Significance
Extinct animals usually had complex acoustic behavior, but fossils reveal little of these details. Here, we report the earliest insect ears and sound-producing system found in Mesozoic katydids. These katydids evolved unexpectedly high acoustic diversity. Our analysis shows that katydids are the earliest known animals to have evolved complex acoustic communication, acoustic niche partitioning, and high-frequency musical calls. Our results not only suggest that acoustic communication might have been an important driver for the early radiation of katydids but also support the hypothesis of the acoustic coevolution of mammals and katydids. These findings unveil acoustic behavioral complexity and evolutionary adaption amongst Mesozoic katydids and contribute to understanding the evolution of Mesozoic soundscape thus far mostly inaccessible from the paleontological record.
Keywords: insect, acoustic communication, soundscape, tympanal ear, Mesozoic
Abstract
Acoustic communication has played a key role in the evolution of a wide variety of vertebrates and insects. However, the reconstruction of ancient acoustic signals is challenging due to the extreme rarity of fossilized organs. Here, we report the earliest tympanal ears and sound-producing system (stridulatory apparatus) found in exceptionally preserved Mesozoic katydids. We present a database of the stridulatory apparatus and wing morphology of Mesozoic katydids and further calculate their probable singing frequencies and analyze the evolution of their acoustic communication. Our suite of analyses demonstrates that katydids evolved complex acoustic communication including mating signals, intermale communication, and directional hearing, at least by the Middle Jurassic. Additionally, katydids evolved a high diversity of singing frequencies including high-frequency musical calls, accompanied by acoustic niche partitioning at least by the Late Triassic, suggesting that acoustic communication might have been an important driver in the early radiation of these insects. The Early—Middle Jurassic katydid transition from Haglidae- to Prophalangopsidae-dominated faunas coincided with the diversification of derived mammalian clades and improvement of hearing in early mammals, supporting the hypothesis of the acoustic coevolution of mammals and katydids. Our findings not only highlight the ecological significance of insects in the Mesozoic soundscape but also contribute to our understanding of how acoustic communication has influenced animal evolution.
The production of acoustic signals is one of the most important behavioral adaptions in animal communication, and the sending and receiving messages using sound is essential for the survival and success of many animals. Acoustic communication can be defined as the transmission of messages via airborne sound waves and is enabled by specialized hearing and sound-producing organs (1–4). It is widespread in two disparate extant animal taxa: insects and vertebrates, the latter including frogs, reptiles, birds, and mammals. Animal acoustic communication serves many purposes, including mating rituals, warning calls, conveying the location of food sources, and social learning, and has led to an amazing diversity and complexity of recent soundscapes (1). Therefore, exploring the evolution of animal acoustics will provide robust evidence for addressing how our modern-day soundscapes came into existence. Moreover, understanding the evolutionary history of animal acoustic behavior is crucial to unveiling how acoustic communication affects large-scale patterns of diversification (1, 2), and knowing how animals have evolved in response to sound pressure (e.g., natural noise) is also a key to the future in predicting how animals will deal with anthropogenic noise stressors (3). Nevertheless, the reconstruction of deep-time acoustic behavior is a major challenge. Using phylogenetic methods based on data from living animals is the most prevalent approach for reconstructing past acoustic behavior (2), although the fossil record of morphological traits permitting the analysis of acoustic communication is rather poor (4).
Insects were the first terrestrial animals to use air-borne sound signals for long-distance communication (5). They display an extremely high diversity of auditory systems and sound-producing organs. For example, tympanal ears have evolved at least 18 times independently in diverse taxa of seven extant insect orders (Orthoptera, Mantodea, Hemiptera, Diptera, Neuroptera, Coleoptera, and Lepidoptera), involving at least 15 body locations (6, 7). The ability to produce a sound that can travel over a long distance using specialized organs, such as a stridulatory (vibration-producing) apparatus or tymbals, has evolved at least in six insect orders, Blattodea, Orthoptera, Mantodea, Hemiptera, Coleoptera, and Lepidoptera (6). These insects provide important clues for understanding the evolutionary processes driving the diversity of acoustic signaling and behavior (1, 6, 8).
Among acoustically signaling insects, katydids (Orthoptera: Tettigoniidae, Prophalangopsidae, and extinct Haglidae) stand out as an ideal model to investigate the evolution of acoustic organs and behavior (1, 8–10). Male katydids produce sounds through friction between specialized veins of the forewings (tegminal stridulation), and these sounds are received by males and females primarily through the ears (auditory tympana) on the protibiae (11) (Fig. 1A). Their sophisticated acoustic communication, an important innovation in sexual selection, has been intensively studied from behavioral, neurological, and evolutionary perspectives (1, 5, 6, 10, 12–22). Although katydids have a rich fossil record dating back to the Triassic, songs have been inferred from only three species: one from the Middle Jurassic (14) and the others from the Early Eocene (13, 15). Therefore, the early evolution of their acoustic communication remains unclear. More importantly, Mesozoic katydid high-frequency songs are thought to be related to hearing improvement in early mammals (5, 12, 23). However, only low-frequency pure-tone (musical) songs have been reported in extinct katydids (13–15), and the origin of high-frequency songs remains unknown (12, 13, 17).
Fig. 1.
Prophalangopsidae and Haglidae from the Jurassic Daohugou Konservat-Lagerstätte. (A) Sigmaboilus sinensis, NND04329, male, with stridulatory files and tympanal ears. (B) Tympanal ears on forelegs (enlargement of boxed area in a). Note the right external (posterior) and left internal (inner) tympana are of similar size. (C) Sigmaboilus sinensis, NND04334, female, tympanal ears (SI Appendix, Fig. S1A). (D) Sigmaboilus sp., NND12z088, female, tympanal ear (SI Appendix, Fig. S1B). (E) Liassophyllum caii, NND12z186, male, stridulatory file (SI Appendix, Fig. S2C). (F) Allaboilus gigantus, NND12z171, male, stridulatory file, and harp (SI Appendix, Fig. S2D). ey, eye; ha, harp; hl, hind leg; sf, stridulatory file; te, tympanal ear. (Scale bars, 10 mm (A), 1 mm (B–F).)
In this study, we describe the exceptionally preserved stridulatory and hearing organs of katydids from the Middle Jurassic Daohugou Konservat-Lagerstätte (approximately 160 Mya) in China. Furthermore, we reexamine some key fossils from the Triassic of South Africa and Kyrgyzstan and compile an updated database of Mesozoic katydids. Using this, we calculate the singing frequencies of Mesozoic katydids, reconstruct their potential acoustic behavior, and analyze the evolution of their acoustic communications. Our results demonstrate that Mesozoic katydids evolved a high diversity of singing frequencies, acoustic niche partitioning, complex acoustic communication, and high-frequency musical calls.
Results
Ear Morphology.
We found well-preserved tympanal ears in 24 prophalangopsids (Abolinae) from the Middle Jurassic Daohugou Konservat-Lagerstätte, consisting of four males in two genera and two species, 19 females in three genera, and one sex unknown (SI Appendix, Table S1). Ears were not observed in the other taxa (e.g., Haglidae) examined. These fossil ears are elliptical structures apparent as a thinning of the leg cuticle, surrounded by a thickened rim, on the anterior (inner) and posterior (external) surfaces of the proximal protibiae (Fig. 1A and SI Appendix, Fig. S1). The size range of the fossil tympana is from 2.1 mm long and 0.8 mm wide to 1.0 mm long and 0.3 mm wide (male and female; all specimens), which is almost the same size as found in extant katydids [from 2.0 mm long and 0.8 mm wide [male, Cyphoderris monstrosa (24)] to 1.2 mm long and 0.5 mm wide (male, Tarragoilus diuturnus and Aboilomimus ornatus)]. Extant katydids and their relatives (Ensifera) exhibit a great deal of variation in the anterior and posterior morphology of the tympanum (11, 25). In our fossils, each tympanum consists of two almost identical (anterior and posterior) membranous areas, exhibiting similarity in size, structure, and position (Fig. 1 B and C). The tympanum comprises two distinct regions: an elliptical, thickened region (tympanal plate) with transverse creases, embedded within a crescent of thinner, transparent membrane (tympanal membrane) (Fig. 1 B–D and SI Appendix, Fig. S1). The entire tympanic membrane is completely exposed without the cuticle, and the tympanic membrane plate is located dorsal to the tympanic membrane.
Sound-Producing and Sound-Radiating Systems.
We examined the sound-producing and sound-radiating systems of forewings in 87 specimens of 31 genera and 40 species from the Middle/Late Triassic of Kyrgyzstan, Late Triassic of South Africa, and Middle Jurassic Daohugou Konservat-Lagerstätte of China. The sound-producing system of these katydids comprises a serrated vein (also known as the stridulatory file), and their sound-radiating system consists of a harp, neck, premirror, and mirror, which are four membranous areas on the wing that serve to amplify and radiate the sounds produced by the file (12, 26; Fig. 1 and SI Appendix, Figs. S2 and S3). Most extant katydids (belonging to Tettigoniidae) exhibit conspicuous wing asymmetry, producing either pure-tone or broadband calls (14, 16). In contrast, hitherto all known Mesozoic katydids have shown highly symmetrical forewings with identical files (SI Appendix, Fig. S2 A and B), consistent with this being a plesiomorphic condition for katydids (14, 27). Thus, these fossil katydids produced only pure-tone songs using a resonant mechanism, like extant Prophalangopsidae (14).
In addition to different sound-producing structures, katydids exhibit a great variety of sound-radiating cells (including harp, neck, premirror, and mirror cells) of the forewings, which also play an important role in their acoustic communication (26). In order to reveal the evolution of sound-radiating cells of Mesozoic katydids, we compiled a database of the forewing area and sound-radiating cells area (70 Mesozoic katydid species and eight extant prophalangopsid species; Dataset S1). Compared to their extant counterparts, fossil katydids have a comparatively small sound-radiating cells area, implying a low efficiency of sound amplification (SI Appendix, Fig. S4). The mirror area/sound-radiating cells area greatly increased through time (SI Appendix, Fig. S4 E and F), suggesting that their sound-radiating efficiency also improved because katydids radiate sound mainly via the mirror (26).
Acoustic Diversity of Jurassic Daohugou Katydids.
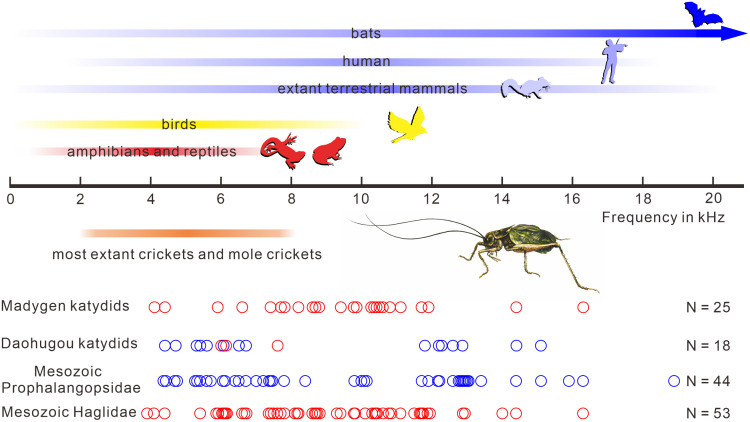
We observed that the file lengths of the Daohugou katydids are mostly restricted to one of two ranges: Haglidae and large Prophalangopsidae (forewing length 40 to 82 mm) have a file 8 to 10 mm long, while small- and medium-sized Prophalangopsidae (forewing length 24 to 41 mm) possess a distinctly shorter file 3.3 to 4.4 mm long (SI Appendix, Table S2). The file teeth are clearly visible in some specimens including Haglidae and Prophalangopsidae. All these teeth are asymmetrical with lateral flaps projecting anteriorly, morphologically similar to those in extant katydids (14). The tooth spacing increases progressively toward the basal end of both files (Fig. 1E and SI Appendix, Fig. S2) and so is just as well organized as that of extant species producing pure-tone calls, suggesting that most of the file was used to generate sounds. A regression model had been proposed for file length and singing frequency based on extant pure-tone producing Tettigoniidae (28) and was further extended to fossil Prophalangopsidae and Haglidae because the tegminal stridulatory apparatus in extinct Prophalangopsidae and Haglidae is similar to extant pure-tone producing Tettigoniidae (14, 29). Fitting the file length of fossil katydids to the regression model indicates that large katydids from Daohugou tuned in at a frequency of 4 to 7 kHz, while small- and medium-sized katydids commonly produced a higher frequency of 12 to 16 kHz (Fig. 2). This result is consistent with a previous observation that frequency is negatively correlated with body size 30.
Fig. 2.
Frequency range of hearing in vertebrates (Above) and frequency range of tones used by extant crickets and fossil katydids (Below). Frequency range of hearing in vertebrates based on ref. 31, and frequency range of tones of extant crickets based on ref. 14. Red triangles indicate the calculated frequency of Haglidae from Madygen. Green and red diamonds indicate the calculated frequency of Prophalangopsidae and Haglidae from Daohugou, respectively. Red and blue circles represent the calculated frequency of Mesozoic Haglidae and Prophalangopsidae, respectively.
Acoustic Diversity of Mesozoic Katydids.
We compiled a database of all fossil occurrences of Mesozoic Haglidae and Prophalangopsidae (16 subfamilies, 201 species), including the geological age, updated taxonomic placement, forewing length and width, and stridulatory file length (Datasets S2–S4). File lengths of Mesozoic Haglidae indicate a uniform distribution mainly between 4.0 and 10.5 mm, but those of Prophalangopsidae show a bimodal distribution with two ranges of 3.0 to 4.5 mm and 6.8 to 15.0 mm. We further calculated their probable singing frequencies based on the regression model (14, 29). Singing frequency is negatively correlated with body size for both Haglidae and Prophalangopsidae, which is consistent with extant katydids (29). Triassic Haglidae showed a diverse song repertoire with frequencies mainly between 4 kHz and 16 kHz (Fig. 2). The Middle/Late Triassic Madygen Konservat-Lagerstätte in Kyrgyzstan yielded the most abundant katydids, comprising 25 species. Their file lengths range from 3 mm to 16.3 mm, with frequencies between 4.1 kHz and 16.3 kHz (Fig. 2). Notably, some Triassic haglids could already produce a high-frequency pure-tone song (SI Appendix, Figs. S5 and S6). In contrast to Haglidae, Mesozoic Prophalangopsidae show a bimodal distribution of frequencies with two ranges of 4 to 8 kHz and 12 to 16 kHz (Fig. 2).
Mesozoic Katydid Faunal Turnover.
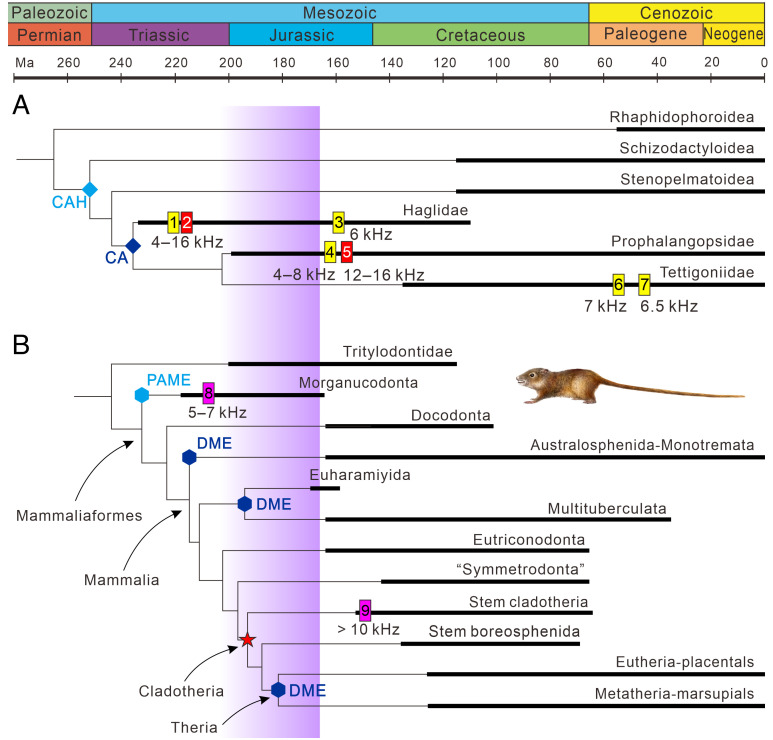
Haglidae probably originated in the Early Triassic and reached the peak of species richness with 47 species described during the Middle–Late Triassic, but they distinctly declined (22 species) from the Early Jurassic and had a low species richness (totally 17 species) from the Middle Jurassic to Cretaceous. In contrast, after the appearance in the Early Jurassic, Prophalangopsidae became more diverse than Haglidae from the Middle Jurassic (35, 27, and 37 species described in the Middle Jurassic, Late Jurassic, and Early Cretaceous, respectively) (SI Appendix, Fig. S7). Therefore, a distinct katydid transition from Haglidae- to Prophalangopsidae-dominated fauna occurred during the Early–Middle Jurassic (Fig. 3).
Fig. 3.
The evolution of katydids and mammaliaforms. (A) Simplified phylogeny of Hagloidea based on refs. 1, 14, and 32. (B) Simplified phylogeny of mammaliaforms based on ref. 33. Black thick lines indicate the known extent of the fossil record. Polygons indicate evolution of auditory organs in katydids and mammals: light blue rhombus, crista acustica homologue (CAH); dark blue rhombus, crista acustica (CA); light blue hexagon, postdentary-attached middle ear (PAME); dark blue hexagon, detached middle ear (DME); and red pentagram, mammal high-frequency hearing in Cladotheria. Vertical yellow bars show the low-frequency sound evidence: 1, Late Triassic fossils from South Africa and Middle/Late Triassic fossils from Madygen, Kyrgyzstan; 3, Archaboilus musicus from the Middle Jurassic of Daohugou (14); 4, Middle Jurassic fossils from Daohugou; 6, Pseudotettigonia amoena from the Early Eocene of Denmark (13); and 7, Pseudotettigonia leona from the Middle Eocene of USA (15). Vertical red bars represent the high-frequency sound evidence: 2, Middle/Late Triassic fossils from Madygen and 5, Middle Jurassic fossils from Daohugou. Vertical pink bars show the evidence of the upper frequency limit of mammal hearing: 8, Morganucodon from the Late Triassic 34 and 9, Henkelotherium guimarotae and Dryolestes leiriensis from the Late Jurassic of Portugal 35, 36. The purple area represents the katydid fauna transition and major radiation of stem mammals in the Early–Middle Jurassic 37.
Discussion
The fossil record of insect ears is exceptionally poor and previously only dated back to the Eocene (13, 25). Our fossils represent the earliest-known insect ears and extend the age range of the modern-type auditory tympana by 100 million years to the Middle Jurassic. In general morphology and size, the fossil ears are identical to those observed in modern Prophalangopsidae and some Stenopelmatidae, representing an ancestral character of the ear (9, 11, 24). Correspondingly, these extinct prophalangopsids probably had evolved similar biomechanics to extant “ancestral” forms of ensiferans like Anoatostomatidae and Prophalangopsidae, the class 2 lever model 38. In this model, the stiff tympanal plate acts as a hinge with the dorsal edge connected to the cuticle of the leg and forms the fulcrum of a lever that can move a load between the fulcrum and the force, effectively transmitting mechanical energy to the fluid environment of the sensory organ 38, 39. Furthermore, katydids probably had evolved the crista acustica also known as Siebold’s organ (an auditory sensory organ of Tettigonioidea and Hagloidea) in the Triassic (Fig. 3A), and this innovation represents a great advance in auditory ability, such as far-field hearing, and provides the precondition for high-frequency acoustic communication (1). Our findings not only represent the oldest tympanal ears in insects but also probably reveal the oldest lever mechanism in the insect auditory system, shedding light on the antiquity and evolution of ancestral insect auditory organs.
Jurassic katydids from Daohugou display both well-preserved ears and sound-producing systems and thus provide compelling evidence for reconstructing early katydid acoustic communication to better understand its roles in evolution. Acoustic signals, as an intraspecific communication channel, are used for a variety of purposes in insects including sexual signaling, courtship signals, aggression, and defense (20, 21, 40). The complex acoustic communication not only implies efficient communication but also provides a new natural selection driver for the speciation and diversification of katydids (16, 17, 27, 41). Male advertisement is widely accepted as the primary function of acoustic signals and the most important means of pair formation in ensiferans (40). The sounds made by Jurassic male katydids were clearly used to advertise the singer’s location and to attract mute females (which only possess ears), like calling songs in extant species (42). Males may be selected by females based on the quality of their calls, such as the temporal patterns and loudness. Some Daohugou katydids produced a comparatively low-frequency song, which is a long-distance advertising signal (43). Such a long-distance communication system requires the evolution of directional sensitivity (44, 45), suggesting that the conspecific females likely had evolved directional hearing, similar to extant Prophalangopsidae (9, 46). In addition, female katydids cannot produce sounds and the ears of male katydids are used for intermale acoustic communication, which is an important way for intermale territorial and aggressive behaviors to be resolved in orthopterans (11, 40). Therefore, the presence of ears in fossil males further suggests that intermale acoustic communication—typical of the acoustic behavior of their extant counterparts (11, 40)—had evolved by the Middle Jurassic including territorial and aggressive signals. Taken together, sophisticated acoustic communication was already established among katydids, at least by the Middle Jurassic.
The Middle/Late Triassic Madygen and Middle Jurassic Daohugou Konservat-Lagerstätte yielded the most diverse fossil katydids. Our analysis shows that both katydid assemblages reveal high diversity and large variation in their singing frequency, suggesting that a high acoustic diversity was widespread in Mesozoic katydids. Interestingly, singing frequencies commonly vary among most species, which is recognized in concurrent katydids from Madygen and Daohugou deposits (Fig. 2). These findings constitute strong support that there is competition for acoustic space in signaling communities, and this has probably resulted in temporal and spectral partitioning of the soundscape. Therefore, acoustic niche partitioning (partitioning of the transmission channel due to acoustic competition) may have been present among these fossil katydids at least by the Late Triassic. If acoustical niches were partitioned, as in extant ecosystems (44), this probably increased the effectiveness of acoustic communication and reduced the probability of interference and masking of overlapping sound signals (44), thus establishing and maintaining the coexistence of ecologically similar katydid species, contributing to the high diversity of Mesozoic katydids. Compared with other orthopteran insects, katydids with acoustic communication experienced a remarkable radiation during the early Mesozoic (47). Although recent studies of extant tetrapods and orthopterans suggest that acoustic communication does not increase diversification rates (1, 2), our results show that acoustic communication might have been an important driver for the early radiation of katydids.
Mesozoic Haglidae show a diverse song repertoire with frequencies mainly between 4 kHz and 16 kHz (Fig. 2). Mesozoic Prophalangopsidae have the same frequency range as that of Mesozoic Haglidae but show a bimodal distribution of frequencies with two ranges of 4 to 8 kHz and 12 to 16 kHz (Fig. 2). Low-frequency songs (<10 kHz) in katydids are better adapted for long-distance communication close to the ground 14, 54. This is because excessive attenuation of pure-tone sound increases linearly with frequency from at least 5 kHz to 40 kHz in habitats with shrubs and trees 55. These songs could also advertise a caller’s position to potential vertebrate predators which home in on sound (5). High-frequency songs of Mesozoic katydids would attenuate rapidly with distance, limiting the communication range 30. They are, however, important for eavesdropping avoidance, because they are above the upper limit of hearing in most Mesozoic animals except some early mammals with developed middle ears (Fig. 2). Mesozoic katydid high-frequency songs are thought to be related to hearing improvement in early mammals (5, 12, 23). However, previously only low-frequency songs have been reported in extinct katydids (13–15). Our analysis shows that high-frequency tone first appeared in the Middle/Late Triassic (Fig. 3). Definite evidence of high-frequency sounds was previously unknown in the Mesozoic or in earlier animals, including insects. Therefore, katydids are currently the earliest known lineage of animals to have evolved high-frequency musical calls.
Our results support the hypothesis of the acoustic coevolution of early mammals (predators) and katydids (prey) (5, 12, 23). Late Triassic stem mammaliaforms had less developed inner and middle ears and a weak high-frequency hearing ability 34, e.g., postdentary-attached middle ear (PAME), functional for both jaw suspension and hearing) in Morganucodon 56, 57 (Fig. 3B). After the emergence of mammals in the Late Triassic, most Jurassic mammals were small insectivores that survived in nocturnal niches not previously used by other vertebrates 58–61. Subsequently, stem mammals underwent a major radiation in the Early–Middle Jurassic 37, 62, with the improvement of hearing in early mammals, e.g., repeated appearance of detached middle ears 56, 57 (Fig. 3B). Some extant nocturnal insectivorous mammals identify and localize their prey via acoustic signals such as some marsupials 63. Similarly, some Early Jurassic stem mammals with developed inner and middle ears (e.g., early cladotherians; Fig. 3B) might have evolved high-frequency hearing 35, 36, which is thought to be useful in locating chorusing prey 5, 23, 64. Mesozoic katydids were probably nocturnal, relying on acoustic contact in vegetated habitats 14, 39, and their large bodies would have been a significant food source for early insectivorous mammals. Haglidae probably appeared in the Early Triassic and diversified during the Middle–Late Triassic (Fig. 3). From the Early to Middle Jurassic, haglids distinctly declined while prophalangopsids appeared and dominated the soundscape of the Pangean supercontinent (Fig. 3A and SI Appendix, Fig. S7). The rise of Jurassic Prophalangopsidae is probably due to their greater flying ability, dispersal being combined with more effective acoustic communication 32. Coinciding with the diversification of early mammalian clades 37, 62 (Fig. 3), the katydid faunal turnover may have been influenced by the early auditory evolution of insectivorous mammals, which probably imposed strong selective stress on katydids. The high-frequency songs of Mesozoic katydids could well have driven the evolution of intricate hearing systems in early mammals, and conversely, mammals with progressive hearing ability could have exerted selective pressure on the evolution of katydids. Thus, the acoustic coevolution of mammals and katydids might have occurred reciprocally and progressively during the Mesozoic (5, 12, 23), partly shaping the complex blueprint of Mesozoic mammalian and katydid evolution (SI Appendix, Fig. S8).
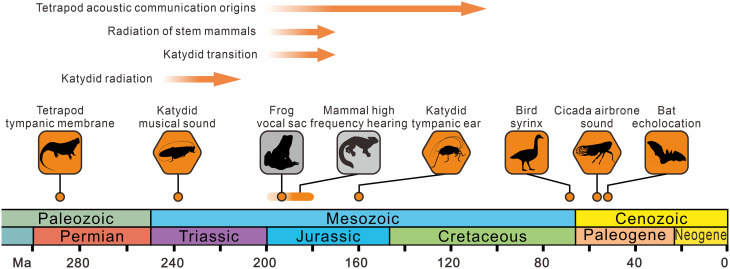
Our results also contribute to understanding the evolution of the Mesozoic soundscape, hitherto mostly inaccessible from the paleontological record. In modern terrestrial ecosystems, insects and frogs tend to dominate the choruses in tropical biomes, but birds dominate the chorus in temperate and boreal biomes 65. The early Mesozoic soundscape was quite different (Fig. 4 and SI Appendix, Fig. S9). During the Triassic, insects especially katydids, dominated the choruses, although some reptiles and amphibians may have made sounds. During the Jurassic, when the world became truly noisy, vertebrate animals had evolved a wide range of vocal abilities (2). Frogs, with their rich repertoire of calls and songs, first appear in the Early Jurassic 50; birds (Avialae) appeared in the Late Jurassic, although their special vocal structure (syrinx) is only reported from the latest Cretaceous 51. In the Cretaceous, the forest soundscape is almost the same as the modern one except lacking the sound of cicadas, which evolved their airborne sound-producing tymbals in the Cenozoic 52. All in all, the Mesozoic soundscape became progressively complex, in which katydids played a pioneering ecological role.
Fig. 4.
The origins of some key acoustic evolutionary events according to fossil evidence. The earliest tetrapod tympanic membrane of the ear comes from the Early Permian (approximately 290 Ma) 48. The earliest orthopteran stridulatory apparatus comes from the Middle/Late Triassic Madygen Konservat-Lagerstätte (approximately 240 Ma) 49. Acoustic communication has arisen repeatedly and independently across major tetrapod groups from 200 Ma to 100 Ma (2). The earliest crown anuran comes from the Early Jurassic (approximately 190 Ma) 50, probably indicating the origin of vocal sacs. The earliest high-frequency hearing ability of mammals probably originated in the Early Jurassic. The earliest orthopteran tympanic ear comes from the Middle Jurassic Daohugou Konservat-Lagerstätte (approximately 160 Ma). The earliest bird vocal organ syrinx comes from the latest Cretaceous (68 to 66 Ma) 51. The earliest record of a cicada tymbal with sac comes from the Paleocene (approximately 55 Ma) 52. The earliest echolocation of bats originates from the Early Eocene (approximately 52 Ma) 53.
Materials and Methods
Geological Settings.
The Daohugou fossil-bearing strata consist of mainly grayish-white tuff, tuffaceous sandstone, siltstone, and shale, with a tuffaceous conglomerate at the base and a thick rhyolitic breccia and andesite at the top. The age of the fossil-bearing strata is considered to be the late Middle Jurassic (66). The fossil insects are commonly preserved as carbonaceous compressions on the surface of gray tuffaceous siltstones (67).
Measurements of Stridulatory Files and Calculation of Acoustic Frequency.
We examined 63 specimens of 9 genera and 18 species (nearly 2% of all Orthoptera specimens) from the Middle Jurassic Daohugou Konservat-Lagerstätte of northeastern China, 21 specimens of 21 genera and 21 species from the Middle/Late Triassic Madygen Formation of Kyrgyzstan, and three specimens of one genus and one species from the Late Triassic Molteno Formation of South Africa. Jurassic specimens from Daohugou are deposited in the Shandong Tianyu Museum of Nature (prefix STMN) and Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (prefix NIGP and NND). Triassic specimens from Kyrgyzstan are deposited in the Palaeontological Institute, Russian Academy of Sciences (prefix PIN), and Triassic specimens from South Africa are deposited in the South African National Botanical Institute (prefix BP). Photographs were taken using a Sony α7 camera and a Zeiss SteREO Discovery V20 microscope system with specimens moistened in 95% alcohol or dry. The figures were prepared with CorelDraw X4 and Adobe Photoshop CS3. We summarized all fossil data of Mesozoic Haglidae and Prophalangopsidae, including the geological age, updated taxonomic placement, forewing length and width, and stridulatory file length (Datasets S2–S4). Almost all taxa are based on forewings, but many forewings are not complete, and their estimated length and width are based on the reconstruction of original researchers. We observed a difference in the forewing length between Mesozoic Haglidae and Prophalangopsidae (M–W test, Z = −2.072, P = 0.038; K–S test, Z = 1.438, P = 0.032; SI Appendix, Fig. S10). Although the M–W test shows no significant difference in the equality of medians of the file lengths of the two families (M–W test, Z = −0.693, P = 0.488), the K–S test reveals that the two distributions of file lengths are significantly different (K–S test, Z = 1.451, P = 0.030; SI Appendix, Fig. S10). For katydids producing pure-tone signals, the response variable carrier frequency with file length was the natural log-transformed: LnFreq = 3.68−0.81 × LnFile (14, 29). The sound frequency of Mesozoic katydids is calculated using this regression model. The Spearman Rank Correlation test shows that sound frequency is negatively correlated with the body size for Mesozoic Haglidae and Prophalangopsidae (Pearson correlation −0.667, P < 0.001 for Haglidae; Pearson correlation −0.793, P < 0.001 for Prophalangopsidae).
Measurements of Sound-Radiating Cells and Calculation of Related Traits.
We examined all Mesozoic haglids and prophalangopsids, among which 38 and 32 species with relatively complete forewings were measured respectively. Additionally, we added measurements of eight recent prophalangopsid species to make a comparison (Dataset S1). Measurements of areas were performed using software tpsDig v.2.16 (68). Six values were measured for each species: forewing area, radiating cells area (the sum of harp, neck, premirror, and mirror areas), harp area, neck area, premirror area, and mirror area. Then, we calculated three traits of sound-radiating cells: percentage of mirror in the forewing area (mirror area/forewing area), percentage of sound-radiating cells in the forewing area (sound-radiating cells area/forewing area), and percentage of mirror in the sound-radiating cells area (mirror area/sound-radiating cells area). We plotted three traits of sound-radiating cells through time, of which “Middle to Late Triassic” were grouped together due to the rarity of Late Triassic values and “Middle to Late Jurassic” were grouped together due to their short geological time range (SI Appendix, Fig. S4). The mirror area/forewing area slightly increased from the Triassic to Jurassic and almost remained stable in the Jurassic and Early Cretaceous (SI Appendix, Fig. S4 A and B). We observed a distinct difference in the mirror area/forewing area between Triassic (mainly from the Madygen Formation) and Jurassic (mainly from the Daohugou Konservat-Lagerstätte) katydids (M–W test, Z = −2.772, P = 0.006; K–S test, Z = 1.418, P = 0.036; SI Appendix, Fig. S10). Compared with Mesozoic species, recent species exhibit a distinct increase in the mirror area/forewing area, which probably indicates their improvement in sound-radiating efficiency. The sound-radiating cells area/forewing area slightly decreased from the Triassic to Early Cretaceous (SI Appendix, Fig. S4 C and D). The mirror area/sound-radiating cells area prominently increased through time (SI Appendix, Fig. S4 E and F).
Statistics.
Differences in the data were tested statistically, assuming a significance level of P < 0.05 for nonparametric tests performed in IBM SPSS Statistics 27. The Spearman rank correlation test was used to compare the rank order of the file length and forewing length of fossil katydids. It was also conducted to compare the rank order of the singing frequency and forewing length of Mesozoic Haglidae and Prophalangopsidae. Both Mann–Whitney (M–W) test and Kolmogorov–Smirnov (K–S) test (both for two independent samples) were conducted to compare differences in the length of forewings and files of the two groups, as well as the mirror area/forewing area between the Triassic (mainly from the Madygen Formation) and Jurassic (mainly from the Daohugou Konservat-Lagerstätte) katydids. The M–W test is used for equality of medians and is, therefore, sensitive to central tendency, and the K–S test is for equality of distributions and is more sensitive to skewness and kurtosis.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Acknowledgments
We are grateful to H. Wang, Z. Luo, T. Martin, and I. Ruf for helpful discussions; D. Yang for the reconstruction; and three anonymous reviewers for careful comments that greatly improved this manuscript. B.W. thanks members of the paleoentomological laboratory of the Palaeontological Institute (Russian Academy of Sciences) for their help during his visit to Moscow (2010). This research was supported by the National Natural Science Foundation of China (NSFC; 42125201, 42288201), NSFC project "Extreme climates and biological responses during the Paleozoic-Mesozoic transition", and Strategic Priority Research Program of the Chinese Academy of Sciences (XDA19050101 and XDB26000000). This work is a contribution to the Deep-time Digital Earth (DDE) Big Science Program. Visits to the Evolutionary Studies Institute (South Africa) were supported by a grant from the DFG [WA 1492/9-1].
Author contributions
B.W. designed research; C.X., B.W., T.W., J.C., D.K., Y.F., and H.Z. performed research; C.X. and B.W. contributed new reagents/analytic tools; C.X., B.W., T.W., J.C., D.K., Y.F., E.A.J., H.Z., and M.S.E. analyzed data; and C.X., B.W., E.A.J., and M.S.E. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Song H., et al. , Phylogenomic analysis sheds light on the evolutionary pathways towards acoustic communication in Orthoptera. Nat. Commun. 11, 4939 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z., Wiens J. J., The origins of acoustic communication in vertebrates. Nat. Commun. 11, 369 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes D. G., Francis C. D., Barber J. R., Using the past to understand the present: Coping with natural and anthropogenic noise. BioScience 71, 223–234 (2021). [Google Scholar]
- 4.Pollack G. S., Mason A. C., Popper A. N., Fay R. R., Insect Hearing (Springer International Publishing, 2016). [Google Scholar]
- 5.Senter P., Voices of the past: A review of Paleozoic and Mesozoic animal sounds. Hist. Biol. 20, 255–287 (2008). [Google Scholar]
- 6.Hedwig B., Insect Hearing and Acoustic Communication (Springer, 2014). [Google Scholar]
- 7.Strauß J., Stumpner A., Selective forces on origin, adaptation and reduction of tympanal ears in insects. J. Comp. Physiol. A 201, 155–169 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Hall M., Robinson D., “Acoustic signalling in Orthoptera” in Advances in Insect Physiology, Jurenka R., Ed. (Academic Press, 2021), pp. 1–99. [Google Scholar]
- 9.Woodrow C., Pulver C., Song H., Montealegre-Z F., Auditory mechanics in the grig (Cyphoderris monstrosa): Tympanal travelling waves and frequency discrimination as a precursor to inner ear tonotopy. Proc. R. Soc. B 289, 20220398 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodrow C., Baker E., Jonsson T., Montealegre-Z F., Reviving the sound of a 150-year-old insect: The bioacoustics of Prophalangopsis obscura (Ensifera: Hagloidea). PLoS One 17, e0270498 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwynne D. T., Katydids and Bush-Crickets: Reproductive Behaviour and Evolution of the Tettigoniidae (Cornell Univ Press, 2001). [Google Scholar]
- 12.Rust J., Choristers of the Jurassic. Proc. Natl. Acad. Sci. U.S.A. 109, 3606–3607 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rust J., Stumpner A., Gottwald J., Singing and hearing in a Tertiary bushcricket. Nature 399, 650 (1999). [Google Scholar]
- 14.Gu J. J., et al. , Wing stridulation in a Jurassic katydid (Insecta, Orthoptera) produced low-pitched musical calls to attract females. Proc. Natl. Acad. Sci. U.S.A. 109, 3868–3873 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwalt D. E., Rust J., A new species of Pseudotettigonia Zeuner (Orthoptera: Tettigoniidae) with an intact stridulatory field and reexamination of the subfamily Pseudotettigoniinae. Syst. Entomol. 39, 256–263 (2014). [Google Scholar]
- 16.Otte D., Evolution of cricket songs. J. Orthoptera Res. 1, 25–49 (1992). [Google Scholar]
- 17.Desutter-Grandcolas L., Phylogeny and the evolution of acoustic communication in extant Ensifera (Insecta, Orthoptera). Zool. Scr. 32, 525–561 (2003). [Google Scholar]
- 18.Béthoux O., Grylloptera – a unique origin of the stridulatory file in katydids, crickets, and their kin (Archaeorthoptera). Arthropod. Syst. Phylo. 70, 43–68 (2012). [Google Scholar]
- 19.Desutter-Grandcolas L., et al. , 3-D imaging reveals four extraordinary cases of convergent evolution of acoustic communication in crickets and allies (Insecta). Sci. Rep. 7, 7099 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey N. W., Pascoal S., Montealegre-Z F., Testing the role of trait reversal in evolutionary diversification using song loss in wild crickets. Proc. Natl. Acad. Sci. U.S.A. 116, 8941–8949 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker C. A., Clemens J., Murthy M., Acoustic pattern recognition and courtship songs: Insights from insects. Annu. Rev. Neurosci. 42, 129–147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubnel T., et al. , Sound vs light: wing-based communication in Carboniferous insects. Commun. Biol. 4, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues P. G., Ruf I., Schultz C. L., Digital reconstruction of the otic region and inner ear of the non-mammalian cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian ear. J. Mammal. Evol. 20, 291–307 (2013). [Google Scholar]
- 24.Mason A. C., Hearing in a primitive ensiferan: The auditory system of Cyphoderris monstrosa (Orthoptera: Haglidae). J. Comp. Physiol. A 168, 351–363 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Plotnick R. E., Smith D. M., Exceptionally preserved fossil insect ears from the Eocene Green River Formation of Colorado. J. Paleontol. 86, 19–24 (2012). [Google Scholar]
- 26.Chivers B. D., et al. , Functional morphology of tegmina-based stridulation in the relict species Cyphoderris monstrosa (Orthoptera: Ensifera: Prophalangopsidae). J. Exp. Biol. 220, 1112–1121 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Jost M. C., Shaw K. L., Phylogeny of Ensifera (Hexapoda: Orthoptera) using three ribosomal loci, with implications for the evolution of acoustic communication. Mol. Phylogenet. Evol. 38, 510–530 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Montealegre-Z F., Scale effects and constraints for sound production in katydids (Orthoptera: Tettigoniidae): Generator morphology constrains signal parameters. J. Evol. Biol. 22, 355–366 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Montealegre-Z F., Ogden J., Jonsson T., Soulsbury C. D., Morphological determinants of signal carrier frequency in katydids (Orthoptera): A comparative analysis using biophysical evidence of wing vibration. J. Evol. Biol. 30, 2068–2078 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Bennet-Clark H. C., Size and scale effects as constraints in insect sound communication. Phil. Trans. R. Soc. Lond. B 353, 407–419 (1998). [Google Scholar]
- 31.Coleman M. N., Boyer D. M., Inner ear evolution in primates through the Cenozoic: Implications for the evolution of hearing. Anat. Rec. 295, 615–631 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Gorochov A. V., New data on taxonomy and evolution of fossil and recent Prophalangopsidae. Acta Zool. Cracov. 46, 117–127 (2003). [Google Scholar]
- 33.Mao F., Zhang C., Liu C., Meng J., Fossoriality and evolutionary development in two Cretaceous mammaliamorphs. Nature 592, 577–582 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Rosowski J. J., Graybeal A., What did Morganucodon hear? Zool. J. Linn. Soc. 101, 131–168 (1991). [Google Scholar]
- 35.Ruf I., Luo Z. X., Wible J. R., Martin T., Petrosal anatomy and inner ear structures of the Late Jurassic Henkelotherium (Mammalia, Cladotheria, Dryolestoidea): Insight into the early evolution of the ear region in cladotherian mammals. J. Anat. 214, 679–693 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo Z. X., Ruf I., Martin T., The petrosal and inner ear of the Late Jurassic cladotherian mammal Dryolestes leiriensis and implications for ear evolution in therian mammals. Zool. J. Linn. Soc. 166, 433–463 (2012). [Google Scholar]
- 37.Grossnickle D. M., Smith S. M., Wilson G. P., Untangling the multiple ecological radiations of early mammals. Trends Ecol. Evol. 34, 936–949 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Bangert M., et al. , Stimulus transmission in the auditory receptor organs of the foreleg of bushcrickets (Tettigoniidae) I. The role of the tympana. Hear. Res. 115, 27–38 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Montealegre Z. F., Robert D., Biomechanics of hearing in katydids. J. Comp. Physiol., A 201, 5–18 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Mason A. C., Territoriality and the function of song in the primitive acoustic insect Cyphoderris monstrosa (Orthoptera: Haglidae). Anim. Behav. 51, 211–224 (1996). [Google Scholar]
- 41.Wilkins M. R., Seddon N., Safran R. J., Evolutionary divergence in acoustic signals: Causes and consequences. Trends Ecol. Evol. 28, 156–166 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Morris G. K., DeLuca P. A., Norton M., Mason A. C., Calling-song function in male haglids (Orthoptera: Haglidae, Cyphoderris). Can. J. Zool. 80, 271–285 (2002). [Google Scholar]
- 43.Römer H., Environmental and biological constraints for the evolution of long range signalling and hearing in acoustic insects. Phil. Trans. R. Soc. Lond. B 340, 179–185 (1993). [Google Scholar]
- 44.Schmidt A. K., Balakrishnan R., Ecology of acoustic signalling and the problem of masking interference in insects. J. Comp. Physiol. A 201, 133–142 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Römer H., Schmidt A. K., Directional hearing in insects with internally coupled ears. Biol. Cybern. 10, 247–254 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Robert D., “Directional hearing in insects” in Sound Source Localization, Popper A. N., Fay R. R., Eds. (Springer, 2005), pp. 6–35. [Google Scholar]
- 47.Rasnitsyn A. P., Quicke D. L. J., History of Insects (Kluwer Academic Publishers, 2002). [Google Scholar]
- 48.Kissel R., Morphology, Phylogeny, and Evolution of Diadectidae (Diadectomorpha University of Toronto, Cotylosauria, 2010). [Google Scholar]
- 49.Sharov A. G., Phylogeny of the Orthopteroidea. Trudy Paleontol. Inst. Akad. Nauk SSSR 118, 1–216 (1968). [Google Scholar]
- 50.Shubin N. H., Jenkins F. A., An early Jurassic jumping frog. Nature 377, 49–52 (1995). [Google Scholar]
- 51.Kingsley E. P., Identity and novelty in the avian syrinx. Proc. Natl. Acad. Sci. U.S.A. 115, 10109–10217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B., Mesozoic Hemiptera and Coleoptera (Insecta) from Northeastern China: Taxonomy, Evolution, and Taphonomy (Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, 2009). [Google Scholar]
- 53.Gunnell G. F., Simmons N. B., Fossil evidence and the origin of bats. J. Mamm. Evol. 12, 209–246 (2005). [Google Scholar]
- 54.Schul J., Patterson A. C., What determines the tuning of hearing organs and the frequency of calls? A comparative study in the katydid genus Neoconocephalus (Orthoptera, Tettigoniidae). J. Exp. Biol. 206, 141–152 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Römer H., Lewald J., High-frequency sound transmission in natural habitats: Implications for the evolution of insect acoustic communication. Behav. Ecol. Sociobiol. 29, 437–444 (1992). [Google Scholar]
- 56.Meng J., Bi S., Zheng X., Wang X., Ear ossicle morphology of the Jurassic euharamiyidan Arboroharamiya and evolution of mammalian middle ear. J. Morphol. 279, 441–457 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Wang J., et al. , A monotreme-like auditory apparatus in a Middle Jurassic haramiyidan. Nature 590, 279–283 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Luo Z. X., Developmental patterns in Mesozoic evolution of mammal ears. Annu. Rev. Ecol. Evol. Syst. 42, 355–380 (2011). [Google Scholar]
- 59.Gill P. G., et al. , Dietary specializations and diversity in feeding ecology of the earliest stem mammals. Nature 512, 303–305 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Maor R., Dayan T., Ferguson-Gow H., Jones K. E., Temporal niche expansion in mammals from a nocturnal ancestor after dinosaur extinction. Nat. Ecol. Evol. 1, 1889–1895 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Morales-García N. M., Gill P. G., Janis C. M., Rayfield E. J., Jaw shape and mechanical advantage are indicative of diet in Mesozoic mammals. Commun. Biol. 4, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schultz J. A., Eat and listen—how chewing and hearing evolved. Science 367, 244–246 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Stannard H. J., Dennington K., Old J. M., The external ear morphology and presence of tragi in Australian marsupials. Ecol. Evol. 10, 9853–9866 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Close R. A., Friedman M., Lloyd G. T., Benson R. B., Evidence for a mid-Jurassic adaptive radiation in mammals. Curr. Biol. 25, 2137–2142 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Farina A., Gage S. H., Ecoacoustics: The Ecological Role of Sounds (John Wiley & Sons, 2017). [Google Scholar]
- 66.Liu Y. Q., Liu X. Y., Ji S. A., Yang Z. Q., U-Pb zircon age for the Daohugou Biota at Ningcheng of Inner Mongolia and comments on related issues. Chin. Sci. Bull. 51, 2634–2644 (2006). [Google Scholar]
- 67.Wang B., et al. , Taphonomic variability of fossil insects: A biostratinomic study of Palaeontinidae and Tettigarctidae (Insecta: Hemiptera) from the Jurassic Daohugou Lagerstätte. Palaios 28, 233–242 (2013). [Google Scholar]
- 68.Rohlf F. J., tpsDig, digitize landmarks and outlines, version 2.16 (Department of Ecology and Evolution (State University of New York, Stony Brook, 2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Data Availability Statement
All study data are included in the article and/or SI Appendix.