This systematic review and meta-analysis assesses the outcomes of 3 types of substitution for sugar-sweetened beverages in adults with overweight or obesity and with or without diabetes.
Key Points
Question
Are low- and no-calorie sweetened beverages (LNCSBs) as the intended substitute for sugar-sweetened beverages (SSBs) associated with improved body weight and cardiometabolic risk factors similar to water replacement?
Findings
In this systematic review and meta-analysis of 17 randomized clinical trials, LNCSBs as a substitute for SSBs were associated with reduced body weight, body mass index, percentage of body fat, and intrahepatocellular lipid, providing benefits that were similar to those of water, the standard-of-care substitution.
Meaning
The findings of this study suggest that over the moderate term, LNCSBs are a viable alternative to water as a replacement strategy in adults with overweight or obesity who are at risk for or have diabetes.
Abstract
Importance
There are concerns that low- and no-calorie sweetened beverages (LNCSBs) do not have established benefits, with major dietary guidelines recommending the use of water and not LNCSBs to replace sugar-sweetened beverages (SSBs). Whether LNCSB as a substitute can yield similar improvements in cardiometabolic risk factors vs water in their intended substitution for SSBs is unclear.
Objective
To assess the association of LNCSBs (using 3 prespecified substitutions of LNCSBs for SSBs, water for SSBs, and LNCSBs for water) with body weight and cardiometabolic risk factors in adults with and without diabetes.
Data Sources
Medline, Embase, and the Cochrane Central Register of Controlled Trials were searched from inception through December 26, 2021.
Study Selection
Randomized clinical trials (RCTs) with at least 2 weeks of interventions comparing LNCSBs, SSBs, and/or water were included.
Data Extraction and Synthesis
Data were extracted and risk of bias was assessed by 2 independent reviewers. A network meta-analysis was performed with data expressed as mean difference (MD) or standardized mean difference (SMD) with 95% CIs. The GRADE (Grading of Recommendations Assessment, Development and Evaluation) system was used to assess the certainty of the evidence.
Main Outcomes and Measures
The primary outcome was body weight. Secondary outcomes were other measures of adiposity, glycemic control, blood lipids, blood pressure, measures of nonalcoholic fatty liver disease, and uric acid.
Results
A total of 17 RCTs with 24 trial comparisons were included, involving 1733 adults (mean [SD] age, 33.1 [6.6] years; 1341 women [77.4%]) with overweight or obesity who were at risk for or had diabetes. Overall, LNCSBs were a substitute for SSBs in 12 RCTs (n = 601 participants), water was a substitute for SSBs in 3 RCTs (n = 429), and LNCSBs were a substitute for water in 9 RCTs (n = 974). Substitution of LNCSBs for SSBs was associated with reduced body weight (MD, −1.06 kg; 95% CI, −1.71 to –0.41 kg), body mass index (MD, −0.32; 95% CI, −0.58 to –0.07), percentage of body fat (MD, −0.60%; 95% CI, −1.03% to –0.18%), and intrahepatocellular lipid (SMD, −0.42; 95% CI, −0.70 to –0.14). Substituting water for SSBs was not associated with any outcome. There was also no association found between substituting LNCSBs for water with any outcome except glycated hemoglobin A1c (MD, 0.21%; 95% CI, 0.02% to 0.40%) and systolic blood pressure (MD, −2.63 mm Hg; 95% CI, −4.71 to −0.55 mm Hg). The certainty of the evidence was moderate (substitution of LNCSBs for SSBs) and low (substitutions of water for SSBs and LNCSBs for water) for body weight and was generally moderate for all other outcomes across all substitutions.
Conclusions and Relevance
This systematic review and meta-analysis found that using LNCSBs as an intended substitute for SSBs was associated with small improvements in body weight and cardiometabolic risk factors without evidence of harm and had a similar direction of benefit as water substitution. The evidence supports the use of LNCSBs as an alternative replacement strategy for SSBs over the moderate term in adults with overweight or obesity who are at risk for or have diabetes.
Introduction
Sugar consumption has emerged as an important public health concern. The evidence on this concern derives largely from consumption of sugar-sweetened beverages (SSBs), with excess intake of SSBs associated with weight gain and downstream cardiometabolic complications.1,2,3,4 Sugar-sweetened beverages have been identified as an important public health target.5,6 It is unclear whether low- and no-calorie sweetened beverages (LNCSBs) as a replacement strategy for SSBs provide the intended benefits. Recent systematic reviews and meta-analyses7 have shown an association between LNCSBs and a higher risk of the conditions that they are intended to prevent, such as weight gain, diabetes, and cardiovascular disease, in prospective cohort studies8 and have reported inconsistent findings for weight loss and improvements in downstream cardiometabolic risk factors in randomized clinical trials (RCTs).7,8 Biological mechanisms involving impaired sensory and endocrine signaling that was mediated by the sweet taste receptor9,10 and changes to the microbiome10,11 have been implicated in support of these observations.
Methodological considerations, however, have been raised that limit the inferences that can be drawn from these data. The available prospective cohort studies are at high risk for reverse causality.12,13,14 Furthermore, the syntheses of RCTs do not fully account for the calories available to be displaced by LNCSBs, with caloric (eg, SSBs) and noncaloric (eg, water and placebo) comparators that are pooled together or with noncaloric comparators that are used as the sole comparator, leading to an underestimation of the outcome of LNCSBs.12,13,14
The prevailing uncertainties have led to mixed recommendations from authoritative bodies. Neither the Dietary Guidelines for Americans nor Canada’s Food Guide supports the use of LNCSBs, and instead both recommend replacing SSBs with water.5,6 The American Heart Association supports a narrow indication for LNCSBs, recommending that LNCSBs should be used as a replacement by only adults who are habitual consumers of SSBs, but emphasizing the use of water or an unsweetened alternative.15 Similarly, diabetes associations in the UK, US, and Canada support LNCSBs insofar as they are used to displace calories from sugars and SSBs.16,17,18 The European Association for the Study of Diabetes has not made any specific recommendations about low- and no-calorie sweeteners (LNCSs) or LNCSBs.19 To update the recommendations of the European Association for the Study of Diabetes, the Diabetes and Nutrition Study Group commissioned the present systematic review and meta-analysis to summarize the evidence from RCTs of the association of LNCSBs, the most important source of LNCSs in a diet and a single food matrix, with intermediate cardiometabolic outcomes.20 Because of the importance of the comparator in drawing inferences about LNCSBs, we conducted network meta-analyses rather than traditional pairwise meta-analyses to assess the association of LNCSBs with body weight and cardiometabolic risk factors in adults with and without diabetes. We used 3 prespecified substitutions: LNCSBs for SSBs (intended substitution with caloric displacement), water for SSBs (standard-of-care substitution with caloric displacement), and LNCSBs for water (reference substitution without caloric displacement).
Methods
This systematic review and network meta-analysis was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions21 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.22 The protocol is registered at ClinicalTrials.gov (NCT02879500).
Data Sources, Searches, and Study Selection
We searched Medline, Embase, and the Cochrane Central Register of Controlled Trials from inception through December 26, 2021. Briefly, for this search, we used variations of the exposure terms (LNCSBs and SSBs), outcome terms (adiposity, glycemia, blood lipids, blood pressure [BP], nonalcoholic fatty liver disease [NAFLD], and uric acid), and study design terms (randomized controlled trial, randomized, and placebo). The full search strategy is presented in eTables 1 to 3 in the Supplement. Manual searches of the reference lists of included studies and reviews were also performed.
eTable 4 in the Supplement shows the PICOTS (Population, Intervention, Comparator, Outcome, Time, and Study) framework.22 We included RCTs of at least 2 weeks that investigated the association of LNCSBs, SSBs, and/or water with cardiometabolic risk factors. We excluded trials that had multimodal interventions, did not use comparator groups containing at least 1 of the other beverage interventions, included children and pregnant or breastfeeding women, or did not provide viable outcome data. Trials of LNCSs in fortified or nutrient-dense beverages (eg, milk and juice) were also excluded because of the presence of other nutrients.
Data Extraction, Risk of Bias Assessment, and Outcomes
Two independent reviewers (N.D.M. and R.Z.) extracted relevant data from each included report (eMethods in the Supplement). Additional information was requested from study authors when necessary. Race and ethnicity data were not collected because the available data were not presented by these variables.
The same independent reviewers (N.D.M. and R.Z.) assessed risk of bias for each included RCT using the Cochrane risk-of-bias tool.23 Five domains of bias were assessed: sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, and selective reporting. Disagreements between the reviewers were resolved by consensus.
The primary outcome was body weight. Secondary outcomes were other measures of adiposity (body mass index [BMI], which was calculated as weight in kilograms divided by height in meters squared; percentage of body fat; and waist circumference), glycemic control (glycated hemoglobin A1c [HbA1c], fasting plasma glucose, 2-hour postprandial glucose during a 75-g oral glucose tolerance test, fasting plasma insulin [FPI], and homeostatic model assessment of insulin resistance), blood lipids (low-density lipoprotein cholesterol, non–high-density lipoprotein cholesterol, triglycerides, high-density lipoprotein cholesterol, and total cholesterol), BP (systolic BP and diastolic BP), measures of NAFLD (intrahepatocellular lipid [IHCL], alanine aminotransferase, and aspartate aminotransferase), and uric acid. Change differences were preferred over end differences. Missing variance data were calculated using established formulas.21
Data Synthesis and Grading the Evidence
This network meta-analysis was based on a frequentist framework and was conducted using the network suite of commands in Stata, version 15 (StataCorp LLC). We used change from baseline values from each study to calculate the mean differences (MDs) between treatments for each substitution (LNCSBs for SSBs, water for SSBs, and LNCSBs for water); otherwise, we used postintervention values (eMethods and eData 1-20 in the Supplement). We performed random-effects network meta-analyses for each outcome to compare the 3 interventions (LNCSBs, SSBs, and water) simultaneously. Inconsistency was assessed in the direct, indirect, and network estimates. We assessed interstudy heterogeneity in the direct (pairwise) estimates using the Cochran Q statistic with quantification by the I2 statistic, where I2 ≥50% and P < .10 were considered to be substantial interstudy heterogeneity. We measured incoherence in the network estimates using both local (loop-specific and side-splitting) and global (design-by-treatment interaction model) approaches.24,25,26 If 10 or more trials were available, we conducted a priori subgroup analyses by age, study duration, type of design, disease status, risk of bias, and funding source. Indirectness was assessed in the indirect comparisons by evaluation of intransitivity across the pairwise comparisons comprising the indirect estimates for the study characteristics of age, study length, sample size, and percentage of male participants. Publication bias was assessed if 10 or more trial comparisons were available; we used comparison-adjusted funnel plots to assess funnel plot asymmetry.24
We assessed the certainty of the evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.20,27,28,29,30 Network estimates of RCTs and the direct and indirect estimates that composed these network estimates started at a high certainty of evidence but were downgraded by established criteria for risk of bias, inconsistency (incoherence), indirectness, imprecision, and publication bias (eMethods in the Supplement).
Results
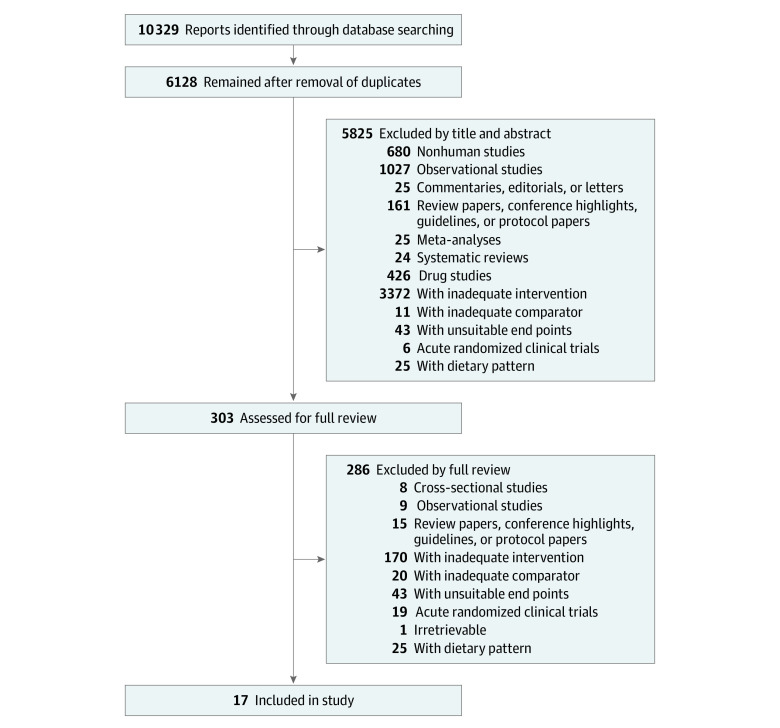
Figure 1 shows the flow of the literature search and selection, and eFigures 27 to 46 in the Supplement show the network diagram for each outcome. We identified 4541 reports, of which 13 met the eligibility criteria. An additional 4 reports were found through manual searching. A total of 17 RCTs with 24 trial comparisons were included that assessed the association of the 3 prespecified substitutions with body weight, other measures of adiposity, and cardiometabolic risk.31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 These RCTs involved 1733 adult participants (mean [SD] age, 33.1 [6.6] years; 1341 women [77.4%] and 392 men [22.6%]) with overweight or obesity who were at risk for or had diabetes.
Figure 1. Literature Search for Randomized Clinical Trials of Low- and No-Calorie Sweetened Beverages .
The Table and eTable 5 in the Supplement provide key trial characteristics.31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 Overall, the RCTs had a medium sample size, with a median (range) number of 72 (27-308) participants, and involved more women than men (23% men to 77% women). Most participants were younger (median [range] age, 34 [23-48] years) and had overweight or obesity (median [range] BMI, 31 [22-36]), with 9 trials31,32,33,34,35,36,38,39,40,41,42,44,45,46 that included only participants with overweight and/or obesity and 1 trial40 that included participants with type 2 diabetes.
Table. Trial Characteristics.
| Source and country | Total No. of participants | Population | Age, mean (SD), y | No. of participants by sex (%) | LNCS type | Beverage dosage, mL/d | Design | Duration, wk | Funding source | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LNCSB | Water | SSB | |||||||||
| Bonnet et al,31 2018; France | 50 | Overweight or healthy weight, otherwise healthy, non- or low-LNCS consumers | 31.1 (10.3) |
|
Aspartame or acesulfame potassium | 660 | 660 | NA | Crossover | 12 | Agency or industry |
| Bruun et al,32 2015; Denmarka | 35 | Overweight or obese, otherwise healthy | 39 (1.1) |
|
Aspartame | 1000 | 1000 | 1000 | Parallel | 26 | Agency or industry |
| Campos et al,34 2015; Switzerland | 27 | Overweight or obese, otherwise healthy, regular SSB consumers | NR |
|
NR | 1300 | NA | 1300 | Parallel | 12 | Agency |
| Ebbeling et al,33 2020; US | 203 | Overweight or obese, otherwise healthy, regular SSB consumers | 27 (5.6) |
|
NR | 355 | 355 | 355 | Parallel | 52 | Agency |
| Engel et al,35 2018; Denmarka | 45 | Overweight or obese, otherwise healthy | 38.6 (7.6) |
|
Aspartame | 1000 | 1000 | 1000 | Parallel | 26 | Agency or industry |
| Hernández-Cordero et al,36 2014; Mexico | 240 | Overweight or obese, otherwise healthy, regular SSB consumers | 33.3 (6.7) |
|
NR | NA | ≥250 | ≥250 | Parallel | 39 | Industry |
| Higgins et al,37 2018; US | 93 | Healthy weight, healthy, non- or low-LNCS consumers | 22.9 (1.0) |
|
Aspartame | 500 | 500 | NA | Parallel | 12 | Industry |
| Higgins and Mattes,38 2019; US | 154 | Overweight or obese, otherwise healthy, non- or low-LNCS consumers | 27.3 (9.6) |
|
Saccharin, aspartame, rebaudioside A, or sucralose | 1250-1750 | NA | 1250-1750 | Parallel | 12 | Agency |
| Madjd et al,39 2015; Iran | 62 | Overweight or obese, otherwise healthy, regular LNCSB consumers | 32 (6.9) |
|
NR | ≥250 | ≥250 | NA | Parallel | 24 | Agency |
| Madjd et al,40 2017; Iran | 81 | Obese, with type 2 diabetes (only on metformin to control diabetes), regular LNCSB consumers | 34.8 (7.2) |
|
NR | ≥250 | ≥250 | NA | Parallel | 24 | Agency |
| Maersk et al,41 2012; Denmark | 35 | Overweight or obese, otherwise healthy | 39 (26) |
|
Aspartame | 1000 | 1000 | 1000 | Parallel | 26 | Agency or industry |
| Peters et al,42 2016; US | 308 | Overweight or obese, otherwise healthy, weight stable, regular LNCSB consumers | 47.8 (10.5) |
|
NR | 710 | 710 | NA | Parallel | 52 | Industry |
| Reid et al,43 2007; England | 133 | Healthy weight, weight watchers and nonweight watchers | 31.8 (9.1) |
|
Aspartame | 1000 | NA | 1000 | Parallel | 4 | Agency |
| Reid et al,44 2010; Scotland | 53 | Overweight, otherwise healthy | 33.7 (9.9) |
|
Aspartame | 1000 | NA | 1000 | Parallel | 4 | Agency |
| Reid et al,45 2014; Scotland | 41 | Obese, otherwise healthy | 35 (9.1) |
|
Aspartame | 1000 | NA | 1000 | Parallel | 4 | Agency or industry |
| Tate et al,46 2012; US | 213 | Overweight or obese, otherwise healthy, regular SSB consumers | 42 (10.7) |
|
NR | 1420-2000 | 1420-2000 | NA | Parallel | 26 | Industry |
| Tordoff and Alleva,47 1990; US | 30 | Healthy weight, healthy | 25.6 (5.3) |
|
Aspartame | 1135 | NA | 1135 | Crossover | 3 | Agency |
Abbreviations: LNCS, low- and no-calorie sweetener; LNCSB, low- and no-calorie sweetened beverage; NA, not applicable; NR, not reported; SSB, sugar-sweetened beverage.
Only 8 trials (11 comparisons)31,32,35,37,38,43,44,45,47 reported the type of LNCS used in the LNCSBs: 7 comparisons for aspartame and 1 comparison each for aspartame and acesulfame potassium blend, saccharin, rebaudioside A, and sucralose. Overall, LNCSBs were a substitute for SSBs in 12 trials (n = 601 participants),33,34,35,38,43,44,45,47 water was a substitute for SSBs in 3 trials (n = 429),33,35,36,41 and LNCSBs were a substitute for water in 9 trials (n = 974).31,33,35,37,39,40,42,46 The median (range) dosages were 1000 (250-2000) mL per day for LNCSBs, 1000 (250-1750) mL per day for SSBs, and 580 (250- 2000) mL per day for water.
Fifteen trials32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 had a parallel design, and 2 trials31,47 had a crossover design. Most RCTs were conducted in Europe (n = 8) and North America (n = 6). The median (range) duration of follow-up was 12 (3-52) weeks. Eight trials33,34,38,39,40,43,44,47 were funded by agencies (government, not-for-profit health agency, or university sources), 4 trials36,37,42,46 were funded by industry, and 5 trials31,32,35,41,45 were funded by a combination of agency and industry. We contacted the authors of 7 studies31,32,34,35,38,44,45 for additional data, and the authors of 2 studies34,38 provided additional data.
eFigures 1 and 2 in the Supplement provide the Cochrane risk-of-bias tool assessments. Eight trial comparisons32,34,35,37,41,43,44,47 received an unclear risk-of-bias rating, and 11 comparisons31,33,36,38,39,40,42,46 were rated as having a low risk of bias. No RCTs were identified as having a high risk of bias, with no evidence of serious summary risk of bias across the trials.
Associations of the Prespecified Substitutions
Figure 2 shows the network meta-analyses of the association of the intended substitution of LNCSBs for SSBs with body weight, other measures of adiposity, and cardiometabolic risk factors. This substitution was associated with reduced body weight (MD, −1.06 kg; 95% CI, −1.71 to –0.41 kg) and lower BMI (MD, −0.32; 95% CI, −0.58 to –0.07), percentage of body fat (MD, −0.60%; 95% CI, −1.03% to –0.18%), and IHCL (standardized MD [SMD], −0.42; 95% CI, −0.70 to –0.14). No other outcomes had significant differences.
Figure 2. Substitution of Low- and No-Calorie Sweetened Beverages (LNCSBs) for Sugar-Sweetened Beverages (SSBs).
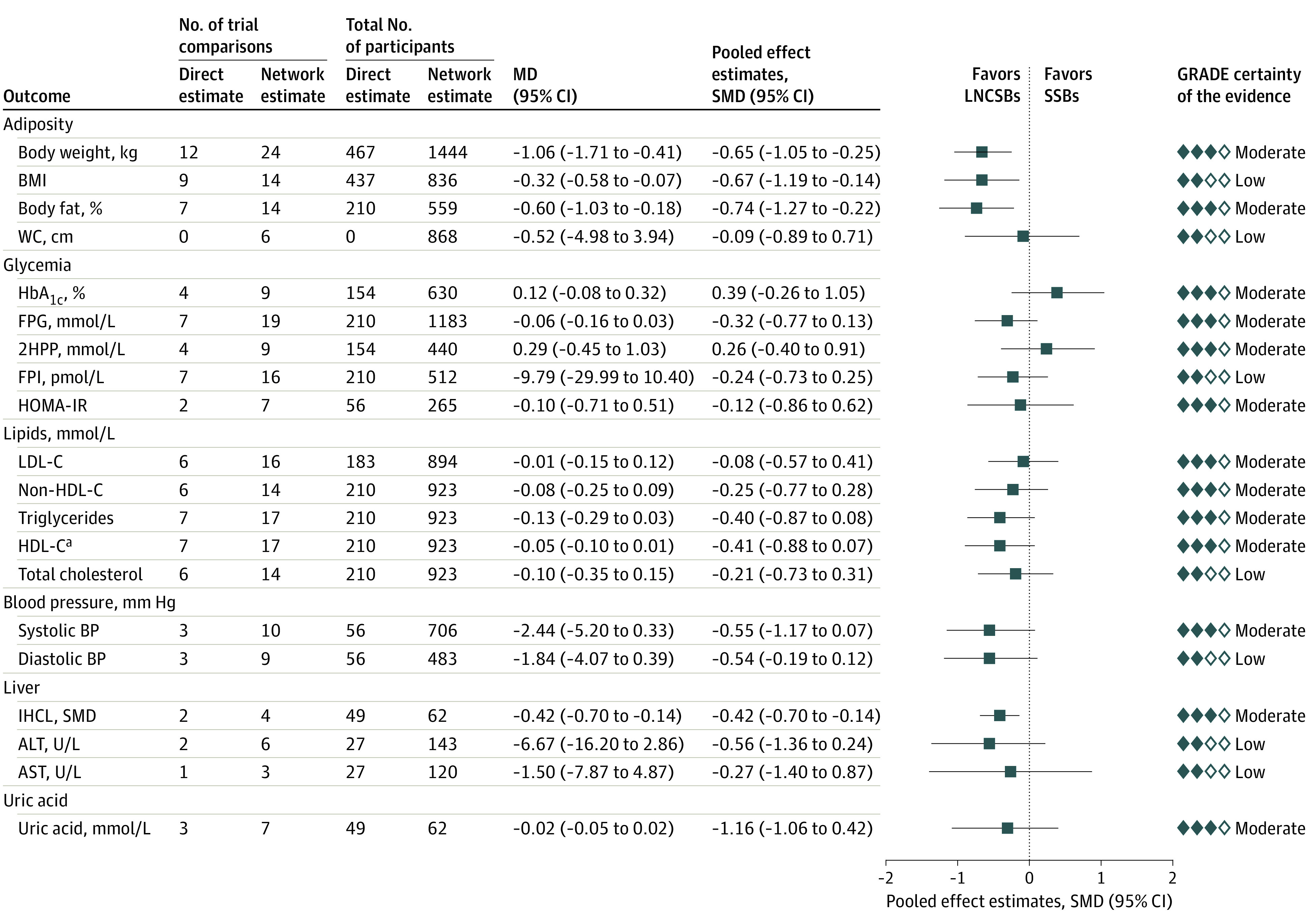
Data were pooled using network random-effects models and expressed as mean differences (MDs) and 95% CIs. To display the results for outcomes on the same plot, standardized mean differences (SMDs, represented by blue squares) and pseudo 95% CIs (represented by black horizontal lines and proportionally scaled to the 95% CIs of the MDs) were calculated. 2HPP indicates 2-hour postprandial glucose; ALT, alanine aminotransferase (to convert to μkat/L, multiply by 0.0167); AST, aspartate aminotransferase (to convert to μkat/L, multiply by 0.0167); BMI, body mass index; FPG; fasting plasma glucose; FPI, fasting plasma insulin; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HbA1c; glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; IHCL, intrahepatocellular lipid; LDL-C, low-density lipoprotein cholesterol; and WC, waist circumference.
aHDL-C result has been reversed for display purposes; that is, a negative MD would mean a positive improvement.
Figure 3 shows the network meta-analyses of the association of the standard-of-care substitution of water for SSBs with body weight, other measures of adiposity, and cardiometabolic risk factors. Neither the primary outcome of body weight (MD, −0.01 kg; 95% CI, −0.95 to 0.98 kg) nor any of the secondary outcomes showed significant differences, although the direction of association favored water for most of the outcomes.
Figure 3. Substitution of Water for Sugar-Sweetened Beverages (SSBs).
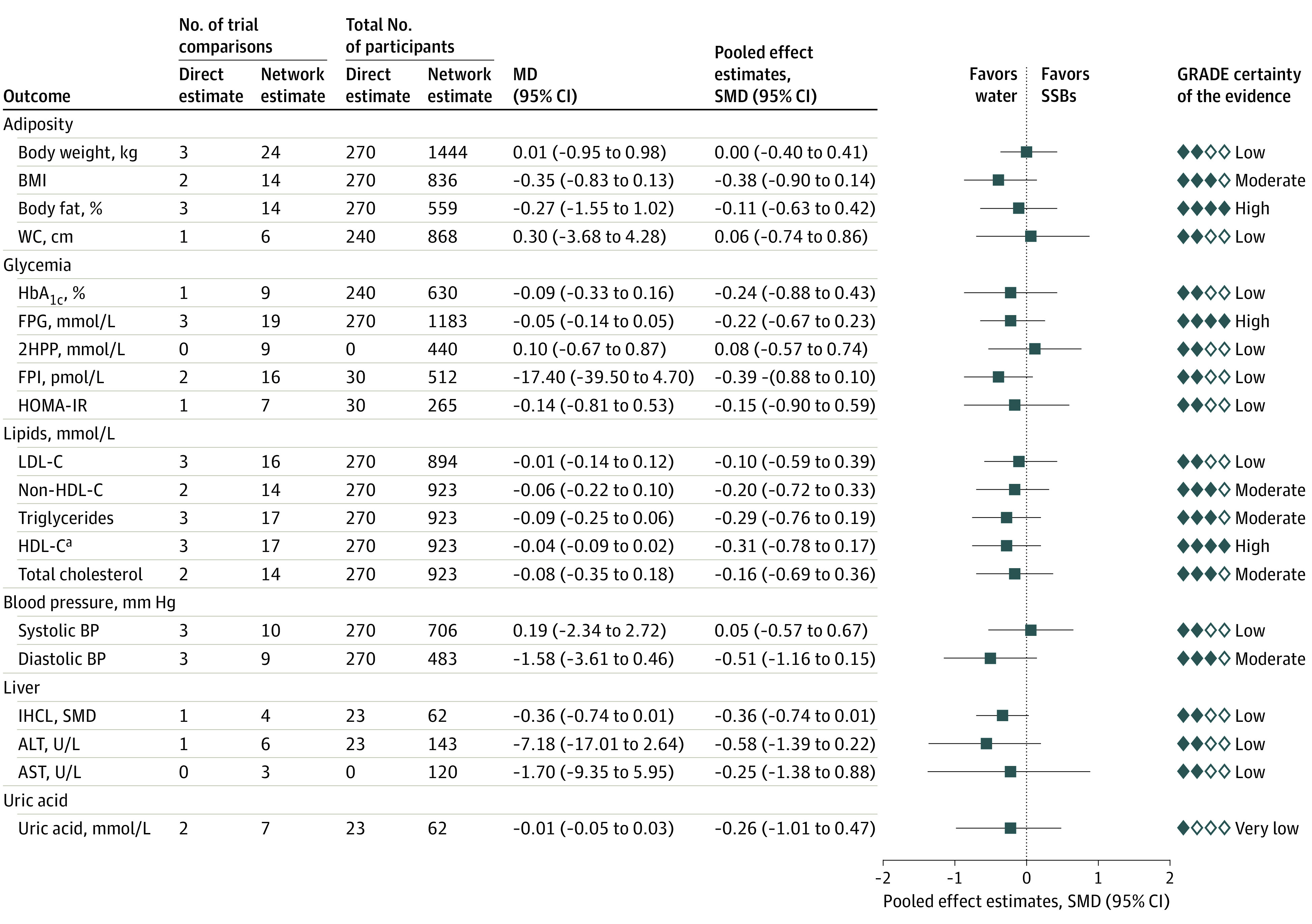
Data were pooled using network random-effects models and expressed as mean differences (MDs) and 95% CIs. To display the results for outcomes on the same plot, standardized mean differences (SMDs, represented by blue squares) and pseudo 95% CIs (represented by black horizontal lines and proportionally scaled to the 95% CIs of the MDs) were calculated. 2HPP indicates 2-hour postprandial glucose; ALT, alanine aminotransferase (to convert to μkat/L, multiply by 0.0167); AST, aspartate aminotransferase (to convert to μkat/L, multiply by 0.0167); BMI, body mass index; FPG; fasting plasma glucose; FPI, fasting plasma insulin; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HbA1c; glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; IHCL, intrahepatocellular lipid; LDL-C, low-density lipoprotein cholesterol; and WC, waist circumference.
aHDL-C result has been reversed for display purposes; that is, a negative MD would mean a positive improvement.
Figure 4 shows the network analyses of the association of the reference substitution of LNCSBs for water with body weight, other measures of adiposity, and cardiometabolic risk factors. Greater reduction in body weight (MD, −1.07 kg; 95% CI, −1.95 to −0.19 kg) was associated with LCSBs compared with water. Among secondary outcomes, water compared with LNCSBs was associated with lower level of HbA1c (MD, 0.21%; 95% CI, 0.02% to 0.40%), and LNCSBs compared with water were associated with a greater decrease in systolic BP (MD, −2.63 mm Hg; 95% CI, −4.71 to −0.55 mm Hg). No secondary outcomes were affected.
Figure 4. Substitution of Low- and No-Calorie Sweetened Beverages (LNCSBs) for Water.
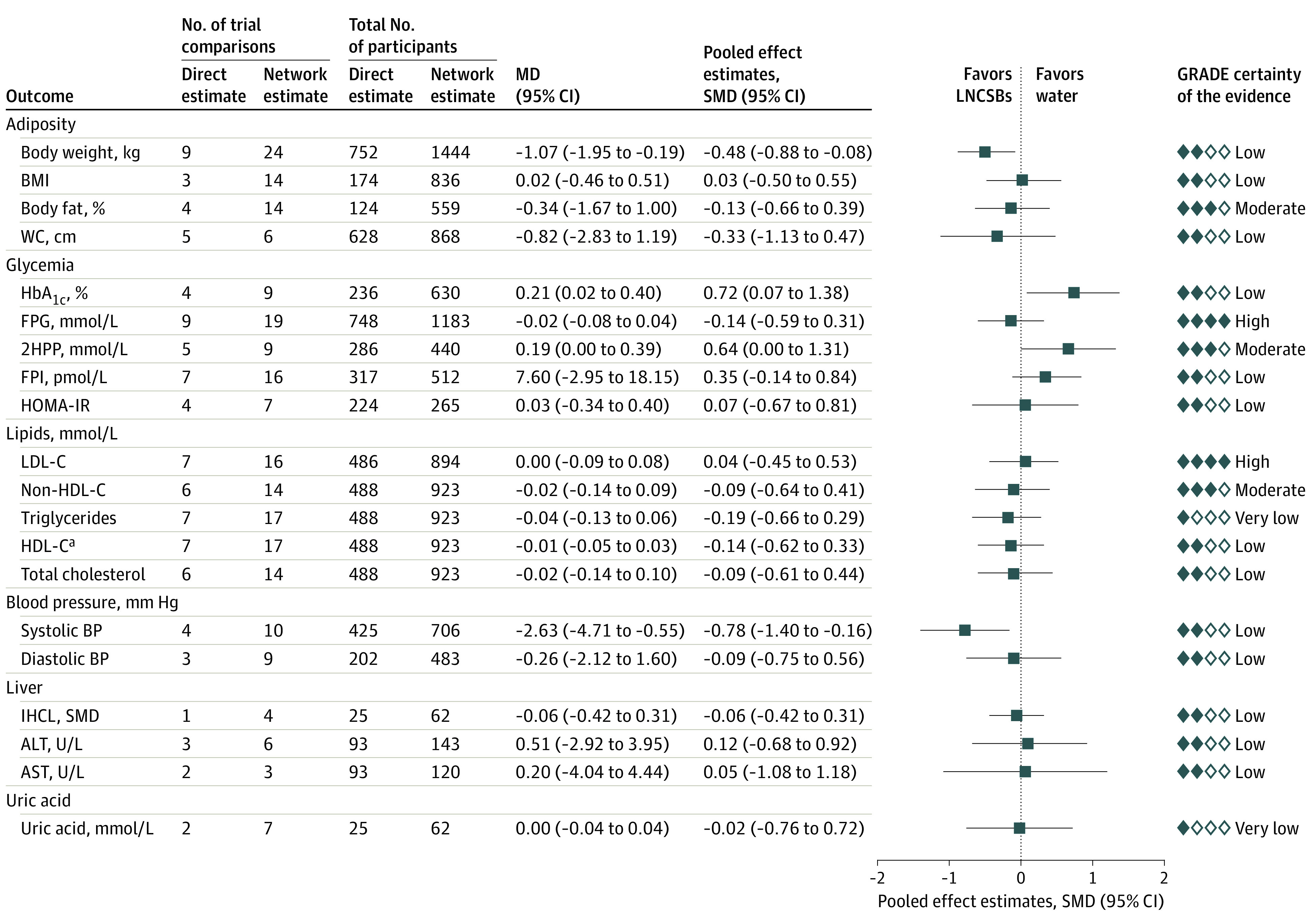
Data were pooled using network random-effects models and expressed as mean differences (MDs) and 95% CIs. To display the results for outcomes on the same plot, standardized mean differences (SMDs, represented by blue squares) and pseudo 95% CIs (represented by black horizontal lines and proportionally scaled to the 95% CIs of the MDs) were calculated. 2HPP indicates 2-hour postprandial glucose; ALT, alanine aminotransferase (to convert to μkat/L, multiply by 0.0167); AST, aspartate aminotransferase (to convert to μkat/L, multiply by 0.0167); BMI, body mass index; FPG; fasting plasma glucose; FPI, fasting plasma insulin; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HbA1c; glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; IHCL, intrahepatocellular lipid; LDL-C, low-density lipoprotein cholesterol; and WC, waist circumference.
aHDL-C result has been reversed for display purposes; that is, a negative MD would mean a positive improvement.
Adverse Events and Inconsistency
Adverse events were reported in 4 trials,33,36,43,44 including tiredness, mood swings, headaches, body aches, nausea, hospitalizations, and weight gain. In all cases, the adverse events were not observed,43,44 deemed to be unrelated to the intervention,33 or not severe enough to be of consequence.36
eTables 6 and 7 in the Supplement show the loop-specific and the design-by-treatment assessment of inconsistency (incoherence) in the network estimates. No significant incoherence was observed by any approach across the 3 substitutions.
eFigures 7 to 26 in the Supplement provide the assessments of network, direct and indirect estimates, inconsistency (heterogeneity) in the direct estimates, and inconsistency (incoherence) between the direct and indirect estimates using side-splitting method. There was evidence of substantial heterogeneity (I2≥50%; P < .10) in the direct pairwise estimates of the association of LNCSBs as a substitute for water with the primary outcome of body weight and secondary outcomes of waist circumference, HbA1c, FPI, homeostatic model assessment of insulin resistance, and triglycerides. Incoherence was not significant for any comparison, but on visual inspection slight instability between direct and indirect measures was present for BMI, percentage of body fat, HbA1c, fasting blood glucose, FPI, homeostatic model assessment of insulin resistance, low-density lipoprotein cholesterol, triglycerides, high-density lipoprotein cholesterol, total cholesterol, systolic BP, diastolic BP, IHCL, alanine aminotransferase, aspartate aminotransferase, and uric acid.
Subgroup Analyses, Intransitivity, and Publication Bias
Because no outcome had 10 or more trials in all 3 comparisons, we did not conduct subgroup analyses.
eFigures 3 to 6 in the Supplement present the evaluation of intransitivity (a domain of indirectness) among the indirect comparisons by comparing the distribution of the potential effect modifiers across the available direct comparisons for age, study length, sample size, and percentage of males. The assumption of transitivity was met for all indirect comparisons as there was no overlap in the range between the pairwise comparisons.
eFigures 47 to 57 in the Supplement show the comparison-adjusted funnel plots for outcomes with 10 or more trial comparisons (body weight, BMI, percentage of body fat, FPI, fasting plasma glucose, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, total cholesterol, and systolic BP). Funnel plot asymmetry was not observed for any of the outcomes.
GRADE Assessment
eFigures 7 to 26 in the Supplement include the GRADE assessment for the network meta-analysis. The certainty of the evidence for body weight was moderate for LNCSBs as a substitute for SSBs (small reduction; downgrade for imprecision), moderate for water as a substitute for SSBs (no difference; downgrades for inconsistency and imprecision), and low for LNCSBs as a substitute for water (small reduction; downgrades for inconsistency and imprecision). The certainty of the evidence for the adiposity and cardiometabolic outcomes was generally moderate, ranging from very low to high for each of the 3 substitutions (downgrades for inconsistency, imprecision, and/or indirectness) and with nearly all directions of the association favoring the use of LNCSBs or water as a substitute for SSBs (small to trivial reductions) and diverging for the use of LNCSBs as a substitute for water (small to no differences).
Discussion
In the present systematic review and meta-analysis, the use of LNCSBs as a substitute for SSBs was associated with reduced body weight, BMI, percentage of body fat, and IHCL, whereas the use of water as a substitute for SSBs was associated with no significant improvements, although the direction of association favored water in all cases. Furthermore, neither LNCSBs nor water as a substitute for SSBs was associated with significant improvements in glycemic control, BP, uric acid, or other aspects of the lipid profile or NAFLD markers, but the directions of the association favored LNCSBs or water in nearly all cases. The use of LNCSBs as a substitute for water did not show significant differences, except for a greater decrease in HbA1c seen with water and in body weight and systolic BP seen with LNCSBs.
Findings in the Context of Existing Studies
The findings in this study are in agreement with those reported in other systematic reviews and meta-analyses,48,49,50,51 which have allowed for the interpretation of results by comparator. Specifically, the findings that (1) reduced body weight, BMI, and body fat were associated with the use LNCSBs as a substitute for SSBs with caloric displacement and (2) neutral outcomes were associated with the use of LNCSBs as a substitute for water without caloric displacement are consistent with the results of other systematic reviews and meta-analyses of RCTs.48,49,50,51
Decreases in body weight,48,49 body weight and BMI,50 and a composite of body weight or BMI51 were observed with the substitution of LNCSs for a caloric comparator (sugars in foods or beverages) predominantly in participants with overweight or obesity. Miller and Perez50 further showed reductions in fat mass and waist circumference. Similarly, Toews et al7 found small reductions in BMI with sucrose in foods or beverages as the caloric comparator in predominantly healthy participants. On the other hand, undifferentiated analyses by Toews et al7 of the outcome of substituting LNCSs for a combination of caloric and noncaloric comparators and another analysis by Azad et al8 that restricted the outcome of substituting LNCSBs for matched noncaloric comparators (placebo, water, or weight loss diet) found no differences in body weight with LNCSs predominantly in participants with overweight or obesity. Overall, these findings are consistent with the mechanism of LNCSBs being associated with weight loss insofar as they were a factor in reducing net energy intake.
The observed improvements in downstream, intermediate cardiometabolic outcomes are also in agreement with findings of previous systematic reviews and meta-analyses. In addition to their association with weight gain,52 fructose-containing sugars that provide excess calories, especially in beverage form, have been associated with increased triglycerides,53,54 glucose,55 insulin,55 uric acid,56 and NAFLD markers.57 Toews et al7 showed that the use of LNCSs as a substitute for caloric sugars (sucrose) were a factor in reduced BP, and the reductions seen in IHCL would be expected through displacement of calories from SSBs.
The findings of this study can inform guidance on the role of LNCSBs in sugar-reduction strategies. There has been a particular focus on SSBs as the most important source of added or free sugars in several countries,58,59,60 given that the overconsumption of sugar has been associated with weight gain, diabetes, and downstream complications of hypertension and coronary heart disease.1,2,3,4 Although water is considered to be the standard-of-care substitution for SSBs by authoritative bodies,5,6,15,16,17,18,19 with many health organizations recommending against the use of LNCSBs, the existing evidence confirms the intended benefits of LNCSBs as a substitute for SSBs over the moderate term. For habitual consumers of SSBs with overweight or obesity, who are at risk for or have type 2 diabetes, and who are unable to switch to water, LNCSBs may provide a viable alternative. This finding is particularly important given that most people in the National Weight Control Registry who are successful at weight loss maintenance consume LNCSBs and report that LNCSBs help in controling caloric intake and weight loss maintenance.61
There is a need for high-quality RCTs that focus on quantifying the outcome of LNCSBs using different LNCS blends as substitutes for SSBs compared with the outcome of water (the standard-of-care substitution). We await the results of the STOP Sugars NOW (Strategies to Oppose Sugars With Non-nutritive Sweeteners or Water) trial and other similar RCTs to help clarify the role of LNCSBs. Future research using a range of designs is warranted to confirm whether the intended benefits of using LNCSBs as a substitute for SSBs are durable and extend to hard clinical outcomes.
Strengths and Limitations
This systematic review and meta-analysis has several strengths. First, the use of network meta-analysis allowed for the simultaneous assessment of the 3 prespecified substitutions (LNCSBs for SSBs, water for SSBs, and LNCSBs for water), leveraging direct and indirect comparisons with a common comparator to increase the information size. Undertaking a network meta-analysis rather than a regular pairwise meta-analysis provided 2 distinct advantages: (1) more precise estimates than single direct or indirect estimates, and (2) the ability to compare interventions that had not been compared before. Second, a comprehensive literature search that included only RCTs provided the greatest protection against bias, no evidence of serious risk of bias among the included trials, and use of the GRADE approach to assess the certainty of the estimates.
This systematic review and meta-analysis also has several limitations. First, evidence of inconsistency was present in the primary outcome of body weight across the substitutions of water for SSBs and LNCSBs for water and in several secondary outcomes across the 3 prespecified substitutions, resulting in downgrades for serious inconsistency. This inconsistency was associated with either unexplained heterogeneity in the direct estimates or incoherence from the difference between direct and indirect estimates. Network estimates closely followed the direct estimate, with indirect estimates improving precision when coherent and only trivially affecting network estimates when incoherent. Second, there was evidence of serious indirectness in several of the analyses. Only 1 RCT of direct comparisons was available for several secondary outcomes, limiting generalizability and leading to downgrades for serious indirectness. The moderate median follow-up duration of 12 weeks was considered to be another potential source of indirectness across the analyses. Although there is some uncertainty about whether the benefits and lack of harm associated with LNCSBs extended beyond the 12-week median follow-up, any harm may have manifested within this time frame. The analyses also included RCTs with up to 1 year of follow-up that showed no evidence of harm or even benefit.33,42 Other large RCTs in children and adolescents (which were not captured in the present analyses) offer further evidence of durable benefit.62,63 Therefore, we did not downgrade the evidence for the lack of long-term follow-up as a source of indirectness and instead made all conclusions specific to the moderate term. Third, there was evidence of serious imprecision in several of the pooled estimates. The 95% CIs crossed the prespecified minimal important differences for the primary outcome of body weight and several secondary outcomes across the 3 prespecified substitutions. Balancing the strengths and limitations, we assessed the certainty of the evidence as generally low to moderate for most outcomes.
Conclusions
In this systematic review and meta-analysis, using LNCSBs as an intended substitute for SSBs appeared to be associated with reductions in body weight and cardiometabolic risk factors, including BMI, percentage of body fat, and IHCL, without evidence of harm. These small improvements were similar in direction to those associated with water substitution, the standard of care. The evidence provides a good indication of the benefits of LNCSBs as an alternative replacement strategy over the moderate term for SSBs in adults with overweight or obesity who are at risk for or have diabetes.
eMethods.
eTable 1. Search Strategy MEDLINE
eTable 2. Search Strategy EMBASE
eTable 3. Search Strategy Cochrane
eTable 4. PICOTSb Framework
eTable 5. Trial Characteristics
eTable 6. Loop-Specific Approach for Inconsistency
eTable 7. Design by Treatment Approach for Inconsistency
eFigure 1. Cochrane Risk of Bias Summary for All Included Trials
eFigure 2. Risk of Bias Proportion for All Included Trials
eFigure 3. Transitivity Analysis Box Plots Showing the Distribution of the Mean Age (Years) of the Trials Across the Available Direct Comparisons
eFigure 4. Transitivity Analysis Box Plots Showing the Distribution of the Study Length (Weeks) of the Trials Across the Available Direct Comparisons
eFigure 5. Transitivity Analysis Box Plots Showing the Distribution of the Sample Size of the Trials Across the Available Direct Comparisons
eFigure 6. Transitivity Analysis Box Plots Showing the Distribution of % Males of the Trials Across the Available Direct Comparisons
eFigure 7. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Body Weight
eFigure 8. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on BMI
eFigure 9. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Body Fat (%)
eFigure 10. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Waist Circumference
eFigure 11. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on HbA1c
eFigure 12. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Fasting Plasma Glucose (FPG)
eFigure 13. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on 2-hour Post-Prandial Glucose (2HPP)
eFigure 14. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Fasting Insulin
eFigure 15. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on HOMA-IR
eFigure 16. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on LDL-C
eFigure 17. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Non-HDL-C
eFigure 18. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Triglycerides
eFigure 19. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on HDL-C
eFigure 20. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Total Cholesterol
eFigure 21. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on SBP
eFigure 22. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on DBP
eFigure 23. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on IHCL
eFigure 24. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on ALT
eFigure 25. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on AST
eFigure 26. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Uric Acid
eFigure 27. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Body Weight
eFigure 28. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With BMI
eFigure 29. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Body Fat %
eFigure 30. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Waist Circumference
eFigure 31. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With HbA1c
eFigure 32. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With FPG
eFigure 33. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With 2h-PP
eFigure 34. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With FPI
eFigure 35. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With HOMA-IR
eFigure 36. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With LDL-C
eFigure 37. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Non-HDL-C
eFigure 38. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With TGs
eFigure 39. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With HDL-C
eFigure 40. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With TC
eFigure 41. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With SBP
eFigure 42. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With DBP
eFigure 43. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With IHCL
eFigure 44. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With ALT
eFigure 45. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With AST
eFigure 46. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Uric Acid
eFigure 47. Comparison Adjusted Funnel Plot for Body Weight
eFigure 48. Comparison Adjusted Funnel Plot for BMI
eFigure 49. Comparison Adjusted Funnel Plot for Body Fat (%)
eFigure 50. Comparison Adjusted Funnel Plot for Fasting Insulin
eFigure 51. Comparison Adjusted Funnel Plot for Fasting Blood Glucose
eFigure 52. Comparison Adjusted Funnel Plot for LDL-Cholesterol
eFigure 53. Comparison Adjusted Funnel Plot for Non-HDL-Cholesterol
eFigure 54. Comparison Adjusted Funnel Plot for Triglycerides
eFigure 55. Comparison Adjusted Funnel Plot for HDL-Cholesterol
eFigure 56. Comparison Adjusted Funnel Plot for Total-Cholesterol
eFigure 57. Comparison Adjusted Funnel Plot for Systolic Blood Pressure
eData 1. Body Weight (Kg)
eData 2. BMI (kg/m2)
eData 3. Body Fat (%)
eData 4. Waist Circumference (cm)
eData 5. HbA1c (%)
eData 6. Fasting Blood Glucose (mmol/L)
eData 7. 2-Hour Post-Prandial Glucose (mmol/L)
eData 8. Fasting Plasma Insulin (pmol/L)
eData 9. HOMA-IR
eData 10. LDL-Cholesterol (mmol/L)
eData 11. Non-HDL-Cholesterol (mmol/L)
eData 12. Triglycerides (mmol/L)
eData 13. HDL-Cholesterol (mmol/L)
eData 14. Total-Cholesterol (mmol/L)
eData 15. Systolic Blood Pressure (mmHg)
eData 16. Diastolic Blood Pressure (mmHg)
eData 17. Intrahepatocellular Lipid/Liver Fat (SMD)
eData 18. Alanine Aminotransferase (U/L)
eData 19. Aspartate Aminotransferase (U/L)
eData 20. Uric Acid (mmol/L)
References
- 1.Imamura F, O’Connor L, Ye Z, et al. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jayalath VH, de Souza RJ, Ha V, et al. Sugar-sweetened beverage consumption and incident hypertension: a systematic review and meta-analysis of prospective cohorts. Am J Clin Nutr. 2015;102(4):914-921. doi: 10.3945/ajcn.115.107243 [DOI] [PubMed] [Google Scholar]
- 3.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084-1102. doi: 10.3945/ajcn.113.058362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi B, Huang Y, Reilly KH, et al. Sugar-sweetened beverages and risk of hypertension and CVD: a dose-response meta-analysis. Br J Nutr. 2015;113(5):709-717. doi: 10.1017/S0007114514004383 [DOI] [PubMed] [Google Scholar]
- 5.US Departments of Agriculture and Health and Human Services. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. US Departments of Agriculture and Health and Human Services; 2015. [Google Scholar]
- 6.Health Canada . Canada's dietary guidelines for health professionals and policy makers. Accessed July 31, 2021. https://publications.gc.ca/site/eng/9.852216/publication.html
- 7.Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;364:k4718. doi: 10.1136/bmj.k4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azad MB, Abou-Setta AM, Chauhan BF, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017;189(28):E929-E939. doi: 10.1503/cmaj.161390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sylvetsky AC, Rother KI. Nonnutritive sweeteners in weight management and chronic disease: a review. Obesity (Silver Spring). 2018;26(4):635-640. doi: 10.1002/oby.22139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter SR, Reister EJ, Cheon E, Mattes RD. Low calorie sweeteners differ in their physiological effects in humans. Nutrients. 2019;11(11):2717. doi: 10.3390/nu11112717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514(7521):181-186. doi: 10.1038/nature13793 [DOI] [PubMed] [Google Scholar]
- 12.Malik VS. Non-sugar sweeteners and health. BMJ. 2019;364:k5005. doi: 10.1136/bmj.k5005 [DOI] [PubMed] [Google Scholar]
- 13.Sievenpiper JL, Khan TA, Ha V, Viguiliouk E, Auyeung R. The importance of study design in the assessment of nonnutritive sweeteners and cardiometabolic health. CMAJ. 2017;189(46):E1424-E1425. doi: 10.1503/cmaj.733381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mela DJ, McLaughlin J, Rogers PJ. Perspective: standards for research and reporting on low-energy (“artificial”) sweeteners. Adv Nutr. 2020;11(3):484-491. doi: 10.1093/advances/nmz137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson RK, Lichtenstein AH, Anderson CAM, et al. ; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research; and Stroke Council . Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation. 2018;138(9):e126-e140. doi: 10.1161/CIR.0000000000000569 [DOI] [PubMed] [Google Scholar]
- 16.Diabetes Canada . Diabetes Canada 2018 Clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;42(supp 1):S1-S326. [DOI] [PubMed] [Google Scholar]
- 17.Diabetes UK. Evidence-based nutrition guidelines for the prevention and management of diabetes. March 2018. Accessed July 31, 2021. https://diabetes-resources-production.s3.eu-west-1.amazonaws.com/resources-s3/2018-03/1373_Nutrition%20guidelines_0.pdf
- 18.American Diabetes Association . 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(suppl 1):S48-S65. doi: 10.2337/dc20-S005 [DOI] [PubMed] [Google Scholar]
- 19.Mann JI, De Leeuw I, Hermansen K, et al. ; Diabetes and Nutrition Study Group (DNSG) of the European Association . Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis. 2004;14(6):373-394. doi: 10.1016/S0939-4753(04)80028-0 [DOI] [PubMed] [Google Scholar]
- 20.Puhan MA, Schünemann HJ, Murad MH, et al. ; GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 21.Higgins J, Thomas, J, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. 2021. Accessed July 31, 2021. https://training.cochrane.org/handbook/current
- 22.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. doi: 10.1371/journal.pone.0076654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932-944. doi: 10.1002/sim.3767 [DOI] [PubMed] [Google Scholar]
- 26.Jackson D, Barrett JK, Rice S, White IR, Higgins JPT. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med. 2014;33(21):3639-3654. doi: 10.1002/sim.6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. doi: 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 28.Brignardello-Petersen R, Bonner A, Alexander PE, et al. ; GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36-44. doi: 10.1016/j.jclinepi.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 29.Brignardello-Petersen R, Murad MH, Walter SD, et al. ; GRADE Working Group . GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. 2019;105:60-67. doi: 10.1016/j.jclinepi.2018.08.022 [DOI] [PubMed] [Google Scholar]
- 30.Brignardello-Petersen R, Mustafa RA, Siemieniuk RAC, et al. ; GRADE Working Group . GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol. 2019;108:77-85. doi: 10.1016/j.jclinepi.2018.11.025 [DOI] [PubMed] [Google Scholar]
- 31.Bonnet F, Tavenard A, Esvan M, et al. Consumption of a carbonated beverage with high-intensity sweeteners has no effect on insulin sensitivity and secretion in nondiabetic adults. J Nutr. 2018;148(8):1293-1299. doi: 10.1093/jn/nxy100 [DOI] [PubMed] [Google Scholar]
- 32.Bruun JM, Maersk M, Belza A, Astrup A, Richelsen B. Consumption of sucrose-sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: a 6-month randomised controlled trial. Eur J Clin Nutr. 2015;69(8):949-953. doi: 10.1038/ejcn.2015.95 [DOI] [PubMed] [Google Scholar]
- 33.Ebbeling CB, Feldman HA, Steltz SK, Quinn NL, Robinson LM, Ludwig DS. Effects of sugar-sweetened, artificially sweetened, and unsweetened beverages on cardiometabolic risk factors, body composition, and sweet taste preference: a randomized controlled trial. J Am Heart Assoc. 2020;9(15):e015668. doi: 10.1161/JAHA.119.015668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campos V, Despland C, Brandejsky V, et al. Sugar- and artificially sweetened beverages and intrahepatic fat: a randomized controlled trial. Obesity (Silver Spring). 2015;23(12):2335-2339. doi: 10.1002/oby.21310 [DOI] [PubMed] [Google Scholar]
- 35.Engel S, Tholstrup T, Bruun JM, Astrup A, Richelsen B, Raben A. Effect of high milk and sugar-sweetened and non-caloric soft drink intake on insulin sensitivity after 6 months in overweight and obese adults: a randomized controlled trial. Eur J Clin Nutr. 2018;72(3):358-366. doi: 10.1038/s41430-017-0006-9 [DOI] [PubMed] [Google Scholar]
- 36.Hernández-Cordero S, Barquera S, Rodríguez-Ramírez S, et al. Substituting water for sugar-sweetened beverages reduces circulating triglycerides and the prevalence of metabolic syndrome in obese but not in overweight Mexican women in a randomized controlled trial. J Nutr. 2014;144(11):1742-1752. doi: 10.3945/jn.114.193490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins KA, Considine RV, Mattes RD. Aspartame consumption for 12 weeks does not affect glycemia, appetite, or body weight of healthy, lean adults in a randomized controlled trial. J Nutr. 2018;148(4):650-657. doi: 10.1093/jn/nxy021 [DOI] [PubMed] [Google Scholar]
- 38.Higgins KA, Mattes RD. A randomized controlled trial contrasting the effects of 4 low-calorie sweeteners and sucrose on body weight in adults with overweight or obesity. Am J Clin Nutr. 2019;109(5):1288-1301. doi: 10.1093/ajcn/nqy381 [DOI] [PubMed] [Google Scholar]
- 39.Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Effects on weight loss in adults of replacing diet beverages with water during a hypoenergetic diet: a randomized, 24-wk clinical trial. Am J Clin Nutr. 2015;102(6):1305-1312. doi: 10.3945/ajcn.115.109397 [DOI] [PubMed] [Google Scholar]
- 40.Madjd A, Taylor MA, Delavari A, Malekzadeh R, Macdonald IA, Farshchi HR. Beneficial effects of replacing diet beverages with water on type 2 diabetic obese women following a hypo-energetic diet: a randomized, 24-week clinical trial. Diabetes Obes Metab. 2017;19(1):125-132. doi: 10.1111/dom.12793 [DOI] [PubMed] [Google Scholar]
- 41.Maersk M, Belza A, Stødkilde-Jørgensen H, et al. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283-289. doi: 10.3945/ajcn.111.022533 [DOI] [PubMed] [Google Scholar]
- 42.Peters JC, Beck J, Cardel M, et al. The effects of water and non-nutritive sweetened beverages on weight loss and weight maintenance: a randomized clinical trial. Obesity (Silver Spring). 2016;24(2):297-304. doi: 10.1002/oby.21327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid M, Hammersley R, Hill AJ, Skidmore P. Long-term dietary compensation for added sugar: effects of supplementary sucrose drinks over a 4-week period. Br J Nutr. 2007;97(1):193-203. doi: 10.1017/S0007114507252705 [DOI] [PubMed] [Google Scholar]
- 44.Reid M, Hammersley R, Duffy M. Effects of sucrose drinks on macronutrient intake, body weight, and mood state in overweight women over 4 weeks. Appetite. 2010;55(1):130-136. doi: 10.1016/j.appet.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 45.Reid M, Hammersley R, Duffy M, Ballantyne C. Effects on obese women of the sugar sucrose added to the diet over 28 d: a quasi-randomised, single-blind, controlled trial. Br J Nutr. 2014;111(3):563-570. doi: 10.1017/S0007114513002687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tate DF, Turner-McGrievy G, Lyons E, et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr. 2012;95(3):555-563. doi: 10.3945/ajcn.111.026278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51(6):963-969. doi: 10.1093/ajcn/51.6.963 [DOI] [PubMed] [Google Scholar]
- 48.Rogers PJ, Hogenkamp PS, de Graaf C, et al. Does low-energy sweetener consumption affect energy intake and body weight? a systematic review, including meta-analyses, of the evidence from human and animal studies. Int J Obes (Lond). 2016;40(3):381-394. doi: 10.1038/ijo.2015.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogers PJ, Appleton KM. The effects of low-calorie sweeteners on energy intake and body weight: a systematic review and meta-analyses of sustained intervention studies. Int J Obes (Lond). 2021;45(3):464-478. doi: 10.1038/s41366-020-00704-2 [DOI] [PubMed] [Google Scholar]
- 50.Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014;100(3):765-777. doi: 10.3945/ajcn.113.082826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laviada-Molina H, Molina-Segui F, Pérez-Gaxiola G, et al. Effects of nonnutritive sweeteners on body weight and BMI in diverse clinical contexts: systematic review and meta-analysis. Obes Rev. 2020;21(7):e13020. doi: 10.1111/obr.13020 [DOI] [PubMed] [Google Scholar]
- 52.Sievenpiper JL, de Souza RJ, Mirrahimi A, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. 2012;156(4):291-304. doi: 10.7326/0003-4819-156-4-201202210-00007 [DOI] [PubMed] [Google Scholar]
- 53.Chiavaroli L, de Souza RJ, Ha V, et al. Effect of fructose on established lipid targets: a systematic review and meta-analysis of controlled feeding trials. J Am Heart Assoc. 2015;4(9):e001700. doi: 10.1161/JAHA.114.001700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.David Wang D, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis. 2014;232(1):125-133. doi: 10.1016/j.atherosclerosis.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 55.Choo VL, Viguiliouk E, Blanco Mejia S, et al. Food sources of fructose-containing sugars and glycaemic control: systematic review and meta-analysis of controlled intervention studies. BMJ. 2018;363:k4644. doi: 10.1136/bmj.k4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang DD, Sievenpiper JL, de Souza RJ, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr. 2012;142(5):916-923. doi: 10.3945/jn.111.151951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu S, Sievenpiper JL, de Souza RJ, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr. 2014;68(4):416-423. doi: 10.1038/ejcn.2014.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kirkpatrick SI, Raffoul A, Lee KM, Jones AC. Top dietary sources of energy, sodium, sugars, and saturated fats among Canadians: insights from the 2015 Canadian Community Health Survey. Appl Physiol Nutr Metab. 2019;44(6):650-658. doi: 10.1139/apnm-2018-0532 [DOI] [PubMed] [Google Scholar]
- 59.Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11(8):1840. doi: 10.3390/nu11081840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popkin BM, Hawkes C. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016;4(2):174-186. doi: 10.1016/S2213-8587(15)00419-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catenacci VA, Pan Z, Thomas JG, et al. Low/no calorie sweetened beverage consumption in the National Weight Control Registry. Obesity (Silver Spring). 2014;22(10):2244-2251. doi: 10.1002/oby.20834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367(15):1397-1406. doi: 10.1056/NEJMoa1203034 [DOI] [PubMed] [Google Scholar]
- 63.Ebbeling CB, Feldman HA, Chomitz VR, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367(15):1407-1416. doi: 10.1056/NEJMoa1203388 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Search Strategy MEDLINE
eTable 2. Search Strategy EMBASE
eTable 3. Search Strategy Cochrane
eTable 4. PICOTSb Framework
eTable 5. Trial Characteristics
eTable 6. Loop-Specific Approach for Inconsistency
eTable 7. Design by Treatment Approach for Inconsistency
eFigure 1. Cochrane Risk of Bias Summary for All Included Trials
eFigure 2. Risk of Bias Proportion for All Included Trials
eFigure 3. Transitivity Analysis Box Plots Showing the Distribution of the Mean Age (Years) of the Trials Across the Available Direct Comparisons
eFigure 4. Transitivity Analysis Box Plots Showing the Distribution of the Study Length (Weeks) of the Trials Across the Available Direct Comparisons
eFigure 5. Transitivity Analysis Box Plots Showing the Distribution of the Sample Size of the Trials Across the Available Direct Comparisons
eFigure 6. Transitivity Analysis Box Plots Showing the Distribution of % Males of the Trials Across the Available Direct Comparisons
eFigure 7. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Body Weight
eFigure 8. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on BMI
eFigure 9. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Body Fat (%)
eFigure 10. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Waist Circumference
eFigure 11. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on HbA1c
eFigure 12. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Fasting Plasma Glucose (FPG)
eFigure 13. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on 2-hour Post-Prandial Glucose (2HPP)
eFigure 14. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Fasting Insulin
eFigure 15. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on HOMA-IR
eFigure 16. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on LDL-C
eFigure 17. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Non-HDL-C
eFigure 18. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Triglycerides
eFigure 19. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on HDL-C
eFigure 20. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Total Cholesterol
eFigure 21. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on SBP
eFigure 22. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on DBP
eFigure 23. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on IHCL
eFigure 24. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on ALT
eFigure 25. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on AST
eFigure 26. Network Analysis With GRADE Assessment of the Certainty of the Evidence Comparing LNCSBs, SSBs and Water on Uric Acid
eFigure 27. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Body Weight
eFigure 28. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With BMI
eFigure 29. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Body Fat %
eFigure 30. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Waist Circumference
eFigure 31. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With HbA1c
eFigure 32. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With FPG
eFigure 33. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With 2h-PP
eFigure 34. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With FPI
eFigure 35. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With HOMA-IR
eFigure 36. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With LDL-C
eFigure 37. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Non-HDL-C
eFigure 38. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With TGs
eFigure 39. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With HDL-C
eFigure 40. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With TC
eFigure 41. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With SBP
eFigure 42. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With DBP
eFigure 43. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With IHCL
eFigure 44. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With ALT
eFigure 45. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With AST
eFigure 46. Network Diagram for Randomized Controlled Trials Investigating the Association of the Substitution of LNCSBs for SSBs, Water for SSBs, and LNCSBs for Water With Uric Acid
eFigure 47. Comparison Adjusted Funnel Plot for Body Weight
eFigure 48. Comparison Adjusted Funnel Plot for BMI
eFigure 49. Comparison Adjusted Funnel Plot for Body Fat (%)
eFigure 50. Comparison Adjusted Funnel Plot for Fasting Insulin
eFigure 51. Comparison Adjusted Funnel Plot for Fasting Blood Glucose
eFigure 52. Comparison Adjusted Funnel Plot for LDL-Cholesterol
eFigure 53. Comparison Adjusted Funnel Plot for Non-HDL-Cholesterol
eFigure 54. Comparison Adjusted Funnel Plot for Triglycerides
eFigure 55. Comparison Adjusted Funnel Plot for HDL-Cholesterol
eFigure 56. Comparison Adjusted Funnel Plot for Total-Cholesterol
eFigure 57. Comparison Adjusted Funnel Plot for Systolic Blood Pressure
eData 1. Body Weight (Kg)
eData 2. BMI (kg/m2)
eData 3. Body Fat (%)
eData 4. Waist Circumference (cm)
eData 5. HbA1c (%)
eData 6. Fasting Blood Glucose (mmol/L)
eData 7. 2-Hour Post-Prandial Glucose (mmol/L)
eData 8. Fasting Plasma Insulin (pmol/L)
eData 9. HOMA-IR
eData 10. LDL-Cholesterol (mmol/L)
eData 11. Non-HDL-Cholesterol (mmol/L)
eData 12. Triglycerides (mmol/L)
eData 13. HDL-Cholesterol (mmol/L)
eData 14. Total-Cholesterol (mmol/L)
eData 15. Systolic Blood Pressure (mmHg)
eData 16. Diastolic Blood Pressure (mmHg)
eData 17. Intrahepatocellular Lipid/Liver Fat (SMD)
eData 18. Alanine Aminotransferase (U/L)
eData 19. Aspartate Aminotransferase (U/L)
eData 20. Uric Acid (mmol/L)