Abstract
Background
An unmet medical need remains for an effective dengue tetravalent vaccine that can be administered irrespective of previous dengue exposure. TAK-003, a dengue tetravalent vaccine, has demonstrated efficacy in an ongoing phase 3 trial in children and adolescents living in dengue-endemic areas, with an acceptable safety profile in both dengue-naive and dengue-exposed individuals.
Methods
Safety findings are presented herein from an integrated analysis of data for healthy 4–60-year-olds from two phase 2 and three phase 3 double-blind, placebo-controlled clinical trials of TAK-003 (TAK-003, n = 14 627; placebo, n = 7167). Safety evaluation included analyses of postinjection reactogenicity, unsolicited adverse events (AEs), serious AEs (SAEs), and deaths. Subgroup analyses were performed by age group, baseline serostatus, and gender.
Results
The most common local and systemic AEs were injection site pain (43% for TAK-003 and 26% for placebo) and headache (34% and 30%, respectively). Injection site AEs were mostly mild and resolved within 1–3 days. Unsolicited AEs and AEs leading to discontinuation occurred with similar frequency across both groups, while SAEs were fewer for TAK-003 recipients (6% vs 8% for placebo). Four of the 5 vaccine-related SAEs (which included hypersensitivity, dengue fever, and dengue hemorrhagic fever) occurred in the placebo group. No deaths were considered vaccine-related. Subgroup analyses showed no differences in safety by baseline serostatus or by gender, albeit analysis by age indicated greater local reactogenicity rates for adolescents (46% for TAK-003 and 28% for placebo) and adults (56% and 19%, respectively) than for children (37% and 25%, respectively).
Conclusions
No important safety risks were identified, and TAK-003 was well tolerated irrespective of age, gender, or baseline dengue serostatus in recipients aged 4–60 years.
Keywords: dengue tetravalent vaccine, clinical safety, TAK-003, adverse events, hospitalization
In this integrated safety analysis of 5 phase 2 and 3 double-blind, randomized, placebo-controlled trials in 4–60-year-olds, TAK-003, a dengue tetravalent vaccine, was well tolerated irrespective of age, sex, or baseline dengue serostatus, with no important safety risks identified.
The incidence of dengue fever has grown dramatically in recent decades, associated with societal changes such as population growth and increasing urbanization, particularly in tropical and subtropical cities with poor waste and water management, leading to proliferation of dengue-transmitting mosquito species such as Aedes aegypti [1]. In addition, rising temperatures and global climate change have led to expansion of the major mosquito vectors into new areas, extension of the transmission season, decrease in extrinsic incubation periods, and increase in mosquito vectorial capacity [2].
Most of the current preventive measures that rely on mosquito control and individual protection are of limited efficacy, complex to implement, and questionable in terms of cost-effectiveness [3–5]. Integration of routine vaccination with other prevention methods shows the highest potential for reduction of dengue disease burden. Thus, in addition to vaccination against dengue, control of the mosquito population to stop disease spread could fit into a multimodal approach to reducing the overall disease burden. Other technologies such as those involving release of Wolbachia-transfected A. aegypti, genetic modification and release of mosquitoes, or reversible sterilization of mosquitoes, have the potential to substantially reduce the spread of disease [6–8].
Vaccine development, as part of an integrated dengue prevention strategy, has assumed the need for tetravalent vaccines against all 4 serotypes, which often cocirculate in endemic regions, to avoid any potential risk of vaccine-induced immune enhancement [9–11]. Although a dengue tetravalent vaccine (CYD-TDV; Dengvaxia, Sanofi-Pasteur) based on a yellow fever backbone is now licensed in many endemic countries, it is recommended only for individuals with previous dengue infection [12, 13]. There remains, therefore, an unmet need for an effective dengue tetravalent vaccine that can be administered irrespective of previous dengue exposure.
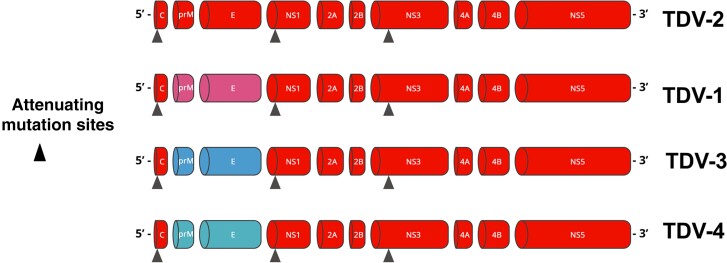
TAK-003, a live attenuated dengue tetravalent vaccine, comprises 4 dengue virus (DENV) strains based on a DENV-2 backbone (Figure 1) [15, 16]. During the TAK-003 clinical development program, safety data have been assessed from approximately 27 000 participants across dengue-endemic and nonendemic regions, with long-term safety follow-up still ongoing in 2 trials. An integrated safety analysis across the double-blind, placebo-controlled, phase 2 and phase 3 trials of TAK-003 is presented herein.
Figure 1.
Genetic structure of the 4 TAK-003 vaccine strains. Abbreviations: C, capsid; E, envelope; NS, nonstructural protein; prM, premembrane; TDV-1/2/3/4, dengue serotype 1/2/3/4 strain. Image: Takeda Pharmaceuticals.
METHODS
Trials Included
Safety data for participants aged 4–60 years from 5 double-blind, randomized, placebo-controlled phase 2 and 3 trials were included in the integrated analysis (Table 1). Participants in all trials received 2 subcutaneous injections of either TAK-003 or placebo 3 months apart. Data for the ongoing DEN-301 trial were included up to the last completed trial milestone of 36 months after the second vaccine dose. Data extracted from DEN-203 trial for the pooled analysis included only those for placebo recipients ≥4 years old because the high-dose TAK-003 formulation used in this trial differed from that used in the other 4 placebo-controlled trials. Likewise, data extracted from DEN-204 trial included only those for participants ≥4 years old from groups 1 and 4 because the exploratory dose regimens investigated for the other 2 trial groups did not conform to the dosing regimen of 2 TAK-003 doses 3 months apart. Safety follow-up periods varied between the trials, from 6 to 48 months after the second vaccine dose. The 5 trials were conducted in adults, adolescents, and children (Table 1). All trials excluded participation from individuals with hypersensitivity to any vaccine component, impaired or altered immune function, planned or ongoing pregnancy or breastfeeding, or previous vaccination against dengue.
Table 1.
Completed and Ongoing Phase 2 and 3 Placebo-Controlled Trials Comprising the Integrated Analysis (Placebo-Controlled Trials Safety Pool, Safety Set)
| Clinical Trial Code and Identifiera | Trial Design | TAK-003 Vaccine Potency, Log10 PFUs per Doseb | Safety Objectives | Trial Population | Trial Location(s) | Safety Follow-up Timeframe | Trial Dates and Status |
|---|---|---|---|---|---|---|---|
| Phase 2 trials | |||||||
| ȃDEN-204c [17–19]; NCT02302066 |
Randomized (1:2:5:1), double-blind, placebo-controlled | TDV-1 to TDV-4: 4.4, 3.8, 4.5, 5.6 | To evaluate safety and tolerability of TAK-003, reactogenicity of TAK-003 in a subset of participants, and to assess safety during long-term follow-up |
Aged 2–17 y | The Dominican Republic, Panama, The Philippines | 48 mo after 1st dose | 2014–2019 Completed |
| ȃDEN-203d [20–22]; NCT01511250 |
Randomized (2:1 for part 1; 3:1 for part 2 [expansion]), double-blind, placebo-controlled, age-descending, expansion trial | TDV-1 to TDV-4: 4.3, 4.7, 5.0, and 5.5 | To evaluate safety and tolerability of HD TAK-003 and assess safety during long-term follow-up |
Part 1: aged 1.5–45 y; part 2: aged 1.5–11 y |
Colombia, Puerto Rico, Singapore, Thailand | 36 mo after 1st dose | 2011–2016 Completed |
| Phase 3 trials | |||||||
| ȃDEN-304; NCT03423173 |
Randomized (2:2:2:1), double-blind, placebo-controlled | TDV-1 to TDV-4: 5.2, 4.5, 5.4, and 6.2 |
To evaluatesafety and tolerability of TAK-003 | Aged 18–60 y | United States of America | 6 mo after 2nd dose | 2018–2019 Completed |
| ȃDEN-315 [23]; NCT03341637 |
Randomized (3:1), double-blind, placebo controlled | TDV-1 to TDV-4: 5.1, 4.5, 5.4, and 5.9 |
To evaluate safety and tolerability of TAK-003 | Aged 12–17 y | Mexico | 6 mo after 2nd dose | 2017–2019 Completed |
| ȃDEN-301e [24–27]; NCT02747927 |
Randomized (2:1), double-blind, placebo-controlled | TDV-1 to TDV-4: 3.6, 4.0, 4.6, and 5.1 |
To evaluate safety and tolerability of TAK-003, describe reactogenicity of TAK-003 in a subset of participants, and to identify any cases of severe dengue (linked to efficacy objective) |
Aged 4–16 y; (booster: 4–11 y old at time of 1st dose) | Thailand, The Philippines, Sri Lanka, Brazil, Panama, The Dominican Republic, Colombia, Nicaragua | 36 mo after 2nd dose; booster dose planned for 48–56 mo with 2 y of follow-up | 2016–Ongoing |
Abbreviations: HD, high dose; PFUs, plaque-forming units; TDV-1/2/3/4, dengue serotype 1/2/3/4 strain.
All trials were approved by the respective institutional review boards/ethics committees and were conducted in accordance with the Declaration of Helsinki and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) harmonized tripartite guidelines for Good Clinical Practice. Written informed consent was obtained from all participants or their parents/legal guardians before enrollment.
TAK-003 vaccine excipients comprise α,α-trehalose dihydrate, Kolliphor P407 (F127), human serum albumin, potassium dihydrogen phosphate, disodium hydrogen phosphate dihydrate, potassium chloride, and sodium chloride. Potency values in this column are listed for TDV-1, TDV-2, TDV-3, and TDV-4 serotype strains, respectively.
Only group 1 (TAK-003 2-dose regimen given 3 months apart) and group 4 (placebo) participants ≥4 years old were included in the integrated analysis. Reactogenicity and unsolicited adverse events were recorded for a randomly selected subset of 562 of 1794 total participants in this trial.
A high-dose formulation of TAK-003 (HD TAK-003), differing from the commercially-intended formulation, was used in this trial: therefore, only placebo recipients ≥4 years old were included in the integrated safety analysis.
Reactogenicity and unsolicited adverse events were recorded for a randomly selected subset of 3992 of 20 071 total participants in this trial.
Excluded Trials
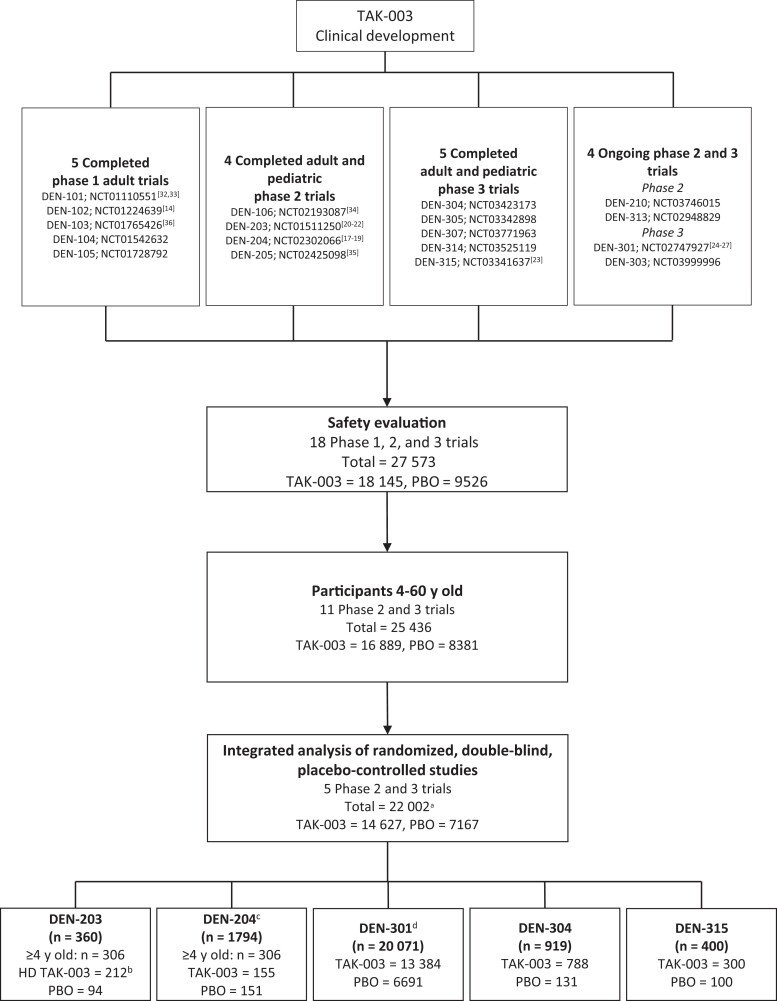
Other clinical trials conducted during TAK-003 development did not include placebo controls or did not use the commercially-intended TAK-003 formulation; these trials constituted an additional 5571 participants who were excluded from the integrated safety analysis (Figure 2).
Figure 2.
Overview of TAK-003 clinical development program and trials included in the current integrated safety evaluation of placebo (PBO)–controlled randomized controlled trials. aAll participants received the commercially intended formulation of TAK-003 except 212 participants in DEN-203 who received a non–commercially-intended formulation of TAK-003 (HD TAK-003). bA high-dose formulation of TAK-003 (HD TAK-003), differing from the commercially intended formulation, was used in this trial: therefore, only PBO recipients ≥4 years old were included in the integrated analysis. cOnly group 1 (TAK-003 2-dose regimen given 3 months apart) and group 4 (PBO) participants ≥4 years old were included in the integrated analysis. Reactogenicity and unsolicited adverse events were recorded for a randomly selected subset of 562 of 1794 total participants in this trial. d4 participants (3 assigned to 2 doses of TAK-003 and 1 assigned to 2 doses of PBO) erroneously received 1 dose of PBO and 1 dose of TAK-003. Reactogenicity and unsolicited adverse events were recorded for a randomly selected subset of 3992 of 20 071 total participants in this trial.
Vaccine
The TAK-003 vaccine potencies varied slightly across the placebo-controlled trials (Table 1). Placebo was 0.5 mL of saline for all included trials.
Safety Assessments
The following safety measures were assessed after each vaccine dose: solicited local and systemic adverse events (AEs) within the first 30 minutes (assessed in the clinic), diary-based solicited local AEs up to 7 days; diary-based solicited systemic AEs up to 14 days, and unsolicited AEs up to 28 days. The severity of solicited and unsolicited AEs was categorized by investigators as mild, moderate, or severe, based on protocol definitions (Supplementary Table 1). Where severity was not recorded, the AE was considered severe. Causal relationship to the trial vaccine or procedures was assessed by investigators (Supplementary Table 2). Solicited and unsolicited AEs were documented for a subset of randomly selected participants from DEN-301 (n = 3992) and DEN-204 (n = 562), and for all participants in the other trials. Deaths, serious AEs (SAEs), and AEs leading to vaccine dose discontinuation or discontinuation from trial were recorded throughout the trials for all participants. Medically attended AEs were recorded in DEN-304 and DEN-315 only, and were defined as spontaneous AEs leading to a consultation with a healthcare professional, including visits to an emergency department, that did not fulfil the seriousness criteria.
Hospitalized Virologically Confirmed Dengue and Severe Dengue
DEN-301 formed the basis for evaluation of all virologically confirmed dengue (VCD) cases that required hospitalization or were severe forms of dengue: all participants were actively monitored for febrile illness, and predefined criteria were used to identify severe forms of dengue (see [25]). VCD was defined as febrile illness or illness clinically suspected to be dengue by the investigator in association with a positive serotype-specific reverse-transcription polymerase chain reaction result [25]. All hospitalized VCD cases were assessed for severity by a masked independent adjudication committee (Dengue Case Severity Adjudication Committee), according to predefined criteria. In addition, a computer algorithm identified cases of dengue hemorrhagic fever (DHF) based on World Health Organization 1997 criteria [28]. All nonhospitalized VCD cases were considered nonsevere.
Statistical Analysis
Safety measures are presented descriptively; subgroup analyses are presented by age group, gender, and baseline dengue serostatus. Seropositive was defined as a reciprocal neutralizing antibody titer ≥10 against ≥1 dengue serotype. Seronegative was defined as no detectable antibody titers against any DENV serotype.
RESULTS
The clinical development program comprised 27 573 participants; 22 002 were included in the placebo-controlled pooled analysis. Results are presented for 21 790 participants who received the commercially-intended TAK-003 formulation or placebo (Figure 2, Table 2, and Supplementary Table 3). Of these, 400 were in DEN-315 (300 in TAK-003 and 100 in placebo group), 919 in DEN-304 (788 and 131, respectively), 20 071 in DEN-301 (13 384 and 6691), 306 in DEN-204 (155 and 151), and 306 in DEN-203 (94 in placebo group). Demographic and baseline characteristics were broadly similar between the TAK-003 and placebo groups. Across groups, 67%–69% of participants were seropositive for ≥1 dengue serotype at baseline (Table 3). Demographic characteristics varied across age groups in accordance with trial enrollment criteria (Supplementary Table 4). The median duration of follow-up after the second vaccine dose was 3.0 years, with 85.9% of participants followed up for up to 36 months, primarily owing to those from DEN-301.
Table 2.
Number and Percentage of Participants Aged 4–60 Years From Each of the 5 Phase 2 or Phase 3 Trials Comprising the Integrated Analysis (Placebo-Controlled Trials Safety Pool, Safety Set)
| Trial | Participants, No. (%) | ||
|---|---|---|---|
| TAK-003 Only (n = 14 623) |
TAK-003 and Placebo (n = 4) |
Placebo Only (n = 7163) |
|
| DEN-301 | 13 380 (91.5) | 4 (100.0)a | 6687 (93.4) |
| DEN-304 | 788 (5.4) | 0 (0.0) | 131 (1.8) |
| DEN-315 | 300 (2.1) | 0 (0.0) | 100 (1.4) |
| DEN-204b | 155 (1.1) | 0 (0.0) | 151 (2.1) |
| DEN-203 | 0 (0.0) | 0 (0.0) | 94 (1.3) |
In DEN-301, 4 participants (3 assigned to 2 doses of TAK-003 and 1 assigned to 2 doses of placebo) erroneously received 1 dose of placebo and 1 dose of TAK-003.
The integrated analysis included only ≥4-year-old participants from DEN-204 trial groups 1 (TAK-003 2-dose regimen given 3 months apart) and group 4 (placebo).
Table 3.
Demographic and Baseline Characteristics for Participants Aged 4–60 Years (Placebo-Controlled Trials Safety Pool, Safety Set)
| Characteristic | Participants, No. (%)a | |
|---|---|---|
| TAK-003 (n = 14 627) | Placebo (n = 7167) | |
| Age, y | ||
| ȃMean (SD) | 11.4 (8.36) | 10.3 (5.66) |
| ȃMedian (range) | 10.0 (4.0–60.0) | 10.0 (4.0–60.0) |
| Age category | ||
| ȃChildren (aged 4–11 y) | 9210 (63.0) | 4728 (66.0) |
| ȃAdolescents (aged 12–17 y) | 4629 (31.6) | 2287 (31.9) |
| ȃAdults (aged 18–60 y) | 788 (5.4) | 152 (2.1) |
| Gender | ||
| ȃMale | 7295 (49.9) | 3659 (51.1) |
| ȃFemale | 7332 (50.1) | 3508 (48.9) |
| Body mass indexb | (n = 14 617) | (n = 7167) |
| ȃMean (SD) | 18.4 (4.49) | 18.0 (3.96) |
| ȃMedian (range) | 17.1 (8.5–64.8) | 16.9 (8.8–42.1) |
| Race | ||
| ȃAsian | 6033 (41.2) | 3069 (42.8) |
| ȃAmerican Indian or Alaska Nativec | 5469 (37.4) | 2762 (38.5) |
| ȃBlack or African American | 1643 (11.2) | 819 (11.4) |
| ȃWhite | 937 (6.4) | 253 (3.5) |
| ȃMultiracial or otherd | 545 (3.7) | 216 (3.0) |
| ȃUnknown race | 0 | 48 (0.7) |
| Region | ||
| ȃLatin America | 7807 (53.4) | 3962 (55.3) |
| ȃAsia Pacific | 6032 (41.2) | 3074 (42.9) |
| ȃNorth America | 788 (5.4) | 131 (1.8) |
| Region endemic status | ||
| ȃEndemic | 13 539 (92.6) | 6936 (96.8) |
| ȃNonendemic | 1088 (7.4) | 231 (3.2) |
| Baseline dengue serostatuse | ||
| ȃSeropositive | 9808 (67.1) | 4975 (69.4) |
| ȃSeronegative | 4472 (30.6) | 2063 (28.8) |
| ȃUnknown | 347 (2.4) | 129 (1.8) |
| Flavivirus vaccination status | ||
| ȃExposed (YF or JE vaccine) | 6030 (41.2) | 2996 (41.8) |
| ȃNot exposed | 8597 (58.8) | 4171 (58.2) |
Abbreviations: JE, Japanese encephalitis; SD, standard deviation; YF, yellow fever.
Data represent no. (%) of participants unless otherwise specified.
Body mass index calculated as weight in kilograms divided by height in meters squared.
Owing to limited selection options on the case report form, this category was most frequently selected as the participant’s race in Latin American countries.
Includes the category “Native Hawaiian or other Pacific Islander.”
Seropositive defined as reciprocal neutralizing antibody titer of ≥10 against ≥1 dengue virus serotype at baseline; seronegative, as no detectable antibody titers against any dengue virus serotype at baseline.
Solicited AEs
Immediate Reactogenicity
Overall, 6.7% (257 of 3830) of TAK-003 and 5.2% (90 of 1725) of placebo recipients experienced local or systemic AEs within 30 minutes after vaccination; the majority experienced injection site pain. Rates were higher after the first vaccination (4.2% and 3.2% for TAK-003 and placebo, respectively) than after the second vaccination (3.1% and 2.3%, respectively).
Local Reactogenicity
Solicited local AEs rates were higher for TAK-003 than for placebo recipients across all age groups, with highest rates observed for adolescents and adults (Supplementary Table 5). AE rates were higher after the first than after the second vaccination in both trial groups (Table 4). The most frequent local reaction after any vaccination was injection site pain for both the TAK-003 (41.8%) and placebo (25.4%) recipients. Most local AEs started on the day of vaccination, were mild in severity, and resolved within 1–3 days. Severe local AEs were reported by 1.3% and 0.7% of TAK-003 and placebo recipients, respectively. Approximately 4% of TAK-003 recipients experienced prolonged solicited local AEs (Supplementary Table 6), defined as those ongoing for ≥7 days after an injection. These events were mostly mild, and all participants recovered.
Table 4.
Solicited Local and Systemic Adverse Events After the First, Second, and Any Vaccination Dose for Participants Aged 4–60 Years (Placebo-Controlled Trials Safety Pool, Safety Set)
| AE | Participants, No. With AE/Total No. With Data | |
|---|---|---|
| TAK-003 (n = 3830a) | Placebo (n = 1725a) | |
| Solicited local AEs within 7 days after dose | ||
| ȃAfter 1st vaccination | ||
| ȃȃAny solicited local AE | 1290/3747 (34.4) | 327/1690 (19.3) |
| ȃȃPain | 1220/3747 (32.6) | 324/1690 (19.2) |
| ȃȃErythema | 218/3729 (5.8) | 3/1680 (0.2) |
| ȃȃSwelling | 98/3723 (2.6) | 8/1680 (0.5) |
| ȃAfter 2nd vaccination | ||
| ȃȃAny solicited local AE | 1056/3654 (28.9) | 222/1647 (13.5) |
| ȃȃPain | 1000/3652 (27.4) | 220/1646 (13.4) |
| ȃȃErythema | 157/3642 (4.3) | 2/1642 (0.1) |
| ȃȃSwelling | 59/3637 (1.6) | 3/1638 (0.2) |
| ȃAfter any vaccination | ||
| ȃȃAny solicited local AE | 1642/3782 (43.4) | 437/1703 (25.7) |
| ȃȃPain | 1581/3782 (41.8) | 432/1703 (25.4) |
| ȃȃErythema | 268/3781 (7.1) | 5/1702 (0.3) |
| ȃȃSwelling | 130/3780 (3.4) | 11/1702 (0.6) |
| Solicited systemic AE within 14 days after dose | ||
| ȃAfter 1st vaccination | ||
| ȃȃAny solicited systemic AE, excluding fever | 1360/3749 (36.3) | 519/1690 (30.7) |
| ȃȃHeadache | 922/3406 (27.1) | 370/1512 (24.5) |
| ȃȃMyalgia | 747/3405 (21.9) | 239/1512 (15.8) |
| ȃȃMalaise | 571/3404 (16.8) | 216/1419 (15.2) |
| ȃȃAsthenia | 490/3406 (14.4) | 189/1418 (13.3) |
| ȃȃIrritability/fussinessb | 30/341 (8.8) | 15/177 (8.5) |
| ȃȃDrowsinessb | 36/341 (10.6) | 18/177 (10.2) |
| ȃȃLoss of appetiteb | 47/341 (13.8) | 16/177 (9.0) |
| ȃȃFever (≥38°C) | 189/3517 (5.4) | 97/1577 (6.2) |
| ȃAfter 2nd vaccination | ||
| ȃȃAny solicited systemic AE, excluding fever | 924/3649 (25.3) | 324/1646 (19.7) |
| ȃȃHeadache | 600/3350 (17.9) | 229/1494 (15.3) |
| ȃȃMyalgia | 532/3349 (15.9) | 154/1494 (10.3) |
| ȃȃMalaise | 402/3349 (12.0) | 142/1402 (10.1) |
| ȃȃAsthenia | 355/3349 (10.6) | 123/1402 (8.8) |
| ȃȃIrritability/fussinessb | 18/299 (6.0) | 4/152 (2.6) |
| ȃȃDrowsinessb | 21/299 (7.0) | 7/152 (4.6) |
| ȃȃLoss of appetiteb | 22/299 (7.4) | 9/152 (5.9) |
| ȃȃFever (≥38°C) | 157/3520 (4.5) | 90/1573 (5.7) |
| ȃAfter any vaccination | ||
| ȃȃAny solicited systemic AEs, excluding fever | 1655/3782 (43.8) | 632/1702 (37.1) |
| ȃȃHeadache | 1176/3476 (33.8) | 465/1545 (30.1) |
| ȃȃMyalgia | 973/3476 (28.0) | 316/1545 (20.5) |
| ȃȃMalaise | 795/3475 (22.9) | 300/1451 (20.7) |
| ȃȃAsthenia | 684/3476 (19.7) | 254/1451 (17.5) |
| ȃȃIrritability/fussinessb | 43/348 (12.4) | 17/178 (9.6) |
| ȃȃDrowsinessb | 46/348 (13.2) | 22/178 (12.4) |
| ȃȃLoss of appetiteb | 59/348 (17.0) | 22/178 (12.4) |
| ȃȃFever (≥38°C) | 331/3704 (8.9) | 175/1667 (10.5) |
Abbreviation: AE, adverse event.
Solicited AEs were recorded for a randomly selected subset of participants from DEN-301 and DEN-204. bAssessed only for those participants aged <6 years.
Systemic Reactogenicity
Solicited systemic AE rates were highest after the first vaccination (Table 4), most commonly headache (34% and 30% for TAK-003 and placebo groups, respectively). Most systemic AEs started on the day of or day after vaccination, were mild, and typically resolved within 3–4 days. After any vaccination dose, 5.4% and 6.1% of TAK-003 and placebo recipients, respectively, experienced fever considered vaccine-related, and, 34.2% and 27.9% of TAK-003 and placebo recipients, respectively, experienced vaccine-related solicited systemic AEs (excluding fever); severe vaccine-related systemic AEs were reported by 3.0% and 2.1% of TAK-003 and placebo recipients, respectively.
Prolonged solicited systemic AEs (ie, those still ongoing ≥14 days after any injection) were reported by 1.8% and 1.4% of TAK-003 and placebo participants, respectively, (Supplementary Table 6); most events were mild or moderate in severity. Prolonged events started after a median of 3 and 9 days after vaccination with TAK-003 and placebo, respectively, with median durations of 15 and 12 days, respectively. Eleven (0.3%) of the TAK-003 and 4 (0.2%) of the placebo recipients experienced severe prolonged systemic events, most frequently headache (0.2% in both groups).
Unsolicited AEs
Overall, 21% of TAK-003 versus 23% of placebo recipients experienced unsolicited AEs, most commonly nasopharyngitis and upper respiratory tract infections (Table 5). The incidence of any vaccine-related unsolicited AEs was higher for TAK-003 than for placebo recipients (3.0% vs 1.7%), owing mostly to higher incidence of mild unsolicited injection site reactions.
Table 5.
Unsolicited Adverse Events, by MedDRA Preferred Term, Reported by ≥0.5% of TAK-003 or Placebo Recipients Aged 4–60 Years, Within 28 Days After Any Vaccine Dose (Placebo-Controlled Trials Safety Pool, Safety Set)
| Unsolicited AE | Participants, No. (%) | |
|---|---|---|
| TAK-003 (n = 3830a) | Placebo (n = 1725a) | |
| Any unsolicited AE | 815 (21.3) | 394 (22.8) |
| Nasopharyngitis | 98 (2.6) | 56 (3.2) |
| Upper respiratory tract infection | 90 (2.3) | 58 (3.4) |
| Viral upper respiratory tract infection | 39 (1.0) | 14 (0.8) |
| Viral infection | 30 (0.8) | 14 (0.8) |
| Pyrexia | 29 (0.8) | 16 (0.9) |
| Gastroenteritis | 27 (0.7) | 20 (1.2) |
| Injection site bruising | 27 (0.7) | 1 (<0.1) |
| Injection site pruritus | 26 (0.7) | 0 (0.0) |
| Headache | 22 (0.6) | 21 (1.2) |
| Systemic viral infection | 22 (0.6) | 14 (0.8) |
| Pharyngitis | 21 (0.5) | 10 (0.6) |
| Pharyngotonsillitis | 20 (0.5) | 12 (0.7) |
| Influenza | 19 (0.5) | 9 (0.5) |
| Tonsillitis | 19 (0.5) | 5 (0.3) |
| Varicella | 15 (0.4) | 13 (0.8) |
| Any vaccine-related unsolicited AEb | 114 (3.0) | 30 (1.7) |
Abbreviations: AE, adverse event; MedDRA, Medical dictionary for regulatory activities.
Unsolicited AEs up to 28 days after each vaccine dose were recorded for a randomly selected subset of participants from DEN-301 and DEN-204.
The trial investigator determined whether an adverse event was related to vaccine administration.
Most unsolicited AEs were mild or moderate in severity (Supplementary Table 7), and most resolved within the 28-day recording period. Nineteen (0.5%) of the TAK-003 versus 3 (0.2%) of the placebo recipients experienced severe unsolicited AEs, with similar rates in children, adolescents, and adults (Supplementary Table 8). Five (0.1%) TAK-003 recipients experienced vaccine-related severe AEs (myalgia, anhedonia, malaise, upper respiratory tract infection, and pyrexia); all AEs were nonserious and resolved within 2–17 days. One placebo recipient experienced dose-related severe pyrexia, which resolved within 2 days. Both pyrexia events were classified as severe owing to missing severity information.
The integrated safety analysis did not reveal any TAK-003–related anaphylactic reactions. Equal numbers of TAK-003 and placebo recipients (4 [0.03%] and 4 [0.06%], respectively) experienced vaccine-related hypersensitivity reactions within the first 4 days after dosing (Supplementary Table 9); all TAK-003–related events were nonserious and resolved within 2 to 7 days.
Serious AEs
After adjustment for the differing durations of follow-up, the overall incidence rate for SAEs after any dose was 2.72 events per 100 person-years for TAK-003 recipients and 3.28 per 100 person-years for placebo recipients, with fewer events of dengue fever, DHF, and viral infections in the TAK-003 group (Table 6). The most frequently reported SAEs were infections and infestations, most commonly dengue fever in the placebo group and appendicitis in both groups.
Table 6.
Serious Adverse Events, by Preferred Term, Reported by ≥0.1% of TAK-003 or Placebo Recipients Aged 4–60 Years After any Vaccine Dose (Placebo-Controlled Trials Safety Pool, Safety Set)
| SAE | TAK-003 (n = 14 627; FT, 43 066.7 PY) | Placebo (n = 7167; FT, 21 647.2 PY) | ||
|---|---|---|---|---|
| Participants With AE, No. (%) | FTAR | Participants With AE, No. (%) | FTAR | |
| Any SAE | 908 (6.21) | 2.72 | 542 (7.56) | 3.28 |
| Appendicitis | 78 (0.53) | 0.18 | 34 (0.47) | 0.16 |
| Dengue fevera | 68 (0.46) | 0.16 | 117 (1.63) | 0.55 |
| Gastroenteritis | 48 (0.33) | 0.12 | 18 (0.25) | 0.08 |
| Viral infection | 39 (0.27) | 0.09 | 39 (0.54) | 0.18 |
| Pneumonia | 32 (0.22) | 0.07 | 23 (0.32) | 0.11 |
| Influenza | 31 (0.21) | 0.07 | 20 (0.28) | 0.10 |
| Urinary tract infection | 27 (0.18) | 0.07 | 18 (0.25) | 0.09 |
| Cellulitis | 22 (0.15) | 0.05 | 6 (0.08) | 0.03 |
| Asthma | 19 (0.13) | 0.05 | 5 (0.07) | 0.02 |
| Humerus fracture | 16 (0.11) | 0.04 | 5 (0.07) | 0.02 |
| Lower respiratory tract infection | 16 (0.11) | 0.04 | 6 (0.08) | 0.03 |
| Lymphadenitis | 15 (0.10) | 0.03 | 1 (0.01) | <0.01 |
| Animal bite | 14 (0.10) | 0.03 | 6 (0.08) | 0.03 |
| Dengue hemorrhagic fever | 14 (0.10) | 0.03 | 34 (0.47) | 0.16 |
| Forearm fracture | 14 (0.10) | 0.03 | 4 (0.06) | 0.02 |
| Road traffic accident | 14 (0.10) | 0.03 | 1 (0.01) | <0.01 |
| Radius fracture | 12 (0.08) | 0.03 | 9 (0.13) | 0.04 |
| Upper respiratory tract infection | 7 (0.05) | 0.02 | 10 (0.14) | 0.05 |
| Any vaccine-related SAEb | 1 (<0.01) | <0.01 | 4 (0.06) | 0.02 |
Abbreviations: FT, total follow-up time; FTAR, FT-adjusted AE rate (AEs per 100 PY); PY, person-years; SAE, serious adverse event.
Events based on investigator reporting, not necessarily virologically confirmed. One participant experienced suspected dengue fever considered attributable to TAK-003; however, no febrile illness blood sample was available to test for vaccine viremia because the participant was afebrile.
The trial investigator determined whether an adverse event was related to vaccine administration.
More than 95% of SAEs occurred >30 days after a vaccine dose, and the majority resolved within 1–2 weeks. Five participants experienced SAEs (hypersensitivity, dengue fever, and DHF) considered attributable to the trial vaccine: 4 placebo recipients, and 1 TAK-003 recipient who had suspected dengue starting in the second week after the first vaccination, but which resolved within 5 days (Table 6).
AEs Leading to Discontinuation
The incidences of AEs leading to vaccine dose discontinuation or participant discontinuation from a trial were 0.16% and 0.15%, respectively, for TAK-003 recipients, and 0.13% and 0.13%, respectively, for placebo recipients (Supplementary Tables 7 and 8). The incidence of vaccine-attributable AEs leading to vaccine dose discontinuation and/or discontinuation from the trial was <0.1% for both groups. Three participants discontinued from their trial before the second dose of TAK-003 because of nonserious vaccine-related AEs (mild angioedema, moderate injection site pruritus, and moderate urticaria). One placebo recipient experienced 2 related SAEs of moderate hypersensitivity and discontinued from their trial before the second dose. In addition, owing to vaccine-attributable AEs after the first dose, the second dose was not administered to 3 TAK-003 recipients (1 each with moderate pruritic rash and mild arthralgia; moderate pruritic rash; and severe myalgia) and 1 placebo recipient (1 SAE of moderate allergic reaction).
Deaths
Twenty-one deaths occurred during the TAK-003 clinical development program, none of which was considered related to vaccine administration. Of those, 14 occurred in the placebo-controlled trials, with similar incidences (<0.1%) in the TAK-003 and placebo groups (Supplementary Tables 7 and 8). Ten deaths occurred in the TAK-003 group because of injuries (2 wounds, 2 road traffic accidents, and 1 craniocerebral injury), malignant ependymoma, suicide, asphyxia, cerebrovascular arteriovenous malformation, and multiple organ dysfunction syndrome. Four deaths in the placebo group were because of traumatic lung injury, adenocarcinoma of the colon, lung squamous cell carcinoma, and aseptic meningitis.
Medically Attended AEs
Overall, 23.2% of TAK-003 and 24.2% of placebo recipients in DEN-304 and DEN-315 experienced a medically attended AE (Supplementary Table 10). Viral upper respiratory infection was the most common medically attended AE for both groups (TAK-003, 3.0%; placebo, 4.3%).
Subgroup Analyses
The safety profile observed when the integrated data were analyzed by subgroups was mostly consistent with the overall 4–60-year-old population.
More solicited local AEs were reported by adolescents (45.8%) and adults (56.3%) than by children (36.5%) in the TAK-003 group (Supplementary Table 5). The overall incidence of reactogenicity was highest for seronegative TAK-003 recipients when assessed by baseline dengue serostatus (Supplementary Table 11), with higher AEs rates for adolescents (61.9% for local and 65.6% for systemic AEs) and adults (60.0% and 49.8%, respectively) than for children (39.1% and 35.0%) (Supplementary Table 12). Baseline seronegative participants reported a greater number of unsolicited AEs with both TAK-003 and placebo (seronegative, 23.7%–24.7%; seropositive, 19.1%–22.0%). SAE rates were similar for TAK-003 and placebo recipients, irrespective of baseline serostatus (seronegative, 5.7%–6.7%; seropositive, 6.6%–8.1%; Supplementary Table 13).
The patterns of solicited and unsolicited AEs when assessed by gender were similar to those observed in the overall analysis population. Female participants experienced solicited AEs more frequently (52.3% vs 34.0% for male participants) after TAK-003 dose, but clinically meaningful gender differences were not evident for unsolicited AEs or SAE rates (Supplementary Table 14). Further subgroup analyses (race, country, region, endemic/nonendemic region, baseline flavivirus vaccination status, or combinations of these variables) yielded neither any clinically meaningful differences between the TAK-003 and placebo recipients nor any new identifiable safety risks.
VCD Leading to Hospitalization and Severe Forms of Dengue
The relative risk of VCD leading to hospitalization up to 36 months after the second TAK-003 dose was 0.14 (95% CI, 0.09–0.22) for baseline dengue seropositive participants and 0.23 (95% CI, 0.13–0.41) for baseline dengue seronegative participants (Supplementary Table 15).
Exploratory analysis for baseline seronegative participants at serotype level revealed more hospitalized dengue cases caused by DENV-3 in TAK-003 recipients (11 of 3714 [0.30%]) than in placebo recipients (2 of 1832 [0.11%]) [27]. No efficacy has been shown against DENV-3 in baseline seronegative participants, and no imbalance in hospitalized dengue cases was observed for the other serotypes; the absence of DENV-4 hospitalized cases did not allow an assessment [27]. When assessed by year of follow-up after the second dose (Supplementary Table 16), it was evident that this imbalance in hospitalized DENV-3 cases occurred in the third year; in the first 2 years, DENV-3 cases were detected only in The Philippines and Thailand, whereas, in the third year, DENV-3 also circulated in Sri Lanka. Higher rates of dengue hospitalizations in Sri Lanka have been implicated as a major reason for the case distribution in the third year (see [27] for in-depth discussion). However, because the observed imbalance was based on few cases derived from interim analyses that are exploratory at serotype level, and, because the trial is still ongoing for long-term follow-up, no firm conclusions can be drawn on possible causes at this stage.
By 3 years after the second dose, 10 of 13 380 (0.07%) TAK-003 recipients and 17 of 6687 (0.25%) placebo recipients had been identified as Dengue Case Severity Adjudication Committee–defined cases of severe dengue and/or DHF. Of the 10 TAK-003 cases, 5 (0.05%) were baseline seropositive and 5 (0.13%) were baseline seronegative; this included 1 baseline seropositive participant and 2 baseline seronegative participants who experienced severe dengue. An imbalance in baseline seronegative participants was observed for severe forms of dengue, albeit based on very small numbers of cases: 5 (0.13%) in the TAK-003 group versus 1 (0.05%) in the placebo group; all were attributable to DENV-3 and occurred across the trial years; severity did not increase over time.
DISCUSSION
The substantial increase in dengue over recent decades means that it remains one of the major threats to global health. In addition to expansion of the disease beyond tropical and subtropical countries, explosive outbreaks are now occurring, with the largest ever number of dengue cases reported recently in 2019 [29]. Given the potential for more severe disease after reexposure, and the cocirculation of serotypes in endemic areas, there is a desperate unmet need for a tetravalent vaccine that can be administered regardless of previous dengue exposure.
CYD-TDV is currently the only licensed dengue tetravalent vaccine recommended for individuals with laboratory-confirmed previous DENV infection, limiting its clinical use and public health benefit [12, 13, 30]. In long-term safety follow-up of TAK-003 to 3 years after the second vaccine dose, the incidences of VCD, hospitalized VCD, and severe dengue were substantially fewer for the TAK-003 than for the placebo recipients. Though there was an imbalance between TAK-003 and placebo groups in hospitalized VCD, including severe forms of dengue caused by DENV-3 in baseline seronegative participants, the overall number of cases remained very small with no evidence of increased severity over time [27]. Some fluctuation in reported VCD cases over time is to be expected because of local epidemiology and varying efficacy of TAK-003 against individual serotypes. Ongoing data collection in the DEN-301 trial and postauthorization vigilance will allow close observation, further characterization, and assessment of possible causes of imbalances. Any hospitalized dengue cases caused by DENV-3 in baseline seronegative vaccinees, including severe forms of dengue, warrant close monitoring and are considered an important potential risk in the TAK-003 Risk Management Plan. The same is true for hospitalized dengue cases caused by DENV-4, as the absence of cases did not allow an assessment.
Data from a single trial, DEN-301, predominate in this integrated analysis. Reactogenicity was higher for TAK-003 than for placebo recipients, led primarily by injection site reactions for adolescents and adults; nevertheless, reactogenicity rates overall were lower after the second than after the first dose of TAK-003. Injection site reactions are commonly observed with many vaccines, particularly live attenuated vaccines, and are generally mild and self-limiting without treatment [31]. The integrated analyses did not reveal any TAK-003–related anaphylactic reactions. As with all vaccines, continual close monitoring for potential occurrence of rare hypersensitivity reactions after vaccination is integral to clinical trials and to postlicensure routine pharmacovigilance activities. Analyses of unsolicited AEs and SAEs up to 36 months after the second vaccine dose did not identify any unusual events considered to be a safety risk or concern.
While this analysis focused on randomized placebo-controlled trials, an additional 5571 participants comprised the TAK-003 clinical development program and participated in non–placebo-controlled trials or trials evaluating a different formulation of TAK-003. Safety data from these trials [14, 32–36], including 2 key trials evaluating coadministration of TAK-003 with yellow fever (DEN-305; NCT03342898) or hepatitis A (DEN-314; NCT03525119) vaccines, did not reveal any new safety risks other than those already presented in this integrated analysis. The safety profile of TAK-003, combined with the favorable efficacy results from the ongoing phase 3 trial [24–27], indicates a promising potential for this vaccine as part of an integrated dengue control program.
In summary, no important safety risks were identified in this integrated safety analysis up to 36 months after the second dose of TAK-003, with a demonstration that it is well tolerated, irrespective of age, gender, or baseline dengue serostatus in recipients aged 4–60 years. While the TAK-003 safety profile is still evolving, with potential risks being closely monitored, these findings provide an encouraging advance in the search for a dengue tetravalent vaccine that is well tolerated by dengue-naive and dengue-exposed individuals and that can be integrated into a multimodal approach for dengue disease prevention in the future.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments . The authors express much gratitude and thank the following: adult participants and parents/legal guardians of children/adolescent participants who enrolled in the various clinical trials; trial investigators and their staff for their dedication and support for conducting the TAK-003 clinical trials; Karin Helsberg PhD (Trilogy Writing and Consulting) for editorial assistance in the preparation of TAK-003 Module 2.7.4, a common technical document comprising the integrated safety analyses; Guy de la Rosa Arosemena MD, Shibadas Biswal MD, and Vianney Tricou, PharmD DPhil, for their stewardship of TAK-003 clinical trials comprising the safety data for integrated analyses; and members of respective institutional review boards and ethics committees who approved and facilitated conduct of the TAK-003 clinical trials in accordance with the Declaration of Helsinki and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) harmonized tripartite guidelines for Good Clinical Practice. Editorial assistance in preparation of this manuscript was provided by Jennifer Engelmoer, PhD (Sula Communications), funded by Takeda Pharmaceuticals.
Financial support . This work was supported by Takeda Pharmaceuticals.
Supplementary Material
Contributor Information
Sanjay S Patel, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Martina Rauscher, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Maria Kudela, Takeda Pharmaceuticals International Inc, Cambridge, Massachusetts, USA.
Hang Pang, Takeda Pharmaceuticals International Inc, Cambridge, Massachusetts, USA.
References
- 1. Guzman MG, Gubler DJ, Izquierdo Aet al. Dengue infection. Nat Rev Dis Primers 2016; 2:16055. 10.1038/nrdp.2016.55 [DOI] [PubMed] [Google Scholar]
- 2. Grange L, Simon-Loriere E, Sakuntabhai Aet al. Epidemiological risk factors associated with high global frequency of inapparent dengue virus infections. Front Immunol 2014; 5:280. 10.3389/fimmu.2014.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maoz D, Ward T, Samuel M, et al. Community effectiveness of pyriproxyfen as a dengue vector control method: A systematic review. PLoS Negl Trop Dis 2017; 11:e0005651. 10.1371/journal.pntd.0005651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samuel M, Maoz D, Manrique P, et al. Community effectiveness of indoor spraying as a dengue vector control method: A systematic review. PLoS Negl Trop Dis 2017; 11:e0005837. 10.1371/journal.pntd.0005837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horstick O, Runge-Ranzinger S, Nathan MBet al. Dengue vector-control services: how do they work? A systematic literature review and country case studies. Trans R Soc Trop Med Hyg 2010; 104:379–86. 10.1016/j.trstmh.2009.07.027 [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Luo J, Wang Y, et al. Suppression of female fertility in Aedes aegypti with a CRISPR-targeted male-sterile mutation. Proc Natl Acad Sci USA 2021; 118:e2105075118. 10.1073/pnas.2105075118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kyrou K, Hammond AM, Galizi R, et al. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol 2018; 36:1062–6. 10.1038/nbt.4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li M, Yang T, Bui M, et al. Suppressing mosquito populations with precision guided sterile males. Nature Commun 2021; 12:5374. 10.1038/s41467-021-25421-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas SJ. Developing a dengue vaccine: progress and future challenges. Ann N Y Acad Sci 2014; 1323:140–59. 10.1111/nyas.12413 [DOI] [PubMed] [Google Scholar]
- 10. Tsai WY, Lin HE, Wang WK. Complexity of human antibody response to dengue virus: implication for vaccine development. Front Microbiol 2017; 8:1372. 10.3389/fmicb.2017.01372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katzelnick LC, Gresh L, Halloran ME, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017; 358:929–32. 10.1126/science.aan6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanofi Pasteur . Dengvaxia (dengue tetravalent vaccine) prescribing information. Available at: https://www.fda.gov/media/124379/download. Accessed 8 June 2022.
- 13. Sanofi Pasteur . Dengvaxia: summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/dengvaxia-epar-product-information_en.pdf. Accessed 8 June 2022.
- 14. Osorio JE, Velez ID, Thomson C, et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14:830–8. 10.1016/S1473-3099(14)70811-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang CY-H, Butrapet S, Tsuchiya KRet al. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol 2003; 77:11436–47. 10.1128/jvi.77.21.11436-11447.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoksan SBN, Halstead SB. Dengue virus vaccine development: study on biological markers of uncloned dengue 1–4 viruses serially passaged in primary kidney cells [abstract 35–8]. In: Arbovirus research in Australia: proceedings of the 4th Symposium. Brisbane,Australia: Commonwealth Scientific and Industrial Research Organization, Division of Tropical Animal Science and Queensland Institute of Medical Research, 1986:35–8. [Google Scholar]
- 17. Sáez-Llorens X, Tricou V, Yu D, et al. Immunogenicity and safety of one versus two doses of tetravalent dengue vaccine in healthy children aged 2–17 years in Asia and Latin America: 18-month interim data from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 2018; 18:162–70. 10.1016/S1473-3099(17)30632-1 [DOI] [PubMed] [Google Scholar]
- 18. Sáez-Llorens X, Tricou V, Yu D, et al. Safety and immunogenicity of one versus two doses of Takeda's tetravalent dengue vaccine in children in Asia and Latin America: interim results from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 2017; 17:615–25. 10.1016/S1473-3099(17)30166-4 [DOI] [PubMed] [Google Scholar]
- 19. Tricou V, Sáez-Llorens X, Yu D, et al. Safety and immunogenicity of a tetravalent dengue vaccine in children aged 2–17 years: a randomised, placebo-controlled, phase 2 trial. Lancet 2020; 395:1434–43. 10.1016/S0140-6736(20)30556-0 [DOI] [PubMed] [Google Scholar]
- 20. Rupp R, Luckasen GJ, Kirstein JL, et al. Safety and immunogenicity of different doses and schedules of a live attenuated tetravalent dengue vaccine (TDV) in healthy adults: a phase 1b randomized study. Vaccine 2015; 33:6351–9. 10.1016/j.vaccine.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 21. Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, et al. Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis 2016; 213:1562–72. 10.1093/infdis/jiv762 [DOI] [PubMed] [Google Scholar]
- 22. Sharma M, Glasner DR, Watkins H, et al. Magnitude and functionality of the NS1-specific antibody response elicited by a live-attenuated tetravalent dengue vaccine candidate. J Infect Dis 2020; 221:867–77. 10.1093/infdis/jiz081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biswal S, Mendez Galvan JF, Macias Parra M, et al. Immunogenicity and safety of a tetravalent dengue vaccine in dengue-naïve adolescents in Mexico City. Rev Panam Salud Publica 2021; 45:e67. 10.26633/RPSP.2021.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biswal S, Borja-Tabora C, Martinez Vargas L, et al. Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: a randomised, placebo-controlled, phase 3 trial. Lancet 2020; 395:1423–33. 10.1016/S0140-6736(20)30414-1 [DOI] [PubMed] [Google Scholar]
- 25. Biswal S, Reynales H, Saez-Llorens X, et al. Efficacy of a tetravalent dengue vaccine in healthy children and adolescents. N Engl J Med 2019; 381:2009–19. 10.1056/NEJMoa1903869 [DOI] [PubMed] [Google Scholar]
- 26. López-Medina E, Biswal S, Saez-Llorens X, et al. Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents two years after vaccination. J Infect Dis 2020; 225:1521–32. 10.1093/infdis/jiaa761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rivera L, Biswal S, Sáez-Llorens X, et al. Three years efficacy and safety of Takeda's dengue vaccine candidate (TAK-003). Clin Infect Dis 2022; 75:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization . Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed. Available at: https://apps.who.int/iris/handle/10665/41988. Accessed 8 June 2022.
- 29. World Health Organization . Dengue and severe dengue fact sheet. Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed 8 June 2022.
- 30. Fongwen N, Delrieu I, Ham LH, et al. Implementation strategies for the first licensed dengue vaccine: a meeting report. Vaccine 2021; 39:4759–65. 10.1016/j.vaccine.2021.06.083 [DOI] [PubMed] [Google Scholar]
- 31. Hervé C, Laupèze B, Del Giudice Get al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines 2019; 4:39. 10.1038/s41541-019-0132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chu H, George SL, Stinchcomb DTet al. CD8+ T-cell responses in flavivirus-naive individuals following immunization with a live-attenuated tetravalent dengue vaccine candidate. J Infect Dis 2015; 212:1618–28. 10.1093/infdis/jiv258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. George SL, Wong MA, Dube TJ, et al. Safety and immunogenicity of a live attenuated tetravalent dengue vaccine candidate in flavivirus-naive adults: a randomized, double-blinded phase 1 clinical trial. J Infect Dis 2015; 212:1032–41. 10.1093/infdis/jiv179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turner M, Papadimitriou A, Winkle P, et al. Immunogenicity and safety of lyophilized and liquid dengue tetravalent vaccine candidate formulations in healthy adults: a randomized, phase 2 clinical trial. Hum Vaccin Immunother 2020; 16:2456–64. 10.1080/21645515.2020.1727697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tricou V, Low JG, Oh HM, et al. Safety and immunogenicity of a single dose of a tetravalent dengue vaccine with two different serotype-2 potencies in adults in Singapore: a phase 2, double-blind, randomised, controlled trial. Vaccine 2020; 38:1513–9. 10.1016/j.vaccine.2019.11.061 [DOI] [PubMed] [Google Scholar]
- 36. Jackson LA, Rupp R, Papadimitriou Aet al. A phase 1 study of safety and immunogenicity following intradermal administration of a tetravalent dengue vaccine candidate. Vaccine 2018; 36:3976–83. 10.1016/j.vaccine.2018.05.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.