal patient presents with delayed-onset of what is normally associated with immediate IgE type hypersensitivity reactions (urticaria, angioedema, diarrhea, shortness of breath, anaphylaxis) in relation to oral intake of red meat, dairy, or gelatin.70 Parenteral exposure, however, results in rapid onset symptoms.66,67,70,72 Most alpha-gal patients do not react to dairy, and even fewer will react to gelatin.70 Cofactors for reactions include exercise, alcohol, and nonsteroidal anti-inflammatory medications.70 Prior to more recent widespread awareness of alpha-gal allergy in endemic areas,80 many patients carried a diagnosis for years as idiopathic anaphylaxis or intermittent urticaria/angioedema.81 Because of the delayed onset of symptoms, one hallmark for which alpha-gal should be considered is allergic reactions that awaken patients from sleep (due to consumption of meat with the evening meal prior to going to bed.) |
|
|
Synopsis:
Excipients are the inactive ingredients in a drug or product, and are there to stabilize, preserve, or enhance the pharmacokinetics and bioavailability of the active ingredients. Excipient allergy is rare and hence often missed or misdiagnosed due to lack of awareness of the need to carefully review all drug ingredients. For the patient, excipient allergy can be frightening and potentially disruptive to healthcare delivery. This narrative review will provide a clinically oriented, international, collaborative perspective on excipient allergy testing, management of future healthcare safety, limitations in our testing modalities, and barriers to optimal care.
Key points:
Excipient allergy is uncommon and often missed or misdiagnosed due to lack of awareness of the need to carefully review the drug ingredients. This is particularly important when clear reactions have occurred to structurally unrelated drugs.
The primary challenge in even evaluating the possibility of an excipient allergy is knowing how to access the necessary information in a drug’s product information and to identify ingredients most likely to be immunogenic. It is important to consider the historical characteristics of the patient being tested as points of information on pre-test probability, along with the limitations of the testing modality being utilized.
Skin testing protocols/panels that are validated, harmonized across healthcare systems, and readily available are needed. In the meantime, it is recommended that future publications to the literature also provide at least some data on skin testing results from healthy controls.
Given how uncommon these reactions are there is a clear need for international collaborations between the Allergists and immunologists who see the patients, excipient allergy researchers, and the laboratory testing industry to meet a need for confirmatory tests.
Clinics Care Points:
Cross-reactivity between PEGs, polysorbate 80 and poloxamers, which share long chains of repeating ethylene oxide units can possibly be determined by skin testing, but how clearly this skin test cross-reactivity translates into clinical cross-reactivity upon drug challenge remains to be determined.
Individuals with parenteral sensitivity to CMC who have demonstrated tolerance to small amounts of oral CMC and other celluloses such as hypromellose can be advised to read labels and avoid injectable medications and high dose mucosal/enteral drugs containing CMC.
Because CMC (carboxymethylcellulose) is a common additive to foods and medications, it is recommended that the rare patients who react at lower doses via the oral route should be provided with an epinephrine autoinjector and counseled on strict avoidance.
In those who are allergic to protamine, avoidance of protamine during cardiac surgery anticoagulation reversal and the use of non-protamine containing insulins are the mainstays of management.
Patients with mammalian gelatin allergy should undergo testing for alpha-gal allergy.
Funding Sources:
Dr. Stone receives funding from AHRQ/PCORI 1K12HS026395-01 and the American Academy of Allergy, Asthma and Immunology Foundation.
Footnotes
Conflicts of Interest: none to disclose
References:
- 1.Lukawska J, Mandaliya D, Chan AWE, et al. Anaphylaxis to trometamol excipient in gadolinium-based contrast agents for clinical imaging. J Allergy Clin Immunol Pract. 2019;7(3):1086–1087. [DOI] [PubMed] [Google Scholar]
- 2.Munk SJ, Heegaard S, Mosbech H, Garvey LH. Two episodes of anaphylaxis following exposure to hydroxypropyl methylcellulose during cataract surgery. J Cataract Refract Surg. 2013;39(6):948–951. [DOI] [PubMed] [Google Scholar]
- 3.Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–922. [DOI] [PubMed] [Google Scholar]
- 4.Medicine USNLo. Daily Med. Daily Med Web site. Published 2021. Accessed 8-17-2021, 2021. [Google Scholar]
- 5.Shah S, Prematta T, Adkinson NF, Ishmael FT. Hypersensitivity to polyethylene glycols. J Clin Pharmacol. 2013;53(3):352–355. [DOI] [PubMed] [Google Scholar]
- 6.Gachoka D. Polyethylene Glycol (PEG)-Induced Anaphylactic Reaction During Bowel Preparation. ACG Case Rep J. 2015;2(4):216–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anton Girones M, Roan Roan J, de la Hoz B, Sanchez Cano M. Immediate allergic reactions by polyethylene glycol 4000: two cases. Allergol Immunopathol (Madr). 2008;36(2):110–112. [DOI] [PubMed] [Google Scholar]
- 8.Pizzimenti S, Heffler E, Gentilcore E, et al. Macrogol hypersensitivity reactions during cleansing preparation for colon endoscopy. J Allergy Clin Immunol Pract. 2014;2(3):353–354. [DOI] [PubMed] [Google Scholar]
- 9.Sellaturay P, Nasser S, Ewan P. Polyethylene Glycol-Induced Systemic Allergic Reactions (Anaphylaxis). J Allergy Clin Immunol Pract. 2021;9(2):670–675. [DOI] [PubMed] [Google Scholar]
- 10.Sohy C, Vandenplas O, Sibille Y. Usefulness of oral macrogol challenge in anaphylaxis after intra-articular injection of corticosteroid preparation. Allergy. 2008;63(4):478–479. [DOI] [PubMed] [Google Scholar]
- 11.Bordere A, Stockman A, Boone B, et al. A case of anaphylaxis caused by macrogol 3350 after injection of a corticosteroid. Contact Dermatitis. 2012;67(6):376–378. [DOI] [PubMed] [Google Scholar]
- 12.Wenande EC, Skov PS, Mosbech H, Poulsen LK, Garvey LH. Inhibition of polyethylene glycol-induced histamine release by monomeric ethylene and diethylene glycol: a case of probable polyethylene glycol allergy. J Allergy Clin Immunol. 2013;131(5):1425–1427. [DOI] [PubMed] [Google Scholar]
- 13.Brandt N, Garvey LH, Bindslev-Jensen U, Kjaer HF, Bindslev-Jensen C, Mortz CG. Three cases of anaphylaxis following injection of a depot corticosteroid with evidence of IgE sensitization to macrogols rather than the active steroid. Clin Transl Allergy. 2017;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewachter P, Mouton-Faivre C. Anaphylaxis to macrogol 4000 after a parenteral corticoid injection. Allergy. 2005;60(5):705–706. [DOI] [PubMed] [Google Scholar]
- 15.Stone CA Jr., Liu Y, Relling MV, et al. Immediate Hypersensitivity to Polyethylene Glycols and Polysorbates: More Common Than We Have Recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533–1540 e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruusgaard-Mouritsen MA, Johansen JD, Garvey LH. Clinical manifestations and impact on daily life of allergy to polyethylene glycol (PEG) in ten patients. Clin Exp Allergy. 2021;51(3):463–470. [DOI] [PubMed] [Google Scholar]
- 17.Caballero ML, Krantz MS, Quirce S, Phillips EJ, Stone CA Jr. Hidden Dangers: Recognizing Excipients as Potential Causes of Drug and Vaccine Hypersensitivity Reactions. J Allergy Clin Immunol Pract. 2021;9(8):2968–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krantz MS, Liu Y, Phillips EJ, Stone CA Jr. Anaphylaxis to PEGylated liposomal echocardiogram contrast in a patient with IgE-mediated macrogol allergy. J Allergy Clin Immunol Pract. 2020;8(4):1416–1419 e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu IN, Rutkowski K, Kennard L, Nakonechna A, Mirakian R, Wagner A. Polyethylene glycol may be the major allergen in depot medroxy-progesterone acetate. J Allergy Clin Immunol Pract. 2020;8(9):3194–3197. [DOI] [PubMed] [Google Scholar]
- 20.Jakubovic BD, Saperia C, Sussman GL. Anaphylaxis following a transvaginal ultrasound. Allergy Asthma Clin Immunol. 2016;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruusgaard-Mouritsen MA, Jensen BM, Poulsen LK, Duus Johansen J, Garvey LH. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol. 2021. [DOI] [PubMed] [Google Scholar]
- 22.Zhou ZH, Stone CA Jr., Jakubovic B, et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9(4):1731–1733 e1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumortier G, Grossiord JL, Agnely F, Chaumeil JC. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm Res. 2006;23(12):2709–2728. [DOI] [PubMed] [Google Scholar]
- 24.Urban-Morlan Z, Castro-Rios R, Chavez-Montes A, et al. Determination of poloxamer 188 and poloxamer 407 using high-performance thin-layer chromatography in pharmaceutical formulations. J Pharm Biomed Anal. 2008;46(4):799–803. [DOI] [PubMed] [Google Scholar]
- 25.Singh-Joy SD, McLain VC. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int J Toxicol. 2008;27 Suppl 2:93–128. [DOI] [PubMed] [Google Scholar]
- 26.Carbonell A, Escudero AI, Miralles JC, et al. Anaphylaxis Due to Poloxamer 238. J Investig Allergol Clin Immunol. 2018;28(6):419–420. [DOI] [PubMed] [Google Scholar]
- 27.Skanjeti A, Darcissac C, Jaulent C, et al. Grade 3 anaphylactic shock after administration of [(99m)Tc]-labeled nanocolloidal albumin (Nanocoll((R))) for sentinel node scintigraphy. Eur J Nucl Med Mol Imaging. 2019;46(6):1214–1215. [DOI] [PubMed] [Google Scholar]
- 28.Jones MT, Mahler HC, Yadav S, et al. Considerations for the Use of Polysorbates in Biopharmaceuticals. Pharm Res. 2018;35(8):148. [DOI] [PubMed] [Google Scholar]
- 29.Stone CA Jr., Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85(12):2694–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badiu I, Geuna M, Heffler E, Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenande E, Kroigaard M, Mosbech H, Garvey LH. Polyethylene glycols (PEG) and related structures: overlooked allergens in the perioperative setting. A A Case Rep. 2015;4(5):61–64. [DOI] [PubMed] [Google Scholar]
- 32.Bergmann KC, Maurer M, Church MK, Zuberbier T. Anaphylaxis to Mepolizumab and Omalizumab in a Single Patient: Is Polysorbate the Culprit? J Investig Allergol Clin Immunol. 2020;30(4):285–287. [DOI] [PubMed] [Google Scholar]
- 33.Steele RH, Limaye S, Cleland B, Chow J, Suranyi MG. Hypersensitivity reactions to the polysorbate contained in recombinant erythropoietin and darbepoietin. Nephrology (Carlton). 2005;10(3):317–320. [DOI] [PubMed] [Google Scholar]
- 34.Tasneem M, Siddique F, Ahmad A, Farooq U. Stabilizers: indispensable substances in dairy products of high rheology. Crit Rev Food Sci Nutr. 2014;54(7):869–879. [DOI] [PubMed] [Google Scholar]
- 35.Muroi N, Nishibori M, Fujii T, et al. Anaphylaxis from the carboxymethylcellulose component of barium sulfate suspension. N Engl J Med. 1997;337(18):1275–1277. [DOI] [PubMed] [Google Scholar]
- 36.Dumond P, Franck P, Morisset M, Sainte Laudy J, Kanny G, Moneret-Vautrin DA. Pre-lethal anaphylaxis to carboxymethylcellulose confirmed by identification of specific IgE--review of the literature. Eur Ann Allergy Clin Immunol. 2009;41(6):171–176. [PubMed] [Google Scholar]
- 37.Brockow K, Bauerdorf F, Kugler C, Darsow U, Biedermann T. “Idiopathic” anaphylaxis caused by carboxymethylcellulose in ice cream. J Allergy Clin Immunol Pract. 2021;9(1):555–557 e551. [DOI] [PubMed] [Google Scholar]
- 38.Ohnishi A, Hashimoto K, Ozono E, et al. Anaphylaxis to Carboxymethylcellulose: Add Food Additives to the List of Elicitors. Pediatrics. 2019;143(3). [DOI] [PubMed] [Google Scholar]
- 39.Calogiuri GF, Muratore L, Nettis E, Casto AM, Di Leo E, Vacca A. Immediate-type hypersensitivity reaction to Mannitol as drug excipient (E421): a case report. Eur Ann Allergy Clin Immunol. 2015;47(3):99–102. [PubMed] [Google Scholar]
- 40.Tenny S, Patel R, Thorell W. Mannitol. In: StatPearls. Treasure Island (FL)2021. [Google Scholar]
- 41.Hegde VL, Venkatesh YP. Anaphylaxis to excipient mannitol: evidence for an immunoglobulin E-mediated mechanism. Clin Exp Allergy. 2004;34(10):1602–1609. [DOI] [PubMed] [Google Scholar]
- 42.Jain SS, Green S, Rose M. Anaphylaxis following intravenous paracetamol: the problem is the solution. Anaesth Intensive Care. 2015;43(6):779–781. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalo Garijo MA, Duran Quintana JA, Bobadilla Gonzalez P, Maiquez Asuero P. Anaphylactic shock following povidone. Ann Pharmacother. 1996;30(1):37–40. [DOI] [PubMed] [Google Scholar]
- 44.Bruusgaard-Mouritsen MA, Mortz C, Winther L, Garvey LH. Repeated idiopathic anaphylaxis caused by povidone. Ann Allergy Asthma Immunol. 2021;126(5):598–600. [DOI] [PubMed] [Google Scholar]
- 45.Liccioli G, Mori F, Barni S, Pucci N, Novembre E. Anaphylaxis to Polyvinylpyrrolidone in Eye Drops Administered to an Adolescent. J Investig Allergol Clin Immunol. 2018;28(4):263–265. [DOI] [PubMed] [Google Scholar]
- 46.Gray PE, Katelaris CH, Lipson D. Recurrent anaphylaxis caused by topical povidone-iodine (Betadine). J Paediatr Child Health. 2013;49(6):506–507. [DOI] [PubMed] [Google Scholar]
- 47.Ronnau AC, Wulferink M, Gleichmann E, et al. Anaphylaxis to polyvinylpyrrolidone in an analgesic preparation. Br J Dermatol. 2000;143(5):1055–1058. [DOI] [PubMed] [Google Scholar]
- 48.Preuss JF, Goddard CE, Clarke RC, Platt PR, Sadleir PH. Anaphylaxis to intravenous paracetamol containing povidone. A case report and narrative review of excipient allergy related to anaesthesia. Anaesth Intensive Care. 2020;48(5):404–408. [DOI] [PubMed] [Google Scholar]
- 49.Lee AY, Chey WY, Choi J, Jeon JS. Insulin-induced drug eruptions and reliability of skin tests. Acta Derm Venereol. 2002;82(2):114–117. [DOI] [PubMed] [Google Scholar]
- 50.Kim R. Anaphylaxis to protamine masquerading as an insulin allergy. Del Med J. 1993;65(1):17–23. [PubMed] [Google Scholar]
- 51.Valchanov K, Falter F, George S, et al. Three Cases of Anaphylaxis to Protamine: Management of Anticoagulation Reversal. J Cardiothorac Vasc Anesth. 2019;33(2):482–486. [DOI] [PubMed] [Google Scholar]
- 52.Chu YQ, Cai LJ, Jiang DC, Jia D, Yan SY, Wang YQ. Allergic shock and death associated with protamine administration in a diabetic patient. Clin Ther. 2010;32(10):1729–1732. [DOI] [PubMed] [Google Scholar]
- 53.Levy JH, Adkinson NF Jr. Anaphylaxis during cardiac surgery: implications for clinicians. Anesth Analg. 2008;106(2):392–403. [DOI] [PubMed] [Google Scholar]
- 54.Levy JH, Schwieger IM, Zaidan JR, Faraj BA, Weintraub WS. Evaluation of patients at risk for protamine reactions. J Thorac Cardiovasc Surg. 1989;98(2):200–204. [PubMed] [Google Scholar]
- 55.Levy JH, Zaidan JR, Faraj B. Prospective evaluation of risk of protamine reactions in patients with NPH insulin-dependent diabetes. Anesth Analg. 1986;65(7):739–742. [PubMed] [Google Scholar]
- 56.Weiss ME, Nyhan D, Peng ZK, et al. Association of protamine IgE and IgG antibodies with life-threatening reactions to intravenous protamine. N Engl J Med. 1989;320(14):886–892. [DOI] [PubMed] [Google Scholar]
- 57.Bollinger ME, Hamilton RG, Wood RA. Protamine allergy as a complication of insulin hypersensitivity: A case report. J Allergy Clin Immunol. 1999;104(2 Pt 1):462–465. [DOI] [PubMed] [Google Scholar]
- 58.Land MH, Piehl MD, Burks AW. Near fatal anaphylaxis from orally administered gelatin capsule. J Allergy Clin Immunol Pract. 2013;1(1):99–100. [DOI] [PubMed] [Google Scholar]
- 59.Khoriaty E, McClain CD, Permaul P, Smith ER, Rachid R. Intraoperative anaphylaxis induced by the gelatin component of thrombin-soaked gelfoam in a pediatric patient. Ann Allergy Asthma Immunol. 2012;108(3):209–210. [DOI] [PubMed] [Google Scholar]
- 60.Robbins KA, Keet CA. Intraoperative anaphylaxis likely due to Gelfoam in a pediatric patient undergoing liver biopsy. Ann Allergy Asthma Immunol. 2015;114(6):531–533. [DOI] [PubMed] [Google Scholar]
- 61.Spencer HT, Hsu JT, McDonald DR, Karlin LI. Intraoperative anaphylaxis to gelatin in topical hemostatic agents during anterior spinal fusion: a case report. Spine J. 2012;12(8):e1–6. [DOI] [PubMed] [Google Scholar]
- 62.Luhmann SJ, Sucato DJ, Bacharier L, Ellis A, Woerz C. Intraoperative anaphylaxis secondary to intraosseous gelatin administration. J Pediatr Orthop. 2013;33(5):e58–60. [DOI] [PubMed] [Google Scholar]
- 63.Agarwal N, Spalding C, Nassef M. Life-threatening intraoperative anaphylaxis to gelatin in Floseal during pediatric spinal surgery. J Allergy Clin Immunol Pract. 2015;3(1):110–111. [DOI] [PubMed] [Google Scholar]
- 64.Uyttebroek A, Sabato V, Bridts CH, De Clerck LS, Ebo DG. Anaphylaxis to succinylated gelatin in a patient with a meat allergy: galactose-alpha(1, 3)-galactose (alpha-gal) as antigenic determinant. J Clin Anesth. 2014;26(7):574–576. [DOI] [PubMed] [Google Scholar]
- 65.Serrier J, Khoy K, Ollivier Y, et al. Recurrent anaphylaxis to a gelatin-based colloid plasma substitute and to cetuximab following sensitisation to galactose-alpha-1,3-galactose. Br J Anaesth. 2021;126(6):e200–e202. [DOI] [PubMed] [Google Scholar]
- 66.Stone CA Jr., Commins SP, Choudhary S, et al. Anaphylaxis after vaccination in a pediatric patient: further implicating alpha-gal allergy. J Allergy Clin Immunol Pract. 2019;7(1):322–324 e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stone CA Jr., Hemler JA, Commins SP, et al. Anaphylaxis after zoster vaccine: Implicating alpha-gal allergy as a possible mechanism. J Allergy Clin Immunol. 2017;139(5):1710–1713 e1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2012;129(5):1334–1342 e1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choudhary SK, Karim S, Iweala OI, et al. Tick salivary gland extract induces alpha-gal syndrome in alpha-gal deficient mice. Immun Inflamm Dis. 2021;9(3):984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Platts-Mills TAE, Commins SP, Biedermann T, et al. On the cause and consequences of IgE to galactose-alpha-1,3-galactose: A report from the National Institute of Allergy and Infectious Diseases Workshop on Understanding IgE-Mediated Mammalian Meat Allergy. J Allergy Clin Immunol. 2020;145(4):1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidle P, Reidenbach K, Kugler C, Eberlein B, Biedermann T, Darsow U. Recall urticaria-A new clinical sign in the diagnosis of alpha-gal syndrome. J Allergy Clin Immunol Pract. 2019;7(2):685–686. [DOI] [PubMed] [Google Scholar]
- 72.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chitnavis M, Stein DJ, Commins S, Schuyler AJ, Behm B. First-dose anaphylaxis to infliximab: a case of mammalian meat allergy. J Allergy Clin Immunol Pract. 2017;5(5):1425–1426. [DOI] [PubMed] [Google Scholar]
- 74.Stone CA Jr., Choudhary S, Patterson MF, et al. Tolerance of porcine pancreatic enzymes despite positive skin testing in alpha-gal allergy. J Allergy Clin Immunol Pract. 2020;8(5):1728–1732 e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hawkins RB, Wilson JM, Mehaffey JH, Platts-Mills TAE, Ailawadi G. Safety of Intravenous Heparin for Cardiac Surgery in Patients With Alpha-Gal Syndrome. Ann Thorac Surg. 2021;111(6):1991–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mullins RJ, Richards C, Walker T. Allergic reactions to oral, surgical and topical bovine collagen. Anaphylactic risk for surgeons. Aust N Z J Ophthalmol. 1996;24(3):257–260. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi H, Chinuki Y, Tanaka A, Morita E. Laminin gamma-1 and collagen alpha-1 (VI) chain are galactose-alpha-1,3-galactose-bound allergens in beef. Allergy. 2014;69(2):199–207. [DOI] [PubMed] [Google Scholar]
- 78.Vidal C, Mendez-Brea P, Lopez-Freire S, Gonzalez-Vidal T. Vaginal Capsules: An Unsuspected Probable Source of Exposure to alpha-Gal. J Investig Allergol Clin Immunol. 2016;26(6):388–389. [DOI] [PubMed] [Google Scholar]
- 79.Muglia C, Kar I, Gong M, Hermes-DeSantis ER, Monteleone C. Anaphylaxis to medications containing meat byproducts in an alpha-gal sensitized individual. J Allergy Clin Immunol Pract. 2015;3(5):796–797. [DOI] [PubMed] [Google Scholar]
- 80.Iglesia EGA, Stone CA Jr., Flaherty MG, Commins SP. Regional and temporal awareness of alpha-gal allergy: An infodemiological analysis using Google Trends. J Allergy Clin Immunol Pract. 2020;8(5):1725–1727 e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carter MC, Ruiz-Esteves KN, Workman L, Lieberman P, Platts-Mills TAE, Metcalfe DD. Identification of alpha-gal sensitivity in patients with a diagnosis of idiopathic anaphylaxis. Allergy. 2018;73(5):1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Campbell E, Peebles RJ, Stone C Jr., et al. Evaluation of Alpha Gal in Vaccines and Medications using a Human Monoclonal IgE Antibody. J Allergy Clin Immunol. 2021;147(2):Supplement, AB7. [Google Scholar]
- 83.Garcia-Menaya JM, Cordobes-Duran C, Gomez-Ulla J, et al. Successful Desensitization to Cetuximab in a Patient With a Positive Skin Test to Cetuximab and Specific IgE to Alpha-gal. J Investig Allergol Clin Immunol. 2016;26(2):132–134. [DOI] [PubMed] [Google Scholar]
- 84.Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Stone CA, Jr., Garvey LH. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: Don’t give up on the second dose! Allergy. 2021;76(9):2916–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biro P, Schmid P, Wuthrich B. [A life-threatening anaphylactic reaction following mannitol]. Anaesthesist. 1992;41(3):130–133. [PubMed] [Google Scholar]
- 86.Commins SP, Satinover SM, Hosen J, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: Total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. 2018;29(8):841–849. [DOI] [PubMed] [Google Scholar]
- 88.Brestoff JR, Zaydman MA, Scott MG, Gronowski AM. Diagnosis of red meat allergy with antigen-specific IgE tests in serum. J Allergy Clin Immunol. 2017;140(2):608–610 e605. [DOI] [PubMed] [Google Scholar]
- 89.Commins SP. Diagnosis & management of alpha-gal syndrome: lessons from 2,500 patients. Expert Rev Clin Immunol. 2020;16(7):667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morisset M, Richard C, Astier C, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67(5):699–704. [DOI] [PubMed] [Google Scholar]


