Abstract
Purpose:
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that affects bulbar functions including speech and voice. Voice onset time (VOT) was examined in speakers with ALS in early and late stages to explore the coordination of the articulatory and phonatory systems during speech production.
Method:
VOT was measured in nonword /bap/ produced by speakers with early-stage ALS (n = 11), late-stage ALS (n = 6), and healthy controls (n = 13), and compared with speech performance decline (a marker of disease progression) in ALS.
Results:
Overall comparison of the VOT values among the three groups showed a significant difference, F(2,27) = 11.71, p < .01. Speakers in late-stage ALS displayed longer voicing lead (negative VOT) than both healthy speakers and speakers in early-stage ALS. VOT was also significantly negatively correlated with speech performance (i.e., Intelligible Speaking Rate), r(15) = .74, p < .01.
Conclusions:
Speakers with more severe ALS showed greater occurrence of voicing lead and longer voicing lead. Findings show voicing precedes articulatory onset with disease progression in the production of bilabial stops, which suggests that the relative timing of coordination between the supralaryngeal structures and the phonatory system is affected in the late stage of ALS.
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by the deterioration of the upper and lower motor neurons in the brain and spinal cord, affecting bulbar motor functions such as speech and voice production. The involvement of the bulbar nerves (trigeminal, facial, hypoglossal, glossopharyngeal, and vagus) is progressive (Janzen et al., 1988) and affects the speech subsystems differentially (Green et al., 2013). Neuroinflammatory, metabolic, and reactive changes occur in the brainstem regions (Foerster et al., 2013), the site of origin for bulbar nerves innervating speech subsystems. Early symptoms of dysarthria are voice changes, velopharyngeal dysfunction, imprecise articulation, and tongue fasciculations (Hanson et al., 2011). As ALS progresses to impair more regions, patients usually show both spastic and flaccid characteristics.
ALS broadly impacts the speech production mechanisms (affecting articulatory, resonatory, respiratory, and phonatory subsystems). However, the rate of impact and the pattern of impairment on different subsystems are variable. Over the years, aerodynamic, acoustic, and kinematic measures have been used to evaluate changes in performance within each subsystem. Prior studies focused on the use of instrument-based measures for each of the speech subsystems and identified markers of bulbar decline in ALS (see Green et al., 2013; Rong et al., 2015; Yunusova et al., 2010). Rong et al. (2015, 2016) used a data modeling approach to better understand the contribution of each subsystem on speech intelligibility decline. The authors showed that changes in the articulatory and phonatory subsystems were more sensitive at predicting early bulbar decline than current standard clinical measures (i.e., speech intelligibility and speaking rate). Among all of the speech subsystems, the articulatory subsystem contributed the most to speech intelligibility decline over the course of the disease, particularly a reduction in lip and jaw movement measures. Deficits in speech production observed, in terms of phonetic features with bulbar function decline, were voicing contrast during syllable-initial/final consonants, place and manner of articulation contrasts for lingual consonants, stop versus nasal manner of production, features related to liquid consonants, and various features related to syllable shape (Kent et al., 1991). Currently, we do not have evidence of changes that may occur in the temporal coordination of the supralaryngeal structures with the larynx as disease progresses.
A widely used measure for temporal coordination is the acoustic voice onset time (VOT; Auzou et al., 2000). VOT is a measure of the temporal difference between an articulatory stop release and the onset of vocal fold vibrations (Lisker & Abramson, 1964). VOT is a frequently used index to describe intergestural coordination between the articulatory and laryngeal systems during speech and is an important acoustic cue for the voiced–voiceless distinction (Baken & Orlikoff, 2000). In healthy English speakers, voiced stops such as /b, d, g/ show voicing lead or short voicing lag (on average 0–25 ms; Auzou et al., 2000). Negative VOT (lead) is described when voicing begins as the obstruent starts and continues past the release. This measure is often observed in phrase-initial position after a pause and is relatively rare (Davidson, 2016). VOT has shown differences across a number of factors including speaking rate, gender, and age (see Rae 2018). Changes in VOT and the variability of VOT across and within speakers have been evaluated and reported across the types of dysarthria in neurogenic disorders such as Parkinson's disease (Auzou et al., 2000). VOT abnormalities in dysarthria can be interpreted as a loss of timing control and variables such as glottal opening size, transglottal pressure, and vocal fold tension affect the values.
Few studies have evaluated VOT in speakers with ALS. Caruso and Burton (1987) studied VOT in eight speakers with ALS and age-matched controls. The authors found significantly longer stop-gap and vowel duration values; however, there was not a significant difference in VOT for the six word-initial stop consonants /p,t,k,b,d,g/. The study participants had speech intelligibility greater than 80% and therefore may not have seen changes in VOT associated with higher levels of severity. Recently, Rowe and Green (2019) studied the proportion of VOT to syllable duration as a coordination measure and the variability of VOT as a measure of consistency across repetitions of a sequential motion rate task (/pataka/) in seven individuals with ALS ranging from mild to severe speech impairment. The findings indicated deficits in the measure of coordination but not for the consistency measure (coefficient of variation of VOT), compared with healthy talkers. As a follow-up, Rowe et al. (2020) reported that the coordination measure (ratio of VOT to syllable duration) was more impaired in both groups with early and late-stage ALS compared with corresponding early- and late-stage participants with Parkinson's disease. Currently, no published study has examined VOT changes in coordination of speech subsystems in different stages of ALS. A direct comparison of VOT between early- and late-stage groups with ALS may reveal how VOT changes in terms of ALS progression. Specifically, VOT may provide useful information regarding changes in the coordination of the articulatory and laryngeal subsystems as speech intelligibility declines.
The purpose of this study was to examine VOT in speakers with early- and late-stage ALS during consonant–vowel–consonant (/CVC/) production. First, the mean VOT was compared among speakers with early-stage ALS, late-stage ALS, and age-matched controls. We hypothesize that speakers with late-stage ALS will show increased VOT (more voicing lead) than healthy speakers due to the changes in temporal coordination in the more disordered group. Second, this study examined the relationship between intelligible speaking rate (i.e., as a marker of bulbar progression) and VOT in speakers with ALS. Intelligible speaking rate is speech intelligibility multiplied by speaking rate, which gives the efficiency of communication. Speaking rate is measured as the number of words produced per minute (wpm) and speech intelligibility as the percentage of words correctly transcribed by a naïve listener out of the total words read. We hypothesize that VOT will be significantly (and inversely) correlated with speech performance (as measured by intelligible speaking rate).
Method
Data Set
This study utilized an existing data set that was collected as a part of a multi-institutional study by The University of Texas at Dallas (the last author's former institution), MGH Institution of Health Professions, and the University of Toronto. The data collection protocols were approved by the institutional review boards of all participating institutions. Participants with ALS were diagnosed by one of the three board-certified clinical neurologists at the three sites following El Escorial criteria (Brooks et al., 2000). The healthy speakers had no reported history of speech, language, hearing, or neurological problems. The original study collected both acoustic and kinematic (tongue and lip motion) data synchronously using an electromagnetic articulograph (Allison et al., 2017; Wang et al., 2018). A high-quality Shure Microflex microphone (at 22 kHz sampling rate) was placed approximately 15 mm from the front of the subject's mouth. The participants were instructed to produce a list of nonwords (CVC; e.g., /bap/) with a short interval and repeated the stimuli 20 times. The data collection procedure and equipment standardized across the three sites. All researchers had the necessary expertise to carry out the protocol. Each site completed about one third of the subject recruitment and data collection tasks. This study used the audio data only. Audio files were retrieved from a secure, shared database across the three sites. Audio data (/bap/ sounds) from 17 participants with ALS (13 men and four women) and 13 healthy speakers' (five women and eight men) were analyzed for this study.
Speech intelligibility and speech rate measures were retrieved from the database, previously calculated by licensed speech-language pathologists at each of the locations. The speech performances (i.e., speech intelligibility and speaking rate) were measured at the phrase level using the Sentence Intelligibility Test software (SIT; Yorkston et al., 2007). During each data collection session, the SIT software generated a random list of 11 sentences with increasing length from five to 15 words. Participants were then asked to read these sentences once while the audio was recorded. The speech-language pathologist was unfamiliar to the speakers and transcribed the words by typing what she heard in the SIT software after the session. The SIT software then calculated speech intelligibility (percentage of correctly perceived words) by comparing how many words were understood correctly and speaking rate by how many (correct or incorrect) words were produced per minute. The measures were calculated locally at each data collection site during the data collection session and uploaded to a shared database between the three sites.
Finally, the intelligible speaking rate was calculated by multiplying the speech intelligibility score by the speaking rate. This is a clinical measure for communication efficiency used previously in ALS research (Wang et al., 2016, 2018; K. M. Yorkston & Beukelman, 1981). Intelligible speaking rate was calculated by the first author using the available speaking rate and speech intelligibility scores for each speaker (intelligible speaking rate [ISR] = speech intelligibility × speaking rate, for example, 200 words per minute × 90% = 180 words per minute).
A total of 34 participants were screened for analysis. Four of them were excluded, because there were no prominent stop release bursts in the spectrograms. One participant's data from the early-stage ALS group and three participants' data from the late-stage ALS group were excluded. Thus, a total of 30 participants were included for data analysis—11 speakers in early-stage ALS, six in late-stage ALS, and 13 healthy controls.
Data Analysis
The participants with ALS were assigned to two groups—early-stage ALS (three women and eight men) and late-stage ALS (one woman and five men) based on the speech performance scores (speaking rate and speech intelligibility) for data analysis. This task was performed by the first author who is trained in speech-language pathology. The criteria for the two groups were based on group characteristics found in a previous study by (Bandini et al., 2018). For the early-stage ALS group, participants had a speaking rate greater than 150 wpm and speech intelligibility greater than 97%. For the late-stage ALS group, speaking rate was less than 150 wpm and speech intelligibility less than 93%. The age, average speaking rate, and speech intelligibility scores for the three groups are reported in Table 1.
Table 1.
Age, speaking rate, and speech intelligibility for the three groups.
| Speaker characteristics | Healthy speakers | Early-stage ALS | Late-stage ALS |
|---|---|---|---|
| Age (M ± SD) | 51–74 years (63.23 ± 8.17) |
52–74 years (59.72 ± 10.27) |
52–81 years (62 ± 11.46) |
| Speaking rate (wpm) | 176–207.23 (M = 187.36) |
154.57–196.43 (M = 172.69) |
63.16–127.5 (M = 99.5) |
| Speech intelligibility (%) | 99–100 (M = 99.6) |
97.27–100 (M = 99.1) |
14.55–92.72 (M = 62.4) |
Note. ALS = Amyotrophic lateral sclerosis; wpm = words per minute.
After obtaining VOT values from each session, we first compared the VOT values among the three groups (early-stage ALS, late-stage ALS, and healthy controls). We then investigated the relationship between VOT and speech performance, using intelligible speaking rate.
VOT Analysis
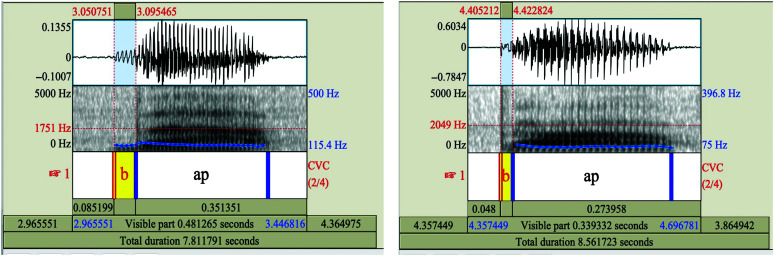
For the analysis of this study, 10 sound files for each participant were examined using the Praat acoustic analysis software (Version 6.1.11; Boersma & Weenink, 2007). Each production of /bap/ was manually analyzed to identify the precise timing of the stop release and the onset of phonation. These time points were determined primarily based on visual inspection of the amplitude signal and spectrogram. Pulsing options were displayed in the spectrogram. The time points for the two features were recorded in milliseconds. If the voicing followed the release of stop closure, VOT was calculated as positive. If the voicing preceded the release of the stop closure, VOT was calculated as negative. Five clear and correct (able to view stop release) productions were analyzed for each participant (Özsancak et al., 2001; Wang et al., 2004). A total of 150 productions for all of the 30 participants (five productions each) were measured. Intraobserver reliability and interobserver reliability were measured for separate sets of randomized 20% of the analyzed VOT data. Intraobserver reliability was measured by the same observer for six participants, two participants from each group. Interobserver reliability was completed by a second observer (second author) in six participants (two healthy, two early stage and two late-stage ALS) for a total of 30 productions. Intraclass correlational analysis estimates and their 95% confidence intervals (CIs) were calculated using Excel analysis toolpak (version 2112) based on a mean-rating (k = 2), absolute agreement, two-way mixed-effects model (Koo & Li, 2016). Our reliability index showed excellent agreement for intraobserver (0.99 with 95% CI being 0.96 to 0.99) and interobserver (0.98 with 95% CI being 0.95 to 0.99) reliability in measuring VOT. Figure 1 gives an example of lead and short lag VOT. Both examples were obtained from data samples of speakers with ALS.
Figure 1.
Example spectrograms showing voicing lead and short voicing lag in word-initial /b/ in stimuli /bap/. Spectrograms show (A) prevoiced/lead and (B) short-lag voice onset time (VOT). The onset of periodicity can be seen on the spectrogram at the first occurrence of the vertical lines, which correspond to the glottal pulses. The stop burst is seen after the voicing or glottal pulsing in the prevoicing or lead VOT while stop burst occurs before voicing onset in short lag VOT.
Statistical Analysis
The data met all assumptions for normal distribution and therefore statistical tests were run. To compare the differences in VOT across three groups, we ran a one-way analysis of variance (ANOVA). Tukey's honestly significant difference (HSD) was used for pairwise comparisons. We performed a post hoc power analysis for the groups (omega-squared, ψ = 0.41), indicating a large effect size (Field, 2013). Pearson's correlation coefficient was computed for the early-stage and late-stage ALS groups, respectively, to analyze the association between ISR and VOT. For both the HSD tests and the correlation analysis, significance was determined using the standard threshold of α = 0.05. All statistical analyses were done in Microsoft Excel 2016 MSO (Version 2112 Build 16.0.14729.20224) 32-bit, using the Analysis Toolpak add-in. An intraclass correlation analysis was performed to compare interobserver and intraobserver measurements.
Results
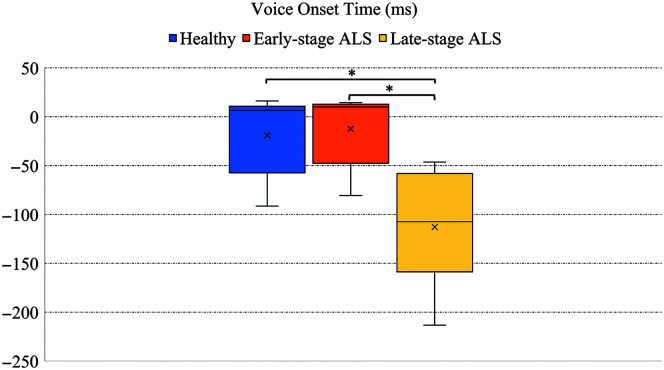
VOT values were measured and compared across three groups: healthy speakers, speakers with early-stage ALS, and speakers with late-stage ALS. On average, all groups showed an average negative VOT or voicing lead, that is, voicing preceded the stop release. The occurrence of negative VOT was 31.5% in healthy speakers, 26.4% in speakers with early-stage ALS, and 96.6% in speakers with late-stage ALS. The overall group comparison using a one-way ANOVA showed a significant difference among the groups, F(2,27) = 11.71, p < .01. Follow-up Tukey's HSD showed that the late-stage ALS group had a significantly longer lead VOT (M = −112.83 ms, SD = 62.78) than healthy speakers (M = −18.86 ms, SD = 39.62) and early-stage ALS group (M = −12.36 ms, SD = 37.06), as shown in Figure 2, but there were no significant differences between healthy and speakers with early stage ALS.
Figure 2.
Voice onset time in healthy, early-stage, and late-stage amyotrophic lateral sclerosis (ALS) groups. Statistically significant comparisons are marked in asterisk.
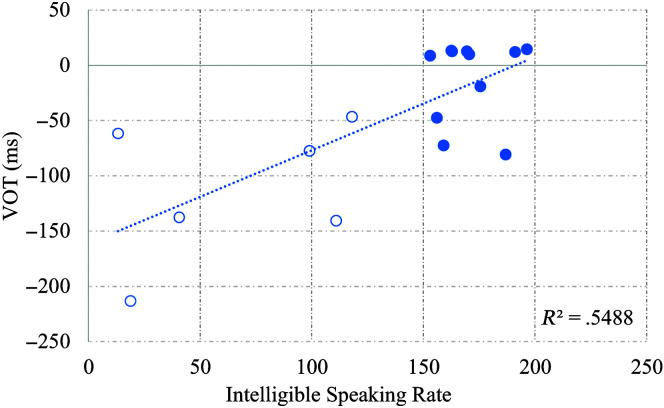
The relationship between ISR and VOT values was examined in both the early-stage and late-stage ALS group as shown in Figure 3. With a decrease in ISR, VOT values became longer, r(15) = .74, p < .01.
Figure 3.
Scatterplot of voice onset time (VOT) for /b/ with intelligible speaking rate in early-stage (filled circles) and late-stage amyotrophic lateral sclerosis (ALS) groups (unfilled circles).
Discussion
VOT measured for voiced word-initial stops was compared among speakers with late-stage ALS, early-stage ALS, and healthy speakers. Speakers in the late-stage ALS group had a significantly longer lead VOT values, whereas the early-stage ALS group did not show differences in VOT from healthy speakers. It has been observed that speech movements are considered as a set of motor programs where the articulators work together as a functional unit to activate and sequence individual phonemes into syllables and words (Gracco & Löfqvist, 1994; Munhall et al., 1994). The subsystems of respiration, phonation, and articulation are three anatomically distinct yet functionally linked components of the speech production mechanism. In healthy speakers, Dromey and Ramig (1998) reported a direct relationship between respiratory and phonatory subsystems, possibly because of shared neural circuitry, where modifying respiration showed more impact on voicing than articulation. The respiratory subsystem, particularly low lung volume has been found to shorten VOT (Hoit et al., 1993). For the production of stops, the difference between intraoral pressure and subglottal pressure determines when the voicing occurs. During the production of the voiced word-initial stops for this study, glottal pulsing preceded the stop release, suggesting that the laryngeal system was activated prior to the buildup of intraoral pressure for the stop. Three possible adjustments to the articulatory-laryngeal subsystems could have occurred while maintaining or initiating phonation during the closure in voiced stops (Rothenberg, 2009): (a) a passive supraglottal expansion; (b) an active enlargement of the cavity by adjustments like larynx lowering, tongue root advancement, tongue body lowering; or (c) nasal airflow through an incomplete velopharyngeal closure. In the group with late-stage ALS, longer negative (lead) VOT may be due to impaired lip and jaw movements (Rong et al., 2016) or due to supraglottal leakage from weakness in the velopharyngeal closure (Rosenfield et al., 1991). Although lung volume changes are typically evident in speakers with late-stage ALS, we were not able to evaluate the impact of respiratory function on VOT. Our current findings show that VOT was not sensitive to early changes (in the-early stage ALS group) while changes in coordination were captured in VOT in the group with late-stage ALS. The finding of no VOT difference between speakers with early stage ALS and healthy speakers is consistent with the literature (Caruso & Burton, 1987; Rowe & Green, 2019).
The occurrence of lead VOT was 31.5% in healthy speakers, and 26.4% in speakers with early-stage ALS. Of the 13 healthy speakers, four speakers displayed both voicing lead and short voicing lag within the five production tokens. In the early stage ALS group (11 speakers), three speakers exhibited both lag and lead mode voicing onset. Switching between voicing mode of voicing lead or short voicing lag based on the context and place of articulation has been reported by Smith (1978) in healthy speakers. Although voiced stops in American English are primarily recognized as short lag or positive VOT (Rae, 2018), there are studies reporting the occurrence of negative VOT (voicing lead) as long as −92 ms in healthy speakers (Dmitrieva et al., 2015; Hunnicutt & Morris, 2016; Smith, 1978). The supralaryngeal mechanisms during the production of bilabial stop consonants have been actively explored (Löfqvist & Gracco, 1997). Lips move at high velocity at the onset of oral closure, resulting in tissue compression and airtight seal for intraoral pressure buildup. There are adjustments that may be enabling healthy speakers to have flexible control using either voicing lead or short lag voicing mode. Ahn (2018) suggested that there may be passive expansions of the pharyngeal wall or active gestures of tongue root retraction to increase the cavity volume during the production of utterance-initial voiced stops in English and higher variations in tongue body/front lowering in labial voiced stops. In group with late-stage ALS, the occurrence of a lead VOT was observed in almost all of the productions (96.7%). One participant used one short voicing lag token, whereas all other five participants produced a lead VOT consistently across their productions. Findings of reduced occurrence of a flexible mode of voicing (lead vs. short lag) in late-stage ALS group and inverse correlation between VOT and intelligible speaking rate may be indicative of the neurodegenerative changes that occur with disease severity in ALS. We speculate that these neurodegenerative changes could be reflected in the longer coordination time between the articulatory and phonatory subsystems. The changes observed in VOT with speech decline in ALS could be what is observed as progressive loss of phonetic features such as voiced–voiceless contrast, oral versus nasal contrasts in later stages of ALS (Kent et al., 1991).
Researchers have previously (see Bóna, 2014) examined VOT in older healthy adults to explore changes in acoustic-phonetic properties of speaking as a result of the aging process. Greater variability in VOT values across sounds and less differentiation between voiced and voiceless stop production has been reported (see Torre & Barlow, 2009), suggesting a decline in supraglottal–glottal coordination. Although age has shown an impact on VOT, our study was controlled for age-related changes in VOT by age-matching participants across the three groups—healthy speakers, early stage, and late-stage speakers.
The coordination between the articulatory and phonatory speech subsystems may be influenced by individual differences in neural degeneration localization (Rong et al., 2016). A recent study (Stipancic et al., 2021) has suggested that this may result in two distinct phenotypes within the late-stage ALS group (bulbar symptomatic—high speech function vs. low speech function groups). Although this is beyond the scope of this study, this may be a potential impact of differences in VOT. Future work should consider other acoustic measures to assess differences in subsystem coordination across the two phenotypes in late-stage ALS.
Clinical Implications
The study based on the findings using VOT suggests the larger relative timing of the coordination between the supralaryngeal and laryngeal systems in nonword utterance initial contexts. Although the direct cause of longer lead VOT is unknown, it may be worth considering treatment options that improve the coordination between the two subsystems, if possible. Findings further reinforce the need for therapy to sustain supraglottal function and speaking modifications (e.g., improve articulatory and velopharyngeal functioning), while the speaker chooses to communicate orally.
Limitations
Although informative, this study has limitations that warrant additional investigations. First, our study only assessed data from a single session, which limits our ability to analyze changes within individual subjects over time. Second, the study findings are limited due to its smaller sample size in the groups. The use of a single and simple speech token, including more complex stimuli would allow us to assess the impact of language context and prosody on VOT and make our findings more generalizable. Third, respiration was not assessed, which prevented us from being able to assess the impact it has on VOT. Future studies should consider measuring VOT for all six English stop consonants in ALS in order to examine differences in laryngeal–supralaryngeal coordination with changing articulatory positioning and voicing. Complementing VOT measures with imaging, tongue kinematic based, or a measure of physiological VOT (e.g., using real-time magnetic resonance imaging) may be a stronger approach for observing the nature of temporal coordination between the subsystems.
Conclusions
VOT was analyzed in speakers with early-stage and late-stage ALS and compared with healthy speakers in relation to speech performance decline. Speakers in late-stage ALS showed longer lead VOT than speakers with early-stage ALS and healthy speakers. With an increase in speech severity symptoms in ALS, VOT duration increased. Our findings suggest that changes in VOT reflect impaired temporal coordination between the laryngeal and supralaryngeal structures. Longer lead VOT evident in late-stage ALS is reflective of the neurodegenerative changes by the disease progression. The occurrence of voicing lead was observed predominantly in late-stage ALS, suggesting that laryngeal and articulatory coordination changes with disease progression.
Acknowledgments
This work was supported by the National Institutes of Health through Grants R01DC016621 (PI: Wang) and R01DC013547 (PI: Green; Co-Is: Campbell, Yunusova, Wang). About a quarter of the data and results has been presented in the Texas Speech-Language-Hearing Association Conference in April 2021. The authors would like to thank Jordan R. Green (MGH Institute of Health Professions and Harvard University), Yana Yunusova (University of Toronto), Thomas Campbell (The University of Texas at Dallas), James Berry (Massachusetts General Hospital and Harvard Medical School), Lorne Zinman (University of Toronto), Jennifer McGlothlin, Brian D. Richburg, Carolina Uzquiano, Alyssa Shrode, Hailey Shuman, and the volunteering participants.
Funding Statement
This work was supported by the National Institutes of Health through Grants R01DC016621 (PI: Wang) and R01DC013547 (PI: Green; Co-Is: Campbell, Yunusova, Wang). About a quarter of the data and results has been presented in the Texas Speech-Language-Hearing Association Conference in April 2021.
References
- Ahn, S. (2018). The role of tongue position in laryngeal contrasts: An ultrasound study of English and Brazilian Portuguese. Journal of Phonetics, 71, 451–467. https://doi.org/10.1016/j.wocn.2018.10.003 [Google Scholar]
- Allison, K. M. , Yunusova, Y. , Campbell, T. F. , Wang, J. , Berry, J. D. , & Green, J. R. (2017). The diagnostic utility of patient-report and speech-language pathologists' ratings for detecting the early onset of bulbar symptoms due to ALS. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 18(5–6), 358–366. https://doi.org/10.1080/21678421.2017.1303515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auzou, P. , Özsancak, C. , Morris, R. J. , Jan, M. , Eustache, F. , & Hannequin, D. (2000). Voice onset time in aphasia, apraxia of speech and dysarthria: A review. Clinical Linguistics & Phonetics , 14(2), 131–150. https://doi.org/10.1080/026992000298878 [Google Scholar]
- Baken, R. J. , & Orlikoff, R. F. (2000). Clinical measurement of speech and voice (2nd ed.). Singular Thomson Learning. [Google Scholar]
- Bandini, A. , Green, J. R. , Wang, J. , Campbell, T. F. , Zinman, L. , & Yunusova, Y. (2018). Kinematic features of jaw and lips distinguish symptomatic from presymptomatic stages of bulbar decline in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 61(5), 1118–1129. https://doi.org/10.1044/2018_JSLHR-S-17-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma, P. , & Weenink, D. (2007). Praat (Version 6.1.11) . https://uvafon.hum.uva.nl/praat/
- Bóna, J. (2014). Voice onset time and speakers' age: Data from Hungarian. Clinical Linguistics & Phonetics, 28(5), 366–372. https://doi.org/10.3109/02699206.2013.875593 [DOI] [PubMed] [Google Scholar]
- Brooks, B. R. , Miller, R. G. , Swash, M. , & Munsat, T. L. (2000). El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1(5), 293–299. https://doi.org/10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
- Caruso, A. J. , & Burton, E. K. (1987). Temporal acoustic measures of dysarthria associated with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 30(1), 80–87. https://doi.org/10.1044/jshr.3001.80 [DOI] [PubMed] [Google Scholar]
- Davidson, L. (2016). Variability in the implementation of voicing in American English obstruents. Journal of Phonetics, 54, 35–50. https://doi.org/10.1016/j.wocn.2015.09.003 [Google Scholar]
- Dmitrieva, O. , Llanos, F. , Shultz, A. A. , & Francis, A. L. (2015). Phonological status, not voice onset time, determines the acoustic realization of onset f0 as a secondary voicing cue in Spanish and English. Journal of Phonetics, 49, 77–95. https://doi.org/10.1016/j.wocn.2014.12.005 [Google Scholar]
- Dromey, C. , & Ramig, L. O. (1998). The effect of lung volume on selected phonatory and articulatory variables. Journal of Speech, Language, and Hearing Research, 41(3), 491–502. https://doi.org/10.1044/jslhr.4103.491 [DOI] [PubMed] [Google Scholar]
- Field, A. (2013). Discovering statistics using IBM SPSS Statistics (4th ed.). Sage. [Google Scholar]
- Foerster, B. R. , Welsh, R. C. , & Feldman, E. L. (2013). 25 years of neuroimaging in amyotrophic lateral sclerosis. Nature Reviews Neurology, 9(9). https://doi.org/10.1038/nrneurol.2013.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracco, V. , & Löfqvist, A. (1994). Speech motor coordination and control: Evidence from lip, jaw, and laryngeal movements. The Journal of Neuroscience, 14(11), 6585–6597. https://doi.org/10.1523/JNEUROSCI.14-11-06585.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J. R. , Yunusova, Y. , Kuruvilla, M. S. , Wang, J. , Pattee, G. L. , Synhorst, L. , Zinman, L. , & Berry, J. D. (2013). Bulbar and speech motor assessment in ALS: Challenges and future directions. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 14(7–8), 494–500. https://doi.org/10.3109/21678421.2013.817585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, E. K. , Yorkston, K. M. , & Britton, D. (2011). Dysarthria in amyotrophic lateral sclerosis: A systematic review of characteristics, speech treatment and augmentative and alternative communication options. Journal of Medical Speech-Language Pathology, 19(3), 12–30. [Google Scholar]
- Hoit, J. D. , Solomon, N. P. , & Hixon, T. J. (1993). Effect of lung volume on voice onset time (VOT). Journal of Speech and Hearing Research, 36(3), 516–520. https://doi.org/10.1044/jshr.3603.516 [DOI] [PubMed] [Google Scholar]
- Hunnicutt, L. , & Morris, P. (2016). Pre-voicing and aspiration in Southern American English. University of Pennsylvania Working Papers in Linguistics, 22(1), 215–224. [Google Scholar]
- Janzen, V. D. , Rae, R. E. , & Hudson, A. J. (1988). Otolaryngologic manifestations of amyotrophic lateral sclerosis. The Journal of Otolaryngology, 17(1), 41–42. [PubMed] [Google Scholar]
- Kent, R. D. , Sufit, R. L. , Rosenbek, J. C. , Kent, J. F. , Weismer, G. , Martin, R. E. , & Brooks, B. R. (1991). Speech deterioration in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 34(6), 1269–1275. https://doi.org/10.1044/jshr.3406.1269 [DOI] [PubMed] [Google Scholar]
- Koo, T. K. , & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. https://doi.org/10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisker, L. , & Abramson, A. S. (1964). A cross-language study of voicing in initial stops: Acoustical measurements. WORD, 20(3), 384–422. https://doi.org/10.1080/00437956.1964.11659830 [Google Scholar]
- Löfqvist, A. , & Gracco, V. L. (1997). Lip and jaw kinematics in bilabial stop consonant production. Journal of Speech, Language, and Hearing Research, 40(4), 877–893. https://doi.org/10.1044/jslhr.4004.877 [DOI] [PubMed] [Google Scholar]
- Munhall, K. G. , Löfqvist, A. , & Kelso, J. A. S. (1994). Lip–larynx coordination in speech: Effects of mechanical perturbations to the lower lip. The Journal of the Acoustical Society of America, 95(6), 3605–3616. https://doi.org/10.1121/1.409929 [DOI] [PubMed] [Google Scholar]
- Özsancak, C. , Auzou, P. , Jan, M. , & Hannequin, D. (2001). Measurement of voice onset time in dysarthric patients: Methodological considerations. Folia Phoniatrica et Logopaedica, 53(1), 48–57. https://doi.org/10.1159/000052653 [DOI] [PubMed] [Google Scholar]
- Rae, R. (2018). Measures of voice onset time: A methodological study. ProQuest Dissertations and Theses, May, 72.
- Rong, P. , Yunusova, Y. , Wang, J. , & Green, J. R. (2015). Predicting early bulbar decline in amyotrophic lateral sclerosis: A speech subsystem approach. Behavioural Neurology, 2015, Article 183027 . https://doi.org/10.1155/2015/183027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, P. , Yunusova, Y. , Wang, J. , Zinman, L. , Pattee, G. L. , Berry, J. D. , Perry, B. , & Green, J. R. (2016). Predicting speech intelligibility decline in amyotrophic lateral sclerosis based on the deterioration of individual speech subsystems. PLOS ONE, 11(5), Article e0154971. https://doi.org/10.1371/journal.pone.0154971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield, D. B. , Viswanath, N. , Herbrich, K. E. , & Nudelman, H. B. (1991). Evaluation of the speech motor control system in amyotrophic lateral sclerosis. Journal of Voice, 5(3), 224–230. https://doi.org/10.1016/S0892-1997(05)80190-0 [Google Scholar]
- Rothenberg, M. (2009). Voice onset time vs. articulatory modelling for stop consonants. Logopedics Phoniatrics Vocology, 34(4), 171–180. https://doi.org/10.3109/14015430903002270 [DOI] [PubMed] [Google Scholar]
- Rowe, H. P. , & Green, J. R. (2019). Profiling speech motor impairments in persons with amyotrophic lateral sclerosis: An acoustic-based approach. Proceedings of the Annual Conference of the International Speech Communication Association, INTERSPEECH, 2019-September, 4509–4513. https://doi.org/10.21437/Interspeech.2019-2911 [Google Scholar]
- Rowe, H. P. , Gutz, S. E. , Maffei, M. F. , & Green, J. R. (2020). Acoustic-based articulatory phenotypes of amyotrophic lateral sclerosis and Parkinson's disease: Towards an interpretable, hypothesis-driven framework of motor control. Proceedings of the Annual Conference of the International Speech Communication Association, INTERSPEECH, 2020-October, 4816–4820. https://doi.org/10.21437/Interspeech.2020-1459 [Google Scholar]
- Smith, B. L. (1978). Effect of place of articulation and vowel environment on voiced stop consonant production. Glossa, 12, 163–175. [Google Scholar]
- Stipancic, K. L. , Yunusova, Y. , Campbell, T. F. , Wang, J. , Berry, J. D. , & Green, J. R. (2021). Two distinct clinical phenotypes of bulbar motor impairment in amyotrophic lateral sclerosis. Frontiers in Neurology, 12. https://doi.org/10.3389/fneur.2021.664713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre, P. , & Barlow, J. A. (2009). Age-related changes in acoustic characteristics of adult speech. Journal of Communication Disorders, 42(5), 324–333. https://doi.org/10.1016/j.jcomdis.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Wang, J. , Kothalkar, P. v. , Kim, M. , Yunusova, Y. , Campbell, T. F. , Heitzman, D. , & Green, J. R. (2016). Predicting intelligible speaking rate in individuals with amyotrophic lateral sclerosis from a small number of speech acoustic and articulatory samples. SLPAT 2016 Workshop on Speech and Language Processing for Assistive Technologies. https://doi.org/10.21437/SLPAT.2016-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Kothalkar, P. V. , Kim, M. , Bandini, A. , Cao, B. , Yunusova, Y. , Campbell, T. F. , Heitzman, D. , & Green, J. R. (2018). Automatic prediction of intelligible speaking rate for individuals with ALS from speech acoustic and articulatory samples. International Journal of Speech-Language Pathology, 20(6), 669–679. https://doi.org/10.1080/17549507.2018.1508499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.-T. , Kent, R. D. , Duffy, J. R. , Thomas, J. E. , & Weismer, G. (2004). Alternating motion rate as an index of speech motor disorder in traumatic brain injury. Clinical Linguistics & Phonetics, 18(1), 57–84. https://doi.org/10.1080/02699200310001596160 [DOI] [PubMed] [Google Scholar]
- Yorkston, K. , Beukelman, D. , Hakel, M. , & Dorsey, M. (2007). Speech intelligibility test. Madonna Rehabilitation Hospital. [Google Scholar]
- Yorkston, K. M. , & Beukelman, D. R. (1981). Communication efficiency of dysarthric speakers as measured by sentence intelligibility and speaking rate. Journal of Speech and Hearing Disorders, 46(3). https://doi.org/10.1044/jshd.4603.296 [DOI] [PubMed] [Google Scholar]
- Yunusova, Y. , Green, J. R. , Wang, J. , Pattee, G. , & Zinman, L. (2010). A protocol for comprehensive assessment of bulbar dysfunction in amyotrophic lateral sclerosis (ALS). Journal of Visualized Experiments, 48, 3–7. https://doi.org/10.3791/2422 [DOI] [PMC free article] [PubMed] [Google Scholar]