Abstract
Background
Diarrhea is the second leading cause of death in children younger than 5 years of age globally. The burden of diarrheal mortality is concentrated in low-resource settings. Little is known about the risk factors for childhood death from diarrheal disease in low- and middle-income countries.
Methods
Data from the World Health Organization (WHO)-coordinated Global Rotavirus and Pediatric Diarrhea Surveillance Networks, which are composed of active, sentinel, hospital-based surveillance sites, were analyzed to assess mortality in children <5 years of age who were hospitalized with diarrhea between 2008 and 2018. Case fatality risks were calculated, and multivariable logistic regression was performed to identify risk factors for mortality.
Results
This analysis comprises 234 781 cases, including 1219 deaths, across 57 countries. The overall case fatality risk was found to be 0.5%. Risk factors for death in the multivariable analysis included younger age (for <6 months compared with older ages, odds ratio [OR] = 3.54; 95% confidence interval [CI], 2.81–4.50), female sex (OR = 1.18; 95% CI, 1.06–1.81), presenting with persistent diarrhea (OR = 1.91; 95% CI, 1.01–3.25), no vomiting (OR = 1.13; 95% CI, .98–1.30), severe dehydration (OR = 3.79; 95% CI, 3.01–4.83), and being negative for rotavirus on an enzyme-linked immunosorbent assay test (OR = 2.29; 95% CI, 1.92–2.74). Cases from the African Region had the highest odds of death compared with other WHO regions (OR = 130.62 comparing the African Region with the European Region; 95% CI, 55.72–422.73), whereas cases from the European Region had the lowest odds of death.
Conclusions
Our findings support known risk factors for childhood diarrheal mortality and highlight the need for interventions to address dehydration and rotavirus-negative diarrheal infections.
Keywords: rotavirus, pediatric, diarrhea, diarrheal mortality, global surveillance
Diarrheal diseases are the second leading cause of death in children younger than 5 years of age worldwide, accounting for approximately 1.7 billion cases and 525 000 deaths each year [1]. Diarrheal deaths disproportionately affect those living in low- and middle-income countries (LMICs), with approximately 90% of diarrheal deaths occurring in sub-Saharan Africa and South Asia [2]. Diarrheal deaths have been decreasing over time largely because of advancements in treatment and introduction of rotavirus vaccines, improvements in nutrition, and improvements in water, sanitation, and hygiene [3]. Known risk factors for pediatric diarrheal mortality include malnutrition, wasting, early cessation of or not breastfeeding, not receiving any dose or the full dose of rotavirus vaccines, poor access to healthcare, and poor sanitation and hygiene [4–7].
The cause of diarrhea may be associated with mortality, and the causes of severe and fatal diarrhea are similar. There are many different pathogens that cause diarrheal infection, including viruses, bacteria, and parasites. Two landmark studies provided insight to the leading etiologies causing pediatric diarrheal disease in LMICs. The Global Enteric Multicenter Study found that the leading pathogens causing moderate-to-severe diarrhea in children <5 years of age were rotavirus, Cryptosporidium, Shigella, and enterotoxigenic Escherichia coli [8]. The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health found that norovirus, rotavirus, Campylobacter, astrovirus, Cryptosporidium, and Shigella were the leading pathogens causing diarrhea in the first 2 years of life [9].
In this paper, we describe case fatality risks and risk factors for mortality from pediatric diarrhea in hospitalized children using data from the World Health Organization (WHO)-coordinated Global Rotavirus Surveillance Network (GRSN) and Global Pediatric Diarrhea Surveillance (GPDS).
METHODS
GRSN and GPDS comprise active, sentinel, hospital-based surveillance of children younger than age 5 years [10, 11]. GRSN, which has been in place since 2008, collects data on children hospitalized with acute watery diarrheal presentation. Diarrhea is defined as more than 3 loose stools in a 24-hour period. Stool specimens are collected within 48 hours of admission and are tested by enzyme-linked immunosorbent assay (ELISA) for rotavirus. All cases meeting the eligibility criteria, who were hospitalized with diarrhea at a sentinel surveillance site, are included in the surveillance network. As of 2018, there were 54 countries participating in GRSN, though the number of countries fluctuates year by year. Beginning in 2017, sites in 28 countries expanded the case definition from acute watery presentation to include bloody and persistent diarrhea to collect information on all pediatric diarrhea cases as part of GPDS. Children with previous hospitalization at the sentinel hospital, or children who transferred from other facilities, were excluded to omit nosocomial diarrhea infections and reenrollment of ongoing/chronic infections. Because GRSN and GPDS are part of routine public health surveillance, they do not require human subjects ethical review.
The outcome of interest was death before discharge from the hospital. Discharge outcomes of transferred, left hospital, unknown, or missing were treated as missing.
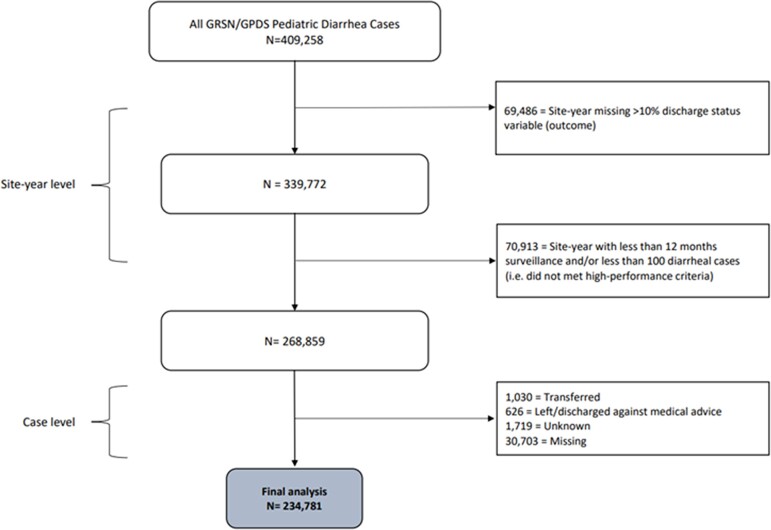
An analysis dataset was created using data from both GRSN and GPDS as follows (Figure 1). For each site in a given year, the site-year data were excluded if more than 10% of the discharge status variable was missing, or if the site had fewer than 100 cases per year and/or cases were not collected in all 12 months of the year. Any cases with the outcome variable missing were excluded.
Figure 1.
Schematic showing creation of dataset used for analysis.
Case fatality risk (CFR) was defined as the number of cases that died divided by the number of cases with known vital status at discharge. Cases missing the outcome variable were excluded under the assumption that they were missing at random. Rotavirus cases were defined as being ELISA positive. Cases with indeterminate or missing rotavirus ELISA results were excluded for CFR calculations based on rotavirus positivity but not for CFR calculations based on all-cause diarrhea.
For regression analyses, cases with unknown vital status at discharge were excluded, under the assumption that they are missing at random. Simple logistic regressions were performed between each covariate of interest and the outcome variable, discharge status. The year variable was treated as linear to adjust for changes in mortality rate because of secular trends.
A multivariable logistic model with vital status at discharge as the outcome was created. Covariates were selected based on scientific and clinical evidence of their association with diarrheal mortality. Acute diarrhea is diarrhea that lasts less than 14 days, whereas persistent diarrhea lasts 14 or more days. Model testing for collinearity (using multiple linear regression to calculate the variance inflation factor), Akaike information criterion (using stepwise regression), and clinically relevant interaction terms (using likelihood ratio tests) was performed. None of the covariates showed collinearity in the model. The final model included the covariates mentioned previously as well as an interaction term between acute/persistent presentation and bloody/watery presentation. Variables with a P value ≤ .05 were considered statistically significant.
Several subanalyses were performed: cases with acute watery presentation only, 2017 and 2018 cases only because the GPDS expanded case definition was introduced in those 2 years, and rotavirus positive cases only. Additionally, a stratified analysis by WHO region was performed to evaluate if there were differences in risk factor by region.
Demographic and clinical features for cases missing the outcome variable were evaluated to see if they were different from cases with the outcome variable. All analyses were performed using R version 3.5.3 software [12].
RESULTS
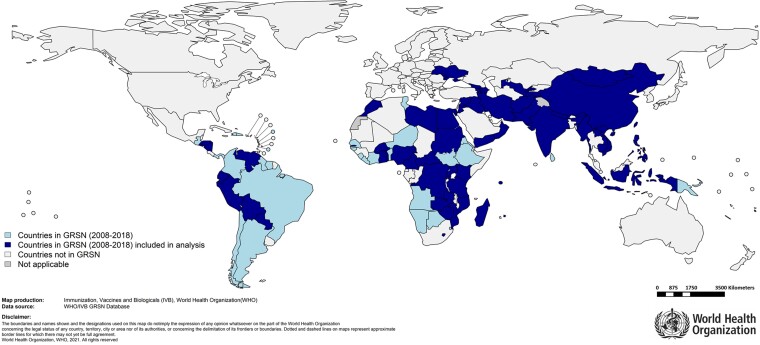
This analysis comprises 234 781 cases, including 1219 deaths, in 57 countries as shown in Figure 2. The total number of cases and years that countries in the analysis dataset contributed data are shown in Supplementary Table 1. The median time from hospital admission to death was 2 days. Children dying within 1 day of admission were similar in characteristics to children dying more than 1 day after admission (Supplementary Table 2).
Figure 2.
Countries that conducted surveillance through the World Health Organization-coordinated Global Rotavirus Surveillance Network (2008–2018) and those that have been included in the analysis.
The overall CFR for any pediatric diarrhea was 0.52% (1219 deaths among 234 781 total cases; 95% confidence interval [CI], .49–.55). The CFR for rotavirus-positive cases was 0.28% (177 deaths among 63 736 total cases; 95% CI, .24–.32). The CFR for pediatric diarrhea cases that were rotavirus negative was 0.61% (1042 deaths among 171 045 total cases; 95% CI, .57–.65). CFRs were highest in the younger ages, female cases, cases from the African Region, cases with persistent and watery presentation, cases with severe dehydration and without vomiting, and cases that tested rotavirus negative by ELISA (Table 1).
Table 1.
Demographic and Clinical Characteristics of Cases
| Variable | Died (n = 1219) | Alive (n = 233 562) | Case Fatality Risk |
|---|---|---|---|
| Age | |||
| ȃ<6 mo | 313 | 38 476 | 0.81% |
| ȃ6 mo–1 y | 522 | 90 769 | 0.58% |
| ȃ1–2 y | 295 | 65 638 | 0.45% |
| ȃ2–5 y | 89 | 38 679 | 0.23% |
| Sex | |||
| ȃMale | 663 | 136 354 | 0.49% |
| ȃFemale | 555 | 97 038 | 0.57% |
| WHO region | |||
| ȃAfrican Region (19 countries) | 811 | 57 619 | 1.41% |
| ȃRegion of the Americas (8 countries) | 139 | 14 364 | 0.97% |
| ȃEastern Mediterranean Region (12 countries) | 162 | 47 132 | 0.34% |
| ȃEuropean Region (7 countries) | 15 | 66 123 | 0.02% |
| ȃSouth East Asia Region (4 countries) | 7 | 5491 | 0.13% |
| ȃWestern Pacific Region (7 countries) | 85 | 42 833 | 0.20% |
| Acute vs persistent | |||
| ȃPersistent (≥14 d) | 28 | 1578 | 1.77% |
| ȃAcute (<14 d) | 1191 | 23 1984 | 0.51% |
| Bloody vs watery | |||
| ȃWatery | 1156 | 22 7821 | 0.51% |
| ȃBloody | 5 | 1726 | 0.29% |
| Vomiting | |||
| ȃNot vomiting | 341 | 51 529 | 0.66% |
| ȃVomiting | 804 | 173 332 | 0.46% |
| Dehydration typea | |||
| ȃNone | 99 | 21 689 | 0.46% |
| ȃSome | 338 | 105 913 | 0.32% |
| ȃSevere | 646 | 48 928 | 1.32% |
| Rotavirus positivity (using ELISA) | |||
| ȃRotavirus positive | 177 | 63 559 | 0.28% |
| ȃRotavirus negative | 1042 | 170 003 | 0.61% |
| Year | |||
| ȃ2008 | 37 | 5387 | 0.69% |
| ȃ2009 | 72 | 8733 | 0.82% |
| ȃ2010 | 167 | 13 914 | 1.20% |
| ȃ2011 | 111 | 13 368 | 0.83% |
| ȃ2012 | 46 | 14 958 | 0.31% |
| ȃ2013 | 86 | 21 617 | 0.40% |
| ȃ2014 | 177 | 30 682 | 0.58% |
| ȃ2015 | 162 | 35 749 | 0.45% |
| ȃ2016 | 166 | 37 387 | 0.44% |
| ȃ2017 | 122 | 29 810 | 0.41% |
| ȃ2018 | 73 | 21 957 | 0.33% |
Abbreviation: ELISA, enzyme-linked immunosorbent assay.
Dehydration severity is categorized based on WHO classification [13].
Use of rotavirus vaccine in the countries included in the analysis increased over time from 9% in 2008 to 68% in 2018. Rotavirus vaccine use in the countries included in this analysis is described in Supplementary Tables 3 and 4 [14]. Individual-level vaccination data were not available for most cases. To examine the effect of rotavirus vaccination, the analysis was stratified by whether rotavirus vaccination had been introduced into the country in the year when the case was enrolled (Supplementary Table 5). Age was identified as a risk factor only for mortality in countries with rotavirus vaccination, but other risk factors were found to be similar regardless of rotavirus vaccine introduction.
Risk factors for death in the multivariable analysis included being of younger age (for <6 months, OR = 3.54; 95% CI, 2.81–4.50), female (OR = 1.18; 95% CI, 1.06–1.81), presenting with persistent diarrhea (OR = 1.91; 95% CI, 1.01–3.25), no vomiting (OR = 1.13; 95% CI, .98–1.30), severe dehydration (OR = 3.79; 95% CI, 3.01–4.83), and being negative for rotavirus on an ELISA test (OR = 2.29; 95% CI, 1.92–2.74; Table 2). Additionally, cases from the African Region had the highest odds of death (OR = 130.62 comparing the African Region with the European Region; 95% CI, 55.72–422.73), whereas cases from the European Region had the lowest odds of death. Increasing year was associated with decreased odds of death (OR = 0.94; 95% CI, .92–.96); however, when included in the model as a categorical variable, year was not statistically significant.
Table 2.
Simple and Multivariable Logistic Regression Analysis of Covariates Association With Mortality
| Variable | Crude | Adjusteda | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | … | <.001 | … | <.001 |
| ȃ<6 mo | 3.54 (2.81–4.50) | 2.07 (1.59, 2.73) | … | |
| ȃ6 mo–1 y | 2.50 (2.01–3.15) | 1.52 (1.19–1.98) | … | |
| ȃ1–2 y | 1.95 (1.55, 2.49) | 1.38 (1.06–1.81) | … | |
| ȃ2–5 y | Ref | Ref | … | |
| Sex | … | .005 | … | .01 |
| ȃMale | Ref | Ref | … | |
| ȃFemale | 1.18 (1.05–1.32) | 1.18 (1.04–1.34) | … | |
| WHO region | … | <.001 | … | <.001 |
| ȃEuropean Region | Ref | Ref | … | |
| ȃAfrican Region | 62.05 (38.70–108.26) | 130.62 (55.75–422.73) | … | |
| ȃRegion of the Americas | 42.65 (25.91–75.82) | 91.03 (38.31–296.76) | … | |
| Eastern Mediterranean Region | 15.20 (9.24–26.84) | 39.44 (16.54–128.84) | … | |
| ȃSouth East Asia Region | 5.62 (2.14–13.33) | 31.33 (9.24–120.01) | … | |
| ȃWestern Pacific Region | 8.75 (5.21–15.76) | 24.12 (9.44–81.62) | … | |
| Acute vs persistent | … | <.001 | … | .03 |
| ȃPersistent (≥14 d) | 3.46 (2.31–4.94) | 1.91 (1.01–3.25) | … | |
| ȃAcute (<14 d) | Ref | Ref | … | |
| Bloody vs watery | … | .24 | … | .95 |
| ȃWatery | Ref | Ref | … | |
| ȃBloody | .59 (.21–1.27) | .97 (.24–2.55) | … | |
| Vomiting | … | <.001 | … | .10 |
| ȃNot vomiting | 1.43 (1.26–1.62) | 1.13 (.98–1.30) | … | |
| ȃVomiting | Ref | Ref | … | |
| Dehydration typeb | … | <.001 | … | <.001 |
| ȃNone | Ref | Ref | … | |
| ȃSome | .71 (.56–.91) | .93 (.72–1.20) | … | |
| ȃSevere | 3.12 (2.50–3.96) | 3.79 (3.01–4.83) | … | |
| Rotavirus positivity (using ELISA) | … | <.001 | … | <.001 |
| ȃRotavirus positive | Ref | Ref | … | |
| ȃRotavirus negative | 2.20 (1.88–2.59) | 2.29 (1.92–2.74) | … | |
| Year | .90 (.88–.92) | .001 | .94 (.92–.96) | <.001 |
The adjusted model includes an interaction term between acute/persistent presentation and watery/bloody presentation.
Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; OR, odds ratio.
Adjusted for all variables.
Dehydration severity is categorized based on WHO classification [13].
Subanalyses gave similar results to those observed in the full analysis (Supplementary Tables 6 and 7); therefore, the full model (as described previously) was used.
Multivariable logistic regression odds ratios stratifying by region were performed for 4 of the regions (Supplementary Table 8); analysis could not be performed for the South East Asia Region or the European Region individually because there were too few deaths in those regions. Across all regions, severe dehydration and a rotavirus-negative ELISA test were significantly associated with increased odds of dying. Younger age was significantly associated with increased odds of dying in the African Region, Region of the Americas, and the Eastern Mediterranean Region. Female sex was significantly associated with increased odds of dying in the Region of the Americas. Not vomiting was significantly associated with an increased odds of dying in the African Region and the Western Pacific Region. The trend of decreasing odds of death over time (years of study) was observed in the Region of the Americas, the Eastern Mediterranean Region, and the Western Pacific Region.
DISCUSSION
Despite advancements in diarrheal treatments, global use of rotavirus vaccines, and general sanitation, diarrhea remains a major contributor to childhood mortality across the globe, especially in LMICs. This analysis characterizes the burden of diarrheal mortality among children that reach care. The overall CFR for children hospitalized with all-cause diarrhea was 0.52%, or 1 in 200 children hospitalized with diarrhea. In low-income countries, where children <3 years of age have an average of 3 diarrheal episodes yearly, this CFR translates to a large absolute number of deaths [1]. We found that younger age, female sex, persistent diarrheal presentation, severe dehydration, and rotavirus-negative ELISA test were all statistically significantly associated with mortality. These findings highlight important risk factors for childhood diarrheal mortality globally, demonstrating the critical need to address dehydration, age- and sex-specific vulnerabilities, and causes of diarrhea other than rotavirus with clinical and public health interventions.
Children younger than 6 months of age had the highest odds of death, being more than twice as likely to die compared with children between 2 and 5 years of age. This is consistent with the literature [5, 8, 15]. Female sex was also associated with increased likelihood of death. This could be due to sex differences in how children are raised and brought to care. Female children may be more likely to have their nutrition neglected, increasing their risk of death [16, 17]. Studies in Bangladesh, Brazil, and Burkina Faso have shown that caregivers are more likely to bring male children to care, so it is plausible that caregivers also are waiting longer to bring female children to care, increasing their likelihood of death from diarrhea [18–20]. Indeed, a study in Ethiopia found that caregivers of female children were almost 2 times more likely to delay care seeking than caregivers of male children [21].
Our finding that the African Region had the highest odds of death and highest burden of deaths is consistent with the literature [2]. For children hospitalized with all-cause diarrhea in the African Region, the CFR was 1.39%, or 1 in 72 children hospitalized with diarrhea. The Region of the Americas, Eastern Mediterranean Region, South-East Asia Region, and Western Pacific Region all had lower odds of death compared with the African Region, but statistically significantly increased odds of death compared with the European Region. Differences by region are hard to interpret because of regional heterogeneity, but variation in mean income level, quality of care, cultural norms including delays in seeking care and discontinuing breastfeeding or feeding, and distance to the closest hospital could explain the large differences in mortality rates between regions.
Persistent diarrheal cases are almost twice as likely to die as acute diarrheal cases. It may be that persistent cases are more commonly associated with malnutrition, which has been shown to be strongly correlated with a higher likelihood of death in studies done in Mozambique and Bangladesh [5, 22]. Additionally, different pathogens cause different presentations, so pathogens causing persistent diarrhea may also cause problems that lead to an increased risk of death. For example, studies have found that enteropathogenic E coli and Clostridium difficile are pathogens commonly associated with persistent diarrhea in LMICs and that these pathogens are also associated with an increased risk of death [23–25].
Severe dehydration was found to be a risk factor for diarrheal death. This finding was not surprising given that dehydration is often the reason of death for children with diarrhea [5, 26].
Mortality from rotavirus is well described in the literature [27]. However, the lower likelihood of death in cases with rotavirus may reflect the successful treatment with hydration in children with diarrhea that reach medical care or reflect the increasing use of rotavirus vaccines in LMICs [5]. A previous analysis of this surveillance network has shown a decrease in hospitalized rotavirus cases after introduction of rotavirus vaccine [28]. Pathogens causing death in ways other than dehydration, for example hemolytic urea syndrome from E coli or complications from Shigella, may be more difficult to treat, leading to higher CFRs [29]. Additionally, these pathogens require treatment with antibiotics, which may not be administered soon enough. As rotavirus vaccines are implemented in more countries and reach more children, it will be increasingly important to shift focus to other diarrheal pathogens that are causing mortality in healthcare facilities.
There are some differences in risk factors for diarrheal death by region. Although dehydration and a rotavirus-negative ELISA test were significant risk factors for death across all regions, female sex was a significant risk factor for death only in the Region of the Americas. Differences in risk factors for diarrheal death by region may be due to variability in diarrheal pathogen etiologies, cultural practices relating to healthcare, or health system capacity. There are several limitations of this analysis. First, the estimated CFRs may be an underestimate. Slightly more than 10% of the cases in the original dataset were missing the outcome variable, vital status at discharge, and were therefore excluded from the analysis. Cases missing the outcome variable are more likely to be younger, be from the African Region or the South East Asia Region, have bloody presentation, have no vomiting, have more severe dehydration, and be rotavirus positive. All of these factors are associated with increased likelihood of death, except for being rotavirus positive, which is associated with a lower likelihood of death. If cases missing vital status at discharge are more likely to have more severe diarrhea, and therefore more likely to have died, then excluding them in the calculation of CFRs and in the regression analysis may bias our estimates toward underestimation.
A second limitation is that the use of WHO region as a covariate ignores heterogeneity within regions and gives higher weight to countries with more cases. WHO regions are based on geography of countries instead of grouping countries with similar characteristics together. Third, there may be other variables that affect likelihood of death that were not collected by the network. For example, continued feeding and breastfeeding before hospital admission, nutritional status, access to healthcare facilities, and concomitant disease such as pneumonia or sepsis, may impact the likelihood of death [30]. Last, given the small proportion of cases with individual-level vaccination data available, we could not draw conclusions regarding vaccination status and rotavirus mortality.
There are also several notable strengths of this analysis. First, the analysis has a large sample size, which allows for the ability to identify risk factors for the rare event of diarrheal death. Second, the population included in the analysis is geographically diverse, allowing for a global perspective of diarrheal disease in LMICs. Third, the data collected from GRSN and GPDS use standardized protocols and laboratory testing, allowing the cases to be readily compared across sites.
Our results show that despite progress, mortality from diarrhea in hospitalized children remains an important public health issue. Knowledge of risk factors of diarrheal mortality will help identify important preventive measures and best interventions for treating children who present to care with diarrhea. The association of severe dehydration with death underscores the importance of hydration in the treatment of children with diarrhea. Furthermore, the association of rotavirus negativity with death suggests the need to broaden the attention from rotavirus to other pathogens causing diarrheal disease, especially those implicated in persistent diarrheal cases. More research is needed to identify the reasons for death of children hospitalized with diarrhea so that interventions can be implemented to save lives.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study personnel from all the sentinel sites and laboratories participating in the WHO-coordinated GRSN and GPDS networks, WHO Regional Offices, WHO Country Offices, Ministries of Health, Regional Reference Laboratories, and the Global Reference Laboratory for all their contributions to the GRSN and GPDS networks. The authors also thank Dr. James Platts-Mills for his contribution to the study. R. M. H. thanks Johns Hopkins Bloomberg School of Public Health’s Program in Applied Vaccine Experiences, especially Dr. Jessica Atwell and Dr. Ruth Karron.
Financial support. The study was funded by the Bill & Melinda Gates Foundation [Grant Number OPP 1149526] and the Gavi Alliance.
Supplementary Material
Contributor Information
Rachel M Hartman, Department of Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland; Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, USA.
Adam L Cohen, Department of Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland.
Sebastien Antoni, Department of Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland.
Jason Mwenda, Department of Vaccine Preventable Diseases Program, World Health Organization Regional Office for Africa, Brazzaville, Congo Republic.
Goitom Weldegebriel, Department of Immunization, Vaccines and Biologicals, World Health Organization Regional Office for Africa, Inter-Support Team for East and South Africa, Harare, Zimbabwe.
Joseph Biey, Department of Vaccine Preventable Diseases, World Health Organization Regional Office for Africa, Inter-Support Team for West Africa, Ouagadougou, Burkina Faso.
Keith Shaba, Department of Vaccine Preventable Diseases Program, World Health Organization Regional Office for Africa, Brazzaville, Congo Republic.
Lucia de Oliveira, Pan American Health Organization/Department of Family, Health Promotion, and Life Course, World Health Organization Regional Office for the Americas, Comprehensive Family Immunization Unit, Washington, DC, USA.
Gloria Rey, Pan American Health Organization/Department of Family, Health Promotion, and Life Course, World Health Organization Regional Office for the Americas, Comprehensive Family Immunization Unit, Washington, DC, USA.
Claudia Ortiz, Pan American Health Organization/Department of Family, Health Promotion, and Life Course, World Health Organization Regional Office for the Americas, Comprehensive Family Immunization Unit, Washington, DC, USA.
Maria Tereza, Pan American Health Organization/Department of Family, Health Promotion, and Life Course, World Health Organization Regional Office for the Americas, Comprehensive Family Immunization Unit, Washington, DC, USA.
Kamal Fahmy, Department of Communicable Diseases, Immunization, Vaccines and Biologicals Unit, World Health Organization Eastern Mediterranean Office, Cairo, Egypt.
Amany Ghoniem, Department of Communicable Diseases, Immunization, Vaccines and Biologicals Unit, World Health Organization Eastern Mediterranean Office, Cairo, Egypt.
Hossam Ashmony, Department of Communicable Diseases, Immunization, Vaccines and Biologicals Unit, World Health Organization Eastern Mediterranean Office, Cairo, Egypt.
Dovile Videbaek, Division of Country Health Programmes, Vaccine-Preventable Diseases and Immunization Unit, World Health Organization European Regional Office, Copenhagen, Denmark.
Simarjit Singh, Division of Country Health Programmes, Vaccine-Preventable Diseases and Immunization Unit, World Health Organization European Regional Office, Copenhagen, Denmark.
Emmanuel Tondo, Department of Immunization and Vaccine Development, World Health Organization South-East Asia Regional Office, New Delhi, India.
Mohammed Sharifuzzaman, Department of Immunization and Vaccine Development, World Health Organization South-East Asia Regional Office, New Delhi, India.
Jayantha Liyanage, Department of Immunization and Vaccine Development, World Health Organization South-East Asia Regional Office, New Delhi, India.
Nyambat Batmunkh, Division of Programmes for Diseases Control, Vaccine Preventable Diseases and Immunization, World Health Organization Western Pacific Regional Office, Manila, Philippines.
Varja Grabovac, Division of Programmes for Diseases Control, Vaccine Preventable Diseases and Immunization, World Health Organization Western Pacific Regional Office, Manila, Philippines.
Josephine Logronio, Division of Programmes for Diseases Control, Vaccine Preventable Diseases and Immunization, World Health Organization Western Pacific Regional Office, Manila, Philippines.
Fatima Serhan, Department of Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland.
Tomoka Nakamura, Department of Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland; Department of Infectious Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, United Kingdom; School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki, Japan.
References
- 1. Diarrhoeal disease . Available at: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease. Accessed 27 January 2020.
- 2. Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC, Levine MM. Global burden of diarrheal diseases among children in developing countries: incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 2017; 35:6783–9. [DOI] [PubMed] [Google Scholar]
- 3. Black R, Fontaine O, Lamberti L, et al. Drivers of the reduction in childhood diarrhea mortality 1980–2015 and interventions to eliminate preventable diarrhea deaths by 2030. J Glob Health 2019; 9:020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Troeger C, Blacker BF, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Acácio S, Mandomando I, Nhampossa T, et al. Risk factors for death among children 0–59 months of age with moderate-to-severe diarrhea in Manhiça district, southern Mozambique. BMC Infect Dis 2019; 19:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al Jarousha AMK, El Jarou MA, El Qouqa IA. Bacterial enteropathogens and risk factors associated with childhood diarrhea. Indian J Pediatr 2011; 78:165–70. [DOI] [PubMed] [Google Scholar]
- 7. Mohan VR, Karthikeyan R, Babji S, et al. Rotavirus infection and disease in a multisite birth cohort: results from the MAL-ED study. J Infect Dis 2017; 216:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 9. Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Heal 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prudden HJ, Hasso-Agopsowicz M, Black RE, et al. Meeting report: WHO workshop on modelling global mortality and aetiology estimates of enteric pathogens in children under five. Cape Town, 28–29th November 2018. Vaccine 2020; 38:4792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The World Health Organization . Rotavirus Surveillance Standards. 2018. Available at: https://cdn.who.int/media/docs/default-source/immunization/vpd_surveillance/vpd-surveillance-standards-publication/who-surveillancevaccinepreventable-19-rotavirus-r2.pdf?sfvrsn=2c43bf06_10&download=true. Accessed 19 June 2022.
- 12. R Core Team . R: A language and environment for statistical computing. 2019; Available at: https://www.r-project.org/.
- 13. The World Health Organization . Integrated Management of Childhood Illness Module 4 Diarrhea. 2014: 17. Available at: https://apps.who.int/iris/bitstream/handle/10665/104772/9789241506823_Module-4_eng.pdf;jsessionid=44E6A802DBBEB67A1087A8323BC3D029?sequence=6. Accessed 19 March 2020. [Google Scholar]
- 14. International Vaccine Access Center (IVAC) , Johns Hopkins Bloomberg School of Public Health. VIEW-hub. Available at: https://view-hub.org/map/?set=current-vaccine-intro-status&group=vaccine-introduction&category=rv. Accessed 19 June 2022.
- 15. Mokomane M, Kasvosve I, de Melo E, Pernica JM, Goldfarb DM. The global problem of childhood diarrhoeal diseases: emerging strategies in prevention and management. Ther Adv Infect Dis 2018; 5:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raj A, McDougal LP, Silverman JG. Gendered effects of siblings on child malnutrition in South Asia: cross-sectional analysis of demographic and health surveys from Bangladesh, India, and Nepal. Matern Child Health J 2014; 19:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kshatriya GK, Acharya SK. Gender disparities in the prevalence of undernutrition and the higher risk among the young women of Indian tribes. PLoS One 2016; 11: e0158308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarker AR, Sultana M, Mahumud RA, Sheikh N, Van Der Meer R, Morton A. Prevalence and health care–seeking behavior for childhood diarrheal disease in Bangladesh. Glob Pediatr Heal 2016; 3:2333794X1668090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konstantyner T, Cláudia T, Oliveira Konstantyner R, et al. Prevalence and hospitalization rates due to diarrhoea in infants: the 2006 Brazilian National Demographic Health Survey. J Epidemiol Res 2016; 2: 29-38. [Google Scholar]
- 20. Wilson SE, Ouédraogo CT, Prince L, et al. Caregiver recognition of childhood diarrhea, care seeking behaviors and home treatment practices in rural Burkina Faso: a cross-sectional survey. PLoS One 2012; 7:e33273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fikire A, Ayele G, Haftu D. Determinants of delay in care seeking for diarrheal diseases among mothers/caregivers with under-five children in public health facilities of Arba Minch town, southern Ethiopia; 2019. PLoS One 2020; 15:e0228558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferdous F, Das SK, Ahmed S, et al. Severity of diarrhea and malnutrition among under five-year-old children in rural Bangladesh. Am J Trop Med Hyg 2013; 89:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pawlowski SW, Warren CA, Guerrant R. Diagnosis and treatment of acute or persistent diarrhea. Gastroenterology 2009; 136:1874–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu J, Torres AG. Enteropathogenic Escherichia coli: foe or innocent bystander? Clin Microbiol Infect 2015; 21:729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell BG, Gardner A. Mortality and Clostridium difficile infection: a review. Antimicrob Resist Infect Control 2012; 1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention . Global Diarrhea Burden. Global Water, Sanitation and Hygiene. Healthy Water. Available at: https://www.cdc.gov/healthywater/global/diarrhea-burden.html. Accessed 18 February 2020.
- 27. Tate JE, Burton AH, Boschi-Pinto Cet al. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis 2016; 62:S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aliabadi N, Antoni S, Mwenda JM, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Heal 2019; 7:e893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khalil IA, Troeger C, Blacker BF, et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis 2018; 18:1229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butler T, Islam M, Azad AK, Islam MR, Speelman P. Causes of death in diarrhoeal diseases after rehydration therapy: an autopsy study of 140 patients in Bangladesh. Bull World Health Organ 1987; 65:323. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.