Abstract
Purpose
C1q and the classical complement cascade are key regulators of synaptic pruning, and their aberrant activation has been implicated in neurodegenerative ophthalmic diseases including geographic atrophy and glaucoma. The antigen-binding fragment antibody ANX007 specifically recognizes globular head groups of C1q to block substrate binding and functionally inhibit classical complement cascade activation. ANX007 was assessed in nonclinical studies of biodistribution and C1q target engagement in the eye following intravitreal (IVT) administration in cynomolgus monkeys.
Methods
Female juvenile cynomolgus monkeys (n = 12) received a single bilateral dose of 1 or 5 mg ANX007/eye, with vitreous and non-perfused tissue samples collected approximately 4 weeks later. In a separate study, male (n = 6/5) and female (n = 6/5) animals received repeat bilateral dosing of 1, 2.5, or 5 mg ANX007/eye on days 1 and 29, with aqueous and vitreous collections on day 44 or day 59. Tissues from the 5 mg/eye repeat-dose group were perfused, and retina, choroid, and optic nerve samples were collected approximately 2 and 4 weeks post-last dose.
Results
Following a single dose of ANX007, vitreous levels of free drug were measurable through 4 weeks at both the 1 and 5 mg dose levels, with approximately 3-day half-life. With repeat dose of 5 mg/eye, free-ANX007 was measurable 4 weeks post-last dose in perfused retina and choroid and up to approximately 2 weeks post-last dose in optic nerve. There was a strong correlation between C1q target engagement and free drug levels in aqueous and vitreous humors and retinal tissue.
Conclusions
Following IVT administration, ANX007 distributes to sites within the retina that are relevant to neurodegenerative ophthalmic disease with clear evidence of C1q target engagement. Based on its mechanism of action inhibiting C1q and its downstream activity, ANX007 is predicted to mitigate tissue damage driven by classical complement activation in the retina. These data support further clinical evaluation of ANX007.
Keywords: ANX007, C1q classical complement cascade, glaucoma, geographic atrophy (GA)
C1q and the classical complement cascade are key regulators of synaptic pruning in neuronal development1–3 but can be aberrantly activated in neurodegenerative diseases.1,4 Complement-mediated neurodegeneration (CMND) is associated with compromised synapse function due to ongoing complement activation on their surface, which leads to inappropriate loss of functional synapses and their neurotrophic support, chronic neuroinflammation, and progressive neuronal loss.2,5 There is mounting evidence that CMND becomes a driver of neurodegeneration regardless of the initiating etiology, and hence is associated with progression of numerous neurologic diseases, such as Alzheimer's disease,6 frontotemporal dementia,7 Huntington disease,5 and schizophrenia,8 and, in particular, neurodegenerative diseases of the retina, including glaucoma9–11 and geographic atrophy (GA).12 In glaucoma, a leading cause of irreversible blindness worldwide,13 increases in intraocular pressure or other factors that damage the optic nerve can trigger activation of complement activation on synapses of the retinal ganglion cells (RGCs), facilitating RGC damage and degeneration in the optic nerve.9–11 Similarly, GA secondary to age-related macular degeneration is a neurodegenerative retinal disease that involves formation of drusen, the hallmark pathology of GA, associated with complement activation and damage to the overlying retinal pigment epithelium, complement activation on photoreceptor cell outer segments, and then complement driven loss of synapses on photoreceptor neurons themselves.12 Due to the progressive destruction of these cells, GA causes irreversible vision loss12,14–16 and is one of the main causes of blindness in people over 50 years of age.17
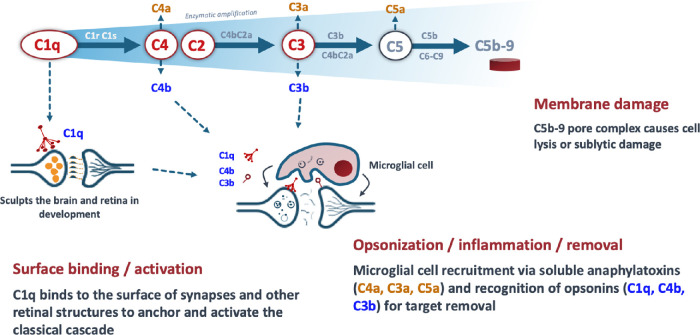
Activation of the complement cascade can occur through three distinct pathways: the classical, lectin, and alternative pathways.3,18–20 C1q is the initiating molecule of the classical pathway and is activated by binding specific substrates that are present on synapses of the RGC within the inner retina in glaucoma, and in multiple layers of outer retina in GA, including drusen, photoreceptor cell outer segments, and photoreceptor cell synapses.12,21 Activation of C1q and downstream components of the classical cascade, including C4 and C3 (Fig. 1), drives tissue damage and inflammation in the retina.1,22 C1q elevation and complement activation have been observed in human glaucomatous retinas4,10 and in mouse, rat, and dog models of glaucoma.9,11,23–25 C1q accumulation and synapse loss occurs early in the disease process in a chronic, spontaneous model of glaucoma in DBA/2J mice.1 C4 and C3 deposition have been documented on outer segments of healthy photoreceptor cells at the leading edge of lesions in retinas from patients with GA, and activation of the complement pathway has been demonstrated to occur on photoreceptor outer segments and to drive retinal atrophy in animal models of GA.15
Figure 1.
Actions associated with C1q activation via the classical complement pathway cascade.
Inhibition of C1q blocks initiation of the classical complement cascade and slows neuronal and synaptic damage by directly reducing nerve cell membrane damage and the inflammatory consequences of complement activation.5,6,26 The blockade of C1q prevents recruitment and activation of all downstream components within the classical pathway, including C4, C3, and C5, that drive a local immune response and tissue destruction in the retina.10 Genetic deletion of C1q or direct injection of a complement inhibitor to the eyes in mice models of glaucoma or outer retinal damage significantly delayed optic nerve damage and protected against degeneration of the inner retina and neuronal cell loss.11,23,27 Further, pharmacologic inhibition of C1q reduced photoreceptor cell loss and preserved outer retinal function in a mouse model of photoreceptor cell loss involving photooxidative damage.26 These results taken together support a role for C1q and early steps in the classical complement cascade as key drivers of neurodegeneration in the eye. Additionally, by selectively targeting C1q, the alternative and lectin pathways are left intact for continued pathogen surveillance and retinal health.20,28
ANX007 is a novel high affinity monoclonal antibody antigen-binding fragment (Fab) that was designed to specifically recognize the substrate-binding head groups of C1q to functionally inhibit the classical complement cascade. ANX007 is being developed as an intravitreal (IVT)-administered treatment to potentially prevent retinal damage associated with ophthalmologic diseases, such as GA and glaucoma (Grover A, et al. IOVS 2021;62:ARVO E-Abstract 219). The IVT route of administration enables local drug exposure at the site of ocular disease by direct delivery into the vitreous and has been safely and effectively used in approved products to treat a variety of ophthalmic conditions.29 IVT injection of the murine parent antibody of ANX007 blocks C1q in C57BL/6 J mice and slows the progression of retinal atrophy in an oxidative model of photoreceptor cell damage.26
ANX007 has been previously evaluated in two phase I clinical studies in patients with primary open-angle glaucoma, without any serious or significant adverse effects. The open-label, single dose, dose-escalation phase I study (NCT03488550) evaluated the safety, tolerability, and pharmacokinetics (PKs) of single IVT-administered doses of ANX007,30 and the randomized, double-masked, sham-controlled phase Ib study (NCT04188015) evaluated the safety, tolerability, and PK of repeat IVT doses of sham and ANX007.31
The purpose of the analyses in this paper is to assess ANX007 biodistribution and C1q target engagement in the eye of cynomolgus monkeys following IVT administration to understand the PKs and pharmacodynamics at sites of ophthalmic neurodegenerative diseases. Additionally, the relationship between aqueous humor, vitreous, and retinal tissue levels of C1q target engagement following ANX007 treatment were assessed to support the use of aqueous humor as a surrogate for retinal target engagement in further nonclinical and clinical studies.
Methods
In Vitro ANX007 Binding Studies
The binding of ANX007 to C1q was assessed using purified human C1q and to cynomolgus monkey and human C1q captured from serum. Binding of ANX007 to purified human C1q was evaluated in an enzyme-linked immunosorbent assay (ELISA) that utilized enzyme-tagged anti-human-kappa antibody followed by enzyme substrate. Human and cynomolgus monkey C1q binding was determined using C1q captured from human and cynomolgus monkey serum in a sandwich ELISA wherein serum C1q was captured with JL-1 antibody (ab71940; Abcam, Waltham, MA, USA) specific to the collagen tail of C1q, and the bound ANX007 is detected with alkaline-phosphatase conjugated anti-human kappa antibodies. ANX007 was titrated 3 times per dilution from a starting concentration of 3000 ng/mL to 8 concentrations. Luminescence counts were fit using a 4-parameter logistic fit to derive binding half-maximal effective concentration (EC50) values and analyzed using GraphPad Prism (version 8.4.0; GraphPad, Inc., La Jolla, CA, USA).
ANX007 Intravitreal Animal Studies
Table 1 summarizes three studies that were completed in cynomolgus monkeys using IVT administration of ANX007.
Table 1.
ANX007 IVT Single Dose and Repeat Dose Study Designs
| Study | Dosing | Collection Timepoints | Samples Collected | PK/PD End Points |
|---|---|---|---|---|
| PK & Ocular Single Dose Tolerability Study | Single dose bilateral: 0, 1, or 5 mg/eye | Days 1 (6 hours postdose), 3, 7, 10, 20, and 30; n = 1-2 per group per timepoint | Vitreous humor, retina, choroid (non-perfused) | Free-ANX007 and free-C1q: vitreous, retina, choroid |
| Repeat Dose Ocular Toxicology Study with 4-Week Recovery | 2 doses, 4 weeks apart bilateral (day 1 and day 29): 0, 1, or 2.5 mg/eye | Days 44 and 59 (approximately 2 and 4 weeks following the second dose); n = 2–6 per group per timepoint | Aqueous humor, vitreous humor, optic nerve (non-perfused) | Free-ANX007 and free-C1q: vitreous, aqueous humor, optic nerve |
| Repeat Dose Ocular Toxicology Study with 4-Week Recovery | 2 doses, 4 weeks apart bilateral (day 1 and day 29): 0 or 5 mg/eye | Days 44 and 59 (approximately 2 and 4 weeks following the second dose); n = 4–6 per group per timepoint | Aqueous humor, vitreous humor, retina, choroid, optic nerve (perfused)* | Free-ANX007 and free-C1q: vitreous, aqueous humor, retina, choroid, optic nerve |
PD, pharmacodynamics (free-C1q); PK, pharmacokinetics (free-ANX007).
Upper body perfusions with saline in the repeat dosed 5 mg/eye group were performed with flushing solution, which ran for approximately 5 minutes at a rate of approximately 250 mL/minute until no blood was visible in the perfusate.
Animals and Housing
Animal studies were in compliance with all applicable sections of the Final Rules of the Animal Welfare Act regulations (Code of Federal Regulations, Title 9),32 the Public Health Service Policy on Humane Care and Use of Laboratory Animals from the Office of Laboratory Animal Welfare,33 the Guide for the Care and Use of Laboratory Animals from the National Research Council,34 and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cynomolgus monkeys were procured from Charles River Laboratories (CRL, Houston, TX, USA) or a CRL-approved vendor, with ages and weights ranging from 2 to 4 years and 2.1 to 3.7 kg, respectively. Only female monkeys (n = 12) were used in the single dose study, and the two repeat dose studies used six male and six female animals and five male and five female animals, respectively. Animals were acclimated to laboratory housing for at least 14 days before the initiation of dosing and were housed in stainless-steel cages equipped with a stainless-steel mesh floor and an automatic watering valve. Room temperature was maintained at 18°C to 29°C with a relative humidity of 30% to 70% and a 12-hour light/12-hour dark cycle. Ten or more air changes per hour with 100% fresh air (no air recirculation) were maintained in the animal rooms.
IVT ANX007 Injections
Animal Sedation for IVT Injection
Buprenorphine analgesia was administered prior to each IVT injection and again within 1-day post-injection. Animals were sedated with ketamine and dexdomitor for the dosing procedure; sedation was reversed with antisedan. Eyes were cleansed with Betadine and rinsed with sterile saline. Prior to dose administration, 1% tropicamide mydriatic was instilled in each eye, followed by proparacaine topical anesthetic.
IVT Injection
Control and ANX007 were filtered before use with a Millex 0.22 µm polyvinylidene fluoride filter into a polyethylene terephthalate glycol container and visually assessed for clarity. IVT studies (bilateral) were performed using a single injection of 50 µL/eye (for doses of 0, 1, and 2.5 mg ANX007/eye), or a double injection of 2 × 50 µL/eye (for the 5 mg ANX007/eye dose) administered approximately 10 minutes apart using 1/2 cc, 27 G × 1/2 inch needles. For double injections, the first injection was made in the superior temporal region and the second injection was in the inferior temporal region, using two syringes per eye. Eyes were examined with an indirect ophthalmoscope to identify any visible post-dosing problems and to confirm test article deposition. Tobramycin ophthalmic solution, USP, topical ocular antibiotic was dispensed onto each eye to prevent infection immediately following dosing and 1 day after dosing.
Clinical Observations and Clinical Pathology
Clinical observations included cage side observations, body weight, food consumption, ophthalmic examinations using slit lamp biomicroscopy and indirect ophthalmoscopy, electroretinograms, tonometry (intraocular pressure measurements), as well as clinical pathology (hematology, clinical chemistry, coagulation, and urinalysis) for the repeat dose studies. Special attention was given to the eyes. Terminal procedures included organ weights and tissue collection and preservation for histology, and histopathology and immunohistochemistry for ocular tissues.
Ocular Tissue Sampling
Prior to enucleation of 1 eyeball, all animals were euthanized by intravenous injection of a commercially available veterinary euthanasia solution containing a mixture of phenytoin and pentobarbital, followed by exsanguination. Tissues and bioanalytical samples were collected after euthanasia and exsanguination in all animals. One eyeball (the left globe) was dissected to isolate the retina, choroid, and optic nerve head. The aqueous humor was removed first via a syringe and placed into two aliquots (each weighed). Following the removal of the lens and cornea, the vitreous humor was removed by gently pulling it out with tweezers and releasing it onto a Petri dish; it was then aliquoted into four vials and shipped to CRL (Skokie, IL, USA) for analysis in validated assays. Extracts of the other ocular tissue samples were prepared in approximately 10 times volume (10 µL/mg tissue) of Tris Buffered saline containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Individual tissue samples were placed in a 2 mL microcentrifuge tube with a 7 mm steel ball and homogenized using the Tissuelyzer II (Qiagen, Germantown, MD, USA), operated at a frequency of 30 Hz for 2 minutes. Extracts were spun at 17,000 G for 20 minutes; supernatants were then removed, aliquoted, and frozen until analysis. Protein concentrations in tissue extracts were assessed using a Pierce Micro BCA protein assay kit (Thermo Fisher, Waltham, MA, USA).
Quantitation of ANX007 and C1q Levels
Free-ANX007
Assays were performed at CRL (Skokie, IL, USA) or at Annexon Biosciences (South San Francisco, CA, USA). Concentrations of free-ANX007 were measured in an indirect ELISA using plates coated with purified human C1q (Complement Technology Inc., Tyler, TX, USA), and ANX007 captured by the bound C1q was detected by incubation with an AffiniPure goat anti-human kappa light chain conjugated to detection agent (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). The summary of bioanalytical methods used to quantify ANX007 is shown in Table 2.
Table 2.
Bioanalytical Methods Summary for ANX007 and C1q
| Species | Analyte | Matrix | Method | LLOQ-ULOQ Range (ng/mL) | Matrix Volume (µL) | Minimum Required Dilution | Inter-Assay Accuracy (CV%)* | Inter-Assay Precision (CV%)* |
|---|---|---|---|---|---|---|---|---|
| Cynomolgus monkeys | Free-ANX007 | Serum | Validated | 1.25–250 | 10 | 1:40 | −4.32, −3.96 | 9.87, 7.84 |
| Free-ANX007 | Vitreous | Validated | 1.25–250 | 10 | 1:40 | 1.28, −3.84 | 11.43, 7.34 | |
| Free-C1q | Vitreous | Validated | 1.953–250 | 60 | 1:5 | −13.21, −12.70 | 27.61, 12.05 | |
| Cynomolgus monkeys | Free-ANX007 | Optic nerve, choroid, retina | Research assays | 0.02–50 | 7.5 | 1:10 | <20% | <20% |
| Free-C1q | Optic nerve, choroid, retina | Research assays | 0.02–50 | 7.5 | 1:10 | <20% | <20% |
Established at the LLOQ and ULOQ.
CV%, % coefficient variation; LLOQ, lower limit of quantitation; ULOQ, upper limit of quantitation.
Free-C1q
Assays were performed at CRL (Skokie, IL, USA) or at Annexon Biosciences (South San Francisco, CA, USA). Concentrations of free-C1q were measured using a sandwich ELISA using plates coated with an antibody against the collagen tail of C1q (JL-1 ab71940; Abcam, Waltham, MA, USA) and C1q captured from different samples was detected using the murine antibody M1, which binds to a distinct epitope on the globular head region on C1q. Because the detection antibody M1 and the treatment antibody ANX007 bind to the same epitope on C1q, their binding to C1q is mutually exclusive; thus, only free C1q will be measured using this assay. The summary of bioanalytical methods used to quantify C1q is shown in Table 2.
Statistical Analysis
Correlation coefficients were determined using the Spearman nonparametric correlation (GraphPad Prism, version 8.4.0; GraphPad, Inc., La Jolla, CA, USA).
Results
In Vitro Binding Affinity of ANX007
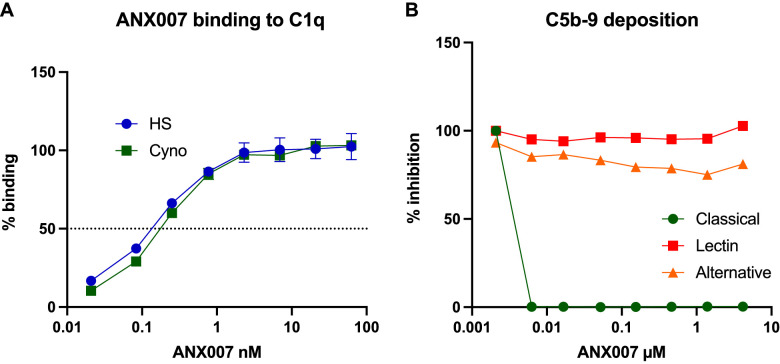
The binding affinity of ANX007 to purified human C1q and to C1q captured from human and cynomolgus monkey serum were similar, with EC50 values of 136 pM (7.7 ng/mL), 147 pM (8 ng/mL), and 179 pM (9.5 ng/mL), respectively (Fig. 2A). These data indicate that the biodistribution and any target-mediated effects are likely to translate from cynomolgus monkeys to humans well. ANX007 specifically inhibited classical complement-mediated activation at 6 nM (300 ng/mL) and had no effect on the lectin or the alternative pathway (Fig. 2B), and thus make ANX007 an ideal candidate for testing effect of C1q inhibition in synaptic function in the eye.
Figure 2.
(A) EC50 of binding to human and cynomolgus monkey C1q is equivalent. ANX007 binding to C1q was determined in vitro using a one-sided enzyme-linked immunosorbent assay with C1q purified from human and cynomolgus serum (maximum luminescence counts to human was 1258074 and to cynomolgus monkey was 1051459). (B) ANX007 inhibits classical but not lectin or alternative complement pathway activation. ANX007 effects on complement activation assays were measured in Wieslab assays (EURO DIAGNOSTICA AB, Sweden) using specific activators of the classical, lectin, and alternative pathways. Cyno, cynomolgus monkey; EC50, half maximal effective concentration; HS, human serum.
ANX007 Tolerability
Parameters studied to evaluate ANX007 tolerability included ophthalmic examinations, electroretinograms, and tonometry. All doses administered were well tolerated, and no adverse ANX007-related changes were noted. Any clinical observations were considered to be unrelated to ANX007 because the observations were not dose dependent, and/or were transient, and/or related to study procedures.
Ocular Pharmacokinetics and Pharmacodynamics
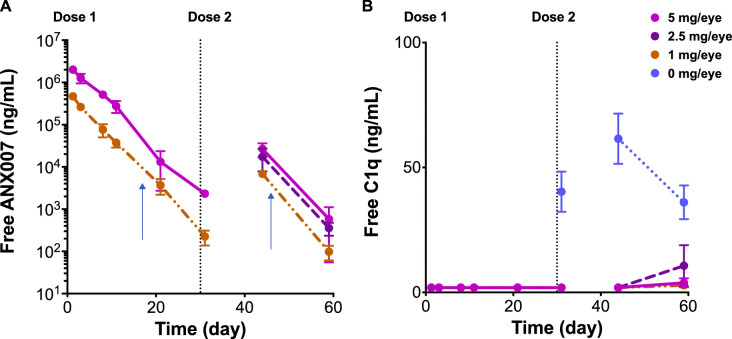
Across studies, ANX007 was dosed at 1, 2.5, or 5 mg/eye as a single dose or as 2 doses on days 1 and 29. Following a single dose, animals were taken down at days 1 (6 hours postdose), 2, 7, 10, 20, or 30, and following 2 doses, on day 44 (approximately 2 weeks post-second dose), or day 59 (approximately 4 weeks post-second dose). At all dose levels tested, vitreous free-ANX007 levels were measurable at approximately 4 weeks following a single dose or repeat doses of ANX007 (Fig. 3A). Likewise, free-C1q levels were inhibited at these timepoints in treated animals (Fig. 3B). The ANX007 half-life was approximately 3 days in the vitreous humor. Aqueous and vitreous levels were highly correlated, and aqueous levels of free-ANX007 were approximately four-fold lower than in the vitreous (data not shown). The PK results for the single dose toxicity study in female cynomolgus monkeys are shown in Table 3.
Figure 3.
Mean vitreous pharmacokinetics and pharmacodynamics at dose levels of 0, 1, 2.5, and 5 mg ANX007 per eye. Doses were administered at day 1 and day 29. Day 44 is approximately 2 weeks after the second dose, and day 59 is approximately 4 weeks post-last dose. N = 2 for 1 to 29 days, and N = 6 at 44 and 59 days. Error bars = standard error of the mean. Graphs represent combined data from single and multiple dose studies. (A) Free-ANX007 levels. (B) Free-C1q levels. Vertical arrows in (A) represent 14 days after the single and repeat-dose intravitreal injections of ANX007, respectively.
Table 3.
Results for a Single Dose Toxicity Study in Female Cynomolgus Monkeys
| Dose (mg/eye) | 1 | 5 |
|---|---|---|
| Theoretical maximum vitreous concentration (mg/mL) | 0.5 | 2.5 |
| Dose volume (µL) | 50 | 100 (two 50 µL doses separated by 10 minutes) |
| No. of main study animals (females) | 12 | 12 |
| Serum TK | ||
| Cmax (ng/mL) | BQL | 2.03 |
| AUClast (h*ng/mL) | BQL | 204 |
| Tmax (h) | NA | 48 |
| Vitreous TK | ||
| Cmax (ng/mL) | 468,000 | 2,010,000 |
| AUClast (h*ng/mL) | 46,500,000 | 247,000,000 |
| Tmax (h) | 6 | 6 |
| Mortality | 0/12 | 0/12 |
| Clinical signs | None | None |
| Histopathology (minimal to mild basophilia*) | 1/12 | 8/12 |
Group mean Cmax and AUC were not reported, thus the group range is listed.
AUClast, area under the concentration versus time curve from the start of dose administration to the last observed quantifiable concentration; BQL, below quantitation limit; Cmax, maximum concentration; NA, not available; TK, toxicokinetics; Tmax, time after dosing at which maximum observed concentration was observed.
This finding occurred in the repeat dose studies in control and treated animals and was determined to be not test article related.
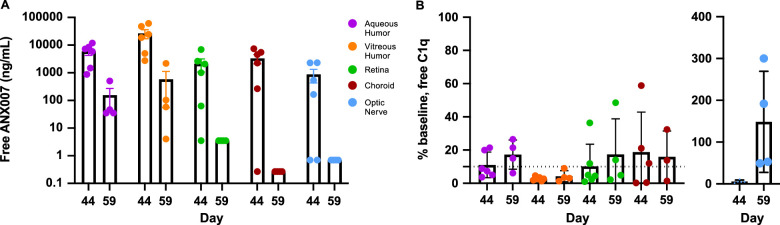
Following bilateral repeat dosing with 5 mg ANX007/eye on day 1 and day 29, free-ANX007 was detected in all sampled tissues (perfused) and fluids (Fig. 4A) at concentrations of 1 µg/mL. At day 59 (approximately 4 weeks post-last dose), free-ANX007 persisted in vitreous and aqueous humor but not in the optic nerve, choroid, or retina. In contrast, suppression of free-C1q levels was maintained at days 44 and 59 in all tissues except the optic nerve. These data indicate that binding of ANX007 to C1q persists to 59 days in all tissues but the optic nerve, and that ANX007 was able to access the back of the eye (Fig. 4B). Importantly, to accurately measure C1q suppression, tissues were perfused to eliminate C1q in blood (approximate concentration in serum is 30 to 60 µg/mL). Notably, the distance for diffusion of ANX007 from the injection site in the vitreous would be highest in the optic nerve. Baseline optic nerve C1q levels were the highest of the perfused tissues assessed which, combined with the furthest distance from the injection site, likely explains the more rapid recovery of C1q levels in the optic nerve at day 59 as compared to other ocular tissues and fluids, which are more proximal to the injection site. The average predose free-C1q serum level in cynomolgus monkeys was 33917.5 ng/mL (range = 16,970 to 44,510 ng/mL). Whereas C1q was fully engaged by ANX007 in the tissues, levels of total-C1q were not changed (data not shown).
Figure 4.
Mean ocular tissue and fluid pharmacokinetics and pharmacodynamics following two 5 mg/eye doses of ANX007 at 1-month intervals. Day 44 is approximately 2 weeks post-last dose, and day 59 is approximately 4 weeks post-last dose. (A) Free-ANX007 levels. (B) Free-C1q (% of baseline using the average of the control group). Baseline free-C1q levels (mean ± standard deviation) were: aqueous humor 3.5 ± 2.3 ng/mL, vitreous humor 67 ± 35 ng/mL, retina 151 ± 165 ng/mL, choroid 35 ± 35 ng/mL, and optic nerve 430 ± 273 ng/mL. Control animals are indicated as day 1/baseline. The 5 mg/eye dose was the only dose that was perfused. N = 3–6 eyes. Assay lower limit of quantitation is shown in (B) with the dotted horizontal line. Error bars = standard error of the mean.
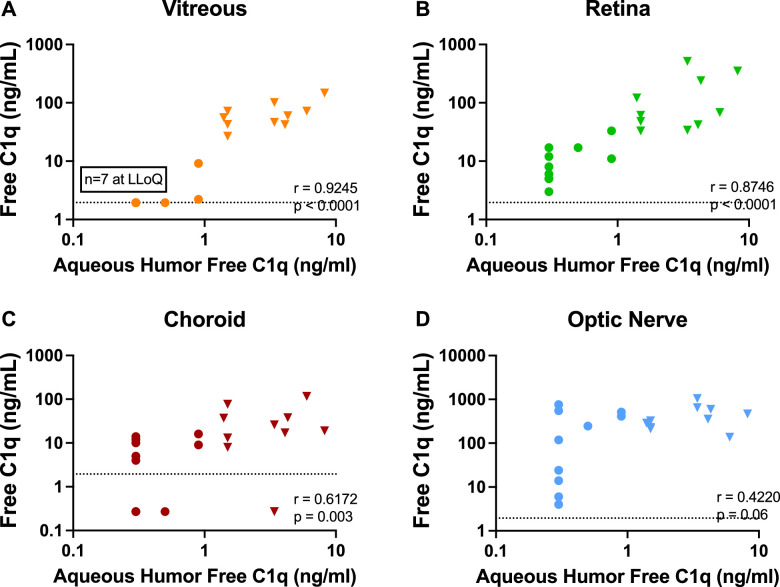
Aqueous humor free-C1q suppression strongly correlated with C1q suppression in perfused tissues, particularly the vitreous and retina (Figs. 5A, 5B; P < 0.0001). These results demonstrate that aqueous humor C1q target engagement is reflected in C1q lowering in both vitreous and the retina. There was a weaker but still significant correlation between aqueous and choroid free-C1q levels (Fig. 5C; P = 0.003), whereas the correlation between optic nerve and aqueous free-C1q levels was less clear (Fig. 5D; P = 0.06). These results demonstrate that intravitreal administration of ANX007 is associated with C1q engagement in the retina, the key target tissue affected in ocular neurodegenerative conditions.
Figure 5.
Correlation of aqueous humor C1q suppression. (A) Vitreous humor. Ocular tissues (B) retina, (C) choroid, and (D) optic nerve. Presented data are a combination of control (triangles) and 5 mg/eye ANX007-treated (circles) animals and show levels of free-C1q in perfused tissues and aqueous humor following 2 ANX007 doses of 5 mg/eye at a 1-month interval. Spearman r = correlation. Assay lower limit of quantitations (LLOQs) are detailed in the Methods section and are shown with dotted horizontal lines.
The generation of anti-drug antibody (ADA) was assessed in a validated assay in the repeat dose 5 mg/eye study, and 9 of the 10 treated animals were ADA positive. However, the effects of ADA on the toxicokinetics of ANX007 were not included in the toxicokinetic evaluation due to the low and transient systemic exposure of ANX007 in serum, and because sample timepoints were limited to a single terminal timepoint for ANX007 in vitreous.
Discussion
ANX007 is a high affinity Fab fragment antibody that shows robust binding to human and cynomolgus monkey C1q. Following IVT administration in cynomolgus monkeys, ANX007 was well tolerated and distributed to relevant sites of neurodegenerative ophthalmic disease within the retina, with clear evidence of C1q target engagement. The presence of free-ANX007 was associated with a reduction of free-C1q, demonstrating target engagement in ocular fluids and perfused ocular tissues. C1q inhibition was near complete in all samples at day 44, approximately 2 weeks after the second dose. The intracellular pool of C1q in microglia35 is not accessible to ANX007, and, therefore, a reduction of greater than 80% in tissues is likely equivalent to complete target engagement in the extracellular space. Although free-Fab was no longer detected, C1q was still bound to ANX007 and thus fully inhibited in the choroid at day 59 (approximately 4 weeks post-last dose). The assays employed cannot measure ANX007 or C1q that is bound to each other.
The half-life of ANX007 in the vitreous following IVT administration was approximately 3 days, which is consistent with published data for other humanized monoclonal antibody fragments administered through this route, including lampalizumab (2.9 days)36 and ranibizumab (approximately 3 days; Lucentis, Genentech).37
The degree of C1q engagement by drug in the retina and the vitreous showed statistically significant correlation with the degree of engagement in the aqueous humor. These results, combined with the mechanism of action and binding properties of ANX007, suggest that ANX007 is able to inhibit classical complement in retinal tissues relevant to neurodegenerative ophthalmic disease following IVT administration. Importantly, in clinical studies where vitreous and ocular tissues cannot be sampled, these data support the relevance of aqueous humor target engagement as a surrogate for retinal target engagement.
In conclusion, these studies demonstrated that IVT-administered ANX007 was well tolerated and distributed to retinal sites of neurodegenerative ophthalmic diseases, with clear evidence of C1q target suppression. Measurement of C1q levels in aqueous humor provides a surrogate approach to represent drug levels at the back of the eye after IVT administration of ANX007. These data support further clinical evaluation of ANX007 as a potential novel treatment for diseases associated with complement-mediated retinal degeneration, such as GA and glaucoma.
Acknowledgments
The authors would like to recognize and thank Susan Pederson for her review of this manuscript; the following Study Directors at Charles River, Reno, for the successful conduct of the nonclinical ocular toxicology studies: Margaret E. Collins, MS, and Andrew Lickteig, PhD, DABT; and Logan Kuhn of Annexon Biosciences, Inc., for providing the Figure 2 binding data.
Funded by Annexon Biosciences, Inc.
Disclosure: A. Grover, Annexon (E); S. Sankaranarayanan, Escape Bio (E), Annexon (E); V. Mathur, Acelot Inc. (E), Annexon (E); P. Suri, Annexon (E); H. Qiu, Annexon (E); Y. Andrews-Zwilling, Annexon (E); K. Mease, Annexon (C), ToxStrategies Inc. (E); L.K. Taylor, Annexon (E); E. Cahir-McFarland, Annexon (E); S. Keswani, Annexon (E); T. Yednock, Annexon (E)
References
- 1. Stevens B, Allen NJ, Vazquez LE, et al.. The classical complement cascade mediates CNS synapse elimination. Cell. 2007; 131: 1164–1178. [DOI] [PubMed] [Google Scholar]
- 2. Schafer DP, Lehrman EK, Kautzman AG, et al.. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron . 2012; 74: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stephan AH, Barres BA, Stevens B.. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012; 35: 369–389. [DOI] [PubMed] [Google Scholar]
- 4. Stasi K, Nagel D, Yang X, et al.. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci . 2006; 47(3): 1024–1029. [DOI] [PubMed] [Google Scholar]
- 5. Ziabska K, Ziemka-Nalecz M, Pawelec P, Sypecka J, Zalewska T.. Aberrant complement system activation in neurological disorders. Int J Mol Sci . 2021; 22: 4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong S, Beja-Glasser VF, Nfonoyim BM, et al.. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016; 352(6286): 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lui H, Zhang J, Makinson SR, et al.. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016; 165(4): 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sekar A, Bialas AR, de Rivera H, et al.. Schizophrenia risk from complex variation of complement component 4. Nature. 2016; 530: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuehn MH, Kim CY, Ostojic J, et al.. Retinal synthesis and deposition of complement components induced by ocular hypertension. Exp Eye Res. 2006; 83: 620–628. [DOI] [PubMed] [Google Scholar]
- 10. Tezel G, Yang X, Luo C, et al.. Oxidative stress and the regulation of the complement activation in human glaucoma. Invest Ophthalmol Vis Sci. 2010; 51(10): 5071–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howell GR, Macalinao DG, Sousa GL, et al.. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest . 2011; 121: 1429–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohlin C, Sandholm K, Ekdahl KN, Nilsson B.. The link between morphology and complement in ocular disease. Mol Immunol. 2017; 89: 84–99. [DOI] [PubMed] [Google Scholar]
- 13. Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmol. 2014; 121(11): 2081–2090. [DOI] [PubMed] [Google Scholar]
- 14. Chirco KR, Potempa LA.. C-reactive protein as a mediator of complement activation and inflammatory activation and inflammatory signaling in age-related macular degeneration. Front Immunol. 2018; 9(539): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katschke KJ, Xi H, Cox C, et al.. Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Sci Rep. 2018; 8(1): 7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curcio CA, Medeiros NE, Millican CL.. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996; 37: 1236–1249. [PubMed] [Google Scholar]
- 17. Miller JW, D'Anieri LL, Husain D, Miller JB, Vavvas DG. Age-related macular degeneration (AMD): a view to the future. J Clin Med . 2021; 10: 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ikeda K, Sannoh T, Kawasaki N, Kawasaki T, Yamashina I.. Serum lectin with known structure activates complement through the classical pathway . J. Biol. Chem. 1987; 262: 7451–7454. [PubMed] [Google Scholar]
- 19. Dommett RM, Klein N, Turner MW.. Mannose-binding lectin in innate immunity: past, present and future. Tissue Antigens. 2006; 68: 193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dunkelberger JR, Song W-C.. Complement and its role in innate and adaptive immune responses. Cell Res . 2010; 20: 34–50. [DOI] [PubMed] [Google Scholar]
- 21. Pauly D, Agarwal D, Dana N, et al.. Cell-type-specific complement expression in the healthy and diseased retina. Cell Rep. 2019; 29(9): 2835–2848.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stephan AH, Madison DV, Mateos JM, et al.. A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013; 33: 13460–13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams PA, Tribble JR, Pepper KW, et al.. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol Neurodegener. 2016; 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grozdanic SD, Kecova H, Harper MH, Nilaweera W, Kuehn MH, Kardon RH.. Functional and structural changes in a canine model of hereditary primary angle-closure glaucoma. Invest Ophthalmol Vis Sci . 2010; 51: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howell GR, Soto I, Ryan M, Graham LC, Smith RS, John SWM.. Deficiency of complement component 5 ameliorates glaucoma in DBA/2J mice. J Neuroinflammation. 2013; 10: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiao H, Rutar M, Fernando N, et al.. Subretinal macrophages produce classical complement activator C1q leading to the progression of focal retinal degeneration. Mol Neurodegener. 2018; 13(1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silverman SM, Kim B-J, Howell GR, et al.. C1q propagates microglial activation and neurodegeneration in the visual axis following retinal ischemia/reperfusion injury. Mol Neurodegener. 2016; 11: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bohlson SS, Fraser DA, Tenner AJ.. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007; 44(1-3): 33–43. [DOI] [PubMed] [Google Scholar]
- 29. Kim HM, Woo SJ.. Ocular drug delivery to the retina: current innovations and future perspectives. Pharmaceutics. 2021; 13: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ClinicalTrials.gov. A phase 1, open-label, single-dose, dose-escalation, safety, tolerability, and PK study of intravitreal ANX007 in participants with primary open-angle glaucoma. NCT04188015 completed August 3, 2018. Available at: https://clinicaltrials.gov/ct2/show/NCT04188015. Accessed April 6, 2021.
- 31. ClinicalTrials.gov. A phase 1b, randomized, double-masked, sham-controlled study of ANX007 administered as intravitreal injections to assess safety and tolerability in participants with primary open-angle glaucoma. NCT03488550 completed June 3, 2019. Available at: https://clinicaltrials.gov/ct2/show/NCT03488550. Accessed April 6, 2021.
- 32. U.S. Department of Agriculture. Final Rules: Animal welfare; 9 CFR Parts 1, 2, and 3. Federal Register. 1989; 54(168): 36112–36163. Available at: https://www.nal.usda.gov/awic/final-rules-animal-welfare-9-cfr-parts-1-2-and-3. Accessed April 8, 2021. [Google Scholar]
- 33. U.S. Department of Health and Human Services, NIH. Public Health Service Policy on Humane Care and Use of Laboratory Animals from the Office of Laboratory Animal Welfare. U.S. Department of Health and Human Services and National Institutes of Health, Office of Laboratory Animal Welfare. Revised 2015. Available at: https://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf. Accessed April 8, 2021. [Google Scholar]
- 34. National Institutes of Health. Guide for the care and use of laboratory animals, 8th ed. Washington, DC. National Academies Press. Available at: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf. Accessed April 8, 2021. [Google Scholar]
- 35. Fonseca MI, Chu S-H, Hernandez MX, et al.. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflammation. 2017; 14: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le KN, Gibiansky L, Good J, et al.. A mechanistic pharmacokinetic/pharmacodynamic model of factor D inhibition in cynomolgus monkeys by lampalizumab for the treatment of geographic atrophy. J Pharmacol Exp Ther. 2015; 355(2): 288–296. [DOI] [PubMed] [Google Scholar]
- 37. Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V.. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005; 46(2): 726–733. [DOI] [PubMed] [Google Scholar]