Abstract
Both cataract and glaucoma significantly affect the quality of life of an individual and they are often found to coexist, either primarily or secondary to one another. Clear-cut guidelines are not available for this subgroup of coexistent morbidities. Through this article, we attempt to discuss the risks and benefits of staged and combined surgery, their short- and long-term effects on the intraocular pressure and pre and postoperative management. The indication and type of surgery will depend on the type, severity and control of glaucoma, and the clinical significance of cataract; and the surgical outcome on the surgical technique used, site of surgery, use of anti-fibrotic agents, and most importantly, the surgeon’s skill and experience.
Keywords: Combined surgery, comorbidity, manual small-incision cataract surgery, MSICS, trabeculectomy
Globally, 2.2 billion people are visually impaired and 94 million are functionally blind because of the untreated cataract and 76 million are affected by glaucoma.[1] Both cataract and glaucoma significantly affect the quality of life of an individual. Cataract and glaucoma are often found to coexist because both affect the elderly.[2,3] The coexistence may be primary or it may be secondary to each other as in phacomorphic glaucoma, phacolytic glaucoma, cataract after glaucoma surgery, anti-glaucoma drug-induced cataract, or iatrogenic. They may even coexist in other conditions, such as pseudoexfoliation (PXF) syndrome, ectopialentis syndromes, congenital anomalies, chronic steroid use, following trauma, or inflammation.[4]
Presence of cataract can influence the accurate assessment of glaucoma, and glaucoma can affect the visual outcomes of cataract surgery. The aim of managing these two conditions is to avoid postoperative spikes in intraocular pressure (IOP), long-term control of IOP, and achieve visual rehabilitation. It is, therefore, essential to have a treatment plan for these patients, especially because clear-cut guidelines are not available for this subgroup. The idea of combining manual small-incision cataract surgery (MSICS) and glaucoma surgery is an old one and with developments in surgical techniques, this combined surgery is emerging as the favored trend. The risks and benefits of staged and combined surgery, their short- and long-term effects on IOP, use of anti-metabolites, and pre and postoperative management are some of the concerns that shall be addressed in this article.
Impact of cataract on glaucoma evaluation
Presence of a lenticular opacity significantly affects the glaucoma as a disease itself and also its evaluation. Intumescent cataracts cause shallowing of the anterior chamber (AC) mimicking primary angle closure disease.[5] The media haze prevents proper visualization of the optic nerve head.[5] It also worsens the mean deviation/defect across all tests, including standard automated perimetry, frequency doubling perimetry, and short wavelength perimetry. It affects glaucoma progression indices as well. A cataract produces generalized reduction of sensitivity and artifacts in visual fields (VF) that may be mistaken as progression in a glaucoma patient, urging the ophthalmologist to step up glaucoma therapy. Hence, interpretation of the VF in such patients should always be done considering the severity of glaucoma as well as the type and location of the lenticular opacity.[5,6] Optical coherence tomography may show false underestimation of the retinal nerve fiber layer (RNFL), and scanning laser polarimetry (SLP) may give erroneous results because of the reduced signal to noise ratio.[7] SLP measurements tend to change significantly after cataract surgery, especially in posterior subcapsular cataracts.[8] In pseudophakics, the type of intraocular lens (IOL) implanted and IOL glistening can also affect the RNFL measurement.[9] Posterior capsular opacification, contrary to cataract, causes overestimation of RNFL thickness.[10] In case of advanced cataract, optic nerve visualization and RNFL imaging may not at all be possible and the patient would be unable to perform reliable VF, making both diagnosis and monitoring of glaucoma more difficult. Hence, it is suggested that a new baseline should always be established after cataract surgery to assess the disc, RNFL, and field defects.
Impact of glaucoma on cataract
Persistently elevated IOP increases the risk of nuclear cataract. Use of miotics such as pilocarpine in patients with posterior subcapsular cataracts would result in a significant drop in vision.[11-17] Miotics have also been implicated in hastening of cataractogenesis.[18] Patients of glaucoma per se, and especially those after trabeculectomy, often have a significantly reduced endothelial count.[19] A trabeculectomy can result in formation or acceleration of cataract in eyes that have postoperative persistent shallow AC and/or inflammatory reaction. Glaucoma also influences the visual prognosis of cataract surgery and may necessitate several modifications of the surgical technique. Multifocal IOLs, because of their influence on contrast sensitivity and imaging, are best avoided in patients with moderate to severe glaucoma.
Preoperative evaluation and management
Apart from the routine preoperative assessment planned for any intraocular surgery, the following also needs to be kept in mind for a patient having cataract and glaucoma:[20]
Information about the number of anti-glaucoma medications being used by the patient will help to decide the type of surgical option best suited for the patient. One also needs to inquire about access to health care, compliance to treatment and follow-up, and socio-economic status. Best corrected distant and near visual acuity should be noted to have a fair idea about the need for cataract surgery and expected visual outcome. The ocular surface especially the conjunctiva should be carefully assessed to select the site for filtering surgery. Any subtle or markedly evident ocular surface hyperemia and or inflammation following chronic use of topical anti-glaucoma therapy should be considered a risk factor for trabeculectomy failure and topical steroids maybe started preoperatively. The AC depth should be gauged and microphthalmos, relative anterior microphthalmos, and nanophthalmos should be ruled out. Shallow AC may necessitate use of highly retentive viscoelastics and use of mannitol. Relative anterior microphthalmos may further be associated with PXF, guttate changes, and poorly dilating pupils. Cilio-lenticular block is common postoperatively in these cases that should be kept in mind. IOL power calculation should be cautiously done in such eyes. The adequacy of pupillary dilatation should be confirmed. Patients on chronic miotic therapy and those with posterior synechiae dilate poorly and the need for pupil expanders may be kept in mind. The type, size, and location of lens opacities should be carefully evaluated to identify field defects mimicking glaucoma. Examination of the eye after dilation is mandatory, as sometimes PXF can be missed in the undilated state. PXF is often associated with poor corneal endothelium, rigid non dilating pupil, zonular instability, and postoperative capsular contractures. In view of the pupillary and zonular status, early cataract surgery may be recommended in such patients. Any zonular weakness may warrant the use of capsular rings/segments and special IOLs.
IOP measurement, setting of individualized target IOP, and if necessary, a diurnal variation test using the Goldmann applanation tonometer need to be done. Gonioscopy is mandatory preoperatively specially to identify the presence and site of synechiae to plan the incision sites. Extensive synechial closure would suggest that cataract surgery alone would not significantly lower the IOP. Compression gonioscopy will indicate if the lens is a confounding factor for the narrow angle configuration. Dilated stereoscopic optic nerve assessment with + 78D/+90D lens is mandatory. If optic nerve photos have been previously taken, a careful comparison can be made. A pale disc may appear pink because of the nuclear sclerosis; therefore, attention should be paid to the contour to determine the extent of cupping rather than color. Anterior segment imaging for angle and lens status maybe done; especially for lens vault that may help to decide whether an early cataract extraction may be helpful. VF should be carefully interpreted in presence of a cataract. A careful VF analysis can help in preventing postoperative surprises because of the extensive glaucomatous disc damage and in appropriate counseling regarding the prognosis. The total deviation/comparison plot crudely correlates to the extent of cataract and the pattern deviation/corrected comparison plot to the extent of glaucoma damage, except in very advanced glaucoma. Presence of split fixation may necessitate further counseling and consent because of the risk of snuff-out effect/washout phenomenon.[21] B-scan ultrasonography to document optic nerve head cupping in cases of dense cataracts also helps in prognostication. Because of the reasons mentioned earlier, RNFL imaging in presence of cataract has a limited role. A specular microscopy may be done in select cases, such as PXF. Biometry should be performed cautiously as extremes of IOP may influence the readings leading to postoperative refractive surprises.[22] Immersion ultrasound is the most accurate.[23]
Syndromes associated with ectopialentis and glaucoma (homocystinuria, Marfan’s), microspherophakia (Weill-Marchesani), congenital anomalies (iridocorneal dysgenesis syndromes), etc., should be looked for.
Anticoagulants and anti-platelet agents, pro inflammatory drugs such as prostaglandin analogues and pilocarpine maybe omitted depending on type of surgery to be done and with physician’s documented opinion in the case of former. Systemic anti-glaucoma drugs should be used when necessary to keep the preoperative IOP low. Irrespective of the type of surgery done, the need for long-term follow-up should be adequately emphasized.
General principles of surgery
Surgery in “cataract with glaucoma” patients remains a controversial subject. The indication and type of surgery depends on the type, severity, and control of glaucoma; and the surgical outcome depends on the surgical technique used, site of surgery, use of anti-fibrotic agents, and surgeon’s experience. Nevertheless, certain basic principles need to be remembered:
Always operate with the IOP as low as possible using as many anti-glaucoma medications as needed. Surgery in an eye with uncontrolled pressures can be disastrous. Any anesthesia according to patient’s convenience and surgeon’s preference may be used; but it is advisable to avoid large volumes of local anesthesia and local massage as these may jeopardize the optic nerve health further. One should expect a poor endothelial count and, hence, during cataract surgery, a dispersive viscoelastic should be liberally used; a soft-shell technique also helps. Any residual viscoelastic should be meticulously removed at the end of surgery. Anticipate increased postoperative inflammation, IOP spikes, and an increased incidence of steroid responsiveness in glaucoma patients.
Options of management
No strict guidelines exist regarding the preferred procedure. Patient and surgeon preference should be considered among several other factors [Fig. 1].[24]
Figure 1.
Empirical guidelines for management of coexistent cataract and glaucoma
The various surgical options include the following:
Simultaneous MSICS and trabeculectomy
Trabeculectomy followed by MSICS
MSICS followed by Trabeculectomy
MSICS only
Laser trabeculoplasty followed by MSICS
Combination of newer anti-glaucoma surgeries and MSICS.
Simultaneous MSICS and trabeculectomy
The combined surgical treatment of cataract and glaucoma has changed dramatically with the technologic advances in both, which has enabled to minimize the incidence and severity of complications and greatly influenced the indications for this surgery.[8] Indications[25]:
Presence of a visually significant cataract with any of the following: moderate glaucomatous damage and poor control in spite of multiple medications, poor compliance/non-compliance, inability to follow-up frequently, intolerance to medications, non-affordability, situations where separate or sequential surgeries cannot be performed, e.g. high-risk anesthesia, multiple systemic co-morbidities, and presence of other risk factors, such as PXF, angle recession, monocular status.[23]
Surgical pearls
Combined surgery is equally effective in treating glaucoma patients with cataracts whether done using a single or a two-site approach.[26,27]
One-site surgery
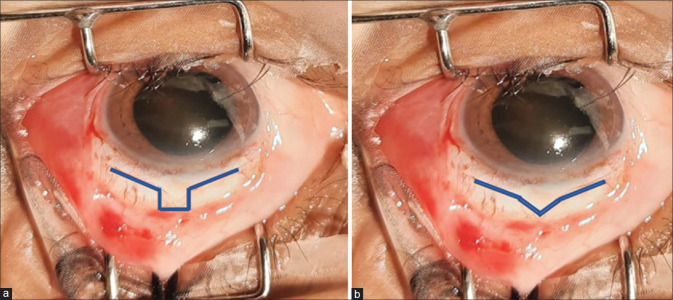
MSICS with trabeculectomy is usually done at one site, i.e. superiorly in routine cases. A fornix-based (for better visualization) superior conjunctival flap is preferred. Mitomycin C is generally used as chances of bleb fibrosis are more, in a dose of 0.2–0.4 mg/ml applied for about 1–2 min, followed by a thorough wash. A ”U” or “V” shaped scleral tunnel is fashioned at 12’o clock position [Fig. 2a and b].
Figure 2.
(a) U-shaped scleral tunnel. (b) V-shaped scleral tunnel
MSICS with IOL implantation can be completed through the scleral tunnel. Use of an AC maintainer is optional. The scleral tunnel is then converted into a partial thickness scleral flap and a conventional trabeculectomy is performed. As an alternative method, the scleral tunnel is not converted into a flap, but the floor of the tunnel is cut using Kelly’s Descemet punch to form a trabeculectomy window and this tunnel may or may not be sutured. Peripheral iridectomy is done after constricting the pupil with intra cameral pilocarpine. The scleral flap is closed with 10-0 nylon with or without releasable sutures, followed by a meticulous conjunctival closure.
Two-site surgery
Here, the MSICS is done temporally and the trabeculectomy superiorly.
Advantages
A simultaneous surgery prevents postoperative IOP elevations that can be detrimental in cases of advanced glaucoma, eliminates the need for glaucoma medications, provides better short- and long-term IOP control, and allows for faster visual rehabilitation. By doing a cost-effective non-machine dependent MSICS and by having only one surgery, it reduces the overall and long-term cost.
Additional advantages of a two-site surgery are less manipulation of sclera and conjunctiva; less risk of excessive coagulation that can cause significant tissue shrinking leading to greater, unpredictable astigmatism and difficult closure; less risk of anti-metabolite entering into the AC; and less chances of any postoperative trabeculectomy suture modification affecting the cataract incision.
Disadvantages
Because two surgeries are done in a single sitting, the surgical time is longer. It is important to remember that a combined surgery has a higher incidence of failure, and is less efficacious than sequential surgery. Complications more commonly arise in combined surgery as the two procedures have contrasting end points; trabeculectomy requires the inner ostium to be patent with aqueous leaking sub-conjunctivally and MSICS on the other hand requires all incisions to seal in a water-tight manner. The common complications noted are hypotony, suprachoroidal hemorrhage, hyphaema, infection, uveitis, and maculopathy secondary to hypotony. Bleb-related complications such as failure, thin-walled bleb, cystic bleb, and blebmigration into cornea can also occur. Blebitis and endophthalmitis can be uncommonly seen with use of anti-metabolites. Refractive outcomes can be unpredictable under the influence of the bleb.
MSICS with trabeculectomy has been found to be non-inferior in PXF patients (if done by an experienced surgeon); with comparable surgical outcomes to phacoemulsification with trabeculectomy; except for more postoperative fibrinous reaction in MSICS with trabeculectomy patients due iris trauma during nucleus delivery.[28] The use of an AC maintainer helps to minimize the complications and fasten the surgery.[28]
Trabeculectomy followed by MSICS
Indications
Visually insignificant cataract with any of the following: need for very low target IOP, advanced glaucoma unlikely to tolerate postoperative IOP spikes, very high IOP not controlled medically or causing significant corneal edema precluding visualization for cataract surgery, and high-risk cases with poor prognosis, e.g. uveitic glaucoma, neovascular glaucoma, scarred ocular surface, etc.
The IOP reduction is rapid, sustained, and greater. Cataract surgery can be planned 4–6 months later once the IOP stabilizes. It needs to be remembered that keratometry, AC depth, and lens position would be altered as a result of the trabeculectomy; therefore, fresh evaluation is essential before the cataract surgery. It is important to always avoid the site of the bleb, and perform the MSICS temporally. Subsequent MSICS in eyes that have undergone filtration surgery is known to affect the bleb adversely, even though the bleb area may have remained untouched.[2] While giving local anesthesia also, one should avoid damage to the bleb and also avoid intensive massage because the IOP may reduce significantly and result in hypotony.
In cases when the bleb the function is poor, bleb needling or revision can be performed during the cataract surgery.
MSICS followed by trabeculectomy
In situations of medically uncontrolled IOP following the MSICS, in early or late postoperative period, a filtration surgery can be planned with routine precautions and use of an anti-metabolite.
MSICS only
In case the patient has advanced cataract and an early glaucoma well controlled with 1–2 tolerated drugs, the cataract should be tackled first. MSICS may also be planned in cases of ocular hypertension and lens-induced glaucoma, e.g. phacomorphic or spherophakia. Cataract surgery alone may also be considered in the treatment of primary angle closure glaucoma (PACG). Recently, primary lens extraction alone gained more acceptance as an alternative surgical approach for glaucoma management, in view of the greater safety and visual recovery associated and the substantial reduction of IOP and deepening of AC and filtration angle. Primary lens extraction in acute PACG eliminates, or at least, reduces the risk of recurrent acute attacks, risk of progression of peripheral anterior synechiae, and development of chronic PACG. Primary lens extraction may be preferred in mild to moderate PACG having appositional angle closure. However, the decrease in IOP after cataract surgery in primary open-angle glaucoma is mild, less predictable, and may return to pre-surgical values after an initial reduction. The decision to do lens extraction as a primary treatment for glaucoma should be based on patient characteristics, target IOP, extent of glaucoma control, type of cataract, type of IOL to be implanted, and surgical skills.[29,30] A good preoperative gonioscopy and anterior segment imaging for evidence of synechiae, their extent, lens vault, and lens thickness may significantly help in the decision making.
Advantages
Doing only MSICS will be easy and fast, with a rapid recovery and lesser possibility of peri-operative complications. The consequent IOP reduction may reduce the future need of glaucoma medications and surgery. A prior cataract removal will aid in proper postoperative disc evaluation, perimetry and imaging. The superior conjunctiva and sclera will remain undisturbed in a temporally done MSICS, should the need for a subsequent trabeculectomy arise.
Disadvantages
Any surgical complications, such as excessive iris manipulation, pigment dispersion, posterior capsular rupture, and vitreous disturbance, will reduce the success rates of future trabeculectomy. Early postoperative IOP spikes may be detrimental to an already vulnerable optic nerve. MSICS done alone will not overcome the diurnal IOP variations, especially those occurring in PXF. The need for anti-glaucoma surgery in such eyes in the future cannot be excluded; hence, monitoring needs to be more frequent.
Laser trabeculoplasty followed by MSICS
Argon laser trabeculoplasty or selective laser trabeculoplasty can be done before the MSICS, but keeping a duration of at least 1 month between the two. A prior laser procedure will not blunt early postoperative IOP spikes that may occur after a subsequent cataract surgery. IOP rise, synechiae formation, hyphema, and uveitis are known complications that can influence the success of the cataract surgery.
Combination of newer anti-glaucoma surgery and MSICS
Minimally invasive glaucoma surgery (MIGS) with MSICS can be done in patients with significant cataract and mild to moderate glaucoma. Tubes, valved or non-valved, can also be done in select high-risk cases. In such surgeries, the tube is secured initially, then MSICS completed in a regular manner and lastly the tube is inserted into the AC, followed by tube fixation and closure.
Advantages
MIGS are conjunctiva and bleb independent surgeries; therefore, they do not preclude future conventional filtration surgery. Those performed through the same incision induce no additional astigmatism and may permit the use of toric IOLs.
Disadvantages
Surgeon expertise is required in MIGS. MSICS on one hand is a cost-effective surgery, whereas MIGS is an expensive affair. There is limited evidence currently about the long-term efficacy of MIGS.
IOL considerations in a glaucoma patient
Aspheric and blue light filtering IOLs may be considered as they improve the contrast sensitivity. Toric IOLs have limitations considering the refractive changes induced by filtering surgery and the resultant blebs. Multifocal (MF) IOLs may augment the photic phenomenon and contrast sensitivity problems in glaucomatous eyes. VF and imaging results may also be inaccurate in eyes implanted with MF IOLs. All these problems are seen more with refractive MF IOLs than diffractive types. Diffractive IOLs that are pupil independent can be considered in miotic pupils. But overall, MF IOLs are best avoided in moderate and advanced disease and should be used with extreme caution in other glaucomas that under all circumstances should be well controlled and stable.[31,32] Extended-range IOLs may be considered because they seem to improve contrast sensitivity. All premium IOLs should be used very cautiously in PXF where decentration is a common phenomenon.
Postoperative care
Frequent steroid and antibiotic drops are advised as in any other intraocular surgery. IOP should be invariably monitored, especially in steroid responders. A cycloplegic, often atropine, is used to prevent malignant glaucoma and deepen the AC. Systemic steroids may be needed to counter uveitis or choroidal effusions. If IOP lowering agents are needed, one should avoid prostaglandin analogs and pilocarpine. Digital massage maybe advised in cases of combined surgery, only after confirmation of the complete healing of the cataract wound. If only MSICS has been done, one needs to re-evaluate the glaucoma, reset a new baseline, and modify treatment accordingly.
Conclusion
There is no single procedure best suitable for eyes with coexistent cataract and glaucoma [Table 1]. Treatment has to be individualized based on surgeon’s comfort, patient’s expectations, and patient’s ocular, systemic, and socioeconomic status. Combined surgery is less efficacious than trabeculectomy alone. Two-site procedure is more effective than a single-site combined surgery. It is important to keep in mind the impact of one disease and surgery on the other and keep re-evaluating the glaucoma and target IOP and modify treatment as needed. It should be counseled strongly to the patient and relatives that glaucoma is a lifetime disease and cataract surgery may improve vision, but not the field loss occurred because of the glaucoma. Meticulous planning and vigilant follow-up will yield gratifying results in these eyes that are at risk of preventable blindness.
Table 1.
Comparison of different surgical options for management of coexistent cataract and glaucoma
| MSICS only | Trabeculectomy only | MSICS with Trabeculectomy | |
|---|---|---|---|
| Visual rehabilitation | Rapid | Slower, may hasten cataract progression | Slow |
| Postoperative course | Short | Long | Much longer |
| IOP reduction | Transient, occasional postoperative spikes may occur especially in presence of surgical complications | Significant | Moderate |
| Need for glaucoma drugs | Usually needed especially in primary open angle glaucoma | Usually not needed | Most often not needed |
| Complications | Few | Few | More |
MSICS=manual small-incision cataract surgery, IOP=intraocular pressure
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World report on vision –Executive summary. [Last accessed on 2022 May 26]. Available from:https://www.who.int/docs/default-source/documents/publications/world-report-on-vision-accessible-executive-summary.pdf .
- 2.Friedman DS, Jampel HD, Lubomski LH, Kempen JH, Quigley H, Congdon N, et al. Surgical strategies for coexisting glaucoma and cataract:An evidence-based update. Ophthalmology. 2002;109:1902–13. doi: 10.1016/s0161-6420(02)01267-8. [DOI] [PubMed] [Google Scholar]
- 3.Lee RK, Gedde SJ. Surgical management of coexisting cataract and glaucoma. Int Ophthalmol Clin. 2004;44:151–66. doi: 10.1097/00004397-200404420-00010. [DOI] [PubMed] [Google Scholar]
- 4.Eid TM, Spaeth GLS. Glaucoma associated with lens disorders. The Glaucomas:Concepts and Fundamentals. Lippincott Williams and Wilkins Philadelphia. 2000:160. [Google Scholar]
- 5.Chung HJ, Choi JH, Lee YC, Kim SY. Effect of cataract opacity type andglaucoma severity on visual field index. Optom Vis Sci. 2016;93:575–8. doi: 10.1097/OPX.0000000000000842. [DOI] [PubMed] [Google Scholar]
- 6.Rao HL, Jonnadula GB, Addepalli UK, Senthil S, Garudadri CS. Effect of cataract extraction on Visual Field Index in glaucoma. J Glaucoma. 2013;22:164–8. doi: 10.1097/IJG.0b013e31822e8e37. [DOI] [PubMed] [Google Scholar]
- 7.Holló G, Naghizadeh F, Hsu S, Filkorn T, Bausz M. Comparison of the current and a new RTVue OCT software version for detection of ganglion cell complex changes due to cataract surgery. Int Ophthalmol. 2015;35:861–7. doi: 10.1007/s10792-015-0064-8. [DOI] [PubMed] [Google Scholar]
- 8.Gazzard G, Foster PJ, Devereux JG, Oen F, Chew PT, Khaw PT. Effect of cataract extraction and intraocular lens implantation on nerve fibre layer thickness measurements by scanning laser polarimeter (GDx) in glaucoma patients. Eye (Lond) 2004;18:163–8. doi: 10.1038/sj.eye.6700600. [DOI] [PubMed] [Google Scholar]
- 9.Park RJ, Chen PP, Karyampudi P, Mills RP, Harrison DA, Kim J. Effects of cataract extraction with intraocular lens placement on scanning laser polarimetry of the peripapillary nerve fiber layer. Am J Ophthalmol. 2001;132:507–11. doi: 10.1016/s0002-9394(01)01110-2. [DOI] [PubMed] [Google Scholar]
- 10.García-Medina JJ, García-Medina M, Dorta SG, Pinazo-Durán MD, Gallego-Pinazo R, Zanón-Moreno VC. Effect of posterior capsular opacification removal on scanning laser polarimetry measurements. Graefes Arch Clin Exp Ophthalmol. 2006;244:1398–405. doi: 10.1007/s00417-005-0244-8. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi K, Hayashi H, Nakao F, Hayashi F. Changes in anterior chamber angle width and depth after intraocular lens implantation in eyes with glaucoma. Ophthalmology. 2000;107:698–703. doi: 10.1016/s0161-6420(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 12.Kim DD, Doyle JW, Smith MF. Intraocular pressure reduction following phacoemulsification cataract extraction with posterior chamber lens implantation in glaucoma patients. Ophthalmic Surg Lasers. 1999;30:37–40. [PubMed] [Google Scholar]
- 13.Tong JT, Miller KM. Intraocular pressure change after sutureless phacoemulsification and foldable posterior chamber lens implantation. J Cataract Refract Surg. 1998;24:256–62. doi: 10.1016/s0886-3350(98)80208-3. [DOI] [PubMed] [Google Scholar]
- 14.Miyake K, Asakura M, Kobayashi H. Effect of intraocular lens fixation on the blood-aqueous barrier. Am J Ophthalmol. 1984;98:451–5. doi: 10.1016/0002-9394(84)90130-2. [DOI] [PubMed] [Google Scholar]
- 15.Johnstone MA. The aqueous outflow system as a mechanical pump:Evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13:421–38. doi: 10.1097/01.ijg.0000131757.63542.24. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound activates the TM ELAM-1/IL-1/NF-kappaB response:A potential mechanism for intraocular pressure reduction after phacoemulsification. Invest Ophthalmol Vis Sci. 2003;44:1977–81. doi: 10.1167/iovs.02-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dada T, Bhartiya S, Begum Baig N. Cataract surgery in eyes with previous glaucoma surgery:Pearls and Pitfalls. J Curr Glaucoma Pract. 2013;7:99–105. doi: 10.5005/jp-journals-10008-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta VB, Rajagopala M, Ravishankar B. Etiopathogenesis of cataract:An appraisal. Indian J Ophthalmol. 2014;62:103–10. doi: 10.4103/0301-4738.121141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soro-Martínez MI, Miralles de Imperial-Ollero JA, Pastor-Montoro M, Arcos-Villegas G, Sobrado-Calvo P, Ruiz-Gómez JM, et al. Corneal endothelial cell loss after trabeculectomy and phacoemulsification in one or two steps:A prospective study. Eye (Lond) 2021;35:2999–3006. doi: 10.1038/s41433-020-01331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy GJ. Challenges in the management of glaucoma coexistent with cataract. Kerala J Ophthalmol. 2010;22:325–9. [Google Scholar]
- 21.Costa VP, Smith M, Spaeth GL, Gandham S, Markovitz B. Loss of visual acuity after trabeculectomy. Ophthalmology. 1993;100:599–612. doi: 10.1016/s0161-6420(93)31597-6. [DOI] [PubMed] [Google Scholar]
- 22.Lumme P, Laatikainen L. Exfoliation syndrome and cataract extraction. Am J Ophthalmol. 1993;116:51–5. doi: 10.1016/s0002-9394(14)71743-x. [DOI] [PubMed] [Google Scholar]
- 23.Swain S, Das S, Subudhi BNR, Dev S. Decision making in surgical management- coexisting cataract and primary open angle glaucoma. Odisha J Ophthalmol. 2015:88–90. [Google Scholar]
- 24.Bhartiya S, Bhagat PR, Deshpande KV, Albis-Donado O. Management of coexistent cataract and glaucoma. Nema HV, Nema N, editors. Gems in Ophthalmology:Glaucoma. Jaypee Brothers Medical Publishers (P) Ltd. 2018:400–27. [Google Scholar]
- 25.Nielsen PJ. Combined small-incision cataract surgery and trabeculectomy:A prospective study with 1 year of follow-up. Ophthalmic Surg Lasers. 1997;28:21–9. [PubMed] [Google Scholar]
- 26.Jindal AP, Al-Aswad L, Watsman SM, Wisner DM, Stelzner SK, Brown EN, et al. Review by Daniel Moore;Techniques for combined cataract and filtering glaucoma surgeries. [Last accessed on 2022 Feb];AAO Eye Wiki February. [Google Scholar]
- 27.Deka R, Das B, Nath A. Efficacy of trabeculectomy and manual small incision cataract surgery with posterior chamber intraocular lens implantation. Int J Health Res Medico Leg Prac. 2019;5:48–51. [Google Scholar]
- 28.Ramyashri S, Rao A, Padhy D, Das G. Small incision cataract surgery with trabeculectomy versus phacoemulsification trabeculectomy in pseudoexfoliation glaucoma. Indian J Ophthalmol. 2020;68:1090–4. doi: 10.4103/ijo.IJO_1319_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eid TM. Primary lens extraction for glaucoma management:A review article. Saudi J Ophthalmol. 2011;25:337–45. doi: 10.1016/j.sjopt.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vizzeri G, Weinreb RN. Cataract surgery and glaucoma. Curr Opin Ophthalmol. 2010;21:20–4. doi: 10.1097/ICU.0b013e328332f562. [DOI] [PubMed] [Google Scholar]
- 31.Teichman JC, Ahmed IK. Intraocular lens choices for patients with glaucoma. Curr Opin Ophthalmol. 2010;21:135–43. doi: 10.1097/ICU.0b013e3283365154. [DOI] [PubMed] [Google Scholar]
- 32.Ichhpujani P, Bhartiya S, Sharma A. Premium IOLs in glaucoma. J Curr Glaucoma Pract. 2013;7:54–7. doi: 10.5005/jp-journals-10008-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]