Abstract
Purpose:
The purpose of the study was to examine the effect of two voice intervention approaches for hypophonia secondary to Parkinson's disease (PD) on self-reported measures of physical demand, mental demand, and vocal performance.
Method:
Thirty-four persons with hypophonia secondary to PD were assigned to one of three groups: Lee Silverman Voice Treatment (LSVT) LOUD (n = 12), SpeechVive (n = 12), and nontreatment clinical control (n = 10). The LSVT LOUD and the SpeechVive participants received 8 weeks of voice intervention following the standardized protocol previously described for each approach. To confirm the effectiveness of each voice intervention, sound pressure level (dB SPL) data were analyzed for the experimental and control participants for a monologue sample obtained pretreatment, midtreatment, and posttreatment. During the voice intervention period, the LSVT LOUD and the SpeechVive participants were instructed to complete a modified version of the National Aeronautics and Space Administration Task Load Index rating scale to indicate the mental and physical demand required to complete the intervention activities, and to indicate how well they performed in completing the assigned vocal tasks.
Results:
The LSVT LOUD and the SpeechVive participants demonstrated a significant posttreatment increase in SPL (dB), in comparison to the clinical controls, thus confirming a positive intervention effect. The LSVT LOUD participants reported significantly higher ratings of physical and mental demand over the course of treatment, in comparison to the SpeechVive participants.
Conclusion:
Consideration of the mental and physical demand associated with two voice intervention approaches, commonly used for PD, may help to foster improved therapeutic compliance and treatment outcomes.
Fatigue is one of the most common and debilitating nonmotor symptoms associated with Parkinson's disease (PD; Friedman & Chou, 2004; Friedman et al., 2016; Garber & Friedman, 2003; Herlofson et al., 2012; Uebelacker et al., 2014), with an estimated prevalence of 33%–81% (Friedman et al., 2007; Havlikova et al., 2008; Stocchi et al., 2014). Despite the high prevalence of fatigue in persons with PD, a universally accepted definition and taxonomy is lacking (Finsterer & Mahjoub, 2014; Kluger et al., 2013), which makes systematic exploration of fatigue a challenge in clinical research. Current methodologies employ the use of self-reported rating scales, including the Fatigue Severity Scale (Krupp et al., 1989) and the Fatigue Impact Scale (Fisk et al., 1994), to identify patients' perception of fatigue associated with a disease state. Many of these instruments, however, do not identify distinct mental and physical fatigue dimensions (H. Ford et al., 1998), or they have not been sensitive to intervention effects in clinical research. In addition, the authors are only aware of a single health care study that has examined the temporal dynamics of fatigue across a period of intervention aimed at smoking cessation (Liu et al., 2013). There continues to be a paucity of longitudinal data, however, on measures of fatigue in persons with PD. Given the progressive nature of PD, perceptions of fatigue may change over time with advances in disease state and potentially in response to our therapeutic approaches for their motor and nonmotor symptoms.
Previous studies have identified increased motor (Friedman & Friedman, 1993; van Hilten et al., 1993) and cognitive fatigue (Alves et al., 2004; Dashtipour et al., 2015) in persons with PD. Despite this important finding, the impact of our voice intervention programs on fatigue has not been systematically explored. This is an important area of study as our voice intervention programs impose varying levels of physical and mental treatment burden (e.g., how much effort is required to achieve a given behavior; Eton et al., 2012). To date, research on treatment burden and treatment fatigue has largely centered on health behaviors such as medication adherence (Claborn et al., 2015) and exercise compliance in patients with chronic disease (Dobkin et al., 2005, 2006; Heckman et al., 2015). These health care outcome data suggest that behavioral interventions that are required frequently or increase patient burden result in reduced adherence and effectiveness.
This study will contemplate the impact of voice intervention on central fatigue, a phenomenon that is reported to frequently occur in persons with PD (Friedman et al., 2007, 2016). Central fatigue encompasses the domains of mental and physical demand, and it has been hypothesized to arise from centrally mediated factors, including biochemical changes in the brain and psychological shifts in motivation and attention (Kluger & Friedman, 2009). In this study, mental demand is operationally defined as the cognitive effects experienced during and/or after completing tasks that require sustained concentration and mental endurance (Chaudhuri & Behan, 2000; Lou et al., 2001), and physical demand is operationally defined as the sense of physical exhaustion or lack of energy required to perform a physical task despite the motivation to do so (Chaudhuri & Behan, 2000; Lou et al., 2001).
Pathophysiology of Fatigue in Parkinson's Disease
It remains unknown how the pathological disturbances associated with PD may cause and/or contribute to central fatigue. It has been postulated, however, that central fatigue is not a consequence of the motor-based disturbances that characterize PD, since fatigue is often reported in the early stage of the disease process (Herlofson et al., 2012), and it often precedes the onset of motor symptoms (Herlofson et al., 2012; Pont-Sunyer et al., 2014; Schrag et al., 2015). Instead, the central fatigue associated with PD is posited to result from central nervous system causes. Chaudhuri and Behan (2004) proposed a general model of fatigue that stems from central nervous system dysfunction, specifically the circuits connecting the basal ganglia and medial frontal areas, such as the anterior cingulate gyrus. Alternate theories have also been proposed to explain central fatigue in persons with PD including alterations in metabolism (Capecci et al., 2013), the presence of inflammatory markers (Lindqvist et al., 2012), and endocrine changes (Kenangil et al., 2009; Lv et al., 2014), which are known or suspected contributors to fatigue in other neurodegenerative conditions, such as multiple sclerosis (Malekzadeh et al., 2015). Another potential agent of central fatigue may be depression (Friedman et al., 2007); however, central fatigue has been shown to persist in patients with PD following successful treatment for depression (Alves et al., 2004). Despite the increased focus on the incidence and prevalence of central fatigue in PD, and its potential pathophysiology, the impact of our voice intervention approaches on central fatigue remains unexplored.
Voice Intervention Approaches and PD
Two evidence-based voice treatments for hypophonia include the Lee Silverman Voice Treatment (LSVT) LOUD program and the SpeechVive prosthesis. The LSVT LOUD program has established efficacy as a voice treatment for persons with PD, and the SpeechVive prosthesis has an emerging evidence base to support its use. While LSVT LOUD and the SpeechVive share the same therapeutic target of increasing vocal intensity, they differ substantially in treatment burden. In the LSVT LOUD program, the maintenance and generalization of the treatment gains is mediated by the patients' ability to perform online vocal intensity monitoring and adjustment, which imposes a substantial cognitive load on the speaker. The use of an internal cueing strategy, such as “Think Loud” in the LSVT LOUD program, requires individuals to consciously manipulate their system to achieve a desired goal.
In addition, the LSVT LOUD program involves high respiratory-phonatory effort, which imposes a high physical load on the speaker. In contrast, the SpeechVive prosthesis elicits increased vocal intensity using an external noise cue, a phenomenon known as the Lombard effect (Lombard, 1911). The Lombard effect is a reflexive response where speakers automatically increase their speech volume when conversing in noise in order to enhance the audibility of the voice. Prior studies have demonstrated a positive Lombard effect in individuals with PD (Adams et al., 2020; Richardson et al., 2014; Shrivastav et al., 2014; Stathopoulos et al., 2014). The use of a natural external cue to elicit Lombard-speech circumvents the patient's need to self-monitor and self-regulate their vocal intensity and, thus, imposes a lower cognitive load. Furthermore, the SpeechVive prosthesis is worn continuously over the course of the day, during opportunities for communication, and as a result, the target vocal intensity is lower than traditionally employed in LSVT LOUD. Targeting a lower vocal intensity prevents the onset of muscle fatigue and vocal hyperfunction that may be associated with speaking at a higher vocal intensity for prolonged periods of time. As a result, the SpeechVive prosthesis induces a lower physical load than other forms of voice therapy. While clinical studies have shown that both the LSVT LOUD program and the SpeechVive prosthesis yield significant improvements in vocal intensity (Ramig, Sapir, Countryman, et al., 2001; Ramig, Sapir, Fox, & Countryman, 2001; Stathopoulos et al., 2014), the treatment loads imposed by each form of therapy may differentially affect the patients' perception of treatment effort.
A recent study on subjective patient experiences reported that high-intensity training exacerbated existing fatigue in some patients with PD (Spurgon et al., 2015). Although this study examined a small sample of people with PD (n = 9), it paves the way for identifying issues that may unduly influence treatment effects. There is a pressing need to examine the physical and psychologic sequelae of our voice intervention programs, particularly for a clinical population that is subject to motor (Berardelli et al., 2001; Sheridan et al., 1987), sensory (Hammer & Barlow, 2010; Mu et al., 2013), and cognitive vulnerabilities (Fox et al., 2002; Zgaljardic et al., 2003, 2006). A prior study of persons with PD reported higher levels of effort, as compared to healthy controls, when completing activities of daily living and speech tasks (Solomon & Robin, 2005). Given this important finding, the constructs of effort should be examined within the context of voice rehabilitation. Understanding how voice intervention programs exacerbate these underlying vulnerabilities will allow clinicians and patients to make informed decisions about the best treatment approaches for them given considerations of fatigue, ensuring strong adherence to a treatment program.
Purpose of the Study
To study the impact of treatment burden on patient perceptions of central fatigue, we can compare two contrasting forms of voice intervention: LSVT LOUD therapy and the SpeechVive prosthesis. The disparate cognitive and physical loads imposed by each intervention approach are likely to contribute to different perceptions of physical and mental demand. This study hypothesized that the patients' perceptions of mental demand would be taxed in response to LSVT LOUD therapy, in part due to the use of internal cuing and the associated increase in the attentional resources required for online vocal intensity monitoring and regulation. It was further hypothesized that the LSVT LOUD therapy would elicit an increased sense of physical demand, as the therapy is designed in accordance with drive activity-dependent neuroplasticity (e.g., modifications in the central nervous system in response to high level of physical activity). Lastly, it was hypothesized that voice intervention delivered through the SpeechVive prosthesis would result in lower ratings of mental and physical demand, due to the use of a natural external cue and the use of a distributed practice schedule and a lower intensity dose of treatment. Ratings of task performance were included to provide a subjective, self-assessment of therapy outcomes.
Materials and Method
The institutional review board at the University of Massachusetts Amherst approved the study procedures, and Purdue University deferred to University of Massachusetts consistent with National Institutes of Health's policy for multisite research. Study participants were paid for their participation and received voice intervention at no cost. Written informed consent was obtained for all study participants.
Participant Description
Forty-one individuals with PD were screened for study eligibility. Seven individuals were excluded from study because they failed to meet the eligibility criteria or they declined to participate. In total, 34 individuals with hypophonia, secondary to idiopathic Parkinson's disease (PD), were enrolled in this multisite study (Purdue University, West Lafayette, Indiana; University of Massachusetts Amherst, Massachusetts). The sample size was chosen to detect a 5-sound pressure level (dB SPL) difference with a study power of 0.8 and significance level of 0.05. Participants were stratified to one of three groups based on hypophonia severity level: LSVT LOUD (n = 12; M age = 68.42, SD = 4.89), SpeechVive (n = 12; M age = 69.58, SD = 7.53), or the clinical control group (n = 10; M age = 66.60, SD = 10.71) where voice intervention was withheld. A balanced distribution of hypophonia severity level was targeted across groups. Judgments of hypophonia were assigned during the study screening procedures and confirmed by the first author (K.R.), who has 13 years of clinical experience with neurological voice disorders. Hypophonia was defined as soft speech noted during conversational speech and/or patient complaint of difficulty being heard by others. Due to the SARS-CoV-2 pandemic, posttreatment data were not collected for three LSVT participants, one SpeechVive participant, and one control participant. The pre- and midtreatment data collected for these participants were included in the statistical analysis.
Criteria for inclusion were (a) a diagnosis of idiopathic Parkinson's disease by a neurologist, (b) presence of hypophonia, (c) no recent history of acute illness per self-report, (d) no comorbid neurological conditions, (e) free of symptoms of depression as reflected by the Geriatric Depression Scale (Yesavage et al., 1982) or under pharmacological management for depressive symptoms, and (f) negative laryngeal pathology as determined by a videolaryngoscopic examination administered at baseline. Hearing was screened for each study participant using standard pure-tone detection measures. The participants demonstrated typical hearing thresholds in at least one ear for octave frequencies between 250 and 4000 Hz presented at 40 dB (Feenaughty et al., 2013). Thirty-two participants were under pharmacological management for their PD-related symptoms. These participants were tested during the “on” state of their medication cycle. One control participant (M35) and one SpeechVive participant (M45) were not under pharmacology management for their PD symptoms at the time of the study.
Table 1 provides a descriptive overview of the participants. The participants presented with mild-to-severe motor involvement as determined by the Hoehn and Yahr staging classification. As reflected in Table 1, the Hoehn and Yahr staging scores were fairly uniform across the LSVT LOUD, the SpeechVive, and the clinical control groups. Motor disease severity, however, was not balanced a priori as prior research has demonstrated that the relationship between disease severity and fatigue in persons with PD is not significant (Ding et al., 2017; Kostić et al., 2016). The Montreal Cognitive Assessment (MoCA; Hoops et al., 2009) was administered at baseline to identify the presence or absence of cognitive impairment. Cognitive deficits are commonly reported in individuals with PD and the inclusion of people with PD, and some level of cognitive deficits enhances the ecological validity of the voice intervention study. The MoCA scores, reported in Table 1, represent the inclusion of 11 participants with mild cognitive impairment (MoCA score: 18–25) and 23 participants with normal cognition (MoCA score ≥ 26). The 11 participants with mild cognitive impairment were uniformly distributed between the LSVT LOUD and the SpeechVive groups (see Table 1). It was determined that all participants recruited for study could fully participate in their assigned group and follow testing instructions in the research laboratory. Furthermore, the participants were asked to identify the presence of speech and/or voice problems using a structured questionnaire that was administered at baseline (see Table 1). A small subset of participants (n = 8) reported a prior history of speech therapy to address speech and/or swallowing concerns. Prior therapy included articulation-based approaches, nonstandardized vocal exercises, and swallowing exercises. Prior therapy occurred at least 12 months prior to enrollment in this study. Five of these eight participants (F31, F37, M35, M38, and M47) were clinical controls, and three of these participants (M43, M45, and M48) were assigned to the SpeechVive group. The three participants assigned to the SpeechVive group had not previously received intervention with the SpeechVive prosthesis. Four participants had previously received a subthalamic nucleus deep brain stimulation; their participant numbers are starred in Table 1.
Table 1.
Participant description.
| ID | Group | Age (years) | Hoehn & Yahr | MoCA score | Hypophonia severity | Chief voice complaints |
|---|---|---|---|---|---|---|
| F03 | LSVT LOUD | 65 | 3 | 30 | Mild | Hoarseness, reduced loudness |
| F04 | LSVT LOUD | 71 | 2 | 27 | Mild | Hoarseness, pitch breaks |
| F05 | LSVT LOUD | 75 | 2 | 26 | Mild/Moderate | Reduced loudness |
| M02 | LSVT LOUD | 62 | 3 | 30 | Moderate | Reduced loudness |
| M03 | LSVT LOUD | 74 | 3 | 26 | Mild | Reduced loudness |
| M04* | LSVT LOUD | 70 | 3 | 24 | Moderate | Reduced loudness, voice tires |
| M08 | LSVT LOUD | 68 | 2 | 21 | Moderate | Reduced loudness |
| M09 | LSVT LOUD | 68 | 4 | 21 | Mild/Moderate | Hoarseness, pitch breaks |
| M11 | LSVT LOUD | 65 | 2 | 29 | Moderate | Hoarseness, reduced loudness |
| M12 | LSVT LOUD | 75 | 3 | 23 | Mild/Moderate | Hoarseness, voice tires |
| M13 | LSVT LOUD | 60 | 1 | 25 | Mild | Reduced loudness, breathiness |
| M14 | LSVT LOUD | 68 | 2 | 27 | Moderate | Hoarseness, voice tires |
| LSVT LOUD (n = 12) | M = 68.42, SD = 4.89 | Range: 1–4 | M = 25.92, SD = 3.12 | |||
| F01 | SpeechVive | 82 | 2 | 23 | Moderate | Hoarseness, reduced loudness |
| F02 | SpeechVive | 77 | 4 | 27 | Mild/Moderate | Breathiness, reduced loudness |
| F46 | SpeechVive | 72 | 2 | 29 | Mild | Reduced loudness, hoarseness |
| M01 | SpeechVive | 75 | 3 | 27 | Mild/Moderate | Breathiness, reduced loudness |
| M05 | SpeechVive | 70 | 2 | 21 | Moderate | Reduced loudness, pitch breaks |
| M06 | SpeechVive | 52 | 3 | 30 | Moderate | Hoarseness, voice tires |
| M07 | SpeechVive | 68 | 1 | 27 | Mild | Voice tires, reduced loudness |
| M10 | SpeechVive | 62 | 3 | 23 | Mild/Moderate | Voice tires, reduced loudness |
| M15 | SpeechVive | 67 | 2 | 26 | Moderate | Reduced loudness, pitch breaks |
| M43 | SpeechVive | 70 | 3 | 23 | Moderate | Reduced loudness |
| M45* | SpeechVive | 69 | 3 | 26 | Mild | Reduced loudness, hoarseness |
| M48 | SpeechVive | 71 | 5 | 24 | Moderate | Reduced loudness, breathiness |
| SpeechVive (n = 12) | M = 69.58, SD = 7.53 | Range: 1–4 | M = 25.50, SD = 2.71 | |||
| F31 | Control | 79 | 3 | 26 | Mild | Reduced loudness, hoarseness |
| F33 | Control | 54 | 2 | 27 | Mild | Reduces loudness, breathiness |
| F34 | Control | 70 | 2 | 27 | Moderate | Reduced loudness |
| F37 | Control | 80 | 2 | 27 | Moderate | Reduced loudness, hoarseness |
| F40* | Control | 58 | 1 | 29 | Mild | Reduced loudness, hoarseness |
| M32* | Control | 54 | 3 | 27 | Moderate | Reduced loudness |
| M35 | Control | 68 | 3 | 27 | Moderate | Reduced loudness, breathiness |
| M38 | Control | 69 | 1 | 27 | Mild | Reduced loudness, hoarseness |
| M39 | Control | 55 | 4 | 26 | Moderate | Reduced loudness |
| M47 | Control | 79 | 5 | 22 | Mild | Reduced loudness |
| Control (n = 10) | M = 66.60, SD = 10.71 | Range: 1–5 | M = 26.50, SD = 1.78 | |||
Note. The first character in the ID column denotes participant sex (M = male; F = female). LSVT = Lee Silverman Voice Treatment.
Deep brain stimulation subthalamic nucleus; MoCA= Montreal Cognitive Assessment; hypophonia severity ratings were assigned or confirmed by the first author (K.R.) during connected speech; voice symptoms were self-reported in a structured health questionnaire.
Intervention Description
The LSVT LOUD and the SpeechVive interventions followed the standardized protocol and treatment dose previously described for each approach (Ramig, Sapir, Fox, & Countryman, 2001; Stathopoulos et al., 2014).
LSVT LOUD. The LSVT LOUD participants (n = 12) received voice treatment at a Massachusetts outpatient clinic and participated in laboratory testing at the University of Massachusetts Amherst. The LSVT LOUD–certified clinician was not involved in any other aspect of the study. The participants received the standard LSVT protocol, which involves 16 voice intervention sessions over 4 weeks (1 hr per session × 4 days per week × 4 weeks) plus the assignment of daily homework and carry-over activities. After the 4-week program concluded, the LSVT LOUD participants were instructed to engage in daily home practice of the vocal exercises for an additional 4 weeks. To facilitate at-home practice, the LSVT LOUD Homework Helper application was installed on each participant's mobile device. A homework log was maintained by the LSVT LOUD participants and reviewed biweekly by research personnel. The LSVT LOUD participants were found to be in compliance with their assigned intervention protocol.
SpeechVive prosthesis. Trained research personnel implemented the SpeechVive intervention over an 8-week period. In accordance with the SpeechVive protocol, participants (n = 12) were instructed to wear the SpeechVive prosthesis daily during communication and during 30 min of oral reading. The SpeechVive prosthesis presented multitalker babble (Auditec of St. Louis) to one ear when the participant was speaking. Multitalker babble has been shown to naturally elicit louder speech due to the Lombard effect (Garnier et al., 2010). The multitalker babble was presented through a small speaker with an open-ear fitting to prevent an occlusion effect. The SpeechVive prosthesis was fit to the ear with the best hearing thresholds as determined by baseline audiometric screening procedures. The detection level was adjusted by the experimenter until the SpeechVive activated and deactivated at the onset and offset of speech, respectively. The amplitude of the multitalker babble was then increased during conversational speech until each participant spoke 3 dB above his/her own comfortable vocal intensity. Six of the participants received intervention with the SpeechVive prosthesis at the University of Massachusetts Amherst, and six participants received the SpeechVive intervention at Purdue University. To monitor compliance with the therapeutic regime, usage data were recorded by the SpeechVive prosthesis and reviewed biweekly by the research personnel. The SpeechVive participants were found to be in compliance with their assigned intervention protocol. The SpeechVive participants did not receive any form of behavioral voice therapy.
Clinical controls. In total, 10 clinical control participants were tested at Purdue University at the same time points as the experimental groups, but voice intervention was withheld. Intervention was offered through an unrelated study to these participants, after their completion of the current study.
Equipment
The acoustic signal was captured using an omnidirectional head-mounted microphone (Sennheiser Model HSP2; Shure Beta 53). The same microphone was used for each participant across sessions and was positioned at a mouth-to-microphone distance of 6 cm at a 45° azimuth. Gain was provided to the acoustic signal through a preamplifier (Denon DN-700R; Marantz PM670) throughout each recording session. The microphone was calibrated for the SPL on the day of testing at a known frequency of 1 kHz and a known decibel level of 94 dB SPL (Sper Scientific Acoustic Calibrator Model 850016; Quest QC-20 calibrator). The same methods of recording and calibration were used for each participant across testing sessions.
Study Variables
Sound pressure level (SPL). To confirm a positive intervention effect, SPL data were captured 1 week before voice intervention was initiated (pre), midtreatment (mid), and within 1 week of treatment end (post). Participants in the control group were tested at their comfortable loudness. Participants in the LSVT LOUD group were tested at their comfortable loudness and at a cued louder voice. Participants in the SpeechVive group were tested with and without the prosthetic in place. For the LSVT LOUD group, the posttreatment, comfortable loudness data were considered in determining a treatment effect since the purpose of LSVT LOUD is to train a louder, clearer voice. Since the SpeechVive is a prosthetic device and it is not intended to elicit a training effect, data from the SpeechVive participants while wearing the device were considered in determining a treatment effect.
For the experimental and the control participants, SPL data were captured for a 30-s monologue (on a neutral topic). Pauses longer than 150 ms were visually identified using a time-aligned spectrogram and acoustic waveform, and these pauses served as utterance boundaries. The SPL data were measured across utterances using a customized script in Praat (Boersma & Weenink, 2019). Calibration values from the microphone calibration were factored into the measurement of SPL. The data measurers were blinded to the participants' group assignment and treatment session. To assess intermeasurer reliability of the SPL data, 10% of the acoustic data were selected for measurement by an independent examiner. A mean intraclass correlation coefficient (ICC) of .985 was reported indicating strong agreement between the original and independent examiner.
Ratings of mental demand, physical demand, and performance. To assess temporal changes in qualitative reports of mental fatigue, physical fatigue, and vocal performance, a daily instrument of self-assessment was completed over the 8-week intervention period.
For the LSVT LOUD and the SpeechVive participants, a modified paper-based version of the National Aeronautics and Space Administration Task Load Index (NASA-TLX; Hart & Staveland, 1988) was used to assess perception of mental demand, physical demand, and vocal performance. The modified NASA-TLX was completed at the end of each clinic visit or home practice session. The NASA-TLX is a widely accepted and validated tool to measure subjective workload after completing a task (Dias et al., 2018; Hart, 2006; Hart & Staveland, 1988). The NASA-TLX was initially created for use in the aviation industry, but has since been expanded for use in other sectors including health care (Fournier et al., 1999; Lowndes et al., 2020; Miyake, 2020; Ruiz-Rabelo et al., 2015; Tubbs-Cooley et al., 2018).
The NASA-TLX contains six predefined dimensions related to mental demand, physical demand, temporal demand, performance, effort, and frustration. To meet the goals of this study, three of these predefined dimensions (mental demand, physical demand, and performance) were examined using a modified NASA-TLX form. For each dimension, the participants were presented with a visual analogue scale with fixed, descriptive endpoints of 0 = “low” and 100 = “high” for the dimensions of mental and physical demand, and 0 = “poor” and 100 = “good” for the dimension of performance. To ensure the participants' interpretation of mental demand, physical demand, and performance were stable over time, written prompts were included. For ratings of mental demand, the prompt was, “How much mental activity was required to perform the treatment tasks (e.g., thinking, decision-making, remembering)?” For ratings of physical demand, the prompt was, “How much physical activity was required to perform the treatment tasks (e.g., muscle fatigue)?” For the performance dimension, the prompt was, “During this treatment session, how successful do you think you were in accomplishing the goals set out by the trainer or yourself?” Visual analogue scales carry low-participant burden, show moderate-to-strong test–retest reliability (Salomon & Murray, 2004; Siegel et al., 1997), and are often used to assess health status in longitudinal studies.
There are two methods of administering and scoring the NASA-TLX. This study used the raw NASA-TLX scores, which are most commonly reported in the literature (Said et al., 2020). The participants rated each of the three predefined dimensions after completing their daily voice program, without weighing the relative contribution of each dimension to a workload score (the alternate scoring method). Distance (in mm) was measured from the left end of the scale to the rating made by the participant. For each participant, an arithmetic mean of each dimension was computed for Intervention Weeks 1, 4, and 8, to reflect the start, middle, and end of treatment. Higher scores reflect perception of increased mental demand, increased physical demand, and increased success in accomplishing their daily therapy goal.
Statistical Analysis
SPL. The mean SPL data for 34 participants were submitted to a 3 × 3 linear mixed model analysis of variance (ANOVA, SAS 9.4). The between-subjects factor was group (three levels: LSVT LOUD, SpeechVive, clinical control group) with a within-subject factor of session (three levels: pre, mid, and post). The SPL data for the clinical control participants were included in the statistical model to confirm an intervention effect.
Ratings of mental demand, physical demand, and performance. The mental demand, physical demand, and performance data for the 24 experimental participants were submitted to a 2 × 3 linear mixed model ANOVA (SAS 9.4). The between-subjects factor was group (two levels: LSVT LOUD, SpeechVive) with a within-subject factor of session (three levels: Week 1, Week 4, and Week 8). Physical demand, mental demand, and performance data were not collected for the clinical control participants, as they did not receive intervention.
For all statistical analyses, participant was included as a repeated effect in the model to account for expected intersubject differences in response to treatment. Tukey post hoc analyses were used to explore all significant main effects and interactions. A Bonferroni-adjusted p value of .01 was used to control for multiple comparisons. Cohen's d effect size statistics are reported for all significant comparisons. Sex was not included as a covariate as no sex-related differences have been reported for perception of fatigue (Said et al., 2020) or SPL in people with PD (Fox & Ramig, 1997; Levitt et al., 2015). Additionally, the effect of sex was mitigated by the balanced distribution of males and females across treatment groups. The inferential statistics reported below are supported by descriptive data.
Results
SPL
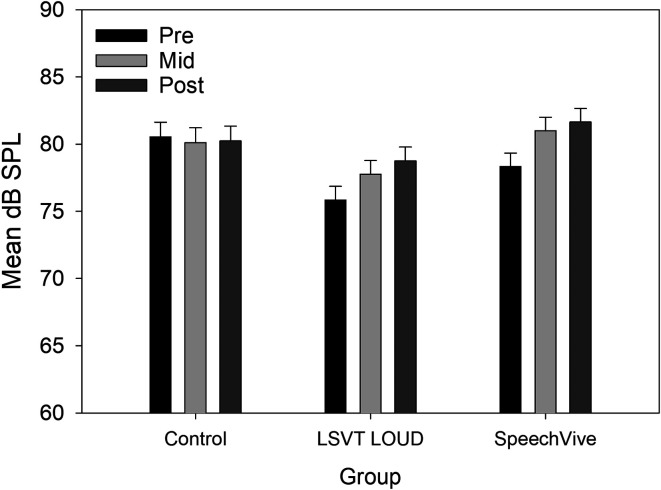
For mean SPL, a significant difference was identified for group, F(2, 31) = 4.34, p = .021, with higher SPLs values reported for the clinical controls (M = 81.07 dB, SD = 4.88), as compared to the LSVT LOUD group, t(31) = 2.78, p = .024 (M = 75.89 dB, SD = 5.12, d = 0.55). Significant differences were identified for session, F(2, 56) = 62.80, p < .001, with higher SPL values reported midtreatment, t(56) = 8.42, p < .001 (M = 80.4, SD = 3.5, d = 0.611), and posttreatment, t(56) = 10.42, p < .001 (M = 81.5, SD = 3.4, d = 0.929), as compared to pretreatment (M = 78.2, SD = 3.7). A significant Group × Session interaction was further identified, F(4, 56) = 31.15, p < .001. Tukey's post hoc analysis indicated that there was no significant change in SPL for the control participants for all session comparisons: Pre- to midtreatment, t(56) = 1.99, p = .557 (M = −0.41 dB); mid- to posttreatment, t(56) = .94, p = .989 (M = +0.12 dB); or pre- to posttreatment, t(56) = .97, p = .987 (M = −0.29 dB). In contrast, the SpeechVive participants demonstrated a significant increase in mean SPL pre- to midintervention, t(56) = 8.91, p < .001, d = 0.227 (M = +2.66 dB), and pre- to postintervention, t(56) = 6.36, p < .001, d = 0.448 (M = +3.32 dB). No significant change in SPL was identified mid- to post-intervention, t(56) = 1.77, p = .700 (M = +0.65 dB). For the LSVT LOUD participants, a significant change in SPL was identified for all session comparisons: Pre- to midtreatment, t(56) = 8.22, p < .001, d = 0.221 (M = +2 dB); mid- to posttreatment, t(56) = 5.13, p = .0001, d = 0.156 (M = +1 dB); and pre- to posttreatment t(56) = 12.51, p < .001, d = 0.377 (M = +3 dB). Group mean SPL data are shown in Figure 1.
Figure 1.
Mean sound pressure level (dB SPL) data are shown for the control, Lee Silverman Voice Treatment (LSVT) LOUD, and SpeechVive groups for the pretreatment (pre), midtreatment (mid), and posttreatment (post) sessions. The bars represent the standard error of the mean.
Ratings of Mental Demand
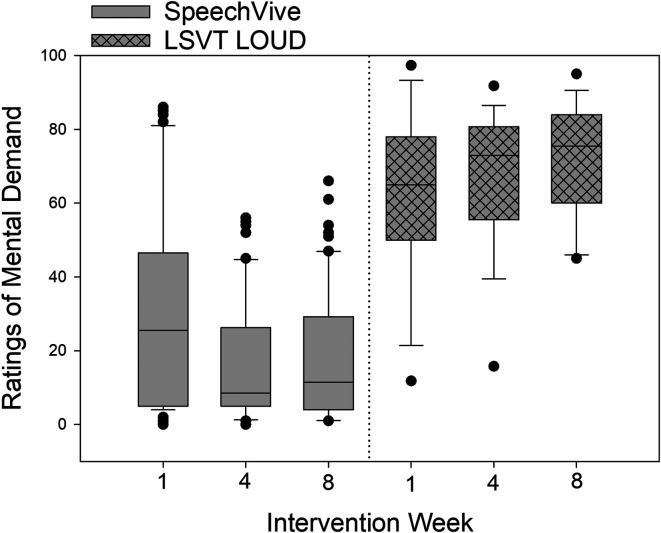
For ratings of mental demand, significant group differences were identified, F(1, 20) = 27.56, p < .001, with the LSVT LOUD participants indicating higher levels of mental demand (M = 66.52, SD = 20.68), as compared to the SpeechVive participants (M = 23.78, SD = 24.86, d = −1.869). A nonsignificant effect of session, F(2, 37) = 4.95, p = .0123, was identified with similar mean ratings of mental demand observed across Week 1 (M = 48.23, SD = 30.23), Week 4 (M = 42.39, SD = 31.56), and Week 8 (M = 40.90, SD = 31.88).
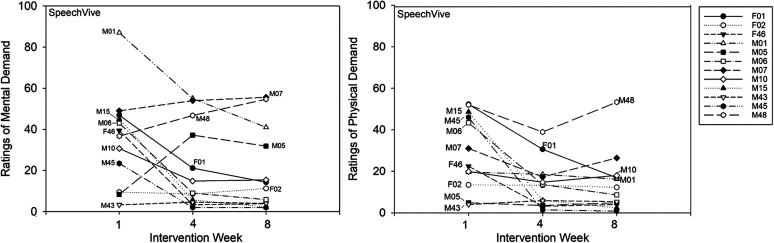
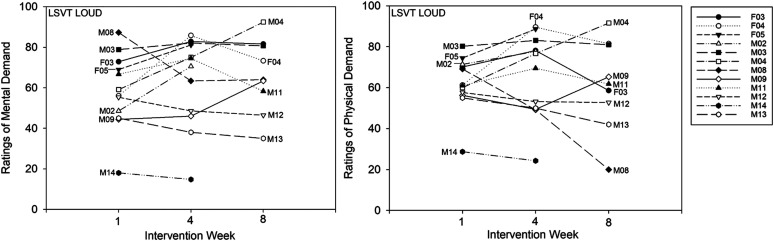
A significant Group × Session interaction was identified, F(2, 37) = 24.74, p < .001. The SpeechVive participants reported significantly lower ratings of mental demand at Week 4, t(37) = 5.83, p < .001 (M = 19.52, SD = 18.03. d = −0.632), and Week 8, t(37) = 6.81, p < .001 (M = 16.59, SD = 17.70, d = −0.753), as compared to Week 1 (M = 35.23, SD = 30.19). Individual subject analysis confirmed that seven of 12 SpeechVive participants followed the group trend. Five speakers (M05, M07, M43, M48, and F02) deviated from this pattern. Speakers M05 indicated higher ratings of mental demand at Week 4 (M = 37.17) and Week 8 (M = 31.83), as compared to Week 1 (M = 8.33). Similarly, speaker M07 reported slightly higher ratings at Week 4 (M = 54) and Week 8 (M = 56), as compared to Week 1 (M = 47). Speaker M48 indicated higher ratings of mental demand at Week 4 (M = 46.67) and Week 8 (M = 54.67), as compared to Week 1 (M = 36.67). Speakers M43 and F02 showed no clinically meaningful change in ratings of mental demand across sessions (see Figure 3). For the LSVT LOUD participants, no significant change in mental demand was reported at Week 4, t(37) = −2.26, p = .235 (M = 66.24, SD = 20.04), or Week 8, t(37) = −2.27, p = .999 (M = 67.03, SD = 15.54), as compared to Week 1 (M = 60.07, SD = 23.53). Individual subject analysis indicated that four of the 12 LSVT LOUD participants (M08, M12, M13, and M14) decreased their ratings of mental demand across treatment with a mean rate of change of 12.40 (100-point scale; SD = 8.89). Five of the twelve LSVT LOUD participants (M02, M04, M09, F03, F05) reported a mean increase of 14.05 (100-point scale; SD = 10.11) across the intervention period. One LSVT LOUD participant (M03) reported no meaningful change in ratings of mental effort across time, and two LSVT LOUD participants (F04 and M11) showed variable ratings of effort across the intervention period. Descriptive group data for ratings of mental demand are shown in Figure 2. Single-subject ratings are depicted in Figure 3 for the SpeechVive participants and Figure 4 for the LSVT LOUD participants.
Figure 3.
Single subject ratings of mental demand and physical demand are shown for Intervention Weeks 1, 4, and 8 for the SpeechVive participants.
Figure 2.
Self-reported ratings of mental demand are shown for Intervention Weeks 1, 4, and 8 for the SpeechVive and Lee Silverman Voice Treatment (LSVT) LOUD participants. The ordinate denotes the self-reported visual analogue scale ratings from 0 (low demand) to 100 (high demand). In the box plots, the horizontal lines denote the median value. The box whiskers extend from the 25th to the 75th percentile. The dots indicate values outside of the 5th/95th percentile.
Figure 4.
Single-subject ratings of mental demand and physical demand are shown for Intervention Weeks 1, 4, and 8 for the Lee Silverman Voice Treatment (LSVT) LOUD participants.
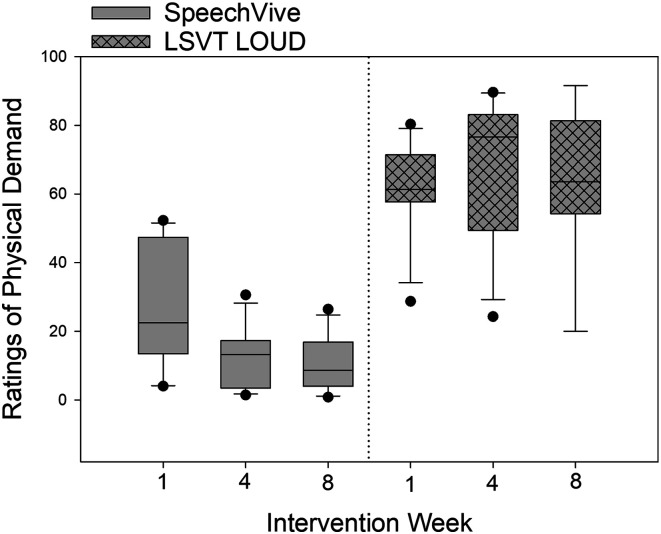
Ratings of Physical Demand
For ratings of physical demand, significant group differences were identified, F(1, 20) = 77.63, p < .001, with the LSVT LOUD participants reporting higher levels of physical demand (M = 65.33, SD = 19.95), as compared to SpeechVive participants (M = 16.68, SD = 18.09, d = −2.555). A significant effect of session, F(2, 37) = 8.45, p < .001, was identified with higher ratings of physical demand reported at Week 1 (M = 45.24, SD = 27.78), as compared to Week 4, t(37) = 3.03, p = .010 (M = 39.69, SD = 33.27, d = −0.181), and Week 8, t(37) =3.84, p < .001 (M = 37.70, SD = 31.96, d = −0.252).
A significant Group × Session interaction was further identified, F(2, 37) = 16.57, p < .001. The SpeechVive participants reported significantly lower levels of physical demand at Week 4, t(37) = 5.82, p < .001 (M = 11.64, SD = 4.03, d = −1.583), and Week 8, t(37) = 6.34, p < .001 (M = 10.5, SD = 8.15, d = −1.51), as compared to Week 1 (M = 26.74, SD = 12.87). Six of the 12 SpeechVive participants followed the group trend. Three SpeechVive participants (F02, M05, and M43) showed no meaningful change in ratings of physical demand across the intervention period, and three SpeechVive users (M48, M10, and M07) showed variable ratings of physical effort across time. For the LSVT LOUD participants, no significant change in ratings of physical demand were identified at Week 4, t(37) = −1.49, p = .672 (M = 68.86, SD = 19.12), or Week 8, t(37) = −0.52, p = .965 (M = 67.59, SD = 20.24), as compared to Week 1 (M = 64.44, SD = 20.58). Individual subject analysis indicated that four of the 12 LSVT LOUD participants (M08, M12, M13, and M14) reported a mean decrease of 18.51 (100-point scale; SD = 9.76) across the intervention period. Furthermore, two of the 12 LSVT LOUD participants (M02 and M04) showed a mean increase of 18.04 (100-point scale; SD = 9.41) across sessions. The remaining six LSVT LOUD participants showed variable ratings of physical demand across the intervention period. Descriptive data for ratings of physical demand are shown at the group level in Figure 5. Single-subject ratings are depicted in Figure 3 for the SpeechVive participants and Figure 4 for the LSVT LOUD participants.
Figure 5.
Self-reported ratings of physical demand are shown for Intervention Weeks 1, 4, and 8 for the Lee Silverman Voice Treatment (LSVT) LOUD and the SpeechVive participants. The ordinate denotes the self-reported visual analogue scale ratings from 0 (low demand) to 100 (high demand). In the box plots, the horizontal lines denote the median value. The box whiskers extend from the 25th to the 75th percentile. The dots indicate values outside of the 5th/95th percentile.
Ratings of Performance
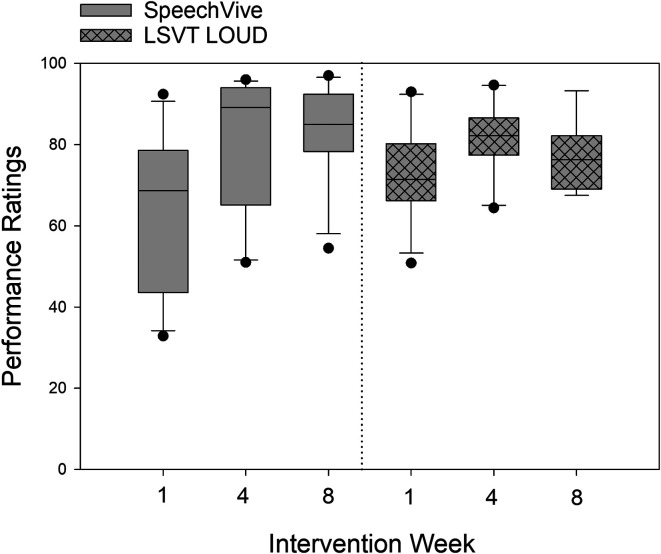
For ratings of performance, no significant group differences were observed, F(1, 20) = 0.11, p = .746, between the LSVT LOUD (M = 76.88 SD = 13.58) and the SpeechVive participants (M = 75.63, SD = 20.86). A significant effect of session, F(2, 37) = 40.12, p < .001, was identified with higher mean performance ratings indicated at Week 4, t(37) = −7.80, p < .001 (M = 81.16, SD = 15.23, d = 0.725) and Week 8, t(37) = −7.44, p < .001 (M = 80.38, SD = 13.71, d = 0.706), as compared to Week 1 (M = 68.29, SD = 19.95).
A significant Group × Session interaction was identified, F(2, 37) = 10.04, p < .001. The SpeechVive participants reported significantly higher levels of performance at Week 4, t(37) = −7.51, p < .001 (M = 81.44 SD = 18.45, d = 0.847), and Week 8, t(37) = −8.87, p < .001 (M = 82.73, SD = 15.21, d = 0.975), as compared to Week 1 (M = 64.07, SD = 22.39). No significant difference was identified between Week 4 and Week 8, t(37) = −1.45, p = .694, for the SpeechVive users. The LSVT LOUD participants reported significantly higher levels of performance at Week 4, t(37) = −3.54, p < .01 (M = 80.89, SD = 11.27, d = 0.59), as compared to Week 1 (M = 72.71, SD = 16.05), but there was no significant difference in performance ratings at Week 8 (M = 78.44, SD = 10.87) as compared to Week 1, t(37) = −2.04, p = .34, and Week 4, t(37) = 1.10, p = .878. Descriptive data for ratings of performance are shown in Figure 6.
Figure 6.
Self-reported ratings of performance are shown for Intervention Weeks 1, 4, and 8 for the Lee Silverman Voice Treatment (LSVT) LOUD and the SpeechVive participants. The ordinate denotes the self-reported visual analogue scale (VAS) ratings from 0 (poor performance) to 100 (good performance). In the box plots, the horizontal lines denote the median value. The box whiskers extend from the 25th to the 75th percentile. The dots indicate values outside of the fifth/95th percentile.
Discussion
The SPL data confirm the intervention effectiveness of the LSVT LOUD and SpeechVive therapies. Pre- to post-intervention, the LSVT LOUD and SpeechVive programs demonstrated a mean vocal intensity gain of approximately 3 dB SPL. The present SPL data are consistent with prior studies reporting vocal intensity gains in monologue speech after completing the LSVT LOUD therapy (Ramig et al., 2018; Ramig, Sapir, Countryman, et al., 2001) and when using the SpeechVive prosthetic (Richardson et al., 2014; Stathopoulos et al., 2014). The present SPL data, however, are not congruent with prior results reported by Ho et al. (1999), who found that individuals with PD did not modulate their vocal intensity in response to background noise, but they did increase their speech volume when explicitly directed by the examiner to read aloud using a louder voice. The difference in study results may be attributed to the different stimuli used in the background noise condition across studies. Ho et al. (1999) used pink noise to elicit Lombard-speech, whereas this study used multitalker babble. Previous psychoacoustic studies have reported a greater Lombard effect for fluctuating noises, such as babble noise, as compared to continuous noise, such as pink noise. For example, the seminal work of Pearson et al. (1976) identified that speakers increased sound pressure level by 0.60 dB for each decibel increase in babble noise. Conversely, the Lombard function for pink noise is approximately 0.4 dB/dB (Giguère et al., 2006; Pearson et al., 1976).
This exploratory study offers preliminary evidence supporting an impact of treatment burden on patient perceptions of central fatigue. The higher ratings of mental demand reported for the LSVT LOUD group are in line with the treatment approach, which requires active self-monitoring and online vocal intensity regulation. The lower ratings of mental demand reported for the SpeechVive group are concordant with an intervention approach that relies on nonvolitional control of vocal intensity. In customizing voice rehabilitation programs, it may be important for clinicians to consider the use of an internal cue, such as “Think LOUD” in LSVT LOUD, versus an external cue, such as the multitalker babble delivered by the SpeechVive prosthesis. Previous studies have shown that some individuals with PD are impaired in decision-making tasks that require internal attentional control (Brown & Marsden, 1988; Dirnberger & Jahanshahi, 2013), which is posited to reflect a resource capacity issue. The influence of internal versus external cueing on task performance has been more extensively explored in the limb literature. The use of an external auditory cue has been shown to significantly increase walking velocity (M. P. Ford et al., 2010), cadence (M. P. Ford et al., 2010), and stride length (M. P. Ford et al., 2010; Rochester et al., 2007) in persons with PD, whereas an internal cue such as “think about taking larger steps” significantly reduced step frequency in persons with PD (Baker et al., 2007). Similar improvements have been shown for the use of external cues to increase handwriting size (Oliveira et al., 1997). The use of an external cue may facilitate a more efficient allocation of attentional resources (Behrmann et al., 2004; Norman & Shallice, 1980; Rochester et al., 2004; Yogev et al., 2005) and may prove beneficial for patients who present with comorbid deficits in executive functioning. Large-scale systematic research is needed to explore these lines of questioning and to determine the patient-specific cognitive parameters that should guide our treatment planning. Lastly, seminal research on cognitive effort has indicated that complex and nonautomatic tasks require increased cognitive control and system resources (Hasher & Zacks, 1979; Schneider & Shiffrin, 1977). As a result, the use of internally mediated voice intervention approaches should strive to automate the desired behavior. LSVT LOUD sets out to accomplish this task through high repetition drills, carry-over activities, and the use of patient-specific functional phrases.
In exploring the temporal dynamics of fatigue in response to intervention, the SpeechVive participants reported significantly lower ratings of mental demand across the intervention period, which may suggest a decrease in listener effort as the speaker adjusted to the external noise cue. There is a plethora of behavioral (Heinrich & Schneider, 2011; Murphy et al., 2000; Pichora-Fuller et al., 1995), pupillometric (Kramer et al., 1997; Zekveld & Kramer, 2014; Zekveld et al., 2010), and neuroimaging (Davis & Johnsrude, 2003) evidence to suggest that cognitive and listener effort increase when listeners are faced with a condition of background noise or a degraded speech signal (Peelle, 2018). It is possible that the SpeechVive users adapted to the noncomprehensibility of the multitalker babble noise over time, and thus, any level of cognitive interference decreased. An examination of individual responses to treatment suggests that the majority of speakers found the SpeechVive prosthesis to be less mentally demanding with continued use, but five speakers reported an increase or no change in mental load with treatment. The factors contributing to this discordant pattern, observed in a small subset of speakers, remains unclear. The authors posit that this finding may reflect impairment in attention-selective processes (Perriol et al., 2005), and/or the speakers may have found the external noise cue to be an auditory distraction. Previous studies have reported that some persons with PD demonstrate reduced inhibition of task-irrelevant information (Canavan et al., 1989; Downes et al., 1989). In contrast, the group data for the LSVT LOUD participants reflected stable ratings in mental demand over the course of treatment. The individual LSVT LOUD data, however, showed that five speakers increased their ratings of mental demand over the intervention period, which is in keeping with the progressive and hierarchical nature of the therapy program. The initial phases of the LSVT LOUD program rely extensively on clinician modeling and external cueing strategies (e.g., “say that again, but louder”), which imposes a lower cognitive load on the patient. As treatment progresses, the patient gradually learns to assume the role of clinician and to independently monitor and adjust their speaking volume. Overall, the higher levels of mental demand reported for the LSVT LOUD participants, in comparison to the SpeechVive participants, reflects important differences in treatment burden. The higher cognitive load imposed by a self-monitoring treatment approach may cause additional stress on already burdened attentional processing and resource allocation system.
This study further supports the hypothesis that the LSVT LOUD participants would indicate higher ratings of physical demand, as compared to the SpeechVive participants. The higher levels of physical demand reported for the LSVT LOUD participants is in keeping with the high level of respiratory and phonatory effort used in this intervention approach. The lower ratings of physical demand observed for the SpeechVive users is in keeping with the intervention approach, where louder speech is elicited during conversation, affording speakers the opportunity for periods of physical rest. Furthermore, while the group data indicated that ratings of physical demand significantly decreased over the intervention period, the SpeechVive users appeared to drive this effect. In comparison to the LSVT LOUD participants, the SpeechVive users showed a larger magnitude of change in their ratings of physical effort over time, while their performance ratings continued to rise, which may suggest motor adaptation and/or muscle conditioning in response to treatment. Prior research has shown significant improvements in aerobic capacity (Schenkman et al., 2012) and muscle strength (Goodwin et al., 2008; Mehrholz et al., 2010), in persons with PD following exercise training. In contrast, the majority of LSVT LOUD participants reported higher ratings of physical demand across the intervention period. These higher levels of physical demand suggest that the LSVT LOUD participants were in compliance with the home therapy program and continued the intensive voice exercises as directed. Motor rehabilitation studies have found similar parallels between high- and low-intensity training programs. Studies have reported similar improvements in gait speed and stride length (Pohl et al., 2003) and aerobic capacity (Ridgel et al., 2009; Shulman et al., 2013) for high-intensity (80% of maximum capacity) and low-intensity (50% of maximum capacity) training programs. Consideration of the patients' current physical conditioning, exercise routine, and general health status may help to inform our voice rehabilitation approach.
Data-driven hypotheses were not formulated for the participants' self-assessment of task performance. Rather, this subjective metric provided insight into the patients' perception of task execution. The performance data were interesting as both treatment groups reported consistent and strong vocal performance, despite significant differences in treatment burden. In comparison to the first week of therapy, performance ratings were significantly higher at Week 4 and Week 8 for the SpeechVive users, and significantly higher at Week 4 for the LSVT LOUD participants. The higher performance rating observed at Week 4, but not Week 8, for the LSVT LOUD speakers is likely attributable to clinician feedback provided during one-on-one therapy in the first 4 weeks of therapy. Furthermore, although not statistically significant, the SpeechVive group started at a lower mean performance rating than the LSVT LOUD group. These data further suggest that clinician feedback may be important for participants to realize their performance gains, particularly in LSVT LOUD therapy.
Limitations and Future Directions
There are several important limitations to consider. First, this study assigned a small sample of patients with mild-to-moderate hypophonia to each intervention group. Therefore, the results cannot be generalized to a broader clinical population with presentation of more severe voice symptoms. Second, baseline vocal intensity was established in a single session. Given the daily motor and nonmotor fluctuations associated with PD, future studies may consider using an extended baseline period. Third, despite matching the groups for hypophonia severity level based on auditory perceptual characteristics, significant differences in vocal intensity were observed for the clinical controls and LSVT LOUD participants. While the effect of hypophonia severity on treatment outcomes was mitigated by the use of a within-subject design, future studies may consider the use of a quantitative metric to establish hypophonia severity or assign subjective ratings of hypophonia by consensus. Furthermore, while this study used SPL to index a treatment effect, future studies may consider exploring individual subjects' ratings of effort in relation to the magnitude of their SPL adjustment. Lastly, given that patients with PD show marked heterogeneity in their clinical phenotype, a more robust participant sample would further elucidate individual responses to intervention fatigue.
While this study demonstrates that persons with PD report increased levels of demand during voice intervention, their ratings of physical and mental demand may be influenced by general health factors, unrelated to the assigned invention program. For example, physical fatigue can be exacerbated by a person's level of physical conditioning and pharmacological regime, and mental fatigue can be exacerbated by clinical depression and/or sleep disturbances, both of which are common in PD. Furthermore, due to the attentional resource allocation issues associated with PD, future directions should explore the potential cognitive burden related to the use of naturalistic external cues like Lombard-elicited speech and internal cueing models. In fostering a better understanding of how cognitive status relates to treatment outcomes, clinicians can better tailor their therapy approach. It should also be noted that the LSVT LOUD participants' daily ratings of effort and performance were recorded in the therapy room for the first 4 weeks of therapy. As a result, the therapy environment and materials may have served as an external cue, which limits the generalization of the findings to everyday speech. Lastly, this study examined patient perceptions of physical demand, but it is not clear how this metric correlates with perception of vocal effort. The authors postulate that as ratings of physical effort increase, vocal effort is also likely to increase, but the two variables may not follow a linear relationship.
While this study offers the first exploratory examination of treatment burden on mental and physical constructs of fatigue in persons with PD, many questions remain unanswered. Future research should seek to examine the threshold of treatment burden that has bearing on voice intervention outcomes, the long-term course of treatment fatigue, and the degree to which the constructs of mental and physical fatigue are influenced by general versus disease-specific variables. While prior research on persons with multiple sclerosis have reported no significant correlation between disease duration and scores on the Fatigue Rating Scale (H. Ford et al., 1998), such disease-related factors should be systematically examined in persons with PD. Future work should also explore the potential association between cognitive status and perception of mental effort in a larger clinical cohort.
Conclusions
In understanding the physical and psychological sequela of our voice intervention approaches, clinicians can be responsive and adaptive in their therapy approach to maximize clinical outcomes and patient adherence to the therapeutic process. Integrating supportive strategies into routine voice care may help patients to overcome treatment burden. These strategies may include cognitive restructuring (recognizing treatment burden), emotion-focus coping strategies (e.g., positive thinking), and problem-based strategies (e.g., routines, planning). The use of supportive strategies to combat treatment burden requires systematic examination, but may prove beneficial.
Author Contributions
Kelly Richardson: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Formal analysis, Writing – review & editing. Jessica E. Huber: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Formal analysis, Writing – review & editing. Brianna Kiefer: Data collection, Writing – review & editing. Sandy Snyder: Data curation, Supervision, Writing – review & editing.
Acknowledgments
This research was supported by National Institute on Deafness and Other Communication Disorders under Award R21 DC016718 (PI: Kelly Richardson, Co-PI: Jessica E. Huber).
Funding Statement
This research was supported by National Institute on Deafness and Other Communication Disorders under Award R21 DC016718 (PI: Kelly Richardson, Co-PI: Jessica E. Huber).
References
- Adams, S. , Kumar, N. , Rizek, P. , Hong, A. , Zhang, J. , Senthinathan, A. , Mancinelli, C. , Knowles, T. , & Jog, M. (2020). Efficacy and acceptance of a Lombard-response device for hypophonia in Parkinson's disease. Canadian Journal of Neurological Sciences, 47(5), 634–641. https://doi.org/10.1017/cjn.2020.90 [DOI] [PubMed] [Google Scholar]
- Alves, G. , Wentzel-Larsen, T. , & Larsen, J. P. (2004). Is fatigue an independent and persistent symptom in patients with Parkinson disease? Neurology, 63(10), 1908–1911. https://doi.org/10.1212/01.wnl.0000144277.06917.cc [DOI] [PubMed] [Google Scholar]
- Baker, K. , Rochester, L. , & Nieuwboer, A. (2007). The immediate effect of attentional, auditory, and a combined cue strategy on gait during single and dual tasks in Parkinson's disease. Archives of Physical Medicine and Rehabilitation, 88(12), 1593–1600. https://doi.org/10.1016/j.apmr.2007.07.026 [DOI] [PubMed] [Google Scholar]
- Behrmann, M. , Geng, J. J. , & Shomstein, S. (2004). Parietal cortex and attention. Current Opinion in Neurobiology, 14(2), 212–217. https://doi.org/10.1016/j.conb.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Berardelli, A. , Rothwell, J. C. , Thompson, P. D. , & Hallett, M. (2001). Pathophysiology of bradykinesia in Parkinson's disease. Brain, 124(Pt. 11), 2131–2146. https://doi.org/10.1093/brain/124.11.2131 [DOI] [PubMed] [Google Scholar]
- Boersma, P. , Weenink, D. (2019). Praat (Version 5.1.05) [Computer program] . http://www.fon.hum.uva.nl/praat/
- Brown, R. G. , & Marsden, C. D. (1988). Internal versus external cues and the control of attention in Parkinson's disease. Brain, 111(2), 323–345. https://doi.org/10.1093/brain/111.2.323 [DOI] [PubMed] [Google Scholar]
- Canavan, A. G. , Passinger, R. E. , Marsden, C. D. , Quinn, N. , Wyke, M. , & Polkey, C. E. (1989). The performance on learning tasks of patients in the early stages of Parkinson's disease. Neuropsychologia, 27(2), 141–156. https://doi.org/10.1016/0028-3932(89)90167-X [DOI] [PubMed] [Google Scholar]
- Capecci, M. , Petrelli, M. , Emanuelli, B. , Millevolte, M. , Nicolai, A. , Provinciali, L. , & Ceravolo, M. G. (2013). Rest energy expenditure in Parkinson's disease: Role of disease progression and dopaminergic therapy. Parkinsonism & Related Disorders, 19(2), 238–241. https://doi.org/10.1016/j.parkreldis.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Chaudhuri, A. , & Behan, P. O. (2000). Fatigue and basal ganglia. Journal of the Neurological Sciences, 179(1–2), 34–42. https://doi.org/10.1016/s0022-510x(00)00411-1 [DOI] [PubMed] [Google Scholar]
- Chaudhuri, A. , & Behan, P. O. (2004). Fatigue in neurological disorders. The Lancet, 363(9413), 978–988. https://doi.org/10.1016/S0140-6736(04)15794-2 [DOI] [PubMed] [Google Scholar]
- Claborn, K. R. , Meier, E. , Miller, M. B. , & Leffingwell, T. R. (2015). A systematic review of treatment fatigue among HIV-infected patients prescribed antiretroviral therapy. Psychology, Health & Medicine, 20(3), 255–265. https://doi.org/10.1080/13548506.2014.945601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtipour, K. , Johnson, E. , Kani, C. , Kani, K. , Hadi, E. , Ghamsary, M. , Pezeshkian, S. , & Chen, J. J. (2015). Effect of exercise on motor and nonmotor symptoms of Parkinson's disease. Parkinson's Disease, 2015, 1–5. https://doi.org/10.1155/2015/586378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. H. , & Johnsrude, I. S. (2003). Hierarchical processing in spoken language comprehension. The Journal of Neuroscience, 23(8), 3423–3431. https://doi.org/10.1523/JNEUROSCI.23-08-03423.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, R. D. , Ngo-Howard, M. C. , Boskovski, M. T. , Zenati, M. A. , & Yule, S. J. (2018). Systematic review of measurement tools to assess surgeons' intraoperative cognitive workload. The British Journal of Surgery, 105(5), 491–501. https://doi.org/10.1002/bjs.10795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, J. , Jiang, S. M. , Yuan, Y. S. , Tong, Q. , Zhang, L. , Xu, Q. R. , & Zhang, K. Z. (2017). The relationship between fatigue and other non-motor symptoms in Parkinson's disease in Chinese population. International Journal of Gerontology, 11(3), 171–175. https://doi.org/10.1016/j.ijge.2016.05.011 [Google Scholar]
- Dirnberger, G. , & Jahanshahi, M. (2013). Executive dysfunction in Parkinson's disease: A review. Journal of Neuropsychology, 7(2), 193–224. https://doi.org/10.1111/jnp.12028 [DOI] [PubMed] [Google Scholar]
- Dobkin, P. L. , Abrahamowicz, M. , Fitzcharles, M. A. , Dritsa, M. , & da Costa, D. (2005). Maintenance of exercise in women with fibromyalgia. Arthritis Care & Research, 53(5), 724–731. https://doi.org/10.1002/art.21470 [DOI] [PubMed] [Google Scholar]
- Dobkin, P. L. , da Costa, D. , Abrahamowicz, M. , Dritsa, M. , Du Berger, R. , Fitzcharles, M. A. , & Lowensteyn, I. (2006). Adherence during an individualized home based 12-week exercise program in women with fibromyalgia. The Journal of Rheumatology, 33(2), 333–341. [PubMed] [Google Scholar]
- Downes, J. J. , Roberts, A. C. , Sahakian, B. J. , Evenden, J. L. , Morris, R. G. , & Robbins, T. W. (1989). Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson's disease: Evidence for a specific attentional dysfunction. Neuropsychologia, 27(11–12), 1329–1343. https://doi.org/10.1016/0028-3932(89)90128-0 [DOI] [PubMed] [Google Scholar]
- Eton, D. T. , Ramalho de Oliveira, D. , Egginton, J. S. , Ridgeway, J. L. , Odell, L. , May, C. R. , & Montori, V. M. (2012). Building a measurement framework of burden of treatment in complex patients with chronic conditions: A qualitative study. Patient Related Outcome Measures, 2012(3), 39–49. https://doi.org/10.2147/PROM.S34681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feenaughty, L. , Tjaden, K. , Benedict, R. H. , & Weinstock-Guttman, B. (2013). Speech and pause characteristics in multiple sclerosis: A preliminary study of speakers with high and low neuropsychological test performance. Clinical Linguistics & Phonetics, 27(2), 134–151. https://doi.org/10.3109/02699206.2012.751624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer, J. , & Mahjoub, S. Z. (2014). Fatigue in healthy and diseased individuals. The American Journal of Hospice and Palliative Care, 31(5), 562–575. https://doi.org/10.1177/1049909113494748 [DOI] [PubMed] [Google Scholar]
- Fisk, J. D. , Ritvo, P. G. , Ross, L. , Haase, D. A. , Marrie, T. J. , & Schlech, W. F. (1994). Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clinical Infectious Diseases, 18(Suppl. 1), S79–S83. https://doi.org/10.1093/clinids/18.supplement_1.s79 [DOI] [PubMed] [Google Scholar]
- Ford, M. P. , Malone, L. A. , Nyikos, I. , Yelisetty, R. , & Bickel, C. S. (2010). Gait training with progressive external auditory cueing in persons with Parkinson's disease. Archives of Physical Medicine and Rehabilitation, 91(8), 1255–1261. https://doi.org/10.1016/j.apmr.2010.04.012 [DOI] [PubMed] [Google Scholar]
- Ford, H. , Trigwell, P. , & Johnson, M. (1998). The nature of fatigue in multiple sclerosis. Journal of Psychosomatic Research, 45(1), 33–38. https://doi.org/10.1016/s0022-3999(98)00004-x [DOI] [PubMed] [Google Scholar]
- Fournier, L. R. , Wilson, G. F. , & Swain, C. R. (1999). Electrophysiological, behavioral, and subjective indexes of workload when performing multiple tasks: Manipulations of task difficulty and training. International Journal of Psychophysiology, 31(2), 129–145. https://doi.org/10.1016/S0167-8760(98)00049-X [DOI] [PubMed] [Google Scholar]
- Fox, C. M. , Morrison, C. E. , Ramig, L. O. , & Sapir, S. (2002). Current perspectives on the Lee Silverman Voice Treatment (LSVT) for individuals with idiopathic Parkinson disease. American Journal of Speech-Language Pathology, 11(2), 111–123. https://doi.org/10.1044/1058-0360(2002/012) [Google Scholar]
- Fox, C. M. , & Ramig, L. O. (1997). Vocal sound pressure level and self-perception of speech and voice in men and women with idiopathic Parkinson disease. American Journal of Speech-Language Pathology, 6(2), 85–94. https://doi.org/10.1044/1058-0360.0602.85 [Google Scholar]
- Friedman, J. H. , Beck, J. C. , Chou, K. L. , Clark, G. , Fagundes, C. P. , Goetz, C. G. , Herlofson, K. , Kluger, B. , Krupp, L. B. , Lang, A. E. , Lou, J. S. , Marsh, L. , Newbould, A. , & Weintraub, D. (2016). Fatigue in Parkinson's disease: Report from a multidisciplinary symposium. NPJ Parkinson's Disease, 2, Article 15025. https://doi.org/10.1038/npjparkd.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, J. H. , Brown, R. G. , Comella, C. , Garber, C. E. , Krupp, L. B. , Lou, J. S. , Marsh, L. , Nail, L. , Shulman, L. , Taylor, C. B. , & Working Group on Fatigue in Parkinson's Disease. (2007). Fatigue in Parkinson's disease: A review. Movement Disorders, 22(3), 297–308. https://doi.org/10.1002/mds.21240 [DOI] [PubMed] [Google Scholar]
- Friedman, J. H. , & Chou, K. L. (2004). Sleep and fatigue in Parkinson's disease. Parkinsonism & Related Disorders, 10(Suppl. 1), S27–S35. https://doi.org/10.1016/j.parkreldis.2003.11.006 [DOI] [PubMed] [Google Scholar]
- Friedman, J. , & Friedman, H. (1993). Fatigue in Parkinson's disease. Neurology, 43(10), 2016–2018. https://doi.org/10.1212/wnl.43.10.2016 [DOI] [PubMed] [Google Scholar]
- Garber, C. E. , & Friedman, J. H. (2003). Effects of fatigue on physical activity and function in patients with Parkinson's disease. Neurology, 60(7), 1119–1124. https://doi.org/10.1212/01.wnl.0000055868.06222.ab [DOI] [PubMed] [Google Scholar]
- Garnier, M. , Henrich, N. , & Dubois, D. (2010). Influence of sound immersion and communicative interaction on the Lombard effect. Journal of Speech, Language, and Hearing Research, 53(3), 588–608. https://doi.org/10.1044/1092-4388(2009/08-0138) [DOI] [PubMed] [Google Scholar]
- Giguère, C. , Laroche, C. , Brault, E. , Ste-Marie, J.-C. , Brosseau-Villeneuve, M. , Philippon, B. , & Vaillancourt, V. (2006). Quantifying the Lombard effect in different background noises. The Journal of the Acoustical Society of America, 120(5), 3378–3378. https://doi.org/10.1121/1.4781616 [Google Scholar]
- Goodwin, V. A. , Richards, S. H. , Taylor, R. S. , Taylor, A. H. , & Campbell, J. L. (2008). The effectiveness of exercise interventions for people with Parkinson's disease: A systematic review and meta-analysis. Movement Disorders, 23(5), 631–640. https://doi.org/10.1002/mds.21922 [DOI] [PubMed] [Google Scholar]
- Hammer, M. J. , & Barlow, S. M. (2010). Laryngeal somatosensory deficits in Parkinson's disease: Implications for speech respiratory and phonatory control. Experimental Brain Research, 201(3), 401–409. https://doi.org/10.1007/s00221-009-2048-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, S. G. (2006). Nasa-Task Load Index (NASA-TLX); 20 years later. Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 50(9), 904–908. https://doi.org/10.1177/154193120605000909 [Google Scholar]
- Hart, S. G. , & Staveland, L. E. (1988). Development of NASA-TLX (Task Load Index): Results of empirical and theoretical research. Advances in Psychology, 52, 139–183. https://doi.org/10.1016/S0166-4115(08)62386-9 [Google Scholar]
- Hasher, L. , & Zacks, R. T. (1979). Automatic and effortful processes in memory. Journal of Experimental Psychology: General, 108(3), 356–388. https://doi.org/10.1037/0096-3445.108.3.356 [Google Scholar]
- Havlikova, E. , Rosenberger, J. , Nagyova, I. , Middel, B. , Dubayova, T. , Gdovinova, Z. , Groothoff, J. W. , & van Dijk, J. P. (2008). Clinical and psychosocial factors associated with fatigue in patients with Parkinson's disease. Parkinsonism & Related Disorders, 14(3), 187–192. https://doi.org/10.1016/j.parkreldis.2007.07.017 [DOI] [PubMed] [Google Scholar]
- Heckman, B. W. , Mathew, A. R. , & Carpenter, M. J. (2015). Treatment burden and treatment fatigue as barriers to health. Current Opinion in Psychology, 5, 31–36. https://doi.org/10.1016/j.copsyc.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, A. , & Schneider, B. A. (2011). Elucidating the effects of ageing on remembering perceptually distorted word pairs. Quarterly Journal of Experimental Psychology, 64(1), 186–205. https://doi.org/10.1080/17470218.2010.492621 [DOI] [PubMed] [Google Scholar]
- Herlofson, K. , Ongre, S. O. , Enger, L. K. , Tysnes, O. B. , & Larsen, J. P. (2012). Fatigue in early Parkinson's disease. Minor inconvenience or major distress? European Journal of Neurology, 19(7), 963–968. https://doi.org/10.1111/j.1468-1331.2012.03663.x [DOI] [PubMed] [Google Scholar]
- Ho, A. K. , Bradshaw, J. L. , Iansek, R. , & Alfredson, R. (1999). Speech volume regulation in Parkinson's disease: Effects of implicit cues and explicit instructions. Neuropsychologia, 37(13), 1453–1460. https://doi.org/10.1016/S0028-3932(99)00067-6 [DOI] [PubMed] [Google Scholar]
- Hoops, S. , Nazem, S. , Siderowf, A. D. , Duda, J. E. , Xie, S. X. , Stern, M. B. , & Weintraub, D. (2009). Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology, 73(21), 1738–1745. https://doi.org/10.1212/WNL.0b013e3181c34b47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenangil, G. , Orken, D. N. , Ur, E. , Forta, H. , & Celik, M. (2009). The relation of testosterone levels with fatigue and apathy in Parkinson's disease. Clinical Neurology and Neurosurgery, 111(5), 412–414. https://doi.org/10.1016/j.clineuro.2008.11.019 [DOI] [PubMed] [Google Scholar]
- Kluger, B. M. , & Friedman, J. H. (2009). Fatigue in Parkinson's disease. In Chaudhuri K. R., Tolosa E., Schapira A., & Poewe W. (Eds.), Non-motor symptoms of Parkinson's disease (2nd ed., pp. 135–146). Oxford University Press. https://doi.org/10.1093/med/9780199684243.003.0011 [Google Scholar]
- Kluger, B. M. , Krupp, L. B. , & Enoka, R. M. (2013). Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology, 80(4), 409–416. https://doi.org/10.1212/WNL.0b013e31827f07be [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostić, V. S. , Tomić, A. , & Ječmenica-Lukić, M. (2016). The pathophysiology of fatigue in Parkinson's disease and its pragmatic management. Movement Disorders Clinical Practice, 3(4), 323–330. https://doi.org/10.1002/mdc3.12343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, S. E. , Kapteyn, T. S. , Festen, J. M. , & Kuik, D. J. (1997). Assessing aspects of auditory handicap by means of pupil dilatation. Audiology, 36(3), 155–164. https://doi.org/10.3109/00206099709071969 [DOI] [PubMed] [Google Scholar]
- Krupp, L. B. , LaRocca, N. G. , Muir-Nash, J. , & Steinberg, A. D. (1989). The fatigue severity scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology, 46(10), 1121–1123. https://doi.org/10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- Levitt, J. , Chitnis, S. , & Walker-Batson, D. (2015). The effects of the “SPEAK OUT! ®” and “LOUD Crowd®” voice programs for Parkinson disease. International Journal of Health Science, 3(2), 13–19. https://doi.org/10.15640/ijhs.v3n2a3 [Google Scholar]
- Lindqvist, D. , Kaufman, E. , Brundin, L. , Hall, S. , Surova, Y. , & Hansson, O. (2012). Non-motor symptoms in patients with Parkinson's disease—Correlations with inflammatory cytokines in serum. PLOS ONE, 7(10), Article e47387. https://doi.org/10.1371/journal.pone.0047387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Li, R. , Lanza, S. T. , Vasilenko, S. A. , & Piper, M. (2013). Understanding the role of cessation fatigue in the smoking cessation process. Drug and Alcohol Dependence, 133(2), 548–555. https://doi.org/10.1016/j.drugalcdep.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard, E. (1911). Le signe de l'élévation de la voix [The sign of raising the voice]. Annales des Maladies de L'Oreille et du Larynx [Annals of Diseases of the Ear and Larynx] , XXXVII, 101–109. [Google Scholar]
- Lou, J. S. , Kearns, G. , Oken, B. , Sexton, G. , & Nutt, J. (2001). Exacerbated physical fatigue and mental fatigue in Parkinson's disease. Movement Disorders, 16(2), 190–196. https://doi.org/10.1002/mds.1042 [DOI] [PubMed] [Google Scholar]
- Lowndes, B. R. , Forsyth, K. L. , Blocker, R. C. , Dean, P. G. , Truty, M. J. , Heller, S. F. , Blackmon, S. , Hallbeck, M. S. , & Nelson, H. (2020). NASA-TLX assessment of surgeon workload variation across specialties. Annals of Surgery, 271(4), 686–692. https://doi.org/10.1097/SLA.0000000000003058 [DOI] [PubMed] [Google Scholar]
- Lv, Z. , Qi, H. , Wang, L. , Fan, X. , Han, F. , Wang, H. , & Bi, S. (2014). Vitamin D status and Parkinson's disease: A systematic review and meta-analysis. Neurological Sciences, 35(11), 1723–1730. https://doi.org/10.1007/s10072-014-1821-6 [DOI] [PubMed] [Google Scholar]
- Malekzadeh, A. , Van de Geer-Peeters, W. , De Groot, V. , Teunissen, C. E. , Beckerman, H. , & TREFAMS-ACE Study Group. (2015). Fatigue in patients with multiple sclerosis: Is it related to pro- and anti-inflammatory cytokines? Disease Markers, 2015, Article 758314. https://doi.org/10.1155/2015/758314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrholz, J. , Friis, R. , Kugler, J. , Twork, S. , Storch, A. , & Pohl, M. (2010). Treadmill training for patients with Parkinson's disease. The Cochrane Database of Systematic Reviews, 1, CD007830. https://doi.org/10.1002/14651858.CD007830.pub2 [DOI] [PubMed] [Google Scholar]
- Miyake, S. (2020). Healthcare worker mental workload assessment by NASA-TLX. Journal of UOEH, 42(1), 63–75. https://doi.org/10.7888/juoeh.42.63 [DOI] [PubMed] [Google Scholar]
- Mu, L. , Sobotka, S. , Chen, J. , Su, H. , Sanders, I. , Nyirenda, T. , Adler, C. H. , Shill, H. A. , Caviness, J. N. , Samanta, J. E. , Sue, L. I. , Beach, T. G. , & Arizona Parkinson's Disease Consortium. (2013). Parkinson disease affects peripheral sensory nerves in the pharynx. Journal of Neuropathology & Experimental Neurology, 72(7), 614–623. https://doi.org/10.1097/NEN.0b013e3182965886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, D. R. , Craik, F. , Li, K. , & Schneider, B. A. (2000). Comparing the effects of aging and background noise on short-term memory performance. Psychology and Aging, 15(2), 323–334. https://doi.org/10.1037/0882-7974.15.2.323 [DOI] [PubMed] [Google Scholar]
- Norman, D. A. , & Shallice, T. (1980). Attention to action: Willed and automatic control of behavior (Technical report no. 99). Center for Human Information Processing. [Google Scholar]
- Oliveira, R. M. , Gurd, J. M. , Nixon, P. , Marshall, J. C. , & Passingham, R. E. (1997). Micrographia in Parkinson's disease: The effect of providing external cues. Journal of Neurology, Neurosurgery, and Psychiatry, 63(4), 429–433. https://doi.org/10.1136/jnnp.63.4.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, K. S. , Bennett, R. L. , & Fidell, S. (1976). Speech levels in various environments (BBC report no. 3281) . Bolt, Beranek and Newman. [Google Scholar]
- Peelle, J. E. (2018). Listening effort: How the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear and Hearing, 39(2), 204–214. https://doi.org/10.1097/AUD.0000000000000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriol, M. P. , Dujardin, K. , Derambure, P. , Marcq, A. , Bourriez, J. L. , Laureau, E. , Pasquier, F. , Defebvre, L. , & Destée, A. (2005). Disturbance of sensory filtering in dementia with Lewy bodies: Comparison with Parkinson's disease dementia and Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry, 76(1), 106–108. https://doi.org/10.1136/jnnp.2003.035022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller, M. K. , Schneider, B. A. , & Daneman, M. (1995). How young and old adults listen to and remember speech in noise. The Journal of the Acoustical Society of America, 97(1), 593–608. https://doi.org/10.1121/1.412282 [DOI] [PubMed] [Google Scholar]
- Pohl, M. , Rockstroh, G. , Rückriem, S. , Mrass, G. , & Mehrholz, J. (2003). Immediate effects of speed-dependent treadmill training on gait parameters in early Parkinson's disease. Archives of Physical Medicine and Rehabilitation, 84(12), 1760–1766. https://doi.org/10.1016/s0003-9993(03)00433-7 [DOI] [PubMed] [Google Scholar]
- Pont-Sunyer, C. , Hotter, A. , Gaig, C. , Seppi, K. , Compta, Y. , Katzenschlager, R. , Mas, N. , Hofeneder, D. , Brücke, T. , Bayés, A. , Wenzel, K. , Infante, J. , Zach, H. , Pirker, W. , Posada, I. J. , Álvarez, R. , Ispierto, L. , De Fàbregues, O. , Callén, A. , … Tolosa, E. (2014). The onset of nonmotor symptoms in Parkinson's disease (the ONSET PD study). Movement Disorders, 30(2), 229–237. https://doi.org/10.1002/mds.26077 [DOI] [PubMed] [Google Scholar]
- Ramig, L. O. , Halpern, A. , Spielman, J. , Fox, C. , & Freeman, K. (2018). Speech treatment in Parkinson's disease: Randomized controlled trial (RCT). Movement Disorders, 33(11), 1777–1791. https://doi.org/10.1002/mds.27460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig, L. O. , Sapir, S. , Countryman, S. , Pawlas, A. A. , O'Brien, C. , Hoehn, M. , & Thompson, L. L. (2001). Intensive voice treatment (LSVT) for patients with Parkinson's disease: A 2 year follow up. Journal of Neurology, Neurosurgery & Psychiatry, 71(4), 493–498. https://doi.org/10.1136/jnnp.71.4.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig, L. O. , Sapir, S. , Fox, C. , & Countryman, S. (2001). Changes in vocal loudness following intensive voice treatment (LSVT) in individuals with Parkinson's disease: A comparison with untreated patients and normal age-matched controls. Movement Disorders, 16(1), 79–83. https://doi.org/10.1002/1531-8257(200101)16:1<79::AID-MDS1013>3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- Richardson, K. , Sussman, J. E. , & Stathopoulos, E. T. (2014). The effect of increased vocal intensity on interarticulator timing in speakers with Parkinson's disease: A preliminary analysis. Journal of Communication Disorders, 52, 44–64. https://doi.org/10.1016/j.jcomdis.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgel, A. L. , Vitek, J. L. , & Alberts, J. L. (2009). Forced, not voluntary, exercise improves motor function in Parkinson's disease patients. Neurorehabilitation and Neural Repair, 23(6), 600–608. https://doi.org/10.1177/1545968308328726 [DOI] [PubMed] [Google Scholar]
- Rochester, L. , Hetherington, V. , Jones, D. , Nieuwboer, A. , Willems, A. M. , Kwakkel, G. , & Van Wegen, E. (2004). Attending to the task: Interference effects of functional tasks on walking in Parkinson's disease and the roles of cognition, depression, fatigue, and balance. Archives of Physical Medicine and Rehabilitation, 85(10), 1578–1585. https://doi.org/10.1016/j.apmr.2004.01.025 [DOI] [PubMed] [Google Scholar]
- Rochester, L. , Nieuwboer, A. , Baker, K. , Hetherington, V. , Willems, A. M. , Chavret, F. , Kwakkel, G. , Van Wegen, E. , Lim, I. , & Jones, D. (2007). The attentional cost of external rhythmical cues and their impact on gait in Parkinson's disease: Effect of cue modality and task complexity. Journal of Neural Transmission, 114(10), 1243–1248. https://doi.org/10.1007/s00702-007-0756-y [DOI] [PubMed] [Google Scholar]
- Ruiz-Rabelo, J. F. , Navarro-Rodriguez, E. , Di-Stasi, L. L. , Diaz-Jimenez, N. , Cabrera-Bermon, J. , Diaz-Iglesias, C. , Gomez-Alvarez, M. , & Briceño-Delgado, J. (2015). Validation of the NASA-TLX score in ongoing assessment of mental workload during a laparoscopic learning curve in bariatric surgery. Obesity Surgery, 25(12), 2451–2456. https://doi.org/10.1007/s11695-015-1922-1 [DOI] [PubMed] [Google Scholar]
- Said, S. , Gozdzik, M. , Roche, T. , Braun, J. , Rössler, J. , Kaserer, A. , Spahn, D. , Nöthiger, C. , & Tscholl, D. (2020). Validation of the raw National Aeronautics And Space Administration Task Load Index (NASA-TLX) questionnaire to assess perceived workload in patient monitoring tasks: Pooled analysis study using mixed models. Journal of Medical Internet Research, 22(9), e19472. https://doi.org/10.2196/19472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, J. A. , & Murray, C. J. (2004). A multi-method approach to measuring health-state valuations. Health Economics, 13(3), 281–290. https://doi.org/10.1002/hec.834 [DOI] [PubMed] [Google Scholar]
- Schenkman, M. , Hall, D. A. , Barón, A. E. , Schwartz, R. S. , Mettler, P. , & Kohrt, W. M. (2012). Exercise for people in early- or mid-stage Parkinson disease: A 16-month randomized controlled trial. Physical Therapy, 92(11), 1395–1410. https://doi.org/10.2522/ptj.20110472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, W. , & Shiffrin, R. M. (1977). Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review, 84(1), 1–66. https://doi.org/10.1037/0033-295X.84.1.1 [Google Scholar]
- Schrag, A. , Horsfall, L. , Walters, K. , Noyce, A. , & Petersen, I. (2015). Prediagnostic presentations of Parkinson's disease in primary care: A case-control study. The Lancet Neurology, 14(1), 57–64. https://doi.org/10.1016/S1474-4422(14)70287-X [DOI] [PubMed] [Google Scholar]
- Sheridan, M. R. , Flowers, K. A. , & Hurrell, J. (1987). Programming and execution of movement in Parkinson's disease. Brain, 110(5), 1247–1271. https://doi.org/10.1093/brain/110.5.1247 [DOI] [PubMed] [Google Scholar]
- Shrivastav, R. , Skowronski, M. , Kopf, L. , & Rakerd, B. (2014). Acoustic characteristics of the Lombard effect from talkers with Parkinson's disease. The Journal of the Acoustical Society of America, 135, 2426. https://doi.org/10.1121/1.4878069 [Google Scholar]
- Shulman, L. M. , Katzel, L. I. , Ivey, F. M. , Sorkin, J. D. , Favors, K. , Anderson, K. E. , Smith, B. A. , Reich, S. G. , Weiner, W. J. , & Macko, R. F. (2013). Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurology, 70(2), 183–190. https://doi.org/10.1001/jamaneurol.2013.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel, J. E. , Torrance, G. W. , Russell, L. B. , Luce, B. R. , Weinstein, M. C. , Gold, M. R. , & the Members of the Panel on Cost Effectiveness in Health and Medicine. (1997). Guidelines for pharmacoeconomic studies. Recommendations from the Panel on Cost Effectiveness in Health and Medicine. PharmacoEconomics, 11(2), 159–168. https://doi.org/10.2165/00019053-199711020-00005 [DOI] [PubMed] [Google Scholar]
- Solomon, N. P. , & Robin, D. A. (2005). Perceptions of effort during handgrip and tongue elevation in Parkinson's disease. Parkinsonism & Related Disorders, 11(6), 353–361. https://doi.org/10.1016/j.parkreldis.2005.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgon, L. , Clarke, C. E. , & Sackley, C. (2015). Subjective experiences of speech and language therapy in patients with Parkinson's disease: A pilot study. Rehabilitation Research and Practice, 2015, Article 839895. https://doi.org/10.1155/2015/839895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos, E. T. , Huber, J. E. , Richardson, K. , Kamphaus, J. , DeCicco, D. , Darling, M. , Fulcher, K. , & Sussman, J. E. (2014). Increased vocal intensity due to the Lombard effect in speakers with Parkinson's disease: Simultaneous laryngeal and respiratory strategies. Journal of Communication Disorders, 48, 1–17. https://doi.org/10.1016/j.jcomdis.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchi, F. , Abbruzzese, G. , Ceravolo, R. , Cortelli, P. , D'Amelio, M. , De Pandis, M. F. , Fabbrini, G. , Pacchetti, C. , Pezzoli, G. , Tessitore, A. , Canesi, M. , Iannacone, C. , Zappia, M. , & FORTE Study Group. (2014). Prevalence of fatigue in Parkinson disease and its clinical correlates. Neurology, 83(3), 215–220. https://doi.org/10.1212/WNL.0000000000000587 [DOI] [PubMed] [Google Scholar]
- Tubbs-Cooley, H. L. , Mara, C. A. , Carle, A. C. , & Gurses, A. P. (2018). The NASA Task Load Index as a measure of overall workload among neonatal, paediatric and adult intensive care nurses. Intensive & Critical Care Nursing, 46, 64–69. https://doi.org/10.1016/j.iccn.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelacker, L. A. , Epstein-Lubow, G. , Lewis, T. , Broughton, M. K. , & Friedman, J. H. (2014). A survey of Parkinson's disease patients: Most bothersome symptoms and coping preferences. Journal of Parkinson's Disease, 4(4), 717–723. https://doi.org/10.3233/JPD-140446 [DOI] [PubMed] [Google Scholar]
- van Hilten, J. J. , Hoogland, G. , van der Velde, E. A. , Middelkoop, H. A. , Kerkhof, G. A. , & Roos, R. A. (1993). Diurnal effects of motor activity and fatigue in Parkinson's disease. Journal of Neurology, Neurosurgery & Psychiatry, 56(8), 874–877. https://doi.org/10.1136/jnnp.56.8.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage, J. A. , Brink, T. L. , Rose, T. L. , Lum, O. , Huang, V. , Adey, M. B. , & Leirer, V. O. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. https://doi.org/10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Yogev, G. , Giladi, N. , Peretz, C. , Springer, S. , Simon, E. S. , & Hausdorff, J. M. (2005). Dual tasking, gait rhythmicity, and Parkinson's disease: Which aspects of gait are attention demanding? European Journal of Neuroscience, 22(5), 1248–1256. https://doi.org/10.1111/j.1460-9568.2005.04298.x [DOI] [PubMed] [Google Scholar]
- Zekveld, A. A. , & Kramer, S. E. (2014). Cognitive processing load across a wide range of listening conditions: Insights from pupillometry. Psychophysiology, 51(3), 277–284. https://doi.org/10.1111/psyp.12151 [DOI] [PubMed] [Google Scholar]
- Zekveld, A. A. , Kramer, S. E. , & Festen, J. M. (2010). Pupil response as an indication of effortful listening: The influence of sentence intelligibility. Ear and Hearing, 31(4), 480–490. https://doi.org/10.1097/AUD.0b013e3181d4f251 [DOI] [PubMed] [Google Scholar]
- Zgaljardic, D. J. , Borod, J. C. , Foldi, N. S. , & Mattis, P. (2003). A review of the cognitive and behavioral sequelae of Parkinson's disease: Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology, 16(4), 193–210. https://doi.org/10.1097/00146965-200312000-00001 [DOI] [PubMed] [Google Scholar]
- Zgaljardic, D. J. , Borod, J. C. , Foldi, N. S. , Mattis, P. J. , Gordon, M. F. , Feigin, A. , & Eidelberg, D. (2006). An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson's disease. Journal of Clinical and Experimental Neuropsychology, 28(7), 1127–1144. https://doi.org/10.1080/13803390500246910 [DOI] [PMC free article] [PubMed] [Google Scholar]