Abstract
Cataract remains a major cause of visual impairment worldwide including in India. The sutureless manual small-incision cataract surgery (MSICS) as an alternative to phacoemulsification, gives equivalent visual results at lower expenses. Still the procedure is often discredited for higher astigmatism due to the larger size of the incision. High astigmatism is an important cause of poor uncorrected visual acuity after cataract surgery. However, there are enough studies in the literature to prove that surgically induced astigmatism (SIA) can be minimized and also eliminated by adopting appropriate wound construction techniques during surgery. Even pre-existing astigmatism if any can be neutralized by changing wound architecture during surgery. Here, we review the various techniques of scleral tunnel construction described in the literature to care for postoperative astigmatism in MSICS.
Keywords: Astigmatism, cataract, manual small-incision cataract surgery
Manual small-incision cataract surgery (MSICS) has gradually evolved to be a popular, safe, and effective method of cataract surgery mostly in developing countries[1] because of its affordable cost, less surgical time, easy learning curve, non-machine dependence, and equivalent visual outcome in comparison to phacoemulsification.[2,3,4,5] But, still it is often discredited for higher postoperative induced stigmatism in comparison to phacoemulsification due to mostly larger sclera corneal section.[5] High astigmatism is an important cause of poor uncorrected visual acuity after cataract surgery.[6] Mean astigmatism reported in literature following MSICS ranges from 0.8D (Ruit et al.),[2] 1.2D (Gogate et al.),[7] and 1 diopter (Muralikrishnan et al.)[8] at 6 weeks. In addition, as cataract surgery has nowadays been considered to be a refractive surgery, the focus of surgery has shifted from just avoiding surgically induced astigmatism (SIA) to modifying pre-existing astigmatism. For refractive cataract surgery, the surgical planning has to be more precise, taking into consideration the size, location, and configuration of the sclerocorneal incision.[9]
Here, we review the various articles published in indexed journals on postoperative astigmatism following MSICS and the various techniques adopted and advised to reduce the same for good quality vision following MSICS.
The scleral tunnel incision in the 12 o’clock position was first created by Colvard et al.[10] in 1980 by making a partial-thickness groove in the sclera about 2 mm behind the limbus and then making the tunnel extend anteriorly. McFarland in 1989 was the one who introduced an incision architecture that is self-sealing.[11] Various modifications of the sclerocorneal section were subsequently designed to reduce the SIA.
Surgical-induced astigmatism in small-incision cataract surgery (SICS)
This is a well-known fact that corneal incision causes the greatest astigmatism, limbal incision the intermediate, and scleral incision the least astigmatism. Also, against-the-rule (ATR) astigmatism yields poorer vision than with-the-rule (WTR) astigmatism, and WTR astigmatism postoperatively seems to be better for unaided distance vision and near vision.[12]
Corneal or keratometric surgical induced astigmatism is the vector difference between the preoperative and postoperative corneal or keratometric astigmatism.[13] Analysis of astigmatism is restricted to keratometric astigmatism in various studies because this is an objective measurement of corneal contour not influenced preoperatively by lenticular astigmatism or postoperatively by subjective patient perception. Change in keratometric cylinder was examined in three ways[14]: (a) the simple subtraction method of calculating cylinder change without regard to the axis; (b) the polar value method of Naeser for determining WTR or ATR change[15]; (c) the vector analysis method of determining the magnitude of the surgically induced astigmatic vector, described by Jaffe and Clayman.[16]
The SIA was compared by Ruit et al.[2] in phaco and SICS at 6 weeks and 6 months postoperatively and at 6-month follow-up, they reported mean astigmatism of 0.7D for the phaco group and 0.88D for the MSICS group with no significant difference in SIA in the two techniques. However, Venkatesh et al. and George et al.[52] reported lesser SIA in phaco,[4,17] and Muralikrishnan et al.[18] conducted a study to compare SIA associated with PHACO and MSICS and reported no significant difference at either the 6-week or 6-month follow-up examination.
The six aspects of a scleral tunnel are site, location, size or length, shape, depth, width, and entry into the anterior chamber.[19] As per available literature, the site, location, size, shape, and depth of sclerocorneal incision along with a surgeon’s experience, influence postoperative SIA significantly.[19,20,21,22] The conclusions of various studies are tabulated in Table 1.
Table 1.
Summary of conclusions of studies related to factors affecting postoperative astigmatism in manual SICS
| Author and year | Conclusions |
|---|---|
| Incision site | |
| Kimura et al. 1999[23] | SICS through a temporal approach provides a better stabilization of refraction with significantly lesser amount of SIA than the superior approach. |
| Gokhale et al. 2005[24] | A shift in the incision site to the superotemporal or temporal the sclera is recommended except in patients with a pre-existing “with the rule” astigmatism of about 1D. |
| Malik et al. 2012[20] | SICS with a temporal approach provides better stabilization of refraction with significantly less SIA than a superior approach. |
| Ayena et al. 2016[25] | Cataract surgery by MSICS technique appears to offer good functional results with a reduction of astigmatism when the incision is made in the temporal superior approach. |
| Arthur et al. 2016[26] | A statistical and clinical significantly greater postoperative corneal astigmatism than preoperative corneal astigmatism was observed for a group of ATR cataract patients who underwent superior approach MSICS. |
| Pawar et al. 2012[27] | SICS which is done with a temporal and a superotemporal approach provides a better quality of vision due to a significantly less SIA than the superior approach. |
| Akura et al. 2000[28] | The incisions on the temporal or superior steep astigmatic axis (with the selective shape) reduced astigmatism in almost all cases. |
| Nielsen et al.[29] | Temporal incisions resulted in a with-the-rule induced change and superior incisions, an against-the-rule-induced change. Preoperative against-the-rule astigmatism was reduced significantly by temporally placed clear corneal incisions and preoperative with-the-rule astigmatism, by superiorly placed clear corneal incisions. |
| Incision location | |
| Archana et al. 2011[30] | Surgically induced astigmatism is significantly higher in clear corneal manual SICS than in sclerocorneal. |
| Olsen et al. 1997[21] | The clear corneal incision induces significantly more regular as well as irregular astigmatism than the scleral tunnel incision. |
| Girard 1995[31] | “With cautery we delineate a 7 mm incision, 3 mm from the limbus and extending from 11 to 1 o’clock”. |
| Incision shape | |
| Pallin et al. 1991[32] | The surgical results in a preliminary survey with an inverted “V” shaped incision show a minimal iatrogenic change in corneal toricity. |
| Singer et al.[33] | The frown incision group consistently had a lower standard deviation from the mean induced astigmatism than the scleral pocket incision group. |
| Akura et al. 2000[28] | In cataract surgery using relatively large scleral self-sealing incisions, the BENT frown incision effectively achieved astigmatic neutrality. The incisions on the temporal or superior steep astigmatic axis (with the selective shape) reduced astigmatism in almost all cases. |
| Jauhari et al. 2014[34] | The authors conclude that a chevron incision gives minimum SIA in manual SICS. |
| Sinskey et al. 1994[35] | 6.0 mm no-stitch frown incision induces a low postoperative astigmatism and remains a relatively stable incision after one month. |
| Burgansky et al. 2002[11] | Enlarging the size of the chevron incision up to 7.0 mm resulted in a small increase in induced astigmatism. |
| Incision length | |
| Sinskey et al. 1994[35] | 6.0 mm no-stitch frown incision induces a low postoperative astigmatism and remains a relatively stable incision after one month. |
| Burgansky et al. 2002[11] | Enlarging the size of the chevron incision up to 7.0 mm resulted in a small increase in induced astigmatism. |
| Sahu et al. 2022[36] | A 2 mm MSICS with phacofracture can deliver low astigmatism and good visual recovery in cataract surgery. |
| Incision width | |
| Girard 1995[31] | “With cautery we delineate a 7 mm incision, 3 mm from the limbus and extending from 11 to 1 o’clock. We make the incision with a N.64 Beaver blade and penetrate approximately 50% of the sclera, carrying the dissection well into clear cornea” |
| Incision depth | |
| Basti et al. 1993[37] | Optimal incision depth is described to be one-half to one-fourth the thickness of the sclera or about 0.3 mm and the scleral flap should neither be too thick nor too thin. |
| Anders et al. 1997[38] | Surprisingly, incision depth did not affect the strength of sclerocorneal incision resulting in astigmatism. |
| Pattanayak et al. 2022[39] | We found a statistically significant effect of depth of sclerocorneal incision on the change of astigmatism following manual SICS, with a superficial incision causing a higher change than a deeper incision. |
| Use of sutures | |
| Eslami et al. 2015[40] | In the MSICS (an acceptable method for cataract surgery in the developing world), the horizontal sutures induced ATR astigmatism and the Xpattern sutures induced mild WTR astigmatism. |
Incision site
Conventionally, cataract surgery done by a superior approach comes with its own advantages like it does not require the surgeon to adapt to a different surgical position along with providing forehead support for the surgeon’s hands while a temporal approach does not and hence is more preferred by the less experienced surgeon over a temporal or superotemporal approach as.[35] Kimura et al.[23] have shown that an oblique incision induces less postoperative astigmatism in comparison to a superior incision. The rate of ATR in the superior approach is quite high.[41] SICS with the temporal approach provides a better stabilization of the refraction with a significantly less SIA than the superior approach.[25] Temporal incision induces a small amount of WTR astigmatism due to the fact that the temporal location is farther from the visual axis than the superior location and hence any flattening due to the wound is less likely to affect the corneal curvature at the visual axis. Also, when the incision is located superiorly, both gravity and eyelid blink tend to create a drag on the incision inducing ATR.[24] Hence, the rate of ATR astigmatism in superior incisions described in the literature is quite high.[42] The temporal incision being farthest from the visual axis is astigmatically neutral and also with temporal incisions, there is no massaging effect of the upper lid nor gravitational drag as occurs in the superior incision.[24] These factors are neutralized well with temporally placed incision because the incision is parallel to the vector of forces.[24] Malik et al.[20] also have described the advantages of the temporal incision over superior incisions such as lesser SIA and better exposure in deep-set eyes and reported a mean SIA value of 0.75 ± 0.4067D for cataract patients with preoperative ATR astigmatism who underwent temporal approach MSICS. Whereas Edmund Arther et al.[26] reported an SIA value of 1.62 ± 0.90D for a similar cohort who underwent superior approach MSICS and concluded that MSICS with the superior-temporal and the temporal approaches provides a better quality of vision due to the significantly less SIA than the superior approach with higher SIA. Gokhale et al.[24] also found temporal and superotemporal tunnels to induce less astigmatism as compared with superior tunnels for MSICS. The mean astigmatism was 1.28D at 2.9° for superior incisions, 0.20D at 23° for superotemporal incisions, and 0.37D at 90° for temporal ones. But Pawar et al.[27] described that superotemporal incision has the advantages of both the locations and approach so it is better than the temporal incision.
Akura et al.[28] reported that pre-existing astigmatism could be reduced by using a steep meridian frown incision in self-sealing extracapsular cataract extraction (ECCE) and also by manipulating the incision’s location and shape, postoperative astigmatism can be controlled with reference to the site of the incision, it is also recommended by Nielsen et al. to place the incision on the steeper corneal meridian based on the preoperative keratometric (K) reading[29] [Fig. 1]. As, there is flattening of the corneal curvature in the meridian on which the incision is placed, with a corresponding steepening to the same degree of the orthogonal meridian.[17] Thus, there will be a reduction in the corneal power of the steeper meridian when an incision is placed on that meridian, with a corresponding increase in the corneal power to the same degree as the flat orthogonal meridian. The postoperative corneal astigmatism decreases as the difference in corneal powers between the flattened steeper meridian (meridian on which the incision was placed) and the steepened flatter meridian is reduced postoperatively. Further, with increasing age, the horizontal corneal meridian becomes more curved than the vertical meridian leading to or increasing existing ATR astigmatism. Thus, with increasing age, there is a shift in ATR.[43] With senile cataract being the most common type of cataract in developing countries[44] and placing an incision on the vertical meridian (superior approach) for a cataract patient with preoperative ATR astigmatism may cause further flattening of the already flat vertical meridian and a corresponding steepening to the same degree of the already steep horizontal meridian leading to high postoperative ATR corneal astigmatism. The WTR astigmatism induced by a temporal incision is advantageous because most elderly patients have preoperative ATR astigmatism.[19] Other advantages of the temporal incision are that the surgery can be easily done in deep-seated eyes, the chances of postoperative ptosis are absent as there is no use of superior rectus fixation suture,[15] there is no accumulation of fluid due to easy drainage of fluid at the lateral canthus, and the superior limbal site can be comfortably used for glaucoma filtration surgery if needed later.
Figure 1.
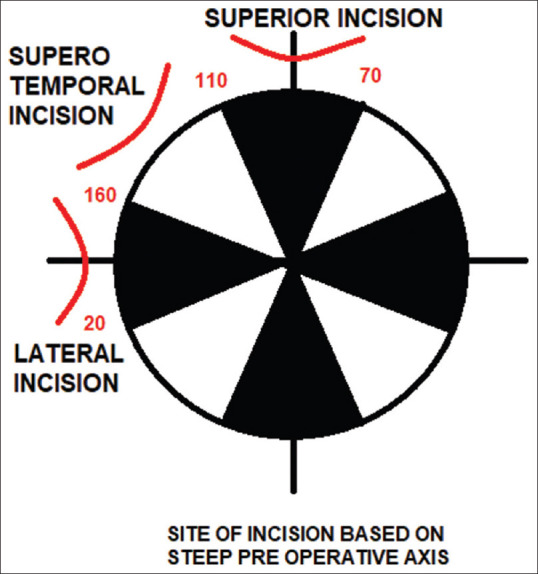
Astigmatism in SICS according to the location of the incision
However, the manual SICS technique from the temporal side has a learning curve as compared to superior incision SICS or conventional ECCE with sutures because it is a different site of the incision.[45] Tunnel construction can have difficulties like premature entry or long corneal tunnel causing decreased visibility; fixation of the eyeball is difficult as there is no fixation suture and inserting two instruments simultaneously for sandwich technique, i.e., vectis and dialer, is challenging for beginners.[45] Other potential pitfalls of the temporal section are oozing during incision construction as the area has a rich vascular supply and increased chances of endophthalmitis as the wound site is exposed to the exterior.[46] However, the absence of a single case of endophthalmitis was reported by Zawar et al.[26] in the immediate postoperative period of 6 weeks, demonstrates the safety of this technique.[45] Studies advocate that surgeons, so long as they are comfortable operating both superiorly and inferiorly, it is better to choose the steep meridian to make the scleral incision to reduce pre-existing astigmatism.
Incision location
According to Haldipurkar et al.,[19] “more the distance of the sclerocorneal incision from the limbus (on sclera), less is the induced astigmatism although tunnel making and maneuverability are difficult in the former” [Fig. 2]. They have suggested the ideal distance of the anterior limit to be around 1–2 mm from the limbus. Archana et al.[30] have proved that SIA is significantly higher in clear corneal manual SICS than in sclerocorneal incisions of 6 mm in length. Olsen et al.[21] also found the amount of astigmatic change to be almost twice as large after a corneal incision than after a scleral incision. Thus, a sclerocorneal tunnel incision 3 mm posterior to the surgical limbus is the ideal choice for MSICS where the incision size is planned to be more than 6 mm.[31]
Figure 2.
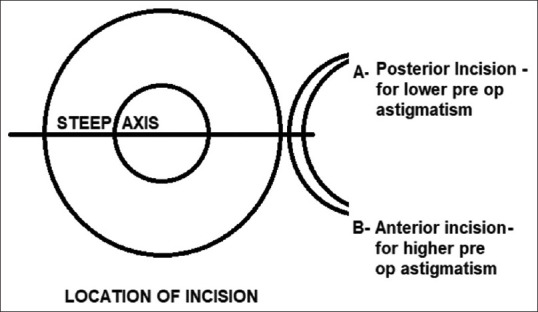
Site of incision based on preoperative astigmatism
Incision shape
The shape of the incision, i.e., an external configuration may either be straight or curved [Fig. 3]. Paul Koch described the incisional funnel as indicating the astigmatic neutral zone.[47] Pallin in 1990 described a chevron-shaped (inverted V) incision, the apex towards the limbus and the limbs are away from it. Though difficult to make, it induces the least astigmatism.[32] In 1991, Singer introduced a frown incision, where each end of the incision is further away from the limbus and produced less astigmatism but slightly more than chevron’s incision. Akura et al.[28] also achieved astigmatic neutrality by frown incision in MSICS with a large self-sealing incision. It was a modified pocket incision curved opposite to the limbus.[33] Blumenthal et al.[48] in 1993 devised a larger pocket tunnel with minimally induced astigmatism called the Blumenthal side cuts. The incision has a straight line and two oblique cuts at its two ends. Jauhari et al.[34] in a prospective comparative study, compared three scleral groove shapes and found the frown and inverted-V incision to have the least amount of astigmatism when compared to the linear incision, and also the chevron-shaped incision (inverted-V) has also been reported to give minimal SIA when compared with straight and frown incisions. Burgansky Z et al.[49] also conclusively proved that enlarging the size of the chevron incision up to 7.0 mm for simplification of operative technique resulted in a small increase in induced astigmatism.[11]
Figure 3.

Shape of incision in manual SICS
Incision length or size
The length of the sclerocorneal incision is not the length along the curvature but the distance between the two ends of the incision and generally varies from 5 to 6 mm for cortical cataracts, and from 7 to 8 mm for nuclear cataracts depending on the size of the nucleus [Fig. 4].[19] Sinskey in 1994 suggested that the 6.0 mm no-stitch frown incision induces low postoperative astigmatism and provides a stable incision.[35] Small-incisions (6 mm) induced the smallest SIA when compared by Burgansky et al.[49] with medium (6.5 mm) and large (7 mm) incisions. They conclusively proved that enlarging the size of the chevron incision up to 7.0 mm for surgical convenience resulted in a small increase in induced astigmatism. Ruit et al.[2] described the straight incision with 6.5–7 mm scleral tunnel 1.5–2 mm posterior to the limbus induced less astigmatism. A 2 mm MSICS with phacofracture can deliver low astigmatism and good visual recovery in cataract surgery.[36] A prospective trial in Japan comparing 3.2-mm incisions of phacoemulsification with 5.5-mm incisions had found a difference in astigmatism of 0.3D.[50]
Figure 4.
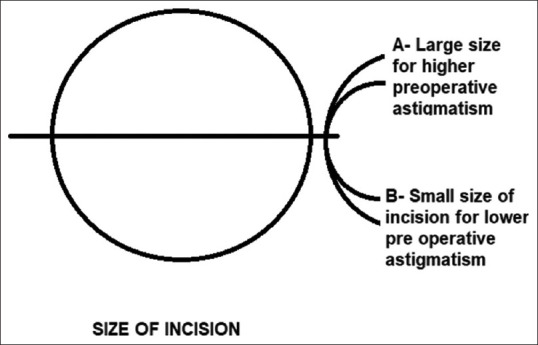
Size of incision and astigmatism in SICS
Incision width
The width of the tunnel is the distance between the external scleral incision and the internal corneal entry incision [Fig. 5] and according to Girard should be at least 4 mm in size.[31] A sclerocorneal tunnel incision, of at least 1–2 mm into the clear cornea, leads to a self-sealing wound with perfect wound integrity which can prevent astigmatic drift in the postoperative period.[19]
Figure 5.
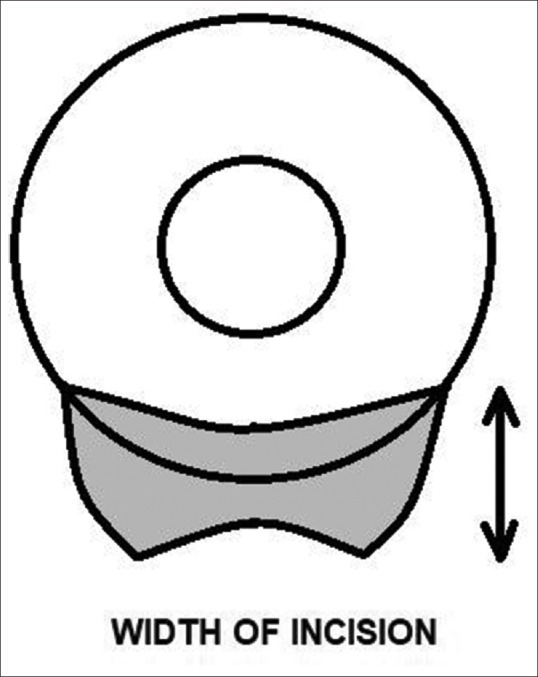
Width of sclerocorneal section
Incision depth
Optimal incision depth is described to be one-half to one-fourth the thickness of the sclera or about 0.3 mm and the scleral flap should neither be too thick nor too thin.[37] Multiple published literature has pointed out that all components of tunnel construction have an effect on the change in astigmatism after MSICS; however, Anders et al.[38] reported that the depth of the incision had no significant effect on induced astigmatism, but a study published by Pattanayak et al.[39] comparing superficial (≤399 mm) vs deep scleral incision (≥ 400 mm) revealed that the depth of sclerocorneal incision had a statistically significant effect on the change of astigmatism following manual SICS, with superficial incision causing a higher change than the deeper incision. Thus, to correct higher preoperative astigmatism, a superficial incision is to be planned than in cases with a lower degree of astigmatism or no astigmatism.
Use of sutures
In cases of poor wound construction requiring sutures for closure, X-pattern sutures were preferred to the horizontal sutures in the patients without significant preoperative steepening in line with the central meridian of the incision. But in cases with significant preoperative steepening, sutureless surgery or horizontal sutures were preferred.[40] But Goel et al.[51] refuted the above observation based on improper case selection, and inappropriate comparison in the study.
Conclusion
MSICS offers similar advantages to phacoemulsification and because of its less surgical time, low cost, minimum complications, and wider applicability, it is more popular in developing and underdeveloped countries, where high-volume surgery is the norm as a huge number of avoidable blindness due to cataract prevailing in those countries. Though it is often discredited for induced astigmatism still using minor modifications like a smaller frown or chevron incisions located either temporally or on the steeper axis away from the limbus, the postoperative astigmatism can be reduced to a great extent, thereby improving the uncorrected visual acuity of patients following cataract surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Venkatesh R, Chang DF, Muralikrishnan R, Hemal K, Gogate P, Sengupta S. Manual small incision cataract surgery:A review. Asia-Pac J Ophthalmol. 2012;1:113–9. doi: 10.1097/APO.0b013e318249f7b9. [DOI] [PubMed] [Google Scholar]
- 2.Ruit S, Tabin G, Chang D, Bajracharya L, Kline DC, Richheimer W, et al. A prospective randomized clinical trial of phacoemulsification vs manual sutureless small-incision extracapsular cataract surgery in Nepal. Am J Ophthalmol. 2007;143:32–8.e2. doi: 10.1016/j.ajo.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Pershing S, Kumar A. Phacoemulsification versus extracapsular cataract extraction:Where do we stand? Curr Opin Ophthalmol. 2011;22:37–42. doi: 10.1097/ICU.0b013e3283414fb3. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh R, Tan CSH, Sengupta S, Ravindran RD, Krishnan KT, Chang DF. Phacoemulsification versus manual small-incision cataract surgery for white cataract. J Cataract Refract Surg. 2010;36:1849–54. doi: 10.1016/j.jcrs.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Gogate P, Deshpande M, Nirmalan PK. Why do phacoemulsification?Manual small-incision cataract surgery is almost as effective, but less expensive. Ophthalmology. 2007;114:965–8. doi: 10.1016/j.ophtha.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 6.Yorston D, Gichuhi S, Wood M, Foster A. Does prospective monitoring improve cataract surgery outcomes in Africa? Br J Ophthalmol. 2002;86:543–7. doi: 10.1136/bjo.86.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogate PM, Kulkarni SR, Krishnaiah S, Deshpande RD, Joshi SA, Palimkar A, et al. Safety and efficacy of phacoemulsification compared with manual small-incision cataract surgery by a randomized controlled clinical trial:Six-week results. Ophthalmology. 2005;112:869–74. doi: 10.1016/j.ophtha.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 8.Muralikrishnan R, Venkatesh R, Venkatesh Prajna N, Kevin Frick. Economic cost of cataract surgery procedures in an established eye care centre in Southern India. Ophthalmic Epidemiol. 2004;11:369–80. doi: 10.1080/09286580490888762. [DOI] [PubMed] [Google Scholar]
- 9.Reddy B, Raj A, Singh VP. Site of incision and corneal astigmatism in conventional SICS versus phacoemulsification. Ann Ophthalmol. 2007;39:209–16. doi: 10.1007/s12009-007-0020-y. [DOI] [PubMed] [Google Scholar]
- 10.Colvard DM, Kratz RP, Mazzocco TR, Davidson B. Clinical evaluation of the Terry surgical keratometer. Am Intra-Ocul Implant Soc J. 1980;6:249–51. doi: 10.1016/s0146-2776(80)80071-1. [DOI] [PubMed] [Google Scholar]
- 11.McFarland M. Small Incision Cataract Surgery, Foldable Lenses, One Stitch Surgery Sutureless Surgery. Thorofare, N. J.:Slack. 1990:107–16. [Google Scholar]
- 12.Bradbury JA, Hillman JS, Cassells-Brown A. Optimal postoperative refraction for good unaided near and distance vision with monofocal intraocular lenses. Br J Ophthalmol. 1992;76:300–2. doi: 10.1136/bjo.76.5.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alpins NA, Goggin M. Practical astigmatism analysis for refractive outcomes in cataract and refractive surgery. Surv Ophthalmol. 2004;49:109–22. doi: 10.1016/j.survophthal.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Huang FC, Tseng SH. Comparison of surgically induced astigmatism after sutureless temporal clear corneal and scleral frown incisions. J Cataract Refract Surg. 1998;24:477–81. doi: 10.1016/s0886-3350(98)80287-3. [DOI] [PubMed] [Google Scholar]
- 15.Naeser K. Conversion of keratometer readings to polar values. J Cataract Refract Surg. 1990;16:741–5. doi: 10.1016/s0886-3350(13)81018-8. [DOI] [PubMed] [Google Scholar]
- 16.Jaffe NS, Clayman HM. The pathophysiology of corneal astigmatism after cataract. Trans Am Acad Ophthalmol Otolaryngol. 1975;79:1615–30. [Google Scholar]
- 17.Nanevicz TM, Prince MR, Gawande AA, Puliafito CA. Excimer laser ablation of the lens. Arch Ophthalmol. 1986;104:1825–9. doi: 10.1001/archopht.1986.01050240099048. [DOI] [PubMed] [Google Scholar]
- 18.Muralikrishnan R, Venkatesh R, Manohar BB, Prajna NV. A comparison of the effectiveness and cost effectiveness of three different methods of cataract extraction in relation to the magnitude of postoperative astigmatism. Asia Pacific J Ophthalmol. 2003;15:5–12. [Google Scholar]
- 19.Haldipurkar SS, Shikari HT, Gokhale V. Wound construction in manual small incision cataract surgery. Indian J Ophthalmol. 2009;57:9–13. doi: 10.4103/0301-4738.44491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malik V, Kumar S, Kamboj R, Jain C, Jain K, Kumar S. Comparison of astigmatism following manual small incision cataract surgery:Superior versus temporal approach. Nepal J Ophthalmol. 2012;4:54–8. doi: 10.3126/nepjoph.v4i1.5851. [DOI] [PubMed] [Google Scholar]
- 21.Olsen T, Dam-Johansen M, Bek T, Hjortdal JØ. Corneal versus scleral tunnel incision in cataract surgery:A randomized study. J Cataract Refract Surg. 1997;23:337–41. doi: 10.1016/s0886-3350(97)80176-9. [DOI] [PubMed] [Google Scholar]
- 22.Ernest PH, Neuhann T. Posterior limbal incision. J Cataract Refract Surg. 1996;22:78–84. doi: 10.1016/s0886-3350(96)80274-4. [DOI] [PubMed] [Google Scholar]
- 23.Kimura H, Kuroda S ichiro, Mizoguchi N, Terauchi H, Matsumura M, Nagata M. Extracapsular cataract extraction with a sutureless incision for dense cataracts. J Cataract Refract Surg. 1999;25:1275–9. doi: 10.1016/s0886-3350(99)00148-0. [DOI] [PubMed] [Google Scholar]
- 24.Gokhale N, Sawhney S. Reduction in astigmatism in manual small incision cataract surgery through change of incision site. Indian J Ophthalmol. 2005;53:201. doi: 10.4103/0301-4738.16684. [DOI] [PubMed] [Google Scholar]
- 25.Ayena KD, Billong J, Vonor K, Diallo JW, Santos MAK, Maneh N, et al. Assessment of astigmatism in manual and sutureless small incision cataract surgery. New Front Ophthalmol. 2015 doi:10.15761/NFO.1000105. [Google Scholar]
- 26.Arthur E, Sadik AA, Kumah DB, Osae EA, Mireku FA, Asiedu FY, et al. Postoperative corneal and surgically induced astigmatism following superior approach manual small incision cataract surgery in patients with preoperative against-the-rule astigmatism. J Ophthalmol. 2016;2016:1–7. doi: 10.1155/2016/9489036. doi:10.1155/2016/9489036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawar VS, Sindal DK. A comparative study on the superior, supero-temporal and the temporal inci- sions in small incision cataract surgeries for post operative astigmatism. J Clin Diagn Res. 2012;6:1229–32. [Google Scholar]
- 28.Akura J, Kaneda S, Hatta S, Matsuura K. Controlling astigmatism in cataract surgery requiring relatively large self-sealing incisions. J Cataract Refract Surg. 2000;26:1650–9. doi: 10.1016/s0886-3350(00)00484-3. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen PJ. Prospective evaluation of surgically induced astigmatism and astigmatic keratotomy effects of various self-sealing small incisions. J Cataract Refract Surg. 1995;21:43–8. doi: 10.1016/s0886-3350(13)80478-6. [DOI] [PubMed] [Google Scholar]
- 30.Archana S, Khurana A, Chawla U. A comparative study of sclerocorneal and clear corneal tunnel incision in manual small-incision cataract surgery. Nepal J Ophthalmol. 1970;3:19–22. doi: 10.3126/nepjoph.v3i1.4273. [DOI] [PubMed] [Google Scholar]
- 31.Girard LJ. Origin of the scleral tunnel incision. J Cataract Refract Surg. 1995;21:6–7. doi: 10.1016/s0886-3350(13)80470-1. [DOI] [PubMed] [Google Scholar]
- 32.Pallin SL. Chevron sutureless closure:A preliminary report. J Cataract Refract Surg. 1991;17((Suppl 1)):706–9. doi: 10.1016/s0886-3350(13)80687-6. [DOI] [PubMed] [Google Scholar]
- 33.Singer JA. Frown incision for minimizing induced astigmatism after small incision cataract surgery with rigid optic intraocular lens implantation. J Cataract Refract Surg. 1991;17((Suppl 1)):677–88. doi: 10.1016/s0886-3350(13)80683-9. [DOI] [PubMed] [Google Scholar]
- 34.Jauhari N, Chopra D, Chaurasia RK, Agarwal A. Comparison of surgically induced astigmatism in various incisions in manual small incision cataract surgery. Int J Ophthalmol. 2014;7:1001–4. doi: 10.3980/j.issn.2222-3959.2014.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinskey RM, Stoppel JO. Induced astigmatism in a 6.0 mm no-stitch frown incision. J Cataract Refract Surg. 1994;20:406–9. doi: 10.1016/s0886-3350(13)80175-7. [DOI] [PubMed] [Google Scholar]
- 36.Sahu A, Bali J, Sahu C, Mishra D, Heda A, Deori N. Early postoperative astigmatism in 2-mm manual small incision cataract surgery with phacofracture. Indian J Ophthalmol. 2022;70:1997–2001. doi: 10.4103/ijo.IJO_308_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basti S, Vasavada AR, Thomas R, Padhmanabhan P. Extracapsular cataract extraction:Surgical techniques. Indian J Ophthalmol. 1993;41:195. [PubMed] [Google Scholar]
- 38.Anders N, Pham DT, Antoni HJ, Wollensak J. Postoperative astigmatism and relative strength of tunnel incisions:A prospective clinical trial. J Cataract Refract Surg. 1997;23:332–6. doi: 10.1016/s0886-3350(97)80175-7. [DOI] [PubMed] [Google Scholar]
- 39.Pattanayak S, Nanda AK, Swain AK. Effect of depth of the sclerocorneal incision on postoperative corneal astigmatism in manual small-incision cataract surgery. Indian J Ophthalmol. 2022;70:1612–6. doi: 10.4103/ijo.IJO_2649_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eslami Y, Mirmohammadsadeghi A. Comparison of surgically induced astigmatism between horizontal and X-pattern sutures in the scleral tunnel incisions for manual small incision cataract surgery. Indian J Ophthalmol. 2015;63:606–10. doi: 10.4103/0301-4738.167113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogate PM, Deshpande M, Wormald RP, Deshpande R, Kulkarni SR. Extracapsular cataract surgery compared with manual small incision cataract surgery in community eye care setting in western India:A randomised controlled trial. Br J Ophthalmol. 2003;87:667–72. doi: 10.1136/bjo.87.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkatesh R, Muralikrishnan R, Balent LC, Prakash SK, Prajna NV. Outcomes of high volume cataract surgeries in a developing country. Br J Ophthalmol. 2005;89:1079–83. doi: 10.1136/bjo.2004.063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyle WM. Changes in corneal astigmatism with age*. Optom Vis Sci. 1971;48:467–78. doi: 10.1097/00006324-197106000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Brian G, Taylor H. Cataract blindness - challenges for the 21st century. Bull World Health Organ. 2001;79:249–56. [PMC free article] [PubMed] [Google Scholar]
- 45.Zawar SV, Gogate P. Safety and efficacy of temporal manual small incision cataract surgery in India. Eur J Ophthalmol. 2011;21:748–53. doi: 10.5301/EJO.2011.6521. [DOI] [PubMed] [Google Scholar]
- 46.Nagaki Y, Hayasaka S, Kadoi C, Matsumoto M, Yanagisawa S, Watanabe K, et al. Bacterial endophthalmitis after small-incision cataract surgery:Effect of incision placement and intraocular lens type. J Cataract Refract Surg. 2003;29:20–6. doi: 10.1016/s0886-3350(02)01483-9. [DOI] [PubMed] [Google Scholar]
- 47.Koch PS. Mastering Phacoemulsification:A Simplified Manual of Strategies for the Spring, Crack and Stop and Chop Technique. Thorofare (N. J.) :Slack. 1994 [Google Scholar]
- 48.Blumenthal M, Ashkenazi I, Fogel R, Assia EI. The gliding nucleus. J Cataract Refract Surg. 1993;19:435–7. doi: 10.1016/s0886-3350(13)80322-7. [DOI] [PubMed] [Google Scholar]
- 49.Burgansky Z, Isakov I, Avizemer H, Bartov E. Minimal astigmatism after sutureless planned extracapsular cataract extraction. J Cataract Refract Surg. 2002;28:499–503. doi: 10.1016/s0886-3350(01)01263-9. [DOI] [PubMed] [Google Scholar]
- 50.Oshika T, Nagahara K, Yaguchi S, Emi K, Takenaka H, Tsuboi S, et al. Three year prospective, randomized evaluation of intraocular lens implantation through 3.2 and 5.5 mm incisions. J Cataract Refract Surg. 1998;24:509–14. doi: 10.1016/s0886-3350(98)80293-9. [DOI] [PubMed] [Google Scholar]
- 51.Goel R, Nagpal S, Malik KPS, Sanoria A. Comparison of surgically induced astigmatism between horizontal and X-pattern sutures in the scleral tunnel incisions for manual small incision cataract surgery. Indian J Ophthalmol. 2016;64:328. doi: 10.4103/0301-4738.182953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.George R, Rupauliha P, Sripriya AV, Rajesh PS, Vahan PV, Praveen S. Comparison of endothelial cell loss and surgically induced astigmatism following conventional extracapsular cataract surgery, manual small-incision surgery and phacoemulsification. Ophthalmic Epidemiol. 2005;12:293–7. doi: 10.1080/09286580591005778. [DOI] [PubMed] [Google Scholar]


