Abstract
Purpose:
The purpose of this study was to evaluate the stability of rotary chair, video head impulse test (vHIT), and vestibular evoked myogenic potential (VEMP) responses in children with normal hearing (NH) and children with cochlear implants (CIs).
Method:
Retrospective analysis of 66 children (33 males, M age = 11.4 years, range: 3–18 years) seen in a tertiary clinic and/or research laboratory who completed rotary chair, VEMP, and vHIT across two test sessions between 2012 and 2019. The stability of these measures was compared between two groups: children with NH (n = 35) and children with CI (n = 31). For each outcome, the session difference was calculated by subtracting Session 1 from Session 2.
Results:
For rotary chair (gain and phase) and vHIT (gain), linear mixed-effects models revealed that there were no significant interactions or main effects for group (CI vs. NH), time between session, gender, or age on the session difference, suggesting that the outcomes of these measures are stable across sessions. For cervical and ocular VEMP amplitude, there was a significant interaction between group and time between sessions on the session difference. Specifically, children with NH demonstrated larger amplitudes at Session 2, whereas children with CI demonstrated smaller amplitudes at Session 2. Next, test findings were classified as normal, unilaterally abnormal, or bilaterally abnormal for Sessions 1 and 2. Misclassification was defined as a mismatch of classification between sessions. Rotary chair and vHIT had the fewest misclassifications, whereas cervical VEMPs had the most misclassifications in children with CI and ocular VEMPs had the most misclassifications in children with NH. Misclassifications in children with CI were mostly consistent with progressive vestibular loss, whereas misclassifications in children with NH were mostly consistent with improved vestibular function.
Conclusions:
Stability and misclassification rates varied between tests and groups. Overall, rotary chair and vHIT outcomes were stable in both groups; however, VEMPs differentially changed between groups, improving in children with NH and declining in children with CI. Furthermore, despite relative stability, some children with CI evidenced progressive vestibular loss on all measures suggesting that vestibular testing should be completed serially due to the possibility of progression.
Rotary chair, video head impulse test (vHIT), and vestibular evoked myogenic potentials (VEMPs) are the most common vestibular assessments used in pediatrics. While all three of these measures are feasible in children, reliability of these measures has primarily been investigated in typically developing children with few studies examining the stability of these measures in children with hearing loss. The likelihood of vestibular loss rises as the severity of hearing loss increases (Brookhouser et al., 1991; Janky, Thomas, et al., 2018). As such, vestibular loss is more common in children with a cochlear implant (CI; Cushing et al., 2013; Janky & Givens, 2015); however, it is unknown if vestibular function is stable in this population or if declines in vestibular function can be seen over time. Therefore, the purpose of this study was to evaluate the stability of rotary chair, vHIT, and VEMP testing over multiple sessions in children with normal hearing (NH) and children with CI.
These three measures provide complementary information regarding vestibular function and may be completed in isolation or in conjunction. Rotary chair assesses midfrequency (.01–.64 Hz) horizontal canal and superior vestibular nerve function; vHIT assesses high-frequency function of each semicircular canal and subsequently both superior and inferior vestibular nerve function; cervical VEMP (cVEMP) assesses saccule and inferior vestibular nerve function; and ocular VEMPs (oVEMP) assess utricle and superior vestibular nerve function. Thus, collectively, these assessments can evaluate the entire vestibular periphery.
Rotary chair can be completed in children as young as 2 months (Janky & Rodriguez, 2018); however, there are conflicting reports of age effects on rotary chair. Abnormal responses in children younger than 1 year have been attributed to maturation (Eviatar & Eviatar, 1979). Both an inverse (Charpiot et al., 2010; Ornitz et al., 1985; Valente, 2007) and linear (Casselbrant et al., 2010) relationship between vestibulo-ocular reflex (VOR) gain and age have been reported. While gain has been found to be reliable in children ages 3–11 years with NH when repeated in a single session (Valente, 2007), no studies have examined test–retest reliability or outcome stability in children with hearing loss over time.
vHIT using goggles can be completed in children as young as 4 years (Bachmann et al., 2018) or with a remote camera system (e.g., Synapsys) in infants < 1 year (Verrecchia et al., 2020; S. R. Wiener-Vacher & Wiener, 2017). Like rotary chair, there are conflicting reports of age effects on vHIT gain. Lower gains are present in children < 3 years, and variability is larger until 6 years (S. R. Wiener-Vacher & Wiener, 2017). While higher VOR gain has been shown in typically developing children compared with adults (Bachmann et al., 2018), others report no significant difference in VOR gain in typically developing children compared with adults (Ross & Helminski, 2016; S. R. Wiener-Vacher & Wiener, 2017). Good test–retest reliability has been found for VOR gain in all six semicircular canals within a single session (intraclass correlation coefficient [ICC] ≥ .821 ≤ .945) in children 4–17 years along with good intrarater reliability between three examiners for the same session (ICC ≥ .800 ≤ .971; Ross & Helminski, 2016). In infants and young children (4–79 months of age) with sensorineural hearing loss, all but three subjects were able to complete vHIT using a remote camera; vHIT was in good agreement with mini ice-water calorics (p < .05), suggesting that vHIT is both a feasible and valid measure of horizontal canal function; however, test–retest reliability or outcome stability were not evaluated (Verrecchia et al., 2020).
cVEMPs have been recorded in infants (Sheykholeslami et al., 2005; Verrecchia et al., 2020; Wang et al., 2008) but are more commonly administered in children 3 years and older (Hsu et al., 2009; Janky & Rodriguez, 2018). oVEMPs can be administered in children after age 2 years (Wang et al., 2013), due to the later developing VOR (Hsu et al., 2009) and ability to maintain upward gaze. In typically developing children, c- and oVEMP amplitudes are not significantly different from adults (Valente, 2007; Wang et al., 2013); however, larger intersubject variability was observed for cVEMPs in children (Valente, 2007). cVEMPs and oVEMPs have been found to be reliable for typically developing children (4–10 years) and adolescents (11–18 years) across two sessions (Fuemmeler et al., 2020; Greenwalt et al., 2021). Verrecchia et al. (2020) completed cVEMPs on infants and young children (4–79 months of age) with sensorineural hearing loss and had 80% compliance rate and 66% of subjects had valid responses defined as repeatable waves (present or absent); however, test–retest reliability or outcome stability were not evaluated.
In our clinic, vestibular assessments are completed in children when there are concerns for dizziness, to assist in determining the etiology of hearing loss and prior to cochlear implantation. Furthermore, our research lab completes vestibular testing to investigate appropriate test techniques (Merchant et al., 2020; Patterson et al., 2020; Rodriguez et al., 2018, 2019) and determine the effects of vestibular loss in children (Janky & Givens, 2015; Janky, Thomas, Al-Salim, et al., 2022; Janky, Thomas, Patterson, et al., 2022). Clinically, children with hearing loss are seen for vestibular testing once and then are only reassessed if concerns arise. In the research lab, some children are seen for vestibular testing serially if they participate in multiple studies. Therefore, the purpose of this study was to determine the stability of vestibular function over time. This is particularly important in children with hearing loss as both developmental and physiologic changes related to the etiology of hearing loss can result in changes to vestibular function.
For children with NH, we hypothesized that despite developmental changes, there would be no significant difference between Sessions 1 and 2 for the three measures, suggesting that these measures are stable, and that classification (i.e., normal vs. unilaterally abnormal vs. bilaterally abnormal) of vestibular function would also remain stable across repeat sessions. For the children with CI, we hypothesized that progressions in vestibular loss may occur, which are etiology dependent, resulting in reduced responses and classification mismatches from Session 1 to Session 2.
Method
Subjects
Data from 66 subjects (33 males, M age = 11.4 years, range: 3–18 years) who completed rotary chair, VEMP, and/or vHIT across two sessions in the Vestibular and Balance Research Laboratory and/or the Vestibular Clinic at Boys Town National Research Hospital (BTNRH) between 2012 and 2019 were retrospectively reviewed. Informed consent was obtained from all subjects for testing approved by the institutional review board at BTNRH. Subjects included 35 children with NH (20 males, M age = 10.9 years, range: 4–18 years) and 31 children with CI (13 males, M age = 11.9 years, range: 3–17 years). All children with NH were seen in the research laboratory as part of multiple research studies and denied any history of dizziness or neurologic complaints. Children with CI were seen as part of multiple research studies or once as part of evaluation in a specialty pediatric hearing clinic and the research laboratory. Tympanometry was completed prior to vestibular testing on all but two subjects; however, both subjects had normal otologic examination with a neurotologist the same day of testing. The remaining subjects had Type A tympanograms, suggesting normal middle ear function. In the children with CI, etiologies of hearing loss included Pendred/large vestibular aqueduct (n = 4), Mondini malformation (n = 2), genetic (n = 2), cytomegalovirus (CMV; n = 3), auditory neuropathy (AN; n = 2), Connexin 26 (n = 2), Waardenburg's syndrome (n = 1), and unknown (n = 15).
Rotary Chair
A total of 33 subjects, 12 subjects with NH (M age = 10.9 years, range: 7–16 years) and 21 subjects with CI (M age = 12.4 years, range: 6–17 years), completed sinusoidal harmonic acceleration (SHA) testing (MicroMedical Technologies) across two sessions. Mean duration between test sessions was 19.9 months (range: 6–56 months). Subjects were seated in a motorized rotary chair in a light-tight booth, preventing ambient light. Eye movements were recorded by either an infrared, two-dimensional video system or with electrodes. Because rotary chair protocols changed within the lab over the course of 7 years, SHA testing was completed in response to a combination of the following frequencies: 0.01, 0.02, 0.04, 0.08, 0.16, and 0.32 Hz with a fixed maximum chair velocity of 60°/s or with variable maximum velocities of 80°/s, 70°/s, 60°/s, 50°/s, 40°/s, and 30°/s, respectively. Thirty subjects completed velocity step testing during one session, at a rate of 100°/s to a set velocity of 100°/s for 45 s followed by deceleration of 100°/s to come to a complete stop. Velocity step testing was only used as needed for this study to categorize bilateral vestibular loss as outlined below. Calibration was attempted for each subject based on manufacturer recommendations. If a valid calibration could not be obtained, default calibration was used. Outcome parameters included gain (eye velocity/chair velocity) and phase (time constant). Because gain and phase are used to determine the presence of vestibular loss and symmetry is used to determine status of central compensation, only the agreement of gain and phase were calculated.
Consistent with Janky and Patterson (2020), rotary chair outcomes were used to categorize vestibular function as
normal: defined as normal gain, phase, and symmetry according to manufacturer normative data
unilaterally abnormal: defined as abnormally low gain (according to manufacturer normative data) and phase lead < 68° at 0.01 Hz
bilaterally abnormal: defined by Strupp et al. (2017): VOR gain < 0.1 at 0.01 Hz, phase lead > 68° at 0.01 Hz or a step time constant < 5 s. When completed, step time constants were < 5 s for all four generated time constants (clockwise, counterclockwise, clockwise stop, counterclockwise stop); (Janky, Patterson, et al., 2018; Judge et al., 2017; MacDougall & Curthoys, 2012; Strupp et al., 2017).
All data were examined to ensure clean data. Tracings with excess noise (e.g., subject crying or excessive movement) requiring mostly qualitative analysis were excluded from this study.
vHIT
A total of 21 subjects, 4 subjects with NH (M age = 10.3 years, range: 7–15 years) and 17 subjects with CI (M age = 12.5 years, range: 6–17: years), completed vHIT testing across two sessions. Mean duration between sessions was 19.9 months (range: 6–49 months). The vHIT was administered using an Otometrics Impulse unit (Natus). Subjects were seated 1 m from a visual target mounted at eye level on the wall and the examiner stood behind the subject to deliver randomized horizontal head impulses (100° to 250°/s peak head velocity). Approximately 20 acceptable head impulses were recorded from the right and left semicircular canals. Calibration was completed for each subject based on manufacturer recommendations. Outcome parameters included gain (eye velocity/head velocity) and presence of corrective saccades.
Consistent with Janky and Patterson (2020), vHIT tracings were used to categorize vestibular function as:
normal: defined as gain ≥ 0.78 with no reproducible corrective saccades (occurring in < 80% of impulses).
unilaterally abnormal: defined as gain < 0.78 with the presence of reproducible corrective saccades (occurring in ≥ 80% of impulses) to either the right or left.
bilaterally abnormal: defined as gain < 0.6 with the presence of reproducible corrective saccades (occurring in ≥ 80% of impulses) to both the right and left (Judge et al., 2017; MacDougall & Curthoys, 2012; Strupp et al., 2017).
VEMP
A total of 51 subjects completed c- and oVEMPs; 24 subjects with NH (M age = 10.4 years, range: 4–18 years) completed c- and oVEMPs, 27 subjects with CI (M age = 12.2 years, range 5–17 years) completed cVEMP, and 22 subjects with CI completed oVEMPs (M age = 11.4 years, range: 5–17 years) across two sessions. Mean duration between sessions was 18 months (range: 2–52 months). VEMPs were completed using an Otometrics Chartr EP 200 (Natus) or a Bio-logic Navigator Pro (Natus). VEMP protocols changed over the 7 years and were combined across data sets; thus, the VEMP methodology varied slightly.
For cVEMPs, all subjects lay in a semirecumbent position. Active electrodes were placed on each sternocleidomastoid muscle (SCM) and aligned with the subject's chin (middle one third of SCM), a reference electrode was placed on the manubrium of the sternum and the ground electrode was placed on either the inner canthi of the eye or the forehead. For 80/106 cVEMP sessions, electromyography (EMG) was monitored directly below the active electrode on the SCMs. EMG was targeted between 100 and 300 μV. For the remaining 26/106 sessions, EMG monitoring was not completed due to equipment capability.
For oVEMPs, the active electrodes were either centered under each pupil or placed mediolaterally under each eye. The reference electrode was placed directly below the active electrode or on the right inner canthi of the eye. The ground electrode was placed on the forehead, the chin, or the manubrium of the sternum. All subjects were instructed to gaze at a target at 30° up-gaze.
For both c- and oVEMP, stimuli were 500 (in 106 of the VEMP sessions) or 750 Hz (in 35 of the VEMP sessions) tone bursts presented at 125 dB SPL at a rate of 5.1/s (Blackman gating, two cycle rise/fall, zero cycle plateau, minimum of 75 sweeps). Gain was set at 5 K with a bandpass filter of 1 – 1 k Hz. After 2017, in subjects with ear canal volumes < 0.8 ml, stimuli were presented at 120 dB SPL (Rodriguez et al., 2018).
VEMP tracings were used to categorize vestibular function as
normal: defined as two VEMP tracings, which replicated and rose 1.5 times out of the noise floor on both the right and left sides.
unilaterally abnormal: defined as absent responses for one ear and two tracings, which replicated and rose 1.5 times out of the noise floor for the remaining ear.
bilaterally abnormal: defined as absent responses, bilaterally.
Statistical Analysis
Descriptive statistics including mean and standard deviation were completed for each measure and each session (SPSS, Version 25). To evaluate the stability of the measures, we conducted linear mixed-effects models in an R environment using the lme4 package to determine the difference in rotary chair gain and phase, VEMP amplitude and vHIT gain at Time 1 compared with Time 2 between subjects with NH and subjects with CI. For each outcome, the session difference was calculated by subtracting Session 1 from Session 2. For VEMPs and vHIT, linear mixed models with repeated measures (i.e., adding a random effect on subjects to model within-subject variation) were applied to handle the outcomes measured repeatedly in left and right ear on the same subject. For all models, the model included group (NH vs. CI), age (continuous), gender, time between Sessions 1 and 2 (continuous) and their interactions as fixed effects. For VEMPs and vHIT, ear was also included as a fixed effect covariate.
Results
Rotary Chair
Descriptive statistics for rotary chair gain and phase at each test frequency across test sessions are included in Table 1. The linear mixed-effects model revealed no significant interactions or main effects for the difference in rotary chair gain or phase from Session 1 to Session 2 at any of the frequencies (p > .05).
Table 1.
Means (SD), mean session difference (SD), sample size for vestibular outcomes across sessions.
| Variable | NH |
CI |
||||
|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session difference | Session 1 | Session 2 | Session difference | |
| Frequency (Hz) | Rotary chair gain | |||||
|
.01 |
0.48 (—), 1 |
0.32 (—), 1 |
−0.16 (—) |
0.14 (0.18), 2 |
0.18 (0.02), 2 |
−0.04 (0.20) |
| .02 | 0.55 (0.08), 11 | 0.56 (0.14), 11 | 0.01 (0.08) | 0.42 (0.17), 16 | 0.41 (0.15), 16 | −0.01 (0.12) |
| .04 | — | — | — | 0.31 (0.16), 2 | 0.32 (0.09), 2 | 0.01 (0.25) |
| .08 | 0.64 (0.1), 12 | 0.62 (0.12), 12 | −0.08 (0.09) | 0.52 (0.18), 19 | 0.51 (0.16), 19 | −0.02 (0.12) |
| .16 | 0.70 (0.08), 11 | 0.67 (0.12), 11 | −0.01 (0.12) | 0.56 (0.16), 19 | 0.53 (0.13), 19 | −0.04 (0.15) |
|
.32
|
0.69 (0.1), 12 |
0.67 (0.11), 12 |
−0.03 (0.08) |
0.63 (0.15), 16 |
0.61 (0.12), 16 |
−0.03 (0.18) |
| Frequency (Hz) | Rotary chair phase | |||||
|
.01 |
40 (—), 1 |
47 (—), 1 |
7 (—) |
52.0 (—), 1 |
— |
— |
| .02 | 25.8 (9.6), 11 | 21.2 (8), 11 | −2.82 (5.34) | 29.5 (10.0), 13 | 35.3 (13.6), 13 | 6.08 (12.01) |
| .04 | — | — | — | 30 (—), 1 | 68 (—), 1 | 38 (—) |
| .08 | 5.3 (3.5), 12 | 6.1 (4.1), 12 | 1.92 (3.23) | 11.2 (7.8), 17 | 10.4 (10.1), 17 | −1 (7.96) |
| .16 | 3.0 (3), 11 | 4.1 (3.2), 11 | 1 (5.2) | 8.6 (8.6), 19 | 10.4 (12.5), 19 | 1.68 (5.44) |
|
.32
|
4.8 (3.8), 12 |
3.9 (2.5), 12 |
.83 (5.47) |
5.9 (4.2), 16 |
4.6 (6.3), 16 |
−2.41 (6.29) |
| Canal | vHIT gain | |||||
|
RH |
1.01 (0.1), 4 |
1.04 (0.1), 4 |
0.02 (0.10) |
0.80 (0.3), 17 |
.80 (0.3), 17 |
−0.0006 (0.06) |
|
LH
|
0.92 (0.05), 4 |
0.96 (0.06), 4 |
0.04 (0.10) |
0.76 (0.3), 17 |
.83 (0.2), 17 |
0.05 (0.22) |
| Ear | cVEMP amplitude | |||||
| Right |
212.5 (133.1), 24 |
306.23 (174.9), 24 |
128.62 (192.11) |
145.5 (215.6), 27 |
62.6 (89.0), 27 |
−82.97 (187.76) |
|
Left
|
239.7 (121.1), 24 |
312.7 (159), 24 |
97.70 (164) |
107.2 (145.0), 27 |
112.0 (159.2), 27 |
4.78 (169.47) |
| Ear | oVEMP amplitude | |||||
|
Right |
8.8 (11.6), 24 |
13.5 (9.7), 24 |
5.03 (8.89) |
1.9 (5.2), 22 |
2.0 (5.0), 22 |
.17 (2.3) |
| Left | 10.8 (11.2), 24 | 10.6 (7), 24 | 0.09 (10.98) | 3.5 (8.4), 22 | 2.9 (6.0), 22 | −.56 (4.81) |
Note. — = too few of cases for analysis; NH = normal hearing; CI = cochlear implant; vHIT = video head impulse test; RH = right horizontal; LH: left horizontal; cVEMP = cervical vestibular evoked myogenic potentials; oVEMP = ocular vestibular evoked myogenic potential.
vHIT
Descriptive statistics for mean vHIT gain in the right and left horizontal canals across test sessions are included in Table 1. The linear mixed-effects model revealed no significant interactions or main effects for the difference in vHIT gain from Session 1 to Session 2 (p > .05).
VEMPs
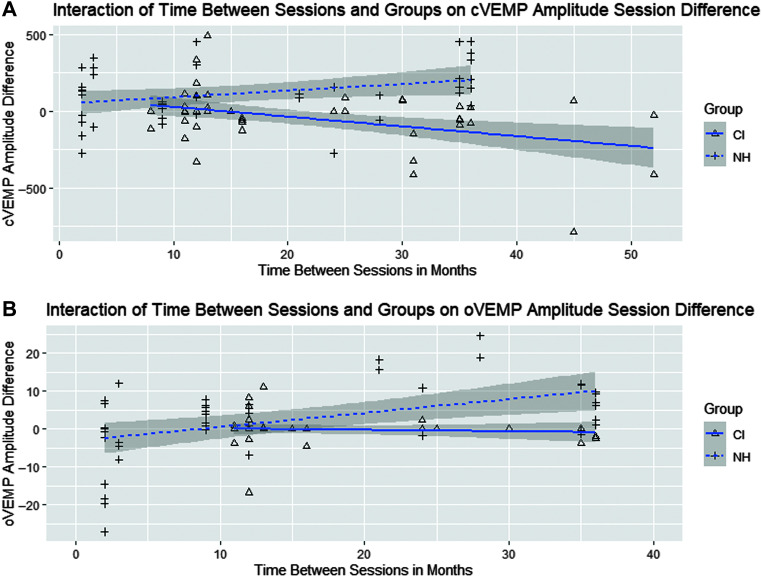
Descriptive statistics for mean cVEMP and oVEMP amplitude across test session are included in Table 1. For cVEMP, the linear mixed-effects model revealed a significant interaction between group and time between sessions (t = −3.701, p < .001; group difference 95% confidence interval [−250.25, −79.44]; see Table 2 and Figure 1a). For children with NH, with every 1 month increase in time between sessions, there is an increase in cVEMP amplitude by 4.5 μV, whereas, for children with CI, with every 1 month increase in time between sessions, there is a decrease in cVEMP amplitude by 5.8 μV. When sessions are 10 months apart, the mean cVEMP amplitude difference in children with CI is 78.7 μV less than that of children with NH (24.3 – 10.3 × 10) and 181.7 μV less for sessions 20 months apart (24.3 – 10.3 × 20).
Table 2.
Model evaluating the difference in cervical and ocular vestibular evoked myogenic potential (cVEMP and oVEMP) amplitude across two sessions.
| Variable | Estimate | Standard error | t value | p value |
|---|---|---|---|---|
|
cVEMP
| ||||
| Intercept | 21.17 | 70.76 | 0.30 | .77 |
| Group a | 24.28 | 63.07 | 0.39 | .70 |
| Age | 5.48 | 5.26 | 1.04 | .30 |
| Gender | −25.77 | 36.94 | −0.70 | .49 |
| Ear | −35.83 | 34.43 | −1.04 | .30 |
| Time between sessions | 4.48 | 2.01 | 2.23 | .03 |
|
Group
a
× Time between sessions
|
−10.26
|
2.77
|
−3.70
|
< .0001
|
|
oVEMP
| ||||
| Intercept | −4.36 | 2.94 | −1.48 | .14 |
| Group a | 3.90 | 2.83 | 1.38 | .17 |
| Age | −0.11 | 0.22 | −0.49 | .62 |
| Gender | 2.01 | 1.54 | 1.31 | .19 |
| Ear | 2.79 | 1.45 | 1.92 | .06 |
| Time between sessions | 0.35 | 0.08 | 4.31 | < .001 |
| Group a × Time between sessions | −0.40 | 0.14 | −2.85 | .005 |
Note. Bold and italicized indicate significant interaction.
Reference group was subjects with normal hearing.
Figure 1.
(A) Scatterplot of cervical vestibular evoked myogenic potential (cVEMP) amplitude session difference based on time between sessions (months) for both subjects with normal hearing (NH) and subjects with cochlear implants (CIs). There was a significant interaction between group and time between session (p < .001), with subjects with NH demonstrating an increase in cVEMP amplitude at Session 2 and subjects with CI demonstrating a decrease in cVEMP amplitude at Session 2. (B) Scatterplot of ocular vestibular evoked myogenic potential (oVEMP) amplitude session difference based on time between sessions (months) for both subjects with NH and subjects with CI. There was a significant interaction between group and time between sessions (p = .005), with subjects with NH demonstrating an increase in oVEMP amplitude at Session 2 and subjects with CI demonstrating a decrease in oVEMP amplitude at Session 2.
For oVEMP, the linear mixed-effects model revealed a significant interaction between group and time between sessions (t = −2.85, p = .005, group difference 95% confidence interval: [−6.53, 0.80]; see Table 2 and Figure 1b). For children with NH, with every 1 month increase in time between sessions, there is an increase in oVEMP amplitude by .35 μV, whereas, for children with CI, with every 1 month increase in time between sessions, there is a decrease in oVEMP amplitude by .06 μV. When sessions are 10 months apart, the mean oVEMP amplitude difference in children with CI is 7.9 μV less than that of children with NH (3.9 – [−0.40] × 10) and 11.9 μV less for sessions 20 months apart (3.9 – [−0.40] × 20).
Vestibular Classification
Rotary chair, vHIT, c- and oVEMP classifications (normal, unilaterally abnormal, and bilaterally abnormal) by subject group (NH vs. CI) across the two sessions are shown in Table 3. A detailed description of the misclassifications is shown in Table 4. In the NH group, there were no misclassifications for rotary chair (n = 12) and vHIT (n = 4) between test sessions. For cVEMP (n = 24), one subject initially had a unilaterally abnormal cVEMP response at Session 1 and normal cVEMP responses at Session 2. For oVEMP (n = 24), five subjects had either unilaterally (n = 2) or bilaterally (n = 3) abnormal oVEMP responses at Session 1 and normal oVEMP responses at Session 2 in all but one subject.
Table 3.
Vestibular test classifications by group across sessions.
| Group | Rotary chair |
cVEMP |
oVEMP |
vHIT |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 1 | Session 2 | Session 1 | Session 2 | Session 1 | Session 2 | ||
| NH | Normal | 12 | 12 | 23 | 24 | 17 | 22 | 4 | 4 |
| Unilaterally abnormal | 0 | 0 | 1 | 0 | 4 | 2 | 0 | 0 | |
| Bilaterally abnormal | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | |
| CI | Normal | 12 | 12 | 13 | 8 | 4 | 5 | 10 | 10 |
| Unilaterally abnormal | 5 | 2 | 7 | 8 | 4 | 2 | 5 | 5 | |
| Bilaterally abnormal | 5 | 8 | 9 | 13 | 14 | 15 | 2 | 2 | |
Note. cVEMP = cervical vestibular evoked myogenic potential; oVEMP = ocular vestibular evoked myogenic potential; vHIT = video head impulse test; NH = normal hearing; CI = cochlear implant.
Table 4.
Vestibular classifications at each session and etiology for subjects with misclassification.
| Test | Subject | Session 1 | Session 2 | Etiology |
|---|---|---|---|---|
| Rotary chair | CI10 | UVL | BVL | Unknown |
| CI13 | UVL | BVL | Unknown | |
| CI14 | UVL | BVL | CMV | |
| vHIT | CI3 | UV | Normal | Genetic |
| CI10 | Normal | UV | Unknown | |
| cVEMP | NH1 | UV | Normal | |
| CI2 | Normal | BV | Pendred | |
| CI5 | Normal | BV | Unknown | |
| CI6 | BV | UV | Unknown | |
| CI7 | Normal | BV | Waardenburg's syndrome | |
| CI8 | Normal | UV | Unknown | |
| CI9 | UV | Normal | Unknown | |
| CI10 | UV | BV | Unknown | |
| CI11 | Normal | UV | Connexin 26 | |
| CI12 | BV | UV | Unknown | |
| CI14 | Normal | BV | CMV | |
| CI15 | UV | BV | Auditory neuropathy | |
| oVEMP | NH1 | UV | Normal | |
| NH2 | BV | Normal | ||
| NH3 | UV | Normal | ||
| NH4 | BV | UV | ||
| NH5 | BV | Normal | ||
| CI2 | BV | Normal | Pendred | |
| CI9 | UV | BV | Unknown | |
| CI14 | UV | BV | CMV |
Note. CI = cochlear implant subject; UVL = unilateral vestibular loss; BVL = bilateral vestibular loss; CMV = cytomegalovirus; vHIT = video head impulse test; UV = unilateral vestibular abnormality; cVEMP = cervical vestibular evoked myogenic potential; NH = normal hearing subject; BV = bilateral vestibular abnormality; oVEMP = ocular vestibular evoked myogenic potential.
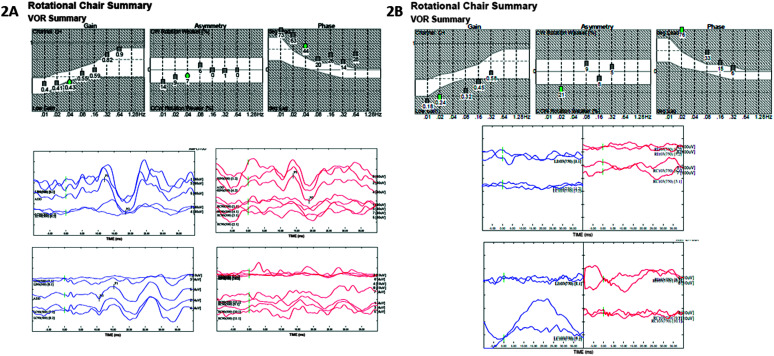
In the CI group, there were three subjects that had misclassifications on rotary chair (n = 22). These three subjects were classified as unilaterally abnormal at Session 1 and then bilaterally abnormal at Session 2. For vHIT (n = 17), there were two misclassifications. One subject was initially classified as unilaterally abnormal at Session 1 and then normal at Session 2. The second subject was initially classified as normal at Session 1 and unilaterally abnormal at Session 2. VEMPs had the largest number of misclassifications, with 11 misclassifications for cVEMPs and three misclassifications for oVEMPs. For cVEMP, eight subjects showed progressive loss and three subjects showed improved responses. Of the eight subjects with progressive loss, six subjects had normal cVEMP at Session 1, four of those subjects had bilaterally abnormal cVEMP, and two of those subjects had unilaterally abnormal cVEMP at Session 2; and two subjects had unilaterally abnormal cVEMP at Session 1 and bilaterally abnormal cVEMP at Session 2. Of the three with improved responses, two subjects had bilaterally abnormal cVEMP at Session 1 and then unilaterally abnormal cVEMP at Session 2 and one subject had unilaterally abnormal cVEMP at Session 1 and normal cVEMP at Session 2. For oVEMP, two subjects showed progressive loss and one subject showed improved responses. Of the two with progressive loss, both subjects had unilaterally abnormal oVEMP at Session 1 and then bilaterally abnormal oVEMP at Session 2. The one subject who showed improvement had bilaterally abnormal oVEMP at Session 1 and then normal oVEMP at Session 2. Results from a subject CI who had misclassifications on rotary chair, cVEMP and oVEMP are shown in Figure 2 (A: Session 1, B: Session 2).
Figure 2.
(A) Session 1 rotary chair and vestibular evoked myogenic potential (VEMP) results from a subject with misclassification. Subject was a child with CI, etiology of hearing loss was cytomegalovirus (CMV). Rotary chair results demonstrate normal gain with a phase lead, consistent with unilateral vestibular loss. Cervical vestibular evoked myogenic potential (cVEMP) demonstrates present cVEMPs, bilaterally. Ocular vestibular evoked myogenic potential (oVEMP) demonstrates present left oVEMP and absent right oVEMP, consistent with unilateral vestibular loss. (B) Session 2 rotary chair and VEMP results from the same subject (time between sessions = 16 months). Rotary chair results demonstrate abnormal gain with a phase lead, consistent with bilateral vestibular loss. cVEMP demonstrates absent cVEMPs bilaterally, consistent with bilateral vestibular loss. oVEMP demonstrates absent oVEMPs bilaterally, consistent with bilateral vestibular loss. VOR = vestibulo-ocular reflex.
Discussion
The purpose of this study was to evaluate the stability of rotary chair, vHIT, and VEMP testing outcomes across two sessions in children with NH and children with CI. Similar to previous literature examining test–retest reliability (Valente, 2007), our results demonstrated good rotary chair stability for subjects with NH and CI (0.02, 0.08, 0.16, and 0.32 Hz) with no significant differences between the groups. The interpretation of vestibular classification using rotary chair was also stable for most subjects with CI (n = 19/22) and all subjects with NH (n = 12/12). The three misclassifications in the CI group each demonstrated a decline in vestibular function, ultimately suggesting bilateral vestibular loss. Two of the subjects had an unknown hearing loss etiology and the last subject was diagnosed with CMV, discussed below.
Previous research has shown good test–retest reliability of vHIT gain in children with NH (Ross & Helminski, 2016). vHIT gain was stable between sessions (i.e., no significant main effects or interactions) for both groups. Furthermore, vHIT had the lowest number of misclassifications (n = 2). One subject with CI was classified as unilaterally abnormal at Session 1 and normal at Session 2; etiology of hearing loss was genetic. The second subject with CI was classified as normal at Session 1 and unilaterally abnormal at Session 2; etiology of hearing loss was unknown. For both subjects, test administration was similar across sessions, including distance to target and head velocity (≥ 150 degrees/second).
Studies completed in our lab revealed excellent reliability for air-conduction cVEMPs and oVEMPs in children (age 4–19 years) with NH, across two sessions (Fuemmeler et al., 2020; Greenwalt et al., 2021). However, results from this study demonstrated a significant interaction in cVEMP amplitudes for the groups and time between sessions. Specifically, children with NH demonstrated increase in cVEMP amplitude at Session 2 compared with Session 1. Conversely, the children with CI demonstrated a decrease in cVEMP amplitude at Session 2 compared with Session 1. Additionally, the mean difference in cVEMP amplitude between children with CI and children with NH continues to increase as the time between sessions gets longer (78.7 μV at 10 months and 181.7 μV at 20 months). Previous studies examined test–retest within days (range: 1–30 days; Fuemmeler et al., 2020; Greenwalt et al., 2021), whereas the mean duration between sessions for this study was 18 months (range: 2–52 months). Despite the longer duration, cVEMP amplitudes remain stable in children with NH. However, due to our larger time between sessions, methodological changes were made. Over the course of the 7 years, EMG recording was introduced. In 80/108 subject's EMG was monitored, only accepting waveforms if EMG was between 100 and 300 μV, allowing for more accurate cVEMP results. Methodological changes were made to enhance the cVEMP amplitude, thus these changes likely resulted in increased amplitudes in subjects with NH, whereas the decreased cVEMP amplitudes in subjects with CI are likely due to progression in vestibular loss.
Regarding classifications, only one subject with NH had a misclassification on cVEMP, where cVEMP was unilaterally absent at Session 1 and normal responses at Session 2. In subjects with CI, cVEMPs demonstrated the largest number of misclassifications (n = 11). Most subjects (8/11) demonstrated a progression of vestibular loss: six subjects progressed to bilaterally abnormal and two subjects progressed to unilaterally abnormal. These misclassifications are attributed to either progressive vestibular loss or differences in methods. For 3/8 subjects with progressive vestibular loss, 750 Hz was used in one session with 500 Hz used in the remaining session. For an additional 3/8 subjects demonstrating progressive vestibular loss, EMG was not monitored at Session 1 but was monitored at Session 2. In these cases, an improvement in VEMP responses with EMG monitoring was expected; thus, progressive vestibular loss is speculated. For the last 2/8 subjects demonstrating progressive vestibular loss, there were no differences between test session methods, suggesting progressive loss. In the remaining misclassified subjects (3/11), cVEMP improved. Two subjects improved from bilaterally abnormal at Session 1 to unilaterally abnormal at Session 2. The last subject improved from unilaterally abnormal at Session 1 to normal at Session 2. All three of these subjects had unknown etiology. For one subject, EMG was not monitored at Session 1 but EMG was monitored at Session 2, which could account for the improvement. For the remaining two subjects, there were no differences in methodology.
Like cVEMPs, previous work in our lab revealed excellent reliability for air-conduction oVEMPs (age 4–19 years) with NH, across two sessions (Fuemmeler et al., 2020; Greenwalt et al., 2021). Results from this study revealed a significant interaction in oVEMP amplitude for the groups and time between sessions. Similar to cVEMPs, subjects with NH had an increase in oVEMP amplitude with increasing time between sessions, whereas subjects with CI had a decrease in oVEMP amplitude as time between sessions increased. Like cVEMP, the mean difference in oVEMP amplitude between children with CI and children with NH continues to increase as the time between sessions gets longer (7.9 μV at 10 months and 11.9 μV at 20 months). Regarding classifications, six children with NH had misclassifications. In four of the subjects with NH, responses went from unilaterally or bilaterally abnormal to present bilaterally, which is consistent with the trend for amplitudes to increase over time. For three of these subjects with NH, the reference electrode was placed directly beneath the active electrode at Session 1 and the reference electrode was place on the right inner canthus (modified belly tendon montage) at Session 2 suggesting that reference electrode contamination is the likely source for the initial abnormal response (Piker et al., 2011). One subject had bilaterally absent responses at Session 1 and unilaterally absent responses at Session 2; however, electrode montages were consistent between sessions. For this subject, the improvement in responses may be due to compliance, rather than improvement in methodology. Alternatively, two subjects with CI demonstrated progression of vestibular loss with unilaterally absent oVEMP responses at Session 1 and bilaterally absent oVEMP responses at Session 2. One of these subjects has the etiology of CMV and is the same subject who demonstrated progression of vestibular loss on rotary chair and cVEMP. The second subject has an unknown etiology and demonstrated normal results at both sessions for rotary chair and vHIT. In regards to maturation, the VOR takes longer to develop compared with the vestibulo-colic reflex (Hsu et al., 2009; Wang et al., 2008) and oVEMPs require more compliance to maintain upward gaze. Because the youngest child in this study was 4 years old, maturation is not felt to be a factor. Rather, compliance, variations in electrode placement, and progressions in vestibular loss are felt to be the major contributing factors for the misclassifications.
There were several limitations to this study. The first limitation was the difference in rotary chair and VEMP protocols over the 7 years. Interestingly, none of the subjects with rotary chair misclassifications (n = 3) had different protocols between sessions and only 4/12 subjects with VEMP misclassifications had different protocols between sessions. While protocol differences do not appear to be explaining the misclassifications, there could be subclinical effects of recording parameters that are reflected in the interactions observed in the linear mixed-effects modeling for VEMPs. There is currently a lack of standardization for VEMP recording parameters, ultimately leading to variability between studies and sites (Rosengren et al., 2019). A second limitation is the unknown status of rotary chair calibration. It is standard practice in our clinic and laboratory to attempt standard calibration on all subjects and only use default when calibration is not valid (typically under age 3 years). Because of the retrospective nature of this study and software limitations, there is no way to confirm calibration status for all subjects. It is unknown how a poor or default calibration may have impacted the results. A third limitation was the absence of bone conduction VEMP testing on the subjects with CI. Recent work in our lab suggests that mechanical changes can abolish VEMP responses, which mimic true vestibular loss (Merchant et al., 2020). While all subjects were tested post-CI, it is unknown whether the progressions observed were true changes in vestibular function or reflect ongoing mechanical changes from the CI over time.
A fourth limitation of the study was the unknown hearing loss etiology for half of the children with CI (15/31). A number of studies have demonstrated vestibular loss in children with CMV (Bernard et al., 2015; Karltorp et al., 2014; Maes et al., 2017), Pendred and enlarged vestibular aqueduct (EVA; Song et al., 2018; S. Wiener-Vacher et al., 2018; Yang et al., 2016; Zhang et al., 2020), and auditory neuropathy (Fujikawa & Starr, 2000; Masuda & Kaga, 2011; Sinha et al., 2013). Furthermore, there is evidence that vestibular loss with these three etiologies is progressive in nature (Bernard et al., 2015; Masuda & Kaga, 2011; Zhang et al., 2020). Therefore, knowledge of the hearing loss etiology would have been helpful in interpreting the misclassifications.
Last, our hearing loss group only included children with CI. Future work is necessary to determine stability of these measures and consistency of vestibular classification in children with varying degree of hearing loss not necessitating a CI.
Conclusions
Rotary chair gain and phase and vHIT gain are stable between Sessions 1 and 2 for subjects with NH and CI. Furthermore, the mean differences between Sessions 1 and 2 were not significantly different between the two groups. However, for c- and oVEMPs, the mean amplitude increased for subjects with NH and decreased for subjects with CI at Session 2 compared with Session 1. The difference between sessions and groups increased as time between sessions increased. From Session 1 to Session 2, children with NH only noted misclassifications for VEMP testing, which are attributed to a combination of changes in protocol and compliance; however, children with CI noted misclassifications across all tests, which are similarly attributed to changes in protocol and compliance, but also progressions in vestibular loss. Because some etiologies of hearing loss are associated with progressive vestibular loss (i.e., CMV, AN, and Pendred, [Bernard et al., 2015; Masuda & Kaga, 2011; Zhang et al., 2020]), vestibular testing should be completed serially in children with CI due to the possibility of progression.
Acknowledgments
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders under Award Number R03DC015318 awarded to Kristen L. Janky. The data that support the findings of this study are openly available in Open Source Framework (OSF) at https://osf.io/cyaq4.
Funding Statement
Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders under Award Number R03DC015318 awarded to Kristen L. Janky. The data that support the findings of this study are openly available in Open Source Framework (OSF) at https://osf.io/cyaq4.
References
- Bachmann, K. , Sipos, K. , Lavender, V. , & Hunter, L. L. (2018). Video head impulse testing in a pediatric population: Normative findings. Journal of the American Academy of Audiology, 29(05), 417–426. https://doi.org/10.3766/jaaa.17076 [DOI] [PubMed] [Google Scholar]
- Bernard, S. , Wiener-Vacher, S. , Van Den Abbeele, T. , & Teissier, N. (2015). Vestibular disorders in children with congenital cytomegalovirus infection. Pediatrics, 136(4), e887–e895. https://doi.org/10.1542/peds.2015-0908 [DOI] [PubMed] [Google Scholar]
- Brookhouser, P. E. , Cyr, D. G. , Peters, J. E. , & Schulte, L. E. (1991). Correlates of vestibular evaluation results during the first year of life. The Laryngoscope, 101(7, Pt. 1), 687–694. https://doi.org/10.1288/00005537-199107000-00001 [DOI] [PubMed] [Google Scholar]
- Casselbrant, M. L. , Mandel, E. M. , Sparto, P. J. , Perera, S. , Redfern, M. S. , Fall, P. A. , & Furman, J. M. (2010). Longitudinal posturography and rotational testing in children three to nine years of age: Normative data. Otolaryngology—Head & Neck Surgery, 142(5), 708–714. https://doi.org/10.1016/j.otohns.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpiot, A. , Tringali, S. , Ionescu, E. , Vital-Durand, F. , & Ferber-Viart, C. (2010). Vestibulo-ocular reflex and balance maturation in healthy children aged from six to twelve years. Audiology & Neuro-Otology, 15(4), 203–210. https://doi.org/10.1159/000255338 [DOI] [PubMed] [Google Scholar]
- Cushing, S. L. , Gordon, K. A. , Rutka, J. A. , James, A. L. , & Papsin, B. C. (2013). Vestibular end-organ dysfunction in children with sensorineural hearing loss and cochlear implants: An expanded cohort and etiologic assessment. Otology & Neurotology, 34(3), 422–428. https://doi.org/10.1097/MAO.0b013e31827b4ba0 [DOI] [PubMed] [Google Scholar]
- Eviatar, L. , & Eviatar, A. (1979). The normal nystagmic response of infants to caloric and perrotatory stimulation. The Laryngoscope, 89(7, Pt. 1), 1036–1045. https://doi.org/10.1288/00005537-197907000-00002 [PubMed] [Google Scholar]
- Fuemmeler, E. , Rodriguez, A. I. , Thomas, M. , Creutz, T. , Fitzpatrick, D. , & Janky, K. L. (2020). Vestibular evoked myogenic potential (VEMP) test-retest reliability in children. Otology & Neurotology, 41(8), e1052–e1059. https://doi.org/10.1097/MAO.0000000000002703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa, S. , & Starr, A. (2000). Vestibular neuropathy accompanying auditory and peripheral neuropathies. JAMA Otolaryngology–Head & Neck Surgery, 126(12), 1453–1456. https://doi.org/10.1001/archotol.126.12.1453 [DOI] [PubMed] [Google Scholar]
- Greenwalt, N. L. , Patterson, J. N. , Rodriguez, A. I. , Fitzpatrick, D. , Gordon, K. R. , & Janky, K. L. (2021). Bone conduction vibration vestibular evoked myogenic potential (VEMP) Testing: Reliability in children, adolescents, and young adults. Ear and Hearing, 42(2), 355–363. https://doi.org/10.1097/AUD.0000000000000925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, Y.-S. , Wang, S.-J. , & Young, Y.-H. (2009). Ocular vestibular-evoked myogenic potentials in children using air conducted sound stimulation. Clinical Neurophysiology, 120(7), 1381–1385. https://doi.org/10.1016/j.clinph.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Janky, K. L. , & Givens, D. (2015). Vestibular, visual acuity, and balance outcomes in children with cochlear implants: A preliminary report. Ear and Hearing, 36(6), e364–e372. https://doi.org/10.1097/AUD.0000000000000194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janky, K. L. , Patterson, J. , Shepard, N. , Thomas, M. , Barin, K. , Creutz, T. , Schmid, K. , & Honaker, J. A. (2018). Video head impulse test (vHIT): The role of corrective saccades in identifying patients with vestibular loss. Otology & Neurotology, 39(4), 467–473. https://doi.org/10.1097/MAO.0000000000001751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janky, K. L. , Thomas, M. L. A. , Al-Salim, S. , & Robinson, S. (2022). Does vestibular loss result in cognitive deficits in children with cochlear implants? Journal of Vestibular Research, 32(3), 245–260. https://doi.org/10.3233/VES-201556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janky, K. L. , Thomas, M. L. A. , High, R. R. , Schmid, K. K. , & Ogun, O. A. (2018). Predictive factors for vestibular loss in children with hearing loss. American Journal of Audiology, 27(1), 137–146. https://doi.org/10.1044/2017_AJA-17-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janky, K. L. , & Rodriguez, A. (2018). Quantitative vestibular function testing in the pediatric population. Seminars in Hearing, 39(3), 257–274. https://doi.org/10.1055/s-0038-1666817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janky, K. L. , Thomas, M. , Patterson, J. , & Givens, D. (2022). Using functional outcomes to predict vestibular loss in children.Otology & Neurotology, 43(3), 352–358. https://doi.org/10.1097/MAO.0000000000003433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge, P. D. , Janky, K. L. , & Barin, K. (2017). Can the video head impulse test define severity of bilateral vestibular hypofunction? Otology & Neurotology, 38(5), 730–736. https://doi.org/10.1097/MAO.0000000000001351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karltorp, E. , Löfkvist, U. , Lewensohn-Fuchs, I. , Lindström, K. , Eriksson Westblad, M. , Teär Fahnehjelm, K. , Verrecchia, L. , & Engman, M.-L. (2014). Impaired balance and neurodevelopmental disabilities among children with congenital cytomegalovirus infection. Acta Paediatrica, 103(11), 1165–1173. https://doi.org/10.1111/apa.12745 [DOI] [PubMed] [Google Scholar]
- MacDougall, H. G. , & Curthoys, I. S. (2012). Plasticity during vestibular compensation: The role of saccades. Frontiers in Neurology, 3. https://doi.org/10.3389/fneur.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes, L. , De Kegel, A. , Van Waelvelde, H. , De Leenheer, E. , Van Hoecke, H. , Goderis, J. , & Dhooge, I. (2017). Comparison of the motor performance and vestibular function in infants with a congenital cytomegalovirus infection or a connexin 26 mutation: A preliminary study. Ear and Hearing, 38(1), e49–e56. https://doi.org/10.1097/AUD.0000000000000364 [DOI] [PubMed] [Google Scholar]
- Masuda, T. , & Kaga, K. (2011). Influence of aging over 10 years on auditory and vestibular functions in three patients with auditory neuropathy. Acta Oto-Laryngologica, 131(5), 562–568. https://doi.org/10.3109/00016489.2010.534112 [DOI] [PubMed] [Google Scholar]
- Merchant, G. R. , Schulz, K. M. , Patterson, J. N. , Fitzpatrick, D. , & Janky, K. L. (2020). Effect of cochlear implantation on vestibular evoked myogenic potentials and wideband acoustic immittance. Ear and Hearing, 41(5), 1111–1124. https://doi.org/10.1097/AUD.0000000000000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz, E. M. , Kaplan, A. R. , & Westlake, J. R. (1985). Development of the vestibulo-ocular reflex from infancy to adulthood. Acta Oto-Laryngologica, 100(3–4), 180–193. https://doi.org/10.3109/00016488509104780 [DOI] [PubMed] [Google Scholar]
- Patterson, J. , Rodriguez, A. , Barin, K. , & Janky, K. L. (2020). Effect of gaze angle during the vertical video head impulse test across two devices in healthy adults and subjects with vestibular loss. Otology & Neurotology, 41(6), e751–e758. https://doi.org/10.1097/MAO.0000000000002652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piker, E. G. , Jacobson, G. P. , McCaslin, D. L. , & Hood, L. J. (2011). Normal characteristics of the ocular vestibular evoked myogenic potential. Journal of the American Academy of Audiology, 22(04), 222–230. https://doi.org/10.3766/jaaa.22.4.5 [DOI] [PubMed] [Google Scholar]
- Rodriguez, A. I. , Thomas, M. L. A. , Fitzpatrick, D. , & Janky, K. L. (2018). Effects of high sound exposure during air-conducted vestibular evoked myogenic potential testing in children and young adults. Ear and Hearing, 39(2), 269–277. https://doi.org/10.1097/AUD.0000000000000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, A. I. , Thomas, M. L. A. , & Janky, K. L. (2019). Air-conducted vestibular evoked myogenic potential testing in children, adolescents, and young adults: Thresholds, frequency tuning, and effects of sound exposure. Ear and Hearing, 40(1), 192–203. https://doi.org/10.1097/AUD.0000000000000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren, S. M. , Colebatch, J. G. , Young, A. S. , Govender, S. , & Welgampola, M. S. (2019). Vestibular evoked myogenic potentials in practice: Methods, pitfalls and clinical applications. Clinical Neurophysiology Practice, 4, 47–68. https://doi.org/10.1016/j.cnp.2019.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, L. M. , & Helminski, J. O. (2016). Test-retest and interrater reliability of the video head impulse test in the pediatric population. Otology & Neurotology, 37(5), 558–563. https://doi.org/10.1097/MAO.0000000000001040 [DOI] [PubMed] [Google Scholar]
- Sheykholeslami, K. , Megerian, C. A. , Arnold, J. E. , & Kaga, K. (2005). Vestibular-evoked myogenic potentials in infancy and early childhood. The Laryngoscope, 115(8), 1440–1444. https://doi.org/10.1097/01.mlg.0000167976.58724.22 [DOI] [PubMed] [Google Scholar]
- Sinha, S. K. , Barman, A. , Singh, N. K. , Rajeshwari, G. , & Sharanya, R. (2013). Vestibular test findings in individuals with auditory neuropathy: Review. The Journal of Laryngology & Otology, 127(5), 448–451. https://doi.org/10.1017/S0022215113000406 [DOI] [PubMed] [Google Scholar]
- Song, J.-J. , Hong, S. K. , Lee, S. Y. , Park, S. J. , Kang, S. I. , An, Y.-H. , Jang, J. H. , Kim, J. S. , & Koo, J.-W. (2018). Vestibular manifestations in subjects with enlarged vestibular aqueduct. Otology & Neurotology, 39(6), e461–e467. https://doi.org/10.1097/MAO.0000000000001817 [DOI] [PubMed] [Google Scholar]
- Strupp, M. , Kim, J.-S. , Murofushi, T. , Straumann, D. , Jen, J. C. , Rosengren, S. M. , Della Santina, C. C. , & Kingma, H. (2017). Bilateral vestibulopathy: Diagnostic criteria consensus document of the Classification Committee of the Bárány Society. Journal of Vestibular Research, 27(4), 177–189. https://doi.org/10.3233/VES-170619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente, M. (2007). Maturational effects of the vestibular system: A study of rotary chair, computerized dynamic posturography, and vestibular evoked myogenic potentials with children. Journal of the American Academy of Audiology, 18(6), 461–481. https://doi.org/10.3766/jaaa.18.6.2 [DOI] [PubMed] [Google Scholar]
- Verrecchia, L. , Galle Barrett, K. , & Karltorp, E. (2020). The feasibility, validity and reliability of a child friendly vestibular assessment in infants and children candidates to cochlear implant. International Journal of Pediatric Otorhinolaryngology, 135, 110093. https://doi.org/10.1016/j.ijporl.2020.110093 [DOI] [PubMed] [Google Scholar]
- Wang, S.-J. , Chen, C.-N. , Hsieh, W.-S. , & Young, Y.-H. (2008). Development of vestibular evoked myogenic potentials in preterm neonates. Audiology & Neuro-Otology, 13(3), 145–152. https://doi.org/10.1159/000112422 [DOI] [PubMed] [Google Scholar]
- Wang, S.-J. , Hsieh, W.-S. , & Young, Y.-H. (2013). Development of ocular vestibular-evoked myogenic potentials in small children. The Laryngoscope, 123(2), 512–517. https://doi.org/10.1002/lary.23535 [DOI] [PubMed] [Google Scholar]
- Wiener-Vacher, S. , Quarez, J. , & Priol, A. (2018). Epidemiology of vestibular impairments in a pediatric population. Seminars in Hearing, 39(3), 229–242. https://doi.org/10.1055/s-0038-1666815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener-Vacher, S. R. , & Wiener, S. I. (2017). Video head impulse tests with a remote camera system: Normative values of semicircular canal vestibulo-ocular reflex gain in infants and children. Frontiers in Neurology, 8, 434. https://doi.org/10.3389/fneur.2017.00434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C. J. , Lavender, V. , Meinzen-Derr, J. K. , Cohen, A. P. , Youssif, M. , Castiglione, M. , Manickam, V. , Bachmann, K. R. , & Greinwald, J. H. (2016). Vestibular pathology in children with enlarged vestibular aqueduct. The Laryngoscope, 126(10), 2344–2350. https://doi.org/10.1002/lary.25890 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Chen, Z. , Zhang, Y. , Hu, J. , Wang, J. , Xu, M. , & Zhang, Q. (2020). Vestibular-evoked myogenic potentials in patients with large vestibular aqueduct syndrome. Acta Oto-Laryngologica, 140(1), 40–45. https://doi.org/10.1080/00016489.2019.1687937 [DOI] [PubMed] [Google Scholar]