Abstract
Bacterial killing in patients with tuberculosis (TB) relapse was compared to that in patients achieving cure, measured by TB molecular bacterial load assay (TB-MBLA) or mycobacteria growth indicator tube (MGIT) time to positivity (TTP). TB-MBLA in 4 relapsed patients was significantly different compared to 132 cured patients after 2 weeks of treatment; MGIT TTP showed a significant difference from week 8.
Keywords: tuberclosis, biomarker, relapse, clinical trial
The majority of patients with tuberculosis (TB) disease can be cured with <6 months of therapy [1, 2], whereas others benefit from an extension to prevent poor treatment outcomes [3]. Markers that identify these subgroups reliably would allow individualized treatment durations, but these are currently not available.
In clinical trials researching new, shorter treatments, such markers could lead to shorter trials by avoiding long-term follow-up, provide a better basis for drug development decisions than culture with its imperfect predictive accuracy, and provide operational advantages such as a shorter time to result and reduced missing data by avoiding culture contamination [4].
The TB molecular bacterial load assay (TB-MBLA) enumerates live Mycobacterium tuberculosis in samples through reverse-transcription polymerase chain reaction of 16S ribosomal RNA, corrected by an internal reference standard [5]. The assay was developed as a replacement of culture media for detecting TB, and so far in clinical trials has shown good correlation to culture, but has not been evaluated against a long-term clinical outcome [6, 7].
We evaluated TB-MBLA as a biomarker for long-term clinical outcomes, especially relapse, in Tanzanian patients of the PanACEA multi-arm, multi-stage trial (PanACEA MAMS) (NCT01785186).
This adds on to previous evaluations that were compared with other quantitative culture measurements over just 2 weeks and 3 months of treatment [7, 8].
METHODS
The PanACEA MAMS TB-01 trial was a 5-arm trial, which assessed 4 experimental treatment regimens including 2 higher doses of rifampicin, moxifloxacin, and SQ109. The trial was conducted in 3 sites in Tanzania and 4 sites in South Africa, and is described elsewhere in more detail [9]. Only Tanzanian patients were included in this TB-MBLA study. All patients who responded well clinically and/or had at least 1 negative culture toward the end of treatment provided the basis for this study; 1 patient with treatment failure was censored from this analysis. Patients were followed up until 6 months after end of treatment by telephone calls or on-site visits if participants were unwell. Recurrence was based on eventual need for retreatment, and assumed to be relapse. An outcome of cure, no information, or relapse was assigned at the end of follow-up. The “no information” outcome was assigned when insufficient follow-up data were available. This work evaluated Tanzanian participants, using sputum samples taken weekly up to week 12, and intermittently up to the end of treatment. Bacterial load was measured by mycobacteria growth indicator tube (MGIT) time to positivity (TTP) and by TB-MBLA, from 2 different sputum samples.
For analyses of long-term endpoint, and differences in killing by treatment arm, the natural log scaled (MBLA mean log colony-forming units +1) and TTP (days) were analyzed by adjusted pairwise Dunn test at observed time points to compare between 3 outcome groups. The summary statistics and the difference between the cured against relapse group with multiple correction adjustment are presented using the Dunn test. The analysis was performed using the PMCMR package in R [10].
Negative cultures were considered to have a TTP of 42 days. Contaminated cultures were considered as missing. For MBLA, single intermediate negative results were considered as missing values in the longitudinal evaluation.
Furthermore, for the longitudinal data analysis of the outcomes of TB-MBLA or TTP over time, linear mixed models were used for estimating inter- and intraindividual variation over time with quadratic time effects to describe bacterial load changes. The quadratic effects were assumed to account for the nonlinear evolution with flattening after a certain time point of observation as seen in the figure. The used model for analysis is reproduced in the Supplementary Materials (supplementary methods).
In analogy to the MAMS primary endpoint, defined as time to the first of 2 negative MGIT cultures at successive visits (see Supplementary Table 2 for visit timing), we similarly assessed time to zero bacterial load, defined as the first of 2 negative/zero results at successive visits. Since the time to event outcomes are typically associated with censoring, we performed a Cox proportional hazards model and describe the difference between the relapse and the cured groups. Similar methods were used for the analysis of time to culture conversion, and the comparison to time to zero TB-MBLA has been presented previously [8].
RESULTS
Of 147 participants with data on TB-MBLA and MGIT TTP over the 26-week treatment period, 4 suffered disease recurrence (assumed to be relapse), 132 achieved cure, and in 11 cases no outcome could be determined.
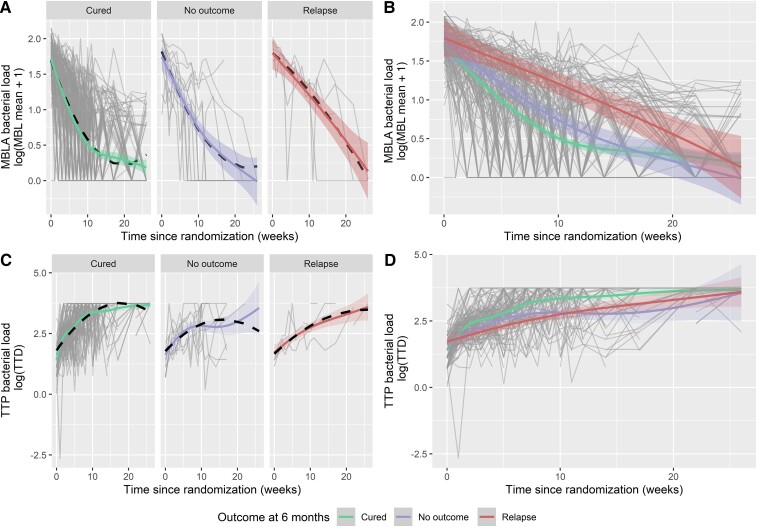
Group-wise modeling of quantitative TB-MBLA using data up to the treatment week specified showed significant differences between the relapse and the healthy groups as early as week 2 and 3, and more consistently from week 6 (Table 1, Supplementary Table 2). For MGIT TTP, the groupwise difference was observed at week 8 of treatment (Table 1, Supplementary Table 3). Additionally, the analysis of the longitudinal model showed the significant effect of both linear and quadratic time effects β5 and β8 for the relapse group compared to the cured group that was statistically significant at 5% level for the outcome of MBLA but not for TTP (Supplementary Tables 3 and 4).
Table 1.
Tuberculosis Molecular Bacterial Load Assay and Time to Positivity Over Time
| Classification | Treatment Week | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 14 | |
| Classification by MGIT TTP vs by TB-MBLA (cured, n = 132; relapse, n = 4) | |||||||||||||
| ȃMissing MGIT | 21 | 27 | 29 | 36 | 42 | 47 | 54 | 44 | 60 | 60 | 57 | 61 | 73 |
| ȃMissing MBLA | 13 | 17 | 10 | 13 | 14 | 13 | 10 | 14 | 17 | 20 | 12 | 20 | 28 |
| ȃCured–relapse: pairwise P value for MGIT TTP | .195 | .384 | .071 | .403 | .612 | .031 | .099 | .028 | .016 | .096 | .013 | .246 | .065 |
| ȃCured–relapse: pairwise P value for MBLA | .461 | .016 | .016 | .231 | .095 | .010 | .004 | .003 | .017 | .002 | .203 | .090 | .110 |
| Classification of cured vs relapse: no. correctly identified of No. observed: (fitted line using the coefficients of the linear mixed-effect model for log[TTP] or log[MBL+1]) | |||||||||||||
| ȃBoundary classification (log[TTP]) | 1.918 | 2.098 | 2.264 | 2.417 | 2.557 | 2.684 | 2.798 | 2.899 | 2.987 | 3.062 | 3.123 | 3.172 | 3.230 |
| ȃCorrectly identified cured (log[TTP]) | 82/113 (73%) |
77/105 (73%) |
73/104 (70%) |
64/96 (67%) |
63/91 (72%) |
62/86 (72%) |
58/80 (73%) |
67/88 (76%) |
58/73 (79%) |
54/72 (75%) |
60/76 (79%) |
58/72 (81%) |
58/60 (97%) |
| ȃCorrectly identified relapse (log[TTP]) | 1/2 | 1/4 | 2/3 | 2/4 | 2/3 | 2/3 | 1/2 | 2/4 | 2/3 | 3/4 | 3/3 | 1/3 | 1/3 |
| ȃBoundary classification (log[MBL+1]) | 1.625 | 1.504 | 1.391 | 1.287 | 1.192 | 1.106 | 1.029 | 0.961 | 0.901 | 0.851 | 0.809 | 0.777 | 0.738 |
| ȃCorrectly identified: cured (log[MBL+1]) | 58/120 (48%) | 57/115 (50%) | 65/123 (53%) | 62/120 (52%) | 62/119 (52%) | 56/119 (47%) | 66/122 (54%) | 67/118 (57%) | 67/115 (58%) | 76/112 (68%) | 76/120 (63%) | 80/112 (71%) | 62/104 (60%) |
| ȃCorrectly identified: relapse (log[MBL+1]) | 2/3 | 4/4 | 3/3 | 2/3 | 3/3 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 2/3 | 2/3 | 3/4 |
Full data can be found in Supplementary Tables 2 and 3. Data are displayed for up to week 14, since no significant changes were observed after this timepoint. The cured vs relapse groups are compared using the Kruskal-Wallis test followed by a post hoc Dunn test to obtain the pairwise difference between groups. The classification of no./No. of correctly identified cured patients/correctly identified relapse patients was done using the upper bound of the 95% credible interval for (log[Mbl + 1]) of the cured group. For TTP, classification the lower bound of the 95% credible interval for (log[TTP]) of the cured group was used. Varying numbers between visits reflect availability of results. Results may be missing due to unavailability of sample, or in the case of MGIT TTP, culture contamination. P values in bold are smaller than 0.05, indicating statistical significance.
Abbreviations: MBL, molecular bacterial load; MBLA, molecular bacterial load assay; MGIT, mycobacteria growth indicator tube; TB-MBLA, tuberculosis molecular bacterial load assay; TTP, time to positivity.
To estimate the precision of differentiation between the cured and relapse groups by the 10-week TB-MBLA decline or MGIT TTP increase, the upper bound of the 95% credible interval (CI) of the cured group was selected after visual inspection of Figure 1 as a cutoff, prioritizing the correct prediction of relapse patients, which meant that 67.9% (76/112) of cured patients and 100% (4/4) of relapsed patients were correctly classified, respectively (Table 1, Supplementary Table 2). For MGIT TTD, using the lower bound of the 95% CI of the cured group, 75% (54/72) of cured and 75% (3/4) of relapsed patients were correctly identified (Table 1, Supplementary Table 3). Besides similar accuracy, the higher amount of missing data in MGIT classification should be noted, which was up to 73 of 136 at week 14; for MBLA, only 29 of 136 were missing (Supplementary Figure 1, Supplementary Tables 1 and 2).
Figure 1.
Quantitative results for bacterial load measured by tuberculosis molecular bacterial load assay over time (A, B), and for mycobacteria growth indicator tube time to positivity (TTP) during treatment (C, D), separated by patient outcome at 12 months postrandomization. Smoothed trend lines for the longitudinal profiles have been described for (log[MBL + 1]) and (log[TTP], days). The black dashed line denotes the regression fit of the marginal component of the linear mixed-effect model, accounting for a linear and quadratic time trend, which is different for the groups cured, relapse, or no outcome in consideration. Abbreviations: MBL, molecular bacterial load; MBLA, molecular bacterial load assay; TTD, time to detection; TTP, time to positivity.
Analyzing the primary endpoint of the MAMS trial, time to at least 2 successive negative MGIT results or 2 consecutive zero TB-MBLA and correlation to long-term unsuccessful outcome showed significant difference between relapse and cured groups (P = .015 for MGIT vs P = .048 for TB-MBLA; Supplementary Figure 2, Supplementary Table 1). Time to first negative measurement was not significantly different between outcome groups for both assays (data not shown). We then assessed TTP increase and TB-MBLA decline by treatment arm and found those to be not substantially different across treatment arms (Supplementary Figure 3). Superiority of the 35 mg/kg rifampicin arm, which had been the main finding from the MAMS trial, had been predominant in South African patients, for whom this assay was not measured.
DISCUSSION
Adding on to previous work that quantified the concordance between TB-MBLA and MGIT measurements [8], we now show encouraging data on quantitative TB-MBLA predicting long-term unfavorable outcome, identifying at-risk patients earlier than MGIT TTP—absence of contamination (missing data) and shorter time-to-result being added advantages. This supports the use of MBLA as a clinical trials endpoint when groupwise means are required, and if found to be sufficiently precise in larger studies, could be used to determine treatment duration for individuals.
TB-MBLA superiority could be due to better precision, but also due to the fact that change in MGIT TTP is a summary measure of postantibiotic lag of growth of still-viable organisms and definite killing, whereas TB-MBLA may be more closely aligned with viable bacterial count, possibly including noncultivable organisms that nevertheless seem important for outcome [11]. However, an observation of TB-MBLA positivity at end of treatment alone was not sufficient to predict adverse outcome, in line with others who found that 37% of cured patients still had detectable TB messenger RNA in their sputum at the end of treatment [12].
The main limitation of this work is the small number of patients with unsuccessful outcome; larger trials will be needed.
Our finding of delayed killing in the group of patients with relapse has further implications. Since new, shorter treatments must prevent relapse to be successful [2], new treatments will have to accelerate bacterial load clearance not only in summary measurements of the entire cohort, but mainly in the slowest responders to be successful and achieve acceptable rates of relapse.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. N. H., N. E. N., and M. H. conceived the study. N. E. N., D. M., W. S., G. K., L. T. M., D. K., K. R., M. J. B., S. H. G., M. H., and N. H. were responsible for acquisition and quality of the data. N. H., A. B., and N. E. N. did the analysis. All authors were involved in interpretation of the data and participated in writing and reviewing the manuscript. N. E. N., A. B., and N. H. wrote the first and the final draft of the manuscript. N. E. N. and A. B. contributed equally to this work.
Financial support. The PanACEA MAMS study was funded by the European and Developing Countries Clinical Trials Partnership (EDCTP1; grant numbers IP.2007.32011.013, IP.2007.32011.012, and IP.2007.32011.011), as well as by the Bundesministerium für Bildung und Forschung (German Ministry of Education and Research; 01KA0901). This analysis was funded by the German Center for Infection Research (grant number 02.702).
Supplementary Material
Contributor Information
Nyanda Elias Ntinginya, National Institute for Medical Research–Mbeya Medical Research Centre, Mbeya, United Republic of Tanzania.
Abhishek Bakuli, Division of Infectious Diseases and Tropical Medicine, University Hospital, LMU Munich, Munich, Germany; German Center for Infection Research, Munich Partner Site, Munich, Germany.
Daniel Mapamba, National Institute for Medical Research–Mbeya Medical Research Centre, Mbeya, United Republic of Tanzania.
Wilber Sabiiti, Division of Infection and Global Health, School of Medicine, University of St Andrews, St Andrews, United Kingdom.
Gibson Kibiki, East African Health Research Commission, Bujumbura, Burundi.
Lilian Tina Minja, Ifakara Health Institute, Dar Es Salaam, United Republic of Tanzania.
Davis Kuchaka, Kilimanjaro Clinical Research Institute, Moshi, United Republic of Tanzania.
Klaus Reither, Ifakara Health Institute, Dar Es Salaam, United Republic of Tanzania; Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland.
Patrick Peter John Phillips, Center for Tuberculosis, University of California, San Francisco, San Francisco, California, USA.
Martin Johan Boeree, Radboud Institute of Health Science, Radboud University Medical Center, Nijmegen, The Netherlands.
Stephen H Gillespie, Division of Infection and Global Health, School of Medicine, University of St Andrews, St Andrews, United Kingdom.
Michael Hoelscher, Division of Infectious Diseases and Tropical Medicine, University Hospital, LMU Munich, Munich, Germany; German Center for Infection Research, Munich Partner Site, Munich, Germany.
Norbert Heinrich, Division of Infectious Diseases and Tropical Medicine, University Hospital, LMU Munich, Munich, Germany; German Center for Infection Research, Munich Partner Site, Munich, Germany.
References
- 1. Imperial MZ, Nahid P, Phillips PPJ, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med 2018; 24:1708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946–1986, with relevant subsequent publications. Int J Tuberc Lung Dis 1999; 3(10 Suppl 2):S231–79. [PubMed] [Google Scholar]
- 3. Aung KJ, Declercq E, Ali MA, et al. Extension of the intensive phase reduces relapse but not failure in a regimen with rifampicin throughout. Int J Tuberc Lung Dis 2012; 16:455–61. [DOI] [PubMed] [Google Scholar]
- 4. Phillips PP, Gillespie SH, Boeree M, et al. Innovative trial designs are practical solutions for improving the treatment of tuberculosis. J Infect Dis 2012; 205(Suppl 2):S250–7. [DOI] [PubMed] [Google Scholar]
- 5. Gillespie SH, Sabiiti W, Oravcova K. Mycobacterial load assay. Methods Mol Biol 2017; 1616:89–105. [DOI] [PubMed] [Google Scholar]
- 6. Honeyborne I, McHugh TD, Phillips PP, et al. Molecular bacterial load assay, a culture-free biomarker for rapid and accurate quantification of sputum Mycobacterium tuberculosis bacillary load during treatment. J Clin Microbiol 2011; 49:3905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Honeyborne I, Mtafya B, Phillips PP, et al. The molecular bacterial load assay replaces solid culture for measuring early bactericidal response to antituberculosis treatment. J Clin Microbiol 2014; 52:3064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sabiiti W, Azam K, Farmer ECW, et al. Tuberculosis bacillary load, an early marker of disease severity: the utility of tuberculosis molecular bacterial load assay. Thorax 2020; 75:606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boeree MJ, Heinrich N, Aarnoutse R, et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CRAN.R Project. The pairwise multiple comparison of mean ranks package (PMCMR) . R package. 2014. Available at: https://CRAN.R-project.org/package=PMCMR. Accessed 31 March 2022.
- 11. Hu Y, Liu A, Ortega-Muro F, Alameda-Martin L, Mitchison D, Coates A. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol 2015; 6:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malherbe ST, Shenai S, Ronacher K, et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med 2016; 22:1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.