Abstract
Background
Antibody responses to non–egg-based standard-dose cell-culture influenza vaccine (containing 15 µg hemagglutinin [HA]/component) and recombinant vaccine (containing 45 µg HA/component) during consecutive seasons have not been studied in the United States.
Methods
In a randomized trial of immunogenicity of quadrivalent influenza vaccines among healthcare personnel (HCP) aged 18–64 years over 2 consecutive seasons, HCP who received recombinant-HA influenza vaccine (RIV) or cell culture–based inactivated influenza vaccine (ccIIV) during the first season (year 1) were re-randomized the second season of 2019–2020 (year 2 [Y2]) to receive ccIIV or RIV, resulting in 4 ccIIV/RIV combinations. In Y2, hemagglutination inhibition antibody titers against reference cell–grown vaccine viruses were compared in each ccIIV/RIV group with titers among HCP randomized both seasons to receive egg-based, standard-dose inactivated influenza vaccine (IIV) using geometric mean titer (GMT) ratios of Y2 post-vaccination titers.
Results
Y2 data from 414 HCP were analyzed per protocol. Compared with 60 IIV/IIV recipients, 74 RIV/RIV and 106 ccIIV/RIV recipients showed significantly elevated GMT ratios (Bonferroni corrected P < .007) against all components except A(H3N2). Post-vaccination GMT ratios for ccIIV/ccIIV and RIV/ccIIV were not significantly elevated compared with IIV/IIV except for RIV/ccIIV against A(H1N1)pdm09.
Conclusions
In adult HCP, receipt of RIV in 2 consecutive seasons or the second season was more immunogenic than consecutive egg-based IIV for 3 of the 4 components of quadrivalent vaccine. Immunogenicity of ccIIV/ccIIV was similar to that of IIV/IIV. Differences in HA antigen content may play a role in immunogenicity of influenza vaccination in consecutive seasons.
Clinical Trials Registration
Keywords: influenza vaccines, immunogenicity, healthcare personnel, Flucelvax Quadrivalent, Flublok Quadrivalent
Healthcare personnel aged 18–64 years who received 2 consecutive seasons of quadrivalent recombinant-hemagglutinin influenza vaccine showed higher post-vaccination hemagglutination-inhibition antibodies against 3 components compared with those who received egg-based inactivated vaccine; those who received the cell-culture vaccines did not.
Annual influenza vaccination, without any preference for type of vaccine, is recommended for healthcare personnel (HCP) in the United States to reduce transmission of influenza in healthcare settings [1]. During the 2019–2020 season, 81% of HCP received influenza vaccination compared with 48% of adults aged ≥18 years [2, 3]. In compliance with employer influenza vaccination policies, most HCP receive an influenza vaccination each season [2]. The majority of seasonal influenza vaccines distributed in the United States are produced in embryonated eggs, which may introduce egg-adapted antigenic changes during vaccine virus propagation [4–6]. Repeated vaccination may boost antibodies to conserved epitopes and those present in egg-adapted vaccines rather than eliciting neutralizing antibodies against vaccine viruses or circulating influenza viruses [7]. Vaccine platforms that do not rely on egg-based production, including standard-dose cell culture–based inactivated influenza vaccine and recombinant-hemagglutinin (HA) protein vaccine with higher HA antigen content, may induce broader and higher immune responses to circulating influenza strains of viruses than egg-based vaccines [8, 9]. Immune responses to annual revaccination may also differ by preexisting antibody levels, antigenic similarity between vaccine viruses in consecutive seasons, and antigen content [10, 11].
During the 2018–2019 influenza season, we initiated a 2-season randomized, open-label immunogenicity trial in a highly vaccinated HCP population to compare immune response to vaccination with egg-based vs non–egg-based quadrivalent vaccines. Antibody responses were measured against influenza antigens produced in cell culture that retain antigenic characteristics of wild-type influenza viruses. In the first study year, quadrivalent recombinant-HA influenza vaccine (RIV) elicited higher antibody titers than either quadrivalent cell culture (ccIIV)– or egg-based quadrivalent, standard-dose, inactivated influenza vaccines (IIVs) against 3 of 4 vaccine components (except B/Victoria) [12]. It is unknown whether the advantages of RIV are maintained or enhanced after repeated vaccination or over multiple seasons. To investigate immune responses to consecutive vaccination with non–egg-based vaccines over 2 seasons, participants who were randomized to ccIIV or RIV or IIV before the 2018–2019 influenza season (study year 1 [Y1]) were re-randomized to receive ccIIV or RIV or IIV in 2019–2020 (study year 2 [Y2]). Antibody responses after vaccination with 4 combinations of ccIIV and RIV receipt during study Y1 and Y2 were compared with responses among participants rerandomized to receive egg-based inactivated influenza vaccines in both seasons.
METHODS
Study Design
A description of the study design in the first season has been published [12]. HCP aged 18–64 years from 2 integrated healthcare systems, Baylor Scott & White Health (BSWH) in Temple, Texas, and Kaiser Permanente Northwest (KPNW) in Portland, Oregon, were enrolled in a 2-year, randomized, open-label immunogenicity trial before the 2018–2019 influenza season (Y1). In Y1, participants were randomized to receive 1 of the following: an egg-based, standard-dose quadrivalent IIV (Fluzone Quadrivalent, Sanofi Pasteur or Fluarix Quadrivalent, GlaxoSmithKline) containing 15 µg of HA protein per strain, quadrivalent ccIIV (Flucelvax Quadrivalent, Seqirus) containing 15 µg of HA per strain, or quadrivalent RIV (Flublok Quadrivalent, Sanofi Pasteur) containing 45 µg of HA per strain [12]. Before the 2019–2020 influenza season (Y2), participants who had been randomized to ccIIV or RIV in Y1 were re-randomized to receive either ccIIV or RIV, resulting in 4 Y1/Y2 combinations of non–egg-based vaccines (ccIIV/ccIIV, ccIIV/RIV, RIV/ccIIV, and RIV/RIV). Randomization was stratified by site and age group (ages 18–44 and 45–64 years at enrollment; Supplementary Figure 1).
Participants randomized to IIV in Y1 were re-randomized to receive IIV (Fluzone Quadrivalent, Sanofi Pasteur), ccIIV, or RIV. Results for the IIV/IIV group are included as a referent in this report. Results for the IIV/ccIIV and IIV/RIV groups that address other trial objectives are not presented here and will be reported separately (Supplementary Figure 1). HCP provided consent for re-randomization and completed an online enrollment survey. Blood samples were collected before vaccination and at approximately 4 weeks post-vaccination (acceptable time range per protocol, 21–62 days) for measurement of serum hemagglutination inhibition (HI) antibody titers.
Influenza Vaccines
The recommended composition of Northern Hemisphere 2019–2020 influenza vaccines included the following strains: A/Brisbane/02/2018 (H1N1)pdm09-like virus, A/Kansas/14/2017 (H3N2), B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage), and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage). Compared with the 2018–2019 influenza vaccine strain composition, 2019–2020 vaccines included updated components for A(H1N1)pdm09 and A(H3N2); A/Kansas/14/2017 (H3N2) is antigenically similar to influenza A(H3N2) clade 3C.3a viruses that circulated during the 2018–2019 season. During 2019–2020, all 4 components of cell-culture and recombinant-HA vaccines were produced from seed viruses that had not been passaged in eggs. During 2018–2019, all 4 components of RIV and 3 components of ccIIV were cell culture–derived except for A(H1N1)pdm09, which was egg-grown. The vaccines were provided in prefilled syringes and administered as a 0.5-mL dose intramuscularly.
Immunogenicity Assays
Laboratory investigators were blinded to randomization arm assignment. HI antibody assays were performed in accordance with the Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza by the World Health Organization [13]. For A(H3N2), HI assays used 0.75% guinea pig erythrocytes against A/Kansas/14/2017 (H3N2) propagated in modified Madin Darby canine kidney (MDCK) cells. For A(H1N1)pdm09 and influenza B, HI assays used 0.5% turkey erythrocytes against cell-propagated A/Idaho/07/2018 (H1N1)pdm09 (A/Brisbane/02/2018-like), B/Colorado/06/2017 (B/Victoria), and B/Phuket/3073/2013 (B/Yamagata) viruses propagated in MDCK cells; both influenza B antigens were ether-treated [14].
Statistical Analyses
Analyses for the second year of the trial were per protocol, excluding participants without post-vaccination serum specimens collected within specified time periods. The primary outcome measures were post-vaccination HI antibody geometric mean titers (GMTs) against cell culture–propagated vaccine reference viruses, mean fold-rise in titer from pre- to post-vaccination for each participant, and GMT ratio comparing Y2 post-vaccination GMTs of each ccIIV/RIV combination group to the IIV/IIV referent group. Based on sample size calculations to detect specified post-vaccination GMT ratios between 2 comparison groups after adjustment for 7 primary comparisons using a Bonferroni correction (Supplementary Table 1), our study had sufficient sample size to detect GMT ratios as low as 1.2. GMTs were compared using the Student t test. Because HCP who received IIV, ccIIV, and RIV were randomized during each of the 2 seasons, we did not adjust for Y2 pre-vaccination HI titer. For GMT ratios comparing non-egg vaccine combinations with the IIV/IIV referent, after applying a Bonferroni correction, statistical significance was defined as 2-tailed P value <.007 or 99.3% confidence interval (CI). Analyses were performed with SAS (version 9.3; SAS Institute, Cary, NC).
Ethical Review
The study protocol was reviewed and approved by the institutional review boards (IRBs) of KPNW, BSWH, and Abt Associates, which provided study site oversight and data management support. The Centers for Disease Control and Prevention relied on BSWH’s IRB. Study findings are reported in accordance with the Consolidated Standards of Reporting Trials statement guidelines.
RESULTS
Participant Characteristics
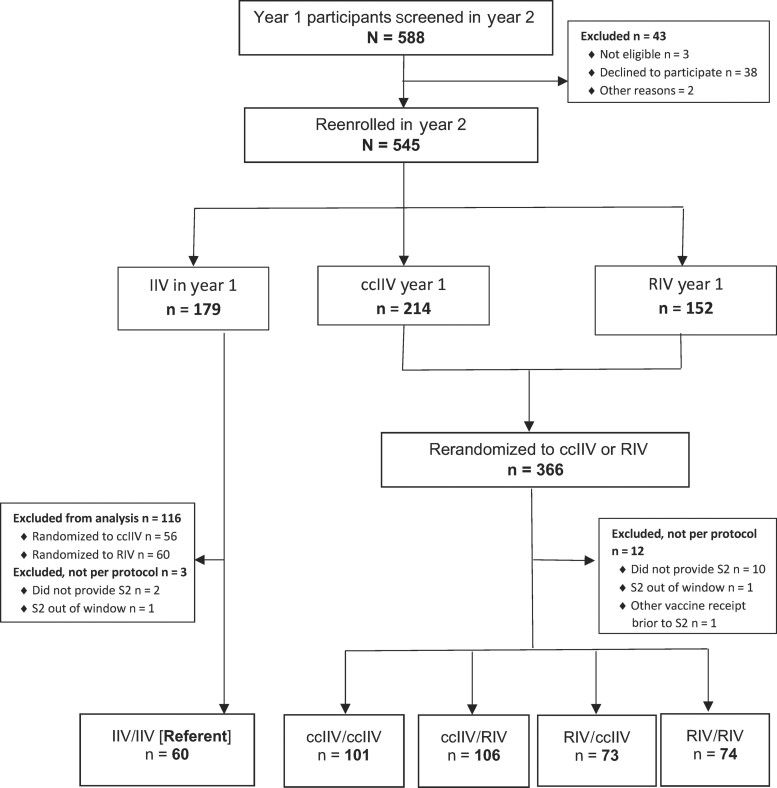
Overall, among 588 participants from Y1 who were screened for eligibility in Y2, 545 re-enrolled (3 were not eligible, 38 declined to participate, and 2 Y1 participants were excluded for other reasons; Figure 1). Among the 545 participants, 179 had received IIV, 214 had received ccIIV, and 152 had received RIV in Y1. After re-randomization in Y2, after excluding participants without sera collected per protocol (n = 15) and those who received IIV in Y1 re-randomized to RIV or ccIIV (n = 116), there were 414 participants in the per-protocol analysis group (60 in the IIV/IIV referent group, 101 in the ccIIV/ccIIV, 106 in the ccIIV/RIV, 73 in the RIV/ccIIV, and 74 in the RIV/RIV groups; Figure 1). Mean participant age was 46 years (Table 1). Participating HCP were predominantly female (82%) and non-Hispanic White (79%); 14% were of Hispanic ethnicity. Eleven percent of participants reported diagnosis or treatment for 1 or more chronic medical conditions during the past year. Most participants had documented influenza vaccinations during the preceding 6 seasons. Across the 5 study arms, participant age, sex, race, Hispanic ethnicity, subjective health status, body mass index, presence of an immunosuppressive condition status, smoking status, pregnancy status, and prior vaccination status were similar (Table 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram for participants re-enrolled in the second study year (2019–2020 influenza season). Abbreviations: ccIIV, cell culture–based inactivated influenza vaccine (Flucelvax Quadrivalent); IIV, inactivated influenza vaccine (Fluzone Quadrivalent); RIV, recombinant-hemagglutinin influenza vaccine (Flublok Quadrivalent); S2, post-vaccination blood (21–62 days after); year 1, 2018–2019; year 2, 2019–2020.
Table 1.
Demographic and Baseline Characteristics of 414 Participants Enrolled and Randomized in Both Seasons of 2-Year Randomized Trial of Quadrivalent Non–Egg-based vs Egg-Based Licensed Influenza Vaccines According to Vaccines Received in Each Study Season
| Characteristic | IIV/IIV | ccIIV/ccIIV | ccIIV/RIV | RIV/ccIIV | RIV/RIV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 60 | n = 101 | n = 106 | n = 73 | n = 74 | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Demographic | ||||||||||
| ȃAge, mean, (SD), years | 46 | (11) | 46 | (11) | 46 | (11) | 47 | (11) | 46 | (11) |
| ȃAge group, years | ||||||||||
| ȃȃ18–44 | 22 | (37) | 42 | (42) | 44 | (42) | 28 | (38) | 30 | (41) |
| ȃȃ45–64 | 38 | (63) | 59 | (58) | 62 | (58) | 45 | (62) | 44 | (59) |
| ȃFemale | 54 | (90) | 83 | (82) | 87 | (82) | 58 | (79) | 56 | (78) |
| ȃWhite | 47 | (78) | 83 | (82) | 88 | (83) | 56 | (77) | 54 | (73) |
| ȃHispanic | 10 | (17) | 10 | (10) | 18 | (17) | 11 | (15) | 10 | (22) |
| ȃSite | ||||||||||
| ȃȃBaylor Scott & White Health | 41 | (68) | 55 | (54) | 61 | (58) | 57 | (78) | 56 | (76) |
| ȃȃKaiser Permanente Northwest | 19 | (32) | 46 | (46) | 45 | (42) | 16 | (22) | 18 | (24) |
| Baseline | ||||||||||
| ȃBody mass index, mean (SD) | 30 | (7) | 29 | (7) | 30 | (7) | 29 | (6) | 30 | (7) |
| ȃSubjective health status, mean (SD)a | 4 | (1) | 4 | (1) | 4 | (1) | 4 | (1) | 4 | (1) |
| ȃDiagnosed or treated for chronic medical condition during the past 12 months | 11 | (18) | 8 | (8) | 13 | (12) | 7 | (10) | 8 | (11) |
| ȃImmunosuppressive condition | 1 | (2) | 0 | (0) | 2 | (2) | 2 | (3) | 1 | (1) |
| ȃSmokerb | 4 | (6) | 5 | (5) | 3 | (3) | 2 | (3) | 6 | (8) |
| ȃPregnantc | 2 | (11) | 0 | (0) | 1 | (3) | 0 | (0) | 1 | (5) |
| ȃPrior influenza vaccination receiptd | ||||||||||
| ȃTotal vaccines received during the preceding 6 seasons, mean (SD)d | 6 | (1) | 6 | (1) | 6 | (1) | 6 | (1) | 6 | (1) |
Abbreviations: ccIIV, cell culture–based inactivated influenza vaccine (Flucelvax Quadrivalent); IIV, quadrivalent inactivated influenza vaccine (Fluzone Quadrivalent); RIV, recombinant-hemagglutinin IIV (Flublok Quadrivalent); SD, standard deviation.
Original answer choice converted to numeric scale where 5 = excellent and 1 = poor. Responses were missing for 5 participants.
Participant was considered a smoker if participant answered currently smokes every day or some days. Responses were missing for 5 participants.
Among 131 female participants with nonmissing responses.
Based on report of vaccination by participant interview or electronic medical record extraction.
Pre-vaccination GMTs in Y2
Compared with the IIV/IIV referent group, the Y2 pre-vaccination GMT in those who received RIV in Y1 tended to be higher and was higher against B/Victoria in the RIV/RIV group (Table 2). The Y2 pre-vaccination GMT tended to be lower against the updated A/Kansas/14/2017(H3N2) among Y1 ccIIV recipients. Compared with participants who received ccIIV in Y1, those who received RIV in Y1 had higher pre-vaccination GMTs in Y2 against A(H3N2).
Table 2.
Pre- and Post-Vaccination Hemagglutination Inhibition Antibody Geometric Mean Titer and Mean Fold-Rise in Titer in the Second Year of the Randomized Immunogenicity Trial According to Quadrivalent Vaccine Combination Received in 2 Study Seasons, With Quadrivalent Inactivated Influenza Vaccine/Quadrivalent Inactivated Influenza Vaccine as Referent Group
| Hemagglutination Inhibition Antibody | IIV/IIV (Ref) | ccIIV/ccIIV | ccIIV/RIV | RIV/ccIIV | RIV/RIV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n = 60 | n = 101 | n = 106 | n = 73 | n = 74 | ||||||
| GMT (99.3% CI)a or Mean Fold-Rise in Titer (99.3% CI)b | ||||||||||
| A/Idaho/07/2018 (H1N1) (cell-propagated A/Brisbane/02/2018-like) | ||||||||||
| ȃPre-vaccine GMT | 27.3 | (17.3–43.2) | 25.4 | (18.7–34.6) | 24.0 | (17.3–33.4) | 35.0 | (22.6–54.3) | 37.5 | (25.4–55.3) |
| ȃPost-vaccine GMT | 38.2 | (23.9–60.9) | 53.0 | (40.5–69.3) | 68.4 | (50.2–93.2) | 75.6 | (52.2–109.3) | 76.3 | (51.9–112.2) |
| ȃMean fold-rise | 1.4 | (1.1–1.7) | 2.1 | (1.7–2.6) | 2.8 | (2.2–3.6) | 2.2 | (1.7–2.8) | 2.0 | (1.6–2.5) |
| A/Kansas/14/2017 (H3N2) | ||||||||||
| ȃPre-vaccine GMT | 102.0 | (72.1–144.3) | 70.6 | (51.3–97.2) | 77.9 | (56.2–108.0) | 132.3 | (95.1–184.1) | 136.4 | (102.8–181.1) |
| ȃPost-vaccine GMT | 216.1 | (165.0–283.0) | 163.4 | (127.1–209.9) | 248.0 | (190.3–323.1) | 181.0 | (129.3–253.4) | 302.5 | (247.2–370.2) |
| ȃMean-fold rise | 2.1 | (1.6–2.9) | 2.3 | (1.7–3.1) | 3.2 | (2.4–4.3) | 1.4 | (1.1–1.8) | 2.2 | (1.6–3.0) |
| B/Colorado/06/2017 (Victoria) | ||||||||||
| ȃPre-vaccine GMT | 34.4 | (26.3–45.0) | 39.7 | (30.2–52.3) | 41.6 | (32.5–53.3) | 47.0 | (32.3–68.5) | 54.0 | (40.9–71.2) |
| ȃPost-vaccine GMT | 44.4 | (34.1–57.8) | 55.6 | (43.1–71.8) | 83.2 | (65.8–105.1) | 66.2 | (45.7–95.8) | 87.0 | (64.1–118.1) |
| ȃMean-fold rise | 1.3 | (1.1–1.5) | 1.4 | (1.1–1.7) | 2.0 | (1.6–2.4) | 1.4 | (1.1–1.7) | 1.6 | (1.3–1.9) |
| B/Phuket/3073/2013 (Yamagata) | ||||||||||
| ȃPre-vaccine GMT | 46.5 | (33.3–64.8) | 47.8 | (36.7–62.3) | 49.0 | (37.1–64.7) | 58.5 | (40.6–84.3) | 65.7 | (47.7–90.5) |
| ȃPost-vaccine GMT | 59.2 | (42.2–83.2) | 73.7 | (57.7–94.1) | 102.6 | (79.3–132.6) | 82.3 | (56.9–119.0) | 108.0 | (81.2–143.5) |
| ȃMean-fold rise | 1.3 | (1.0–1.6) | 1.5 | (1.3–1.8) | 2.1 | (1.7–2.6) | 1.4 | (1.1–1.7) | 1.6 | (1.3–2.0) |
Data shown for participants in per-protocol analysis according to 2-year vaccine combination actually received. Post-vaccination antibody titers were obtained approximately 28 days after year 2 vaccination.
Abbreviations: ccIIV, cell culture–based inactivated influenza vaccine (Flucelvax Quadrivalent, Seqirus); CI, confidence interval; GMT, geometric mean titer; IIV, quadrivalent inactivated influenza vaccine (Fluzone Quadrivalent, Sanofi Pasteur); RIV, recombinant IIV (Flublok, Sanofi Pasteur).
GMTs and 99.3% CIs were calculated using the t distribution applied to log-transformed titers.
Mean fold-rise and 99.3% CIs were defined as the geometric mean of the ratio of post-vaccination titer and pre-vaccination titer for each participant.
GMT Ratios
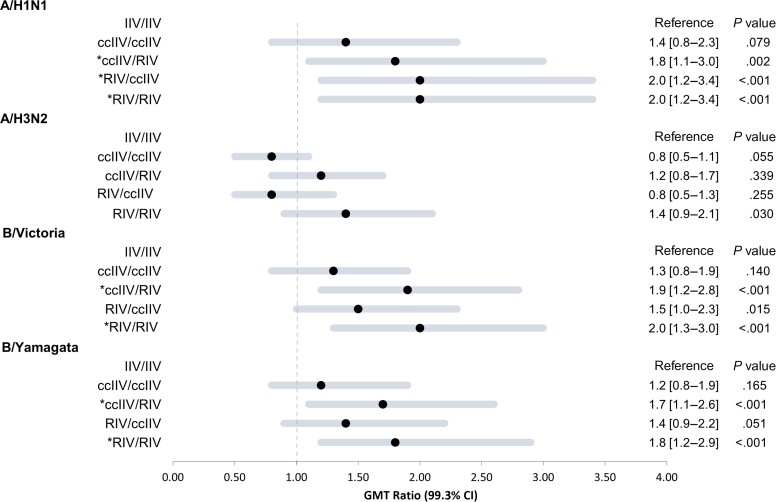
Compared with post-vaccination titers in the IIV/IIV referent group, statistically significant GMT ratios (P <.007) were observed for 3 of 4 cell-propagated antigens in the RIV/RIV group (Figure 2), ranging from 1.8 (CI, 1.2–2.9) against B/Yamagata to 2.0 (CI, 1.2–3.4) against A(H1N1)pdm09; A(H1N1pdm09) was updated in Y2 (2019–2020). In the ccIIV/RIV crossover group, GMT ratios were also statistically significantly greater than 1.0 against all antigens except A(H3N2). Compared with the IIV/IIV referent, the RIV/ccIIV crossover group had a GMT ratio >1.0 against A(H1N1)pdm09, but P values for GMT ratios for A(H3N2) and influenza B antigens were >.007. For repeat ccIIV vaccination (ccIIV/ccIIV), GMT ratios ranged from 0.8 (CI, .5–1.1) against A(H3N2) to 1.4 (CI, .8–2.3) against A(H1N1)pdm09; P values for GMT ratios were >.007.
Figure 2.
Forest plot of geometric mean hemagglutination inhibition (HI) antibody titer ratios (geometric mean titer [GMT] ratios) at 1 month post-vaccination by 2-season vaccine combination. Figure shows ratios comparing year 2 post-vaccination HI antibody titers in each non–egg-based 2-season vaccine combination group to HI titers among participants who received standard-dose egg-based inactivated influenza vaccine both seasons (IIV/IIV; referent group). HI antibody titers were measured against cell-propagated antigens of 2019–2020 vaccine reference strains A/Idaho/07/2018 (H1N1)pdm09 (A/Brisbane/02/2018-like), A/Kansas/14/2017 (H3N2), B/Colorado/06/2017(Victoria), and B/Phuket/3073/2013(Yamagata). The forest plot depicts GMT ratios for each year 1/year 2 vaccine combination, calculated using log-transformed GMTs. Horizontal lines represent the 99.3% CI, and solid circles represent the point estimates. All estimates to the right of the null value of 1 favor the corresponding year 1/year 2 vaccine combination over the IIV/IIV referent group. *Applying a Bonferroni correction for multiple comparisons, P <.007 for GMT ratio is statistically significant. Abbreviations: ccIIV, cell culture–based inactivated influenza vaccine (Flucelvax Quadrivalent); CI, confidence interval; IIV, inactivated influenza vaccine (Fluzone Quadrivalent); RIV, recombinant-hemagglutinin influenza vaccine (Flublok Quadrivalent).
Compared with the ccIIV/ccIIV group, RIV/RIV recipients achieved higher post-vaccination GMTs against A(H3N2) (Table 2).
Mean Fold-Rise in Titer in Y2
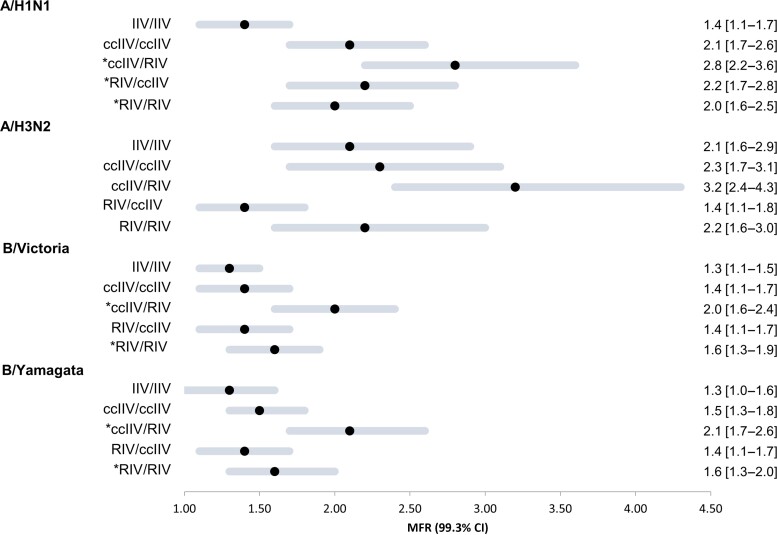
Compared with the IIV/IIV referent group, mean fold-rises in titer for all non–egg-based vaccine combinations (ccIIV/ccIIV, ccIIV/RIV, RIV/ccIIV, and RIV/RIV) tended to be higher against the updated A(H1N1)pdm09 and were significantly higher against updated A(H1N1)pdm09 and both unchanged influenza B antigens in the ccIIV/RIV group (Figure 3). For A(H3N2), the crossover RIV/ccIIV group tended to have higher pre-vaccination GMT and lower mean fold-rise than the IIV/IIV referent group. Compared with participants in the crossover RIV/ccIIV group, those in the ccIIV/RIV group tended to have lower pre-vaccine GMTs with overlapping CIs against all 4 antigens. However, compared with the RIV/ccIIV group, the mean fold-rise was significantly higher at 3.2 (CI, 2.4–4.3) against A(H3N2) among the ccIIV/RIV group and tended to be higher for the other 3 antigens. There were no differences in the Y1/Y2 GMT mean fold-rises in ccIIV/ccIIV and RIV/RIV groups for all 4 vaccine components.
Figure 3.
Forest plot of MFR in geometric mean hemagglutination inhibition (HI) antibody titer (geometric mean titer [GMT]) at 1 month post-vaccination by 2-season vaccination combination. MFR and 99.3% CIs were defined as the geometric mean of the ratio of post-vaccination titer and pre-vaccination titer for each participant. *Applying a Bonferroni correction for multiple comparisons, P <.007 for GMT ratio is statistically significant. Abbreviations: ccIIV, cell culture–based inactivated influenza vaccine (Flucelvax Quadrivalent); CI, confidence interval; MFR, mean fold-rise; IIV, inactivated influenza vaccine (Fluzone Quadrivalent); RIV, recombinant-hemagglutinin influenza vaccine (Flublok Quadrivalent).
DISCUSSION
In this highly influenza-vaccinated population of HCP, receipt of RIV during 2 consecutive seasons elicited higher post-vaccination HI antibody titer ratios against 3 of 4 components than receipt of standard-dose egg-based influenza vaccine during 2 consecutive seasons. Similar to Y1 [12], we also found that receipt of RIV in Y2 elicited higher HI GMTs against 3 of 4 cell-propagated vaccine antigens compared with receipt of consecutive IIV regardless of whether participants received RIV in Y1. Among participants who received RIV in Y1, pre-vaccination GMT in the second year of the trial tended to be higher than among participants who received ccIIV or IIV in Y1, consistent with higher titers observed 6 months after vaccination in Y1 [12]. Against the A(H3N2) vaccine component that was updated in 2019–2020, pre-vaccination titers were not significantly higher among participants who received RIV vs IIV in the first season but tended to be higher than in Y1 ccIIV recipients. Participants who received RIV in consecutive seasons also had higher post-vaccination antibody titers against the updated A(H3N2) than those who received ccIIV in consecutive seasons. Across vaccine combinations, mean fold-rise was generally higher among participants with lower pre-vaccination titers, as expected.
Higher HI antibody titers to consecutive seasons of quadrivalent RIV compared with standard-dose egg-based IIV may be related to lack of egg-adapted mutations or higher antigen content (45 µg of HA/per component vs 15 µg each) or better antigenicity of RIV. Because we found similar HI antibody responses as IIV/IIV to consecutive season doses of standard-dose ccIIV, our findings support that the higher HA antigen content of recombinant-HA vaccine may have contributed to higher antibody titers after RIV in the second season. A trend for higher Y2 pre-vaccination HI titers in Y1 RIV recipients, possibly related to an increase in the magnitude and persistence of functional antibodies observed 6 months after Y1 vaccination [12], may be due to differences in immunological recognition of HA antigenic epitopes when delivered as a recombinant protein. Differences between the antigenicity of recombinant-HA produced in insect cells in RIV and HA from viruses grown in mammalian cell culture in ccIIV may also play a role in eliciting different immune responses, although HI titers for all groups were measured against antigens from viruses grown in cell culture. Pre-vaccination antibody titers against the updated 2019–2020 A(H3N2) vaccine component (A/Kansas/14/2017), which was genetically and antigenically distinct from the 2018–2019 A(H3N2) component, were not significantly higher among Y1 RIV recipients compared with the egg-based IIV referent group but were significantly lower among Y1 ccIIV compared with RIV recipients. In this highly vaccinated population, complex antibody landscapes reflecting early exposures, boosting from natural infections, and repeated vaccination may affect influenza immune responses to egg and non-egg HA antigens. Predicting which vaccine antigens or combinations of vaccines will provide better protection against circulating viruses remains challenging.
Results from observational studies comparing effectiveness of cell culture–based or recombinant vaccines to egg-based vaccines against influenza-associated illness based on administrative diagnostic codes or laboratory-confirmed influenza-related outcomes in different age groups and settings have been inconsistent, with some analyses suggesting little to no difference in effectiveness between vaccines [15–17]. However, one observational study [16] during the 2019–2020 season found significantly higher effectiveness for RIV than for all other vaccines (standard-dose, quadrivalent IIV and ccIIV, and each of trivalent adjuvanted and high-dose IIV) against influenza-related inpatient stays among US Medicare beneficiaries aged ≥65 years [16]. Immunogenicity of influenza vaccines is often measured using antigens similar to those used to make the vaccine rather than antigens from cell culture more similar to wild-type or circulating influenza viruses. Further, egg-grown antigens have often been used to compare immunogenicity of egg-based vs non–egg-based vaccines. HI antibody titers against cell-propagated antigens may provide a better measure of antibody-mediated protection against disease than titers against egg-grown antigens, but data to test this hypothesis further are needed [14]. Results of 2 immunogenicity studies that used antigens grown in cell culture reported similar antibody responses following recombinant-HA vaccine compared with 3 egg-based vaccines (standard-dose, high-dose, and adjuvanted IIV) in adults aged ≥65 years [18] and highly vaccinated active military personnel aged 18–64 years [19]. However, in the open-label immunogenicity trial during 2017–2018, RIV produced a greater cross-protective response against circulating and antigenically evolved 2019–2020 A(H3N2) vaccine strains [18]. A recent randomized head-to-head comparison trial of enhanced vaccines in Hong Kong in community-dwelling adults aged 65–82 years showed that quadrivalent RIV, trivalent, high-dose IIV, and adjuvanted IIV were more immunogenic than standard-dose quadrivalent IIV, including improved humoral and cell-mediated immunity response [20]. Repeated vaccination with egg-based vaccines has been shown to elicit and boost antigens to epitopes related to adaptation to growth in eggs that do not neutralize circulating viruses [21, 22]. For repeatedly vaccinated individuals, benefits of current-season influenza vaccines may include eliciting antibodies to novel epitopes on antigenically distinct or drifted circulating viruses and boosting cross-reactive antibodies recognizing conserved epitopes similar to prior vaccines or infections. Although this immunogenicity trial was conducted over 2 seasons with both unchanged and updated vaccine components, comparisons of immune responses to egg-based and non–egg-based vaccines may require longer studies and depend on antigenic differences among sequential vaccine viruses. In addition to comparing immunogenicity, randomized trials are needed to compare relative effectiveness of egg-based and non–egg-based vaccines against laboratory-confirmed influenza. Multiseason studies will be needed to assess relative effectiveness of combinations of egg and non-egg vaccines against influenza.
The protective role of neuraminidase (NA) antibodies in limiting viral replication, shedding, and severity of illness along with a slower rate of antigenic drift compared with HA makes it a possible vaccine target [23]. Each standard-dose vaccine (IIV and ccIIV) contains residual NA that is not standardized and may vary with each vaccine, whereas RIV lacks NA; there may be unmeasured immunogenicity and protection from the NA antigen in IIV and ccIIV. However, comparative effectiveness studies in US adults aged ≥65 years suggest RIV that lacks NA and contains 3 times as much recombinant HA as standard-dose IIV may be as or more effective than other NA-containing enhanced vaccines during recent influenza seasons [16, 24]. Multiseason comparative effectiveness studies of RIV vs NA-containing, enhanced vaccines are needed.
This study has several limitations. Antibody titers to influenza HA surface protein responsible for inhibition of hemagglutination of red blood cells are considered a surrogate immunological correlate of protection but do not always translate to vaccine effectiveness against laboratory-confirmed influenza [25, 26]. While antibody titers measured in HI assays correlate with viral microneutralization assays, immune responses should also be evaluated using microneutralization and other functional assays, as well as assessment of NA antibody and cell-mediated immune responses.
In the second year of this immunogenicity trial among highly vaccinated adult HCP, recombinant vaccine elicited higher antibody titers than egg-based or cell culture–based vaccines. Receipt of RIV in the preceding or current season elicited more robust antibody response against 3 of 4 vaccine components except A(H3N2). Whether this effect is explained by the 3-fold higher antigen content of recombinant vaccine compared with standard-dose vaccines or possibly due to differences in immune response mechanisms such as HA antigen recognition or activation by B cells requires further study. Repeat vaccination with non–egg-based vaccines in general may lead to higher antibody titers to cell-grown viruses that lack egg-adapted mutations and may represent circulating strains better than the viruses included in egg-based vaccines, although this may vary by vaccine formulation and influenza virus subtype. Additional longitudinal data about the relative immunogenicity and effectiveness of combinations of egg-based and non–egg-based vaccines are needed to inform vaccine recommendations for populations at increased risk for influenza morbidity or mortality such as pregnant persons, those aged ≥65 years, and with underlying asthma or diabetes. Adequately immunogenic and broadly protective, universal influenza vaccines are needed for protecting highly vaccinated HCP and high-risk populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Manjusha Gaglani, Department of Pediatrics, Baylor Scott & White Health, Temple, Texas, USA; Department of Medical Education, Texas A & M University College of Medicine, Temple, Texas, USA.
Sara S Kim, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Allison L Naleway, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, USA.
Min Z Levine, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Laura Edwards, Abt Associates, Atlanta, Georgia, USA.
Kempapura Murthy, Department of Pediatrics, Baylor Scott & White Health, Temple, Texas, USA.
Kayan Dunnigan, Department of Pediatrics, Baylor Scott & White Health, Temple, Texas, USA.
Tnelda Zunie, Department of Pediatrics, Baylor Scott & White Health, Temple, Texas, USA.
Holly Groom, Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon, USA.
Sarah Ball, Abt Associates, Atlanta, Georgia, USA.
Zuha Jeddy, Abt Associates, Atlanta, Georgia, USA.
Danielle Hunt, Abt Associates, Atlanta, Georgia, USA.
Meredith G Wesley, Abt Associates, Atlanta, Georgia, USA.
Suryaprakash Sambhara, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Shivaprakash Gangappa, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Lauren Grant, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Weiping Cao, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
F Liaini Gross, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Margarita Mishina, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Alicia M Fry, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Mark G Thompson, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Fatimah S Dawood, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Brendan Flannery, Influenza Division of the National Center for Immunization and Respiratory Diseases (NCIRD), Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Notes
Acknowledgments. For contributions to this study, the authors thank Judy Herrick, Patricia Sleeth, Mary Jo Koss, Barbara Hairston, Lydia Clipper, Michael Smith, Chandni Raiyani, Martha Zayed, Melissa Zdroik, Rinska Flores, Chengyu Wu, Marcelo Pando Rigal, Sheila Dobin, Natalie Settele, Jason Ettlinger, Jennifer Thomas, Muralidhar Jatla, Madhava Beeram, and Alejandro Arroliga with Baylor Scott & White Health and Kristi Bays, Kimberley Berame, Cathleen Bourdoin, Kenni Graham, Matt Hornbrook, Willa Jones, Dorothy Kurdyla, Mi Lee, Danielle Millay, Yolanda Prado, and Sperry Robinson with Kaiser Permanente Northwest. The authors also thank Nedzad Music and Giuseppe Palladino with Seqirus for providing the cell grown influenza B antigens used for hemagglutination inhibition (HI) testing and Stacie Jefferson, Centers for Disease Control and Prevention (CDC), for assistance with preparation of antigens used for HI testing.
Disclaimer. The findings and conclusions presented here are those of the authors and do not necessarily represent the views of the CDC.
Financial support . This study was funded by the CDC through contract 75D30118F02850 with Abt Associates, Inc. M. G. received support from CDC–Abt Associates (the BSWRI– HCP FluVax Trial). A. L. N. received support from CDC–Abt Associates (the KPNW FluVax Trial). L. E. received support from CDC–Abt Associates (the FluVax Trial). S. B. and Z. J. received support from the CDC (work performed under a contract with employer at the time, Abt Associates).
References
- 1. Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention . Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60(RR-7):1–45. [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Influenza vaccination coverage among health care personnel—United States, 2019–20 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/hcp-coverage_1920estimates.htm. Accessed 9 April 2022.
- 3. Centers for Disease Control and Prevention . Flu vaccination coverage, United States, 2019–20 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1920estimates. Accessed 9 April 2022.
- 4. Zost SJ, Parkhouse K, Gumina ME, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A 2017; 114:12578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gambaryan AS, Robertson JS, Matrosovich MN. Effects of egg-adaptation on the receptor-binding properties of human influenza A and B viruses. Virology 1999; 258:232–9. [DOI] [PubMed] [Google Scholar]
- 6. Xu X, Kilbourne ED, Hall HE, Cox NJ. Nonimmunoselected intrastrain genetic variation detected in pairs of high-yielding influenza A (H3N2) vaccine and parental viruses. J Infect Dis 1994; 170:1432–8. [DOI] [PubMed] [Google Scholar]
- 7. Raymond DD, Stewart SM, Lee J, et al. Influenza immunization elicits antibodies specific for an egg-adapted vaccine strain. Nat Med 2016; 22:1465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richards KA, Moritzky S, Shannon I, et al. Recombinant-HA-based vaccine outperforms split and subunit vaccines in elicitation of influenza-specific CD4 T cells and CD4 T cell-dependent antibody responses in humans. NPJ Vaccines 2020; 5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu ED, Grifoni A, Sutherland A, et al. Balanced cellular and humoral immune responses targeting multiple antigens in adults receiving a quadrivalent inactivated influenza vaccine. Vaccines (Basel) 2021; 9:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richards KA, Shannon I, Treanor JJ, Yang H, Nayak JL, Sant AJ. Evidence that blunted CD4 T-cell responses underlie deficient protective antibody responses to influenza vaccines in repeatedly vaccinated human subjects. J Infect Dis 2020; 222:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stacey HD, Miller MS. Repeated seasonal influenza vaccination: how much is too much of a good thing? J Infect Dis 2020; 222:173–5. [DOI] [PubMed] [Google Scholar]
- 12. Dawood FS, Naleway AL, Flannery B, et al. Comparison of the immunogenicity of cell culture-based and recombinant quadrivalent influenza vaccines to conventional egg-based quadrivalent influenza vaccines among healthcare personnel aged 18–64 seasons: a randomized open-label trial. Clin Infect Dis 2021; 73:1973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Manual for the laboratory diagnosis and virological surveillance of influenza. Available at: https://apps.who.int/iris/bitstream/handle/10665/44518/9789241548090_eng.pdf?sequence=1. Accessed 19 March 2022.
- 14. Levine MZ, Martin ET, Petrie JG, et al. Antibodies against egg- and cell-grown influenza A(H3N2) viruses in adults hospitalized during the 2017–2018 influenza season. J Infect Dis 2019; 219:1904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puig-Barberà J, Tamames-Gómez S, Plans-Rubio P, Eiros-Bouza JM. Relative effectiveness of cell-cultured versus egg-based seasonal influenza vaccines in preventing influenza-related outcomes in subjects 18 years old or older: a systematic review and meta-analysis. Int J Environ Res Public Health 2022; 19:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Izurieta HS, Lu M, Kelman J, et al. Comparative effectiveness of influenza vaccines among US Medicare beneficiaries ages 65 years and older during the 2019–2020 season. Clin Infect Dis 2021; 73:e4251–9. [DOI] [PubMed] [Google Scholar]
- 17. Klein NP, Fireman B, Goddard K, et al. Vaccine effectiveness of cell-culture relative to egg-based inactivated influenza vaccine during the 2017–18 influenza season. PLoS One 2020; 15:e0229279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belongia EA, Levine MZ, Olaiya O, et al. Clinical trial to assess immunogenicity of high-dose, adjuvanted, and recombinant influenza vaccines against cell-grown A(H3N2) viruses in adults 65 to 74 years, 2017–2018. Vaccine 2020; 38:3121–8. [DOI] [PubMed] [Google Scholar]
- 19. Wang W, Alvarado-Facundo E, Vassell R, et al. Comparison of A(H3N2) neutralizing antibody responses elicited by 2018–2019 season quadrivalent influenza vaccines derived from eggs, cells, and recombinant hemagglutinin. Clin Infect Dis 2021; 73:e4312–20. [DOI] [PubMed] [Google Scholar]
- 20. Cowling BJ, Perera RAPM, Valkenburg SA, et al. Comparative immunogenicity of several enhanced influenza vaccine options for older adults: a randomized, controlled trial. Clin Infect Dis 2020; 71:1704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu F, Gross FL, Jefferson SN, et al. Age-specific effects of vaccine egg adaptation and immune priming on A(H3N2) antibody responses following influenza vaccination. J Clin Invest 2021; 131:e146138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu F, Tzeng WP, Horner L, et al. Influence of immune priming and egg adaptation in the vaccine on antibody responses to circulating A(H1N1)pdm09 viruses after influenza vaccination in adults. J Infect Dis 2018; 218:1571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rajendran M, Krammer F, McMahon M. The human antibody response to the influenza virus neuraminidase following infection or vaccination. Vaccines (Basel) 2021; 9:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018–2019. J Infect Dis 2020; 222:278–87. [DOI] [PubMed] [Google Scholar]
- 25. Ward BJ, Pillet S, Charland N, Trepanier S, Couillard J, Landry N. The establishment of surrogates and correlates of protection: useful tools for the licensure of effective influenza vaccines? Hum Vaccin Immunother 2018; 14:647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trombetta CM, Montomoli E. Influenza immunology evaluation and correlates of protection: a focus on vaccines. Expert Rev Vaccines 2016; 15:967–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.